Abstract
Ca2+-triggered exocytosis in neurons and neuroendocrine cells is regulated by the Ca2+-binding protein synaptotagmin (syt) I. Sixteen additional isoforms of syt have been identified, but little is known concerning their biochemical or functional properties. Here, we assessed the abilities of fourteen syt isoforms to directly regulate SNARE (soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein receptor)-catalyzed membrane fusion. One group of isoforms stimulated neuronal SNARE-mediated fusion in response to Ca2+, while another set inhibited SNARE catalyzed fusion in both the absence and presence of Ca2+. Biochemical analysis revealed a strong correlation between the ability of syt isoforms to bind 1,2-dioleoyl phosphatidylserine (PS) and t-SNAREs in a Ca2+-promoted manner with their abilities to enhance fusion, further establishing PS and SNAREs as critical effectors for syt action. The ability of syt I to efficiently stimulate fusion was specific for certain SNARE pairs, suggesting that syts might contribute to the specificity of intracellular membrane fusion reactions. Finally, a subset of inhibitory syts down-regulated the ability of syt I to activate fusion, demonstrating that syt isoforms can modulate the function of each other.
Over the last decade significant progress has been made in defining the molecular machinery that mediates Ca2+-triggered exocytosis from neurons and neuroendocrine cells. A key discovery was the observation that the clostridial neurotoxins, botulinum (serotypes A-G) and tetanus toxin, block neurotransmitter and hormone release by cleaving a group of proteins called soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein receptors (SNAREs),4 revealing that SNAREs are required for secretion (1). SNAREs are conserved from yeast to humans (2), and are classified into two categories: vesicle SNAREs (v-SNAREs) and target membrane SNAREs (t-SNAREs). In the case of synaptic vesicle exocytosis, the v-SNARE is synaptobrevin (syb) 2 (also referred to as vesicle-associated membrane protein (VAMP) II) and the t-SNAREs are syntaxin 1A and SNAP-25B. These three SNARE proteins spontaneously assemble together into highly stable ternary complexes, suggesting that interactions between v- and t-SNAREs might link the vesicle and target membrane to drive fusion (reviewed in Ref. 2). Indeed, Weber et al. (3) reconstituted neuronal v-SNAREs and t-SNAREs into two populations of liposomes and utilized a fluorescence dequenching assay to demonstrate that the assembly of trans-SNARE pairs was in fact sufficient to drive membrane fusion in vitro. Hence, SNAREs have emerged as the core of a conserved membrane fusion machine.
The Ca2+-binding protein, synaptotagmin (syt) I, serves as a major Ca2+ sensor that regulates SNARE-mediated fusion (4-6). Syt I was identified as an abundant constituent of synaptic vesicles and large dense core vesicles (7, 8). Biochemical studies established that Ca2+ triggers the rapid penetration of two conserved Ca2+-binding motifs in the cytoplasmic domain of syt I, called C2 domains (Fig. 1A), into membranes that harbor anionic phospholipids (9-11). This interaction might help to draw the two bilayers together to facilitate fusion, or might buckle the plasma membrane toward the vesicle membrane, lowering the energy barrier for fusion (6, 12).
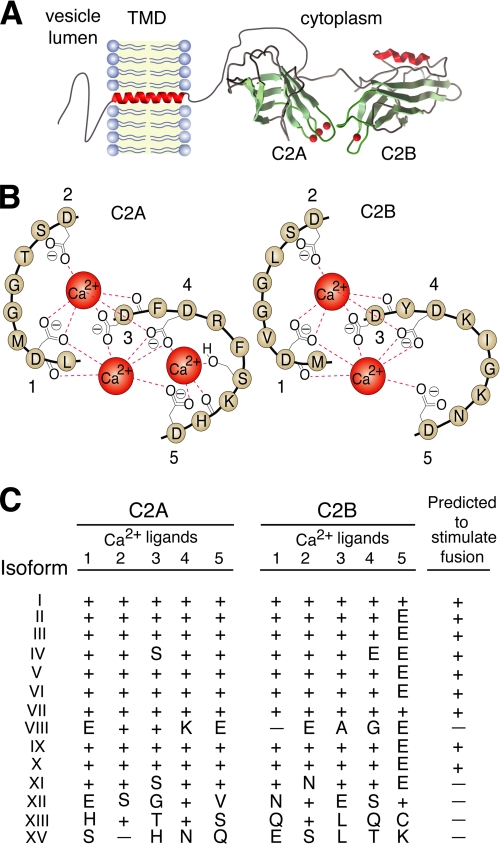
FIGURE 1.
Conservation of the putative Ca2+ ligands among different isoforms of syt. A, model depicting the structure of syt. The image was generated using the crystal structure of the cytoplasmic domain of syt III (37); remaining segments were added with a drawing program. B, schematic diagram showing the five Ca2+-coordinating residues in the C2A and C2B domains of syt I. The diagram is modified and reproduced with permission from the authors of Ref. 51. C, conservation among the Ca2+ ligands in the C2A and C2B domains of syt I-XIII and XV. Numbers correspond to the order of the five aspartate residues that function as Ca2+ ligands in the C2A domain of syt I (D172, 178, 230, 232, 238) and C2B (D303, 309, 363, 365, 371) (6). Single letter codes indicate substitutions of conserved aspartate residues; (+) denotes conservation of an aspartate, and (-) indicates a gap. Based on conservation of the Ca2+ ligands, we predicted which syts might (+) and might not (-) stimulate in vitro SNARE-mediated fusion in response to Ca2+.
Syt I also binds to the t-SNAREs syntaxin 1A (13) and SNAP-25B (14), at all stages of SNARE complex assembly, and these interactions are promoted by Ca2+ on very fast time scales (15). These findings established a biochemical link between the putative Ca2+ sensor and the fusion machinery. Biochemical experiments also revealed that Ca2+·syt I can drive the folding of SNARE proteins into functional complexes (16), and cell-based experiments support the idea that syt operates, in part, via direct interactions with SNAREs (15, 17-19). However, another group has concluded that Ca2+-regulated interactions between syt I (20, 21) and IX (22, 23) with SNARE proteins does not play a role in excitation-secretion coupling, and this issue remains a subject of debate.
