Abstract
We have recently shown that the pharmacological mechanisms through which cannabinoid and opioid drugs influence social play behavior in adolescent rats can be partially dissociated. Here, we characterize the effects of the direct cannabinoid agonist WIN55,212-2, the indirect cannabinoid agonist URB597 and the opioid agonist morphine on social play at the behavioral level. By treating either one or both partners of the test dyad, we show that these drugs differentially affect play solicitation and play responsiveness. By testing these drugs in animals which were either familiar or unfamiliar to the test cage, we show that environmental factors differentially modulate the effects of cannabinoid and opioid drugs on social play. These results support and extend our previous findings suggesting that, although cannabinoid and opioid systems interact in the modulation of social play behavior in adolescent rats, they do so through partially dissociable behavioral and pharmacological mechanisms.
Keywords: Social behavior; Adolescence; Cannabinoid; Opioid; URB597; WIN55,212-2; Morphine
INTRODUCTION
From young baboons grappling playfully in mangrove trees to post-weanling rats wrestling and chasing each other, nearly all young mammals engage in some form of social play, spending time in activities that seem to have no obvious function other than having fun. Clearly, it is quite unlikely that a behavior without any proximal or distal function has been conserved in evolution. Rather, social play takes plenty of time and energy, and evolution demands that activities costing energy provide survival value in return. It has been suggested that, by varying, repeating, and/or recombining subsequences of behavior outside their primary context, play serves to develop physical, cognitive and social capacities, and especially to acquire the ability to flexibly use these capacities under changeable circumstances (Fagen, 1981; Špinka et al., 2001) The notion that social play is important for development is supported by the observations that rats housed in isolation during adolescence, when social play behavior is most abundant, show a variety of behavioral impairments during adulthood (Hol et al., 1999; Potegal and Einon, 1989; van den Berg et al., 1999a; van den Berg et al., 1999b). However, despite its importance for behavioral development, the neural substrates of social play are incompletely understood.
Social play behavior in rats displays an inverted U-shaped curve in ontogeny, being highest around day 30 of life and declining following sexual maturation. Thus, social play is considered the most characteristic expression of social activity in adolescent animals (Panksepp et al., 1984; Vanderschuren et al., 1997; Pellis and Pellis., 1998). Social play is rewarding for adolescent rats (Calcagnetti and Schechter, 1992; Humphreys and Einon, 1981; Normansell and Panksepp, 1990; van den Berg et al., 1999b) and it is modulated by neural systems involved in reward and motivation, such as the opioid and dopaminergic systems (Vanderschuren et al., 1997; Siviy, 1998). Thus, treatment with morphine enhances (Niesink and Van Ree, 1989; Panksepp et al., 1985; Vanderschuren et al., 1995a) and treatment with opioid antagonists decreases social play behavior (Beatty and Costello, 1982; Panksepp et al., 1985; Siegel and Jensen, 1986; Siegel et al., 1985; Vanderschuren et al., 1995c). The role of dopaminergic neurotransmission in the modulation of social play is less clear, as both increases and decreases in social play behavior have been reported after treatment with dopamine receptor agonists (Niesink and Van Ree, 1989; Siviy et al., 1996; Vanderschuren et al., 1997, -2008; Siviy, 1998).
The cannabinoid system has also been implicated in reward processes, and it interacts with opioid neurotransmission in the modulation of drug and food reward (Fattore et al., 2005; Maldonado et al., 2006; Solinas and Goldberg, 2005). We have recently shown that cannabinoid neurotransmission plays an important role in the modulation of social play behavior, with opposite behavioral outcomes depending on how the endocannabinoid system is stimulated (Trezza and Vanderschuren, 2007). Thus, the direct CB1 cannabinoid receptor agonist WIN55,212-2 reduced social play behavior. In contrast, the indirect cannabinoid agonist URB597, which increases endocannabinoid signalling by inhibiting fatty acid amide hydrolase (FAAH), the enzyme that catabolises the endocannabinoid anandamide, enhanced social play. This effect of URB597 depended on opioid and dopaminergic neurotransmission, because it was blocked by the opioid receptor antagonist naloxone and the dopamine receptor antagonist alpha-flupenthixol. Moreover, combined treatment with low, ineffective doses of morphine and URB597 enhanced social play. Interestingly, the play-enhancing effect of morphine was reduced by the CB1 receptor antagonist SR141716, but not by alpha-flupenthixol. This suggests that endocannabinoid and opioid systems jointly facilitate social play, but through partially dissociable mechanisms.
The purpose of the present study was to test the hypothesis that since opioids and cannabinoids influence social play behavior through distinct neural mechanisms, the effects of these drugs on social play are also behaviorally different. To that aim, we performed a behavioral analysis of the effects of WIN55,212-2, URB597 and morphine on social play.