Genetic studies support the idea that syt I plays a critical role in triggering rapid exocytosis (5, 6). Direct support for this model stems from the finding that syt I imparts Ca2+ sensitivity to SNARE-catalyzed membrane fusion reactions that have been reconstituted in vitro (24). In this reduced system, both phosphatidylserine (PS) and t-SNAREs were critical effectors for the action of syt I (16, 25). While these findings have helped to provide a general understanding of how syt I might function to regulate exocytosis, its detailed mechanism of action is still far from understood. Moreover, sixteen additional isoforms of syt have been identified (26), and little is known concerning the functional and biochemical properties for many isoforms of syt. A number of studies have shown that a subset of syt isoforms bind PS-containing liposomes in a Ca2+-promoted manner (27, 28) and that some isoforms of syt interact with the syx-SNAP-25 t-SNARE heterodimer in the absence of Ca2+ (27). However, a comprehensive analysis of whether or not all isoforms of syt bind membrane-embedded t-SNAREs in a Ca2+-promoted manner has not been undertaken. Moreover, the functional properties of most isoforms of syt remain unknown, and it is unclear if all syt isoforms function as Ca2+ sensors for SNARE-catalyzed membrane fusion, in a manner similar to syt I, or whether some isoforms display unique Ca2+-independent functions.
Previously, we found that two other isoforms, syt VII and syt IX, also stimulated SNARE-catalyzed membrane fusion in response to Ca2+, but with markedly different [Ca2+]½ values (25). Hence, different isoforms of syt might underlie, at least in part, the distinct Ca2+ requirements for neurotransmitter release observed for different cell types (4). In addition, all isoforms of syt might not act to stimulate membrane fusion or secretion. For example, syt IV, an isoform that is up-regulated in response to activity (29), does not engage in any known Ca2+-dependent effector interactions (30, 31), and down-regulates large dense core exocytosis in PC12 cells (31-33).
Here, we examined the functional and biochemical properties of fourteen isoforms of syt. One group of syts bound to PS and target membrane SNAREs in a Ca2+-promoted manner and activated fusion. A second group of syts failed to respond to Ca2+ but were able to inhibit SNARE-mediated fusion. Furthermore, a subset of this latter group of syts reduced the ability of syt I to activate fusion by competing for syt-SNARE interactions, providing evidence that syts can modulate the function of one another. Finally, the ability of syt I to efficiently stimulate fusion was specific for certain SNARE pairs, suggesting that syts might contribute to the specificity of intracellular membrane fusion reactions.
EXPERIMENTAL PROCEDURES
Plasmids and Protein Purification—For all experiments, the cytoplasmic domain of the indicated syt isoform was used. cDNA encoding syts I-XII are described in Ref. 28, and cDNA encoding the cytoplasmic domains of syt XIII and XV were provided by M. Craxton (Laboratory of Molecular Biology, Cambridge, UK). The amino acid residues encoding these cytoplasmic domains (C2A-C2B) are as follows: I, 96-421; II, 139-423; III, 290-569; IV, 152-425; V, 218-491; VI, 143-426; VII, 134-403; VIII, 97-395; IX, 104-386; X, 223-501; XI, 150-430; XII, 114-421; XIII, 158-427; XV, 139-421. Each isoform was subcloned into either pTrc-HisA (Invitrogen) or pET28a (Novagen).
Syts were expressed in Escherichia coli and purified as previously described (25). Briefly, bacterial pellets were resuspended in 25 mm HEPES-KOH, 400 mm KCl, 20 mm imidazole, and 5 mm β-mercaptoethanol, and then lysed by sonication followed by addition of Triton X-100 (final 2% v/v). Bacteria were pelleted, and extracts were mixed with Ni-nitrilotriacetic acid-agarose (Qiagen) for 2 h at 4 °C. Beads were washed two times in wash buffer (25 mm HEPES-KOH, 400 mm KCl, 20 mm imidazole, and 5 mm β-mercaptoethanol, 1 mm MgCl2) plus 10 μg/ml DNase and RNase (Roche Applied Science) to remove any bound RNA/DNA. Two more washes were carried out in the resuspension buffer. Proteins were eluted from beads in the resuspension buffer with 500 mm imidazole and 10% glycerol (w/v) and dialyzed overnight against 25 mm HEPES pH 7.4, 200 mm KCl, 10% glycerol (w/v), 1 mm dithiothreitol (Buffer A). Because of technical difficulties, we were unable to express and purify appropriate quantities of recombinant syt XIV and XVI from E. coli.
Plasmids to generate recombinant full-length synaptobrevin 2 and the full-length t-SNARE heterodimer (syntaxin 1A and SNAP-25B), were provided by J. E. Rothman (Columbia University), and proteins were expressed and purified as described (3, 24). cDNA encoding full-length rat syntaxin 1A and syntaxin 4 were provided by R. H. Scheller (Genentech, San Francisco, CA) and J. M. Edwardson (Cambridge, UK), respectively. cDNA encoding full-length SNAP-25B was provided by M. C. Wilson (University of New Mexico). cDNA encoding SNAP-23 and SNAP-29 were provided by R. Jahn (Max Planck Institute, Gottingen, Germany). The following t-SNARE heterodimer pairs were subcloned into pRSF-Duet (Novagen) and expressed as above: syntaxin1A/SNAP-23, syntaxin1A/SNAP-29, syntaxin4/SNAP-23, and syntaxin4/SNAP-25B. Primary references for syb 2, SNAP-25B, and syntaxin 1A can be found in Ref. 16.
Preparation of Protein-free and SNARE-bearing Vesicles—All lipids were obtained from Avanti Polar Lipids (Alabaster, AL). Reconstitution of v-SNARE and t-SNARE vesicles was carried out as previously described (3, 24). v-SNAREs were reconstituted using a lipid mix composed of 30% 1-palmitoyl, 2-oleoyl phosphatidylethanolamine (PE), 52% 1-palmitoyl, 2-oleoyl phosphatidylcholine (PC), 15% 1,2-dioleoyl phosphatidylserine (PS), 1.5% N-(7-nitro-2-1,3-benzoxadiazol-4-yl)-1,2-dipalmitoyl phosphatidylethanolamine (NBD-PE, donor), and 1.5% N-(lissamine rhodamine B sulfonyl)-1,2-dipalmitoyl phosphatidylethanolamine (Rhodamine-PE, acceptor). t-SNAREs were reconstituted in 30% PE, 55% PC, and 15% PS (mol mol-1). When PS was omitted, PC was increased to 70% for the t-SNARE vesicles. v-SNARE and t-SNARE vesicles were reconstituted to give ∼100 copies or ∼80 copies per vesicle, respectively, as described (24). At ∼80 copies/vesicle, the t-SNARE concentration in the fusion assay is ∼3 μm. Protein-free (pf) vesicles were prepared as described previously (24).
Flotation Assays—Flotation assays were carried out as described previously with modifications (24, 25). Briefly, pf vesicles with (30% PE/15% PS/55% PC) or without (30% PE/70% PC) PS, or t-SNARE vesicles (30% PE/70% PC), were mixed with syt (10 μm). All binding reactions were carried out in a total volume of 100 μl of Buffer A with either 0.2 mm EGTA or 1 mm Ca2+. Samples were incubated with shaking for 1 h at room temperature and were then mixed with an equal volume of 80% Accudenz medium, transferred to a Beckman ultracentrifuge tube and layered with 150 μl each of 35 and 30% Accudenz media in Buffer A. Finally, samples were layered with 20 μl of Buffer A lacking glycerol and centrifuged at 280,000 × g for 2.5 h at 4 °C. All buffers/media contained either 0.2 mm EGTA or 1 mm Ca2+.40 μl of vesicles from the 0%/30% interface were collected from each tube, and one-third of the collected sample was resolved by SDS-PAGE and stained with Coomassie Blue. All gels contain standards (stds) that serve as references for the electrophoretic mobility of the proteins used in the flotation assays. For competition experiments in Fig. 6, the indicated concentrations of syt IV, VIII, and XII were included in reactions along with 10 μm syt I. All Coomassie-stained gels are representative examples from ≥3 trials.
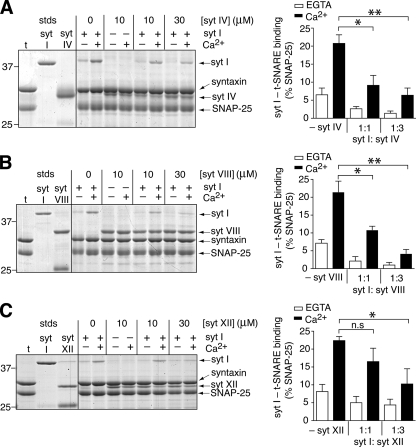
FIGURE 6.
Syt isoforms compete with one another for binding to t-SNAREs. A, left, binding of syt I and IV to t-SNARE (t) vesicles was carried out in the absence (-) or presence (+) of Ca2+. Syts were assayed individually and in the indicated combinations using 10 μm each syt, or 10 μm syt I and 30 μm syt IV (representing a 1:1 and 1:3 molar ratio of syt I:competitor syt, respectively). One-third of each sample was subjected to SDS-PAGE; gels were stained with Coomassie Blue. Right, the amount of syt I bound to t-SNAREs was quantified by densitometry, normalized to the SNAP-25 band in each lane, and plotted as a percentage of SNAP-25. Data represent the average and S.E. from n ≥ 3. B, experiments were carried out as in panel A, except syt VIII was used in place of syt IV. C, experiments were carried out as in panel A, except syt XII was used in place of syt IV. Statistical analysis was carried out using a Student's t test; *, p < 0.05; **, p < 0.01; n.s. (not significant) p > 0.05. All gels are representative from ≥3 trials. In panel A only, the line on the gel (left panel) separates groups of lanes that were originally spaced further apart on the same gel and were combined for the figure.
Fusion Assays—Each reaction consisted of 45 μl of purified t-SNARE vesicles (30% PE/15% PS/55% PC) and 5 μl of purified, v-SNARE vesicles (30% PE/1.5% NBD-PE/1.5% Rhodamine-PE/15% PS/52% PC) plus 0.2 mm EGTA or 1 mm Ca2+ in a total volume of 75 μl of Buffer A. 10 μm syt was added where indicated. For competition experiments in Fig. 5, the indicated concentrations of syt IV, VIII, and XII were included in reactions along with 10 μm syt I. Fusion reactions were carried out in 96-well plates, and NBD fluorescence was monitored at 37 °C in a plate reader (Biotek Instruments; excitation 460 nm, emission 538 nm). After 2 h, 0.5% n-dodecylmaltoside (w/v, Roche Applied Science) was added to maximally dequench the NBD fluorescence. Raw fluorescence was normalized to obtain % maximum fluorescence as described (24, 34). Error bars represent S.E. from n ≥ 3. Percent stimulation in Ca2+ in Fig. 3B was calculated by Equation 1.
 |
(Eq.1) |
Percent stimulation over (-)syt was calculated by Equation 2.
 |
(Eq.2) |
FIGURE 5.
Syts IV and VIII, but not XII, inhibit syt I-mediated stimulation of membrane fusion. Syt I (10 μm) stimulates fusion between v- and t-SNARE vesicles in response to Ca2+ (panels A, C, and E, red trace). A, increasing concentrations of syt IV were added to fusion reactions containing 10 μm syt I and 1 mm Ca2+. Fusion was monitored for 120 min at 37 °C and plotted as % max Fl over time. B, average extent of fusion from the data in panel A (% max Fl) at t = 120 min was plotted as a function of [syt IV]. C, experiments were carried out as in panel A except syt VIII was used in place of syt IV. D, data from panel C were plotted as a function of [syt VIII]. E, experiments were carried out as in panel A except syt XII was used in place of syt IV. F, data from E were plotted as a function of [syt XII]. In all plots, error bars represent S.E. (n = 3).
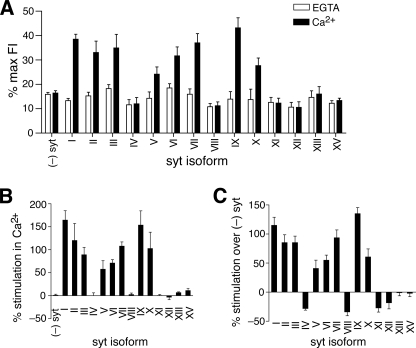
FIGURE 3.
Syt isoforms differentially regulate neuronal SNARE-catalyzed membrane fusion. A, average extent of fusion (% max FI) at t = 120 min, in either 0.2 mm EGTA (open bars) or 1 mm Ca2+ (filled bars), was plotted for each syt isoform. (-)syt indicates fusion between v- and t-SNARE vesicles in the absence of syt. The data plotted here are averages across all trials. Error bars represent S.E. (n ≥ 3). B, average % stimulation in Ca2+ (fusion in Ca2+ compared with fusion in EGTA within each individual trial and then averaged; Equation 1 in “;Experimental Procedures”) was plotted for v- and t-SNARE-mediated fusion and for each syt isoform. Error bars represent S.E. (n ≥ 3). C, average % stimulation over (-)syt (fusion by Ca2+·syt compared with fusion mediated by SNAREs in the absence of syt within each individual trial and then averaged; Equation 2 in “;Experimental Procedures”) was plotted for each syt isoform. Error bars represent S.E. (n ≥ 3).
RESULTS
Conservation of the Ca2+ Ligands in the C2A and C2B Domains of Syt—To date, seventeen isoforms of syt have been identified in vertebrates (26). All members of this protein family share similar overall domain structures (shown in Fig. 1A); the cytoplasmic domains are comprised of tandem C2 domains (C2A and C2B) that are tethered together by a short linker (Fig. 1A) (35-37). C2 domains, first identified in protein kinase C, are now found in a variety of proteins where they often, but not always, mediate binding to Ca2+ and phospholipids (38). In syt I, both C2 domains mediate Ca2+-regulated interactions with anionic phospholipids and t-SNAREs (6). However, the five aspartic acid residues that coordinate Ca2+ ions in each C2 domain of syt I (Fig. 1B) are conserved in only a subset of isoforms (Fig. 1C, see also Refs. 27, 39). The strict conservation of these Ca2+ ligands in syt I-VII and IX-X suggest that these isoforms bind Ca2+ and therefore might be able to stimulate fusion mediated by neuronal SNARE proteins. In contrast, syt VIII, XI-XIII, and XV harbor substitutions that are expected to disrupt Ca2+ binding activity in both C2 domains, suggesting that these isoforms would be incapable of coupling Ca2+ to fusion.
Divergent Activities of syt Isoforms during Neuronal SNARE-mediated Membrane Fusion—In vitro studies revealed that in response to Ca2+, syt I stimulates SNARE-catalyzed membrane fusion (12, 24), providing direct evidence that syt I can act as a Ca2+ sensor for exocytosis in vivo (4-6). More recently, this observation was extended to two other syt family members, syt VII and IX (25). In contrast, syt IV failed to stimulate membrane fusion in reconstituted fusion assays (12), consistent with its putative role as a negative regulator of secretion from neuroendocrine cells (31-33). However, the ability of the other thirteen isoforms of syt to regulate membrane fusion remained unknown.
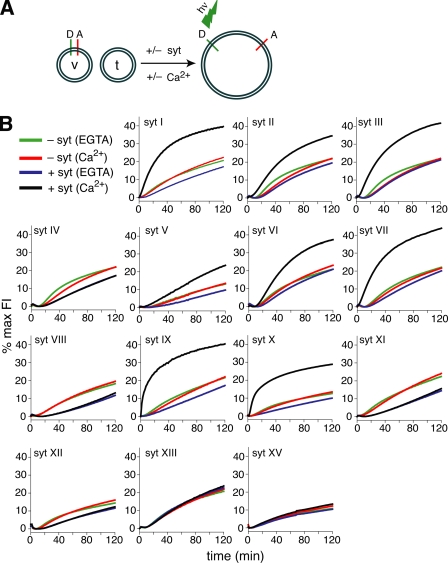
Using the in vitro membrane fusion assay, we screened fourteen syt isoforms for their abilities to regulate fusion between v-SNARE vesicles (v), that harbor synaptobrevin 2, and t-SNARE vesicles (t), that harbor syntaxin 1A/SNAP-25B heterodimers (Fig. 2A). Raw fusion data are shown in Fig. 2B; only a subset of syt isoforms stimulated SNARE-catalyzed fusion in the presence of Ca2+: syt I-III, V-VII, and IX-X. This group of syts corresponded to the isoforms that were predicted to serve as Ca2+ sensors (Fig. 1C), with the exception of syt IV, which has one Asp to Ser substitution in the C2A domain but harbors an intact set of putative Ca2+ ligands in C2B. These data were quantified in Fig. 3A, where the average extent of fusion (% maximum fluorescence intensity), from all trials was plotted for each syt isoform.
FIGURE 2.
Normalized fusion data from fourteen isoforms of syt. A, illustration depicting the in vitro fusion assay used in this study. Fusion of v-SNARE (syb; v) vesicles, containing a donor (D) and acceptor (A) FRET pair, with unlabeled t-SNARE vesicles (syntaxin 1A/SNAP-25B; t), results in dilution of the FRET pair and an increase in donor fluorescence. B, syt isoforms differentially regulate fusion between v- and t-SNARE vesicles. Fusion was monitored for 120 min at 37 °C in the absence (-syt) or presence (+syt, 10 μm) of different syt isoforms, normalized to the maximum donor fluorescence signal (% max Fl), and plotted as a function of time. Reactions were carried out in 0.2 mm EGTA or 1 mm Ca2+. Representative traces for the different syt isoforms are shown from ≥3 independent trials.
To better visualize the impact of syt isoforms on fusion and to highlight differences that might have been obscured by averaging across all trials (because of sample-to-sample differences in the overall extent of fusion), we compared the extent of fusion obtained in Ca2+ to the extent of fusion obtained in EGTA within each individual trial (Fig. 3B, Equation 1 under “;Experimental Procedures”), then averaged the data. In addition, we compared the extent of fusion obtained by Ca2+·syt to fusion mediated by v- and t-SNAREs in the absence of syt (Fig. 3C, Equation 2 under “;Experimental Procedures”), again within each individual trial, and then calculated the average values. The former plot shows the range of stimulation mediated by syts under our experimental conditions, whereas the latter plot indicates the extent to which each syt isoform was able to stimulate or suppress fusion mediated by SNAREs themselves. A key finding was that syts IV, VIII, XI, and XII were unable to respond to Ca2+, but were able to inhibit fusion mediated by SNAREs alone (Fig. 3C).
Stimulation of Membrane Fusion Is Related to the Ability of syts to Engage PS and t-SNAREs in Response to Ca2+—In response to Ca2+, the tandem C2 domains of syt I engage t-SNAREs and the anionic phospholipid PS, and previous studies indicate that these interactions are critical for the regulation of membrane fusion (15, 16, 18, 24, 25). However, others have concluded that syt I operates independently of its Ca2+-promoted interactions with SNAREs (20, 21), and that syt IX functions without binding SNAREs at all (22, 23). To further explore the mechanism of action of syts, we carried out experiments to relate their activity in the fusion assay with their abilities to engage both PS and t-SNAREs.
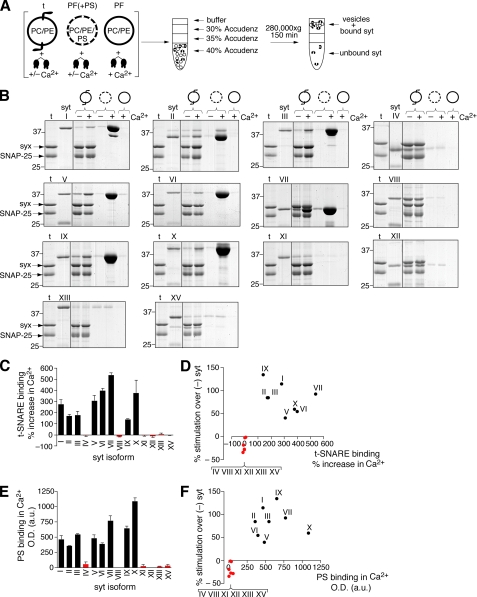
We utilized a co-flotation assay (Fig. 4A and Ref. 24) to determine which of the syt isoforms that had been screened in the fusion assay interact with full-length, membrane-embedded, syntaxin 1A/SNAP-25B heterodimers, and PS. All of the syt isoforms tested exhibited some degree of t-SNARE and PS binding activity in the absence of Ca2+ (Fig. 4, B, C, and E). However, only the stimulatory syts I-III, V-VII, and IX-X bound t-SNAREs and PS in a Ca2+-promoted manner (Fig. 4B). The syts that failed to stimulate fusion, isoforms IV, VIII, XI, XII, XIII, and XV, also bound t-SNAREs and PS, but these interactions were not enhanced by Ca2+. The t-SNARE binding data are in agreement with previously observed results for syts I, VII, and IX (25) (but see also Ref. 22), and the PS binding data are in agreement with a study focused on syts I-XII (28). Protein-free PC/PE vesicles were used as a negative control; no binding of any isoform of syt to these vesicles was detected, indicating syt·lipid interactions are specific for PS under our experimental conditions.
FIGURE 4.
Syts differ in their ability to bind PS and t-SNAREs. A, diagram of the flotation assay used to monitor binding of the different syt isoforms to t-SNARE vesicles (t), or protein free vesicles, with (PF(+PS)) or without PS (PF). Syt will float to the top of the gradient only if it interacts with vesicles. After centrifugation, samples are collected from the top of the gradient and analyzed by SDS-PAGE; proteins are visualized by staining with Coomassie Blue. B, flotation assays were carried out as depicted in panel A. Binding was monitored in either 0.2 mm EGTA (-) or 1 mm Ca2+(+). A representative gel from three independent trials is shown for each syt isoform. For the syt IV gel only, a line was added between the t-SNARE and syt IV standards to indicate lanes that were originally spaced further apart on the same gel and were combined for this figure. C, amount of syt that co-floated with t-SNARE vesicles in 0.2 mm EGTA or 1 mm Ca2+ from panel B was quantified using densitometry and normalized to the syntaxin band in each lane. The average % increase in t-SNARE binding in the presence of Ca2+ versus EGTA was calculated using normalized data and plotted for each isoform. Error bars represent S.E. (n = 3). In panels C-F, red is used to denote the inhibitory syts indicated in Fig. 3C. D, for each syt isoform, % stimulation over syt (from Fig. 3C) was plotted against the average % increase in t-SNARE binding for each syt isoform from panel C. E, the amount of syt bound to PS-harboring vesicles in 1 mmCa2+ from panel B was quantified using densitometry. The average total optical density is plotted for each isoform (because there is no other band in each lane against which the syt signal can be normalized as in panel C). Error bars represent S.E. (n ≥ 3). F, % stimulation over (-)syt (from Fig. 3C) was plotted against the average amount of syt bound to PS-harboring vesicles in 1 mm Ca2+ from panel E.
The extent to which syt associated with t-SNARE vesicles in EGTA or Ca2+ was quantified using densitometry and normalized to the syntaxin band in the corresponding lane; the % change in t-SNARE binding in response to Ca2+ was plotted (Fig. 4C). The amount of syt that bound to PS-containing vesicles in the presence of Ca2+ was also plotted (Fig. 4E). These two plots (Fig. 4, C and E) exhibited similar patterns; syts that bind PS in response to Ca2+ also bind t-SNAREs in response to Ca2+, and vice versa. These findings agree with the notion that syt acts by engaging membranes and SNAREs at the same time and that these interactions are coupled (10, 16).
We then plotted the effect syts had on SNARE-mediated fusion (% stimulation over (-)syt from Fig. 3C) against the % increase in Ca2+-promoted binding to t-SNAREs (Fig. 4D) and against the extent of PS binding in the presence of Ca2+ (Fig. 4F). These plots illustrate a strong correlation between the ability of a syt isoform to bind both of these effectors in a Ca2+-dependent manner with their abilities to stimulate SNARE-catalyzed fusion in response to Ca2+. Similarly, there is a strong correlation between syts that interact with t-SNAREs and PS in a Ca2+-independent manner with their abilities to inhibit SNARE-catalyzed fusion. In no case did a syt isoform stimulate fusion without binding to PS and t-SNAREs in a Ca2+-promoted manner, supporting a model in which syts stimulate fusion through Ca2+-dependent interactions with both of these effectors.
Syt Isoforms Can Modulate the Activity of Each Other—In the course of screening syts I-XIII and XV, we identified a class of syts (IV, VIII, XI, and XII) that failed to respond to Ca2+ in our biochemical assays and that inhibited SNARE-catalyzed fusion (Fig. 3C). These findings prompted the idea that these inhibitory syts might antagonize the action of syt isoforms that stimulate fusion in response to Ca2+ (e.g. see Ref. 33). To test this idea, we asked whether a subset of inhibitory syts could affect the ability of syt I to stimulate SNARE-catalyzed membrane fusion. We fixed the amount of syt I in the fusion assay and titrated increasing amounts of syts IV (Fig. 5, A and B), VIII (Fig. 5, C and D), and XII (Fig. 5, E and F). Syt IV and VIII inhibited syt-stimulated fusion in a dose-dependent manner, whereas syt XII had no appreciable effect over the range of concentrations tested. We note that higher concentrations of syt IV, VIII, and XII could not be tested because of low protein yields and volume constraints of the fusion assay. Syt XI, another inhibitory syt, behaved in a similar manner to syt IV and VIII, i.e. it inhibited syt I-stimulated fusion in a dose-dependent manner.5 Because syt I and IV have been co-localized to large dense core vesicles (LDCVs) in PC12 cells (29, 33), these findings support the idea that different syt isoforms on the same secretory organelle might serve to fine-tune the efficiency of exocytosis.
Next, we found that syts IV and VIII significantly reduced Ca2+-promoted binding of syt I to t-SNAREs (Fig. 6, A and B, left and right panels). In contrast, syt XII, the inhibitory syt that failed to suppress syt I-stimulated fusion, significantly reduced syt I·t-SNARE interactions only when added to the assay in a 3-fold molar excess of syt I (Fig. 6C, left and right panels). Thus, it appears that inhibitory isoforms of syt can impact fusion in two ways: first, they directly inhibit SNARE-mediated fusion, and second, they displace syt I from the fusion complex. These properties are clearly apparent for syt IV and VIII; syt XII differs to some extent as this isoform exhibits less inhibitory activity in terms of both shutting down SNARE function and displacing syt I. Moreover, these experiments provide further support for the idea that syts act, at least in part, via direct interactions with t-SNAREs to regulate fusion.
Specificity of Functional Pairing between syts and t-SNAREs during Membrane Fusion—At present, approximately thirty-six SNARE proteins have been identified in humans (2). Different SNAREs are targeted to distinct subcellular compartments where they catalyze many, and perhaps all, intracellular membrane fusion events (40). It was proposed that pairing between specific v- and t-SNAREs encodes the specificity of intracellular membrane fusion reactions (41). While SNARE pairing clearly plays a central role in the specificity of fusion, it is now established that other mechanisms also come into play (42).
Utilizing a subset of the plasma membrane SNAREs (SNAP-25B, SNAP-23, syntaxin 1A, and syntaxin 4) and a ubiquitously expressed SNARE (SNAP-29), we asked whether syt·t-SNARE pairing might also contribute to the specificity of membrane fusion reactions. First, we observed that the extent of fusion between syb 2 vesicles and target vesicles that harbored distinct sets of t-SNAREs differed for some t-SNARE pairs. Namely, syntaxin 1A/SNAP-29 and syntaxin 4/SNAP-25B did not fuse as efficiently as did syntaxin 1A/SNAP-25B, syntaxin 1A/SNAP-23, or syntaxin 4/SNAP-23 vesicles, all of which exhibited similar levels of fusion (Fig. 7, red and green traces).
FIGURE 7.
Specificity of functional pairing between syt I and t-SNAREs. Fusion reactions were carried out as described in the legend to Fig. 2A using t-SNARE vesicles that harbored the indicated t-SNARE pairs and v-SNARE vesicles than harbored syb 2. Fusion was monitored in the absence (-syt) or presence (+syt) of 10 μm syt I in either 0.2 mm EGTA or 1 mm Ca2+ and plotted as the % max fluorescence intensity over time. Representative traces from three independent trials are shown.
We then tested the ability of syt I to stimulate fusion mediated by these different SNARE pairs. We found that syt I stimulated fusion whenever syntaxin 1A or SNAP-25B were present on the t-SNARE vesicle, but failed to efficiently stimulate fusion when both syntaxin 1A and SNAP-25B had been replaced with syntaxin 4 and SNAP-23 (see also Ref. 43). In addition, Ca2+·syt was able to activate otherwise weak SNARE-mediated fusion reactions (e.g. syntaxin 1A/SNAP-29 and syntaxin 4/SNAP-25B t-SNARE vesicles) by severalfold (Fig. 7). Moreover, syt I had only a slight effect on fusion between syb 2 vesicles and syntaxin 4/SNAP-23 vesicles even though this particular SNARE pair catalyzed fusion in a manner that was similar to reactions mediated by syntaxin 1A/SNAP-25B and syntaxin 1A/SNAP-23. Thus, syt I does not stimulate all SNARE-mediated fusion reactions equally, but rather, discriminates between t-SNAREs, potentially adding a level of specificity to SNARE-mediated fusion reactions.
DISCUSSION
Previous studies characterized the effect of syts I, IV, VII, and IX on fusion mediated by neuronal SNAREs (12, 24, 25). In the current study we extended this analysis to include fourteen isoforms of syt. Most of these isoforms inhibited fusion, to a limited extent under our experimental conditions, in the absence of Ca2+ (e.g. syt I; see also Ref. 24). We note that all isoforms of syt tested bound t-SNAREs to some extent in EGTA. These Ca2+-independent syt-t-SNARE interactions may function by halting SNARE complex assembly and inhibiting fusion in the absence of Ca2+. Syts I-III, V-VII, IX, and X stimulated fusion in response to Ca2+, whereas syts IV, VIII, XI-XIII, and XV failed to facilitate fusion in the presence or absence of Ca2+. Furthermore, some of the syts that failed to stimulate fusion actually inhibited fusion (Figs. 2 and 3). Without exception, stimulatory syts bound to PS and t-SNAREs in response to Ca2+ whereas inhibitory syts did not (Fig. 4). Moreover, a subset of inhibitory syts reduced the extent of syt I-stimulated fusion. These data indicate that the presence of different isoforms of syts on the same secretory organelle might fine-tune the efficiency (Fig. 5), Ca2+-sensitivity (25), and mode of exocytosis (31) by competing for interactions with effectors. Below, we relate these in vitro findings with studies aimed at understanding the function of syt isoforms in cells.
A recent flurry of reports addressed the role of syt II, which is highly homologous to syt I, during exocytosis at synapses. In specific subsets of neurons, syt II plays a critical role in Ca2+-triggered neurotransmitter release (23, 44-46) and can restore rapid release when expressed in syt I knock-out cortical neurons (23). However, the mechanism by which syt II functions had not been explored in detail. Syt II binds PS in response to Ca2+ and here we show that this isoform also interacts with the t-SNAREs syntaxin 1A and SNAP-25B in a Ca2+-promoted manner, and stimulates SNARE-catalyzed fusion. So, as expected, syt II likely shares the same mechanism of action as syt I (27, 46).
It was previously reported that syt IX does not bind to neuronal t-SNAREs (22) but is able to rescue fast neurotransmitter release in syt I knock-out neurons (23). Together, these studies would seem to rule out both Ca2+-independent and Ca2+-dependent syt-SNARE interactions during exocytosis. However, another study employing three different kinds of binding assays demonstrated that syt IX does in fact bind neuronal t-SNAREs in a Ca2+-promoted manner (25). Here, we confirm these observations, as well as the fact that syt IX can directly stimulate SNARE-catalyzed fusion in response to Ca2+. So, analogous to syts I and II, syt IX is likely to regulate membrane fusion via Ca2+-promoted interactions with both PS and t-SNAREs.
Syt IV, a seizure-induced isoform with a single Asp to Ser substitution of one of the Ca2+ ligands in the C2A domain (29) is unable to bind PS and t-SNAREs in a Ca2+-dependent manner (Fig. 4, B, C, and E and Refs. 30, 31). Syt I and IV are co-localized to large dense core vesicles in PC12 cells, where up-regulation of syt IV was shown to decrease the frequency of large dense core vesicle membrane fusion events and to destabilize fusion pores (31, 33). Syt IV also favored the formation of tiny fusion pores, with one-fifth the transmitter flux of full-sized kiss-and-run fusion pores, that always close without dilating (31, 33). So, syt IV negatively regulates the rate of exocytosis and also affects fusion pore structure and dynamics. The inhibitory role of syt IV is important in neurons as well. Activity-induced up-regulation of syt IV negatively regulates the release of neurotrophic factors from dense core vesicles, thereby regulating synaptic plasticity. Up-regulation of syt IV might act homeostatically to dampen overactive circuits.6 Here, we demonstrate that syt IV itself inhibits SNARE-catalyzed membrane fusion (Figs. 2 and 3; see also Ref. 12). We also show that syt IV down-regulates Ca2+·syt I-stimulated membrane fusion in our reduced assay system (Fig. 5, A and B), in part by competing with syt I for interactions with t-SNAREs (Fig. 6A). Therefore, using a reconstituted system, we have recapitulated the negative regulatory role syt IV plays during dense core vesicle exocytosis. It should be noted that syt IV might play a positive role during other kinds of fusion events. For example, syt IV appears to be required for homotypic fusion of immature secretory granules (ISGs) in PC12 cells (47), perhaps by operating on a distinct set of SNAREs. Syt IV was also reported to play a positive role in secretion from glial cells (48).
Syt IV (29) and syt X (49) are of particular interest because their expression levels are induced by activity. Interestingly, these two isoforms had opposite effects in the in vitro fusion assay; syt IV inhibited SNARE-catalyzed fusion, whereas syt X stimulated fusion (Figs. 2 and 3). These two isoforms might be expressed in distinct regions of the brain. For example, in situ hybridization experiments in mouse brain have shown that the syt IV and X mRNA signals are very strong in the hippocampus and dentate gyrus, respectively, but show little overlap within these regions (Allen Brain Atlas). On the other hand, the syt I mRNA signal overlaps significantly with both the syt IV and syt X mRNA signals. A detailed analysis of the tissue distribution and subcellular localization of syt IV and X will provide insight into why these two activity-induced isoforms have such different effects on fusion mediated by neuronal SNARE proteins. One possibility is that syt X is preferentially expressed in inhibitory neurons and facilitates inhibitory synaptic transmission. In this scenario, up-regulation of syt X could serve to dampen overactive neuronal circuits. Finally, it was reported that syt XII was expressed on synaptic vesicles but failed to co-immunoprecipitate with neuronal t-SNAREs (50). In contrast, our co-flotation assays clearly demonstrate significant levels of Ca2+-independent binding of syt XII to membrane-embedded t-SNAREs (Fig. 4). Failure to detect binding by Maximov et al. (50) could be because of differences in experimental conditions; i.e. using purified components as opposed to co-immunoprecipitation from brain extracts where the low abundance of syt XII might fall below the limits of detection. If syt I and XII are indeed expressed on the same synaptic vesicles at similar levels, our results indicate that syt XII might not be able to modulate the function of syt I during synaptic transmission.
In conclusion, we have shown that the ability of syts to mediate Ca2+-triggered membrane fusion is strictly related to their abilities to engage PS and t-SNAREs in a Ca2+-dependent manner. We also identified a subset of inhibitory syts that antagonized syt I function during fusion by competing for syt·SNARE interactions, further establishing t-SNAREs as critical syt effectors. Finally, we further confirm that syt I is selective for certain SNARE pairs over others (Fig. 7 and Refs. 16, 43), supporting the hypothesis that during intracellular membrane transport and exocytosis, the action of syts on specific t-SNAREs might contribute to the specificity of SNARE-mediated membrane fusion events.
Whether the syt isoforms that are inhibitory in our fusion assay screen (using neuronal SNAREs) stimulate fusion mediated by other SNARE pairs is an interesting question that remains to be addressed. Elucidation of the expression pattern and subcellular localization of each isoform of syt is needed to determine which t-SNAREs a given syt isoform will encounter in vivo.
Acknowledgments
We thank A. Riccio for conducting preliminary experiments at the early stages of this study and Jon Rehfuss for helping with protein purifications. We also thank the members of the Chapman laboratory for helpful discussions.
Author's Choice—Final version full access.
This work was supported, in whole or in part, by the National Institutes of Health. This work was also supported by the American Heart Association (to E. R. C.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “;advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: SNARE, soluble NSF (N-ethylmaleimide-sensitive factor) attachment receptor; max FI, maximum fluorescence intensity; PE, 1-palmitoyl, 2-oleoyl phosphatidylethanolamine; PC, 1-palmitoyl, 2-oleoyl phosphatidylcholine; PS, 1,2-dioleoyl phosphatidylserine; syb, synaptobrevin; syt, synaptotagmin.
A. Bhalla, unpublished data.
C. Dean, H. Liu, F. M. Dunning, P. Y. Chang, M. B. Jackson, and E. R. Chapman, submitted manuscript.
References
- 1.Niemann, H., Blasi, J., and Jahn, R. (1994) Trends Cell Biol. 4 179-185 [DOI] [PubMed] [Google Scholar]
- 2.Jahn, R., and Scheller, R. H. (2006) Nat. Rev. Mol. Cell Biol. 7 631-643 [DOI] [PubMed] [Google Scholar]
- 3.Weber, T., Zemelman, B. V., McNew, J. A., Westermann, B., Gmachl, M., Parlati, F., Sollner, T. H., and Rothman, J. E. (1998) Cell 92 759-772 [DOI] [PubMed] [Google Scholar]
- 4.Augustine, G. J. (2001) Curr. Opin. Neurobiol. 11 320-326 [DOI] [PubMed] [Google Scholar]
- 5.Koh, T. W., and Bellen, H. J. (2003) Trends Neurosci. 26 413-422 [DOI] [PubMed] [Google Scholar]
- 6.Chapman, E. R. (2008) Annu. Rev. Biochem. 77 615-641 [DOI] [PubMed] [Google Scholar]
- 7.Matthew, W. D., Tsavaler, L., and Reichardt, L. F. (1981) J. Cell Biol. 91 257-269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perin, M. S., Fried, V. A., Mignery, G. A., Jahn, R., and Sudhof, T. C. (1990) Nature 345 260-263 [DOI] [PubMed] [Google Scholar]
- 9.Chapman, E. R., and Davis, A. F. (1998) J. Biol. Chem. 273 13995-14001 [DOI] [PubMed] [Google Scholar]
- 10.Davis, A. F., Bai, J., Fasshauer, D., Wolowick, M. J., Lewis, J. L., and Chapman, E. R. (1999) Neuron 24 363-376 [DOI] [PubMed] [Google Scholar]
- 11.Hui, E., Bai, J., and Chapman, E. R. (2006) Biophys. J. 91 1767-1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martens, S., Kozlov, M. M., and McMahon, H. T. (2007) Science 316 1205-1208 [DOI] [PubMed] [Google Scholar]
- 13.Bennett, M. K., Calakos, N., and Scheller, R. H. (1992) Science 257 255-259 [DOI] [PubMed] [Google Scholar]
- 14.Schiavo, G., Stenbeck, G., Rothman, J. E., and Sollner, T. H. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 997-1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bai, J., Wang, C. T., Richards, D. A., Jackson, M. B., and Chapman, E. R. (2004) Neuron 41 929-942 [DOI] [PubMed] [Google Scholar]
- 16.Bhalla, A., Chicka, M. C., Tucker, W. C., and Chapman, E. R. (2006) Nat. Struct. Mol. Biol. 13 323-330 [DOI] [PubMed] [Google Scholar]
- 17.Earles, C. A., Bai, J., Wang, P., and Chapman, E. R. (2001) J. Cell Biol. 154 1117-1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang, X., Kim-Miller, M. J., Fukuda, M., Kowalchyk, J. A., and Martin, T. F. (2002) Neuron 34 599-611 [DOI] [PubMed] [Google Scholar]
- 19.Tucker, W. C., Edwardson, J. M., Bai, J., Kim, H. J., Martin, T. F., and Chapman, E. R. (2003) J. Cell Biol. 162 199-209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shin, O. H., Rizo, J., and Sudhof, T. C. (2002) Nat. Neurosci. 5 649-656 [DOI] [PubMed] [Google Scholar]
- 21.Shin, O. H., Rhee, J. S., Tang, J., Sugita, S., Rosenmund, C., and Sudhof, T. C. (2003) Neuron 37 99-108 [DOI] [PubMed] [Google Scholar]
- 22.Shin, O. H., Maximov, A., Lim, B. K., Rizo, J., and Sudhof, T. C. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 2554-2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu, J., Mashimo, T., and Sudhof, T. C. (2007) Neuron 54 567-581 [DOI] [PubMed] [Google Scholar]
- 24.Tucker, W. C., Weber, T., and Chapman, E. R. (2004) Science 304 435-438 [DOI] [PubMed] [Google Scholar]
- 25.Bhalla, A., Tucker, W. T., and Chapman, E. R. (2005) Mol. Biol. Cell 16 4755-4764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Craxton, M. (2007) BMC Genomics 8 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rickman, C., Craxton, M., Osborne, S., and Davletov, B. (2004) Biochem. J. 378 681-686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hui, E., Bai, J., Wang, P., Sugimori, M., Llinas, R. R., and Chapman, E. R. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 5210-5214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vician, L., Lim, I. K., Ferguson, G., Tocco, G., Baudry, M., and Herschman, H. R. (1995) Proc. Natl. Acad. Sci. U. S. A. 92 2164-2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chapman, E. R., Desai, R. C., Davis, A. F., and Tornehl, C. K. (1998) J. Biol. Chem. 273 32966-32972 [DOI] [PubMed] [Google Scholar]
- 31.Wang, C. T., Lu, J. C., Bai, J., Chang, P. Y., Martin, T. F., Chapman, E. R., and Jackson, M. B. (2003) Nature 424 943-947 [DOI] [PubMed] [Google Scholar]
- 32.Machado, H. B., Liu, W., Vician, L. J., and Herschman, H. R. (2004) J. Neurosci. Res. 76 334-341 [DOI] [PubMed] [Google Scholar]
- 33.Wang, C. T., Grishanin, R., Earles, C. A., Chang, P. Y., Martin, T. F., Chapman, E. R., and Jackson, M. B. (2001) Science 294 1111-1115 [DOI] [PubMed] [Google Scholar]
- 34.Parlati, F., Weber, T., McNew, J. A., Westermann, B., Sollner, T. H., and Rothman, J. E. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 12565-12570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perin, M. S., Brose, N., Jahn, R., and Sudhof, T. C. (1991) J. Biol. Chem. 266 623-629 [PubMed] [Google Scholar]
- 36.Sutton, R. B., Davletov, B. A., Berghuis, A. M., Sudhof, T. C., and Sprang, S. R. (1995) Cell 80 929-938 [DOI] [PubMed] [Google Scholar]
- 37.Sutton, R. B., Ernst, J. A., and Brunger, A. T. (1999) J. Cell Biol. 147 589-598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nalefski, E. A., Wisner, M. A., Chen, J. Z., Sprang, S. R., Fukuda, M., Mikoshiba, K., and Falke, J. J. (2001) Biochemistry 40 3089-3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Craxton, M. (2004) BMC Genomics 5 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bock, J. B., Matern, H. T., Peden, A. A., and Scheller, R. H. (2001) Nature 409 839-841 [DOI] [PubMed] [Google Scholar]
- 41.Rothman, J. E. (1994) Nature 372 55-63 [DOI] [PubMed] [Google Scholar]
- 42.Bonifacino, J. S., and Glick, B. S. (2004) Cell 116 153-166 [DOI] [PubMed] [Google Scholar]
- 43.Stein, A., Radhakrishnan, A., Riedel, D., Fasshauer, D., and Jahn, R. (2007) Nat. Struct. Mol. Biol. 14 904-911 [DOI] [PubMed] [Google Scholar]
- 44.Pang, Z. P., Melicoff, E., Padgett, D., Liu, Y., Teich, A. F., Dickey, B. F., Lin, W., Adachi, R., and Sudhof, T. C. (2006) J. Neurosci. 26 13493-13504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pang, Z. P., Sun, J., Rizo, J., Maximov, A., and Sudhof, T. C. (2006) EMBO J. 25 2039-2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagy, G., Kim, J. H., Pang, Z. P., Matti, U., Rettig, J., Sudhof, T. C., and Sorensen, J. B. (2006) J. Neurosci. 26 632-643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahras, M., Otto, G. P., and Tooze, S. A. (2006) J. Cell Biol. 173 241-251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang, Q., Fukuda, M., Van Bockstaele, E., Pascual, O., and Haydon, P. G. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 9441-9446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Babity, J. M., Armstrong, J. N., Plumier, J. C., Currie, R. W., and Robertson, H. A. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 2638-2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maximov, A., Shin, O. H., Liu, X., and Sudhof, T. C. (2007) J. Cell Biol. 176 113-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chapman, E. R. (2002) Nat. Rev. Mol. Cell Biol. 3 498-508 [DOI] [PubMed] [Google Scholar]