In our previous study (Trezza and Vanderschuren, 2007), both members of a test dyad received the same treatment, making it difficult to evaluate whether cannabinoid and opioid drugs affected the initiation to play, the responsivity to play initiation, or both. This can be investigated by treating one partner in a dyad, and by scoring behaviors both per pair of animals and each individual animal of a pair separately. By investigating the situation where a vehicle-treated animal is paired with a drug-treated test partner, the effect of a drug on playfulness can be assessed in more detail. In addition, it can be determined whether being confronted with a drug-treated test partner displaying altered social behavior influences, in turn, the behavior of the vehicle-treated animal.
The amount of social activity exhibited by the partner is not the only factor which modulates social play behavior in adolescent rats. Social play is performed only when primary needs are met and environmental conditions are perceived as relatively safe. For example, when animals are tested in an unfamiliar environment, social play is initially suppressed (Vanderschuren et al., 1995b). This observation prompted us to investigate whether the effects of cannabinoid and opioid drugs on social play would change if rats were tested in an unfamiliar tet cage.
Thus, by using pharmacological manipulations directed at either one or both partners of the test dyad, our first aim was to analyze the effects of cannabinoid and opioid drugs on the motivation to solicit play and the responsiveness to play solicitation. By testing the effects of WIN55,212-2, URB597 and morphine in animals which were either familiar or unfamiliar to the test cage, our second aim was to investigate whether environmental factors differentially modulate the effects of these drugs on social play behavior. Detailed analysis of the effects of cannabinoid and opioid drugs on social play behavior may help to understand the behavioral mechanisms and neural circuits involved in this behavior. Since abnormal social behavior is an integral part of the pathophysiology of disorders such as schizophrenia and autism, getting more information about how the brain processes social information could aid our understanding and treatment of impaired social abilities in humans.
METHODS
Animals
Male Wistar rats (Charles River, Sulzfeld, Germany) arrived in our animal facility at 21 days of age and were housed in groups of four in 40 × 26 × 20 (l × w × h) Macrolon cages under controlled conditions (i.e. temperature 20–21 °C, 60–65% relative humidity and 12/12 h light cycle with lights on at 7.00 a.m.). Food and water were available ad libitum.
All experiments were approved by the Animal Ethics Committee of the University Medical Center Utrecht and were conducted in agreement with Dutch laws (Wet op de Dierproeven, 1996) and European regulations (Guideline 86/609/EEC).
Drugs
WIN55,212-2 (Tocris Cookson, Avonmouth, UK) and URB597 (Cayman Chemical, Ann Arbor, MI, USA) were dissolved in 5% Tween-80/5% polyethylene glycol/saline. Morphine (O.P.G. Utrecht, The Netherlands) was dissolved in saline. WIN55,212-2 (0.3 mg/kg) and URB597 (0.1 mg/kg) were given intraperitoneally (i.p.) 30 min and 2 h before testing, respectively; morphine (1 mg/kg) was given subcutaneously (s.c.) 1 h before testing. Solutions were freshly prepared on the day of the experiment and were administered in a volume of 2 ml/kg. The doses of drugs and timing of their administration were based on our previous study (Trezza and Vanderschuren, 2007). Because of the importance of the neck area in the expression of social play behavior (Pellis and Pellis, 1987; Siviy and Panksepp, 1987), s.c. injections were administered in the flank.
Social play behavior
All the experiments were performed in a sound attenuated chamber under dim light conditions. The testing arena consisted of a Plexiglas cage measuring 40 × 40 × 60 cm (l × w × h), with approximately 2 cm of wood shavings covering the floor. The behaviors of the animals were videotaped using a video camera with zoom lens, video tape recorder and television monitor.
Effects of the CB1 cannabinoid receptor agonist WIN55,212-2, the FAAH inhibitor URB597 and the opioid receptor agonist morphine on social play behavior when one or both partners of a dyad were treated
At 26–28 days of age, rats were individually habituated to the test cage for 10 min on each of the two days prior to testing. On the test day, the animals were socially isolated for 3.5 h before testing. This isolation period has been shown to induce a half-maximal increase in the amount of social play behavior (Niesink and Van Ree, 1989). At the appropriate time before testing, pairs of rats were assigned to one of the following treatments: (a) both rats injected with vehicle; (b) both rats injected with a drug (WIN55,212-2, URB597 or morphine); (c) one rat injected with a drug (WIN55,212-2, URB597 or morphine) and the other with the corresponding vehicle. Each pair was then placed into the test cage for 15 min. The animals in a test pair were no cagemates and did not differ more than 10 g in body weight.
Analysis from the video tape recordings was performed afterwards. Coding of the drug solutions ensured that both during experimentation and behavior analysis, the experimenter was unaware of the treatment of the animals. Behavior was assessed both per pair of animals (panels a–c in figure 1–figure 3) and each individual animal of a pair separately (which was labelled as “Treatment”, panels d–f in figure 1–figure 3) using the Observer 3.0 software (Noldus Information Technology B.V., Wageningen, The Netherlands).
Fig. 1.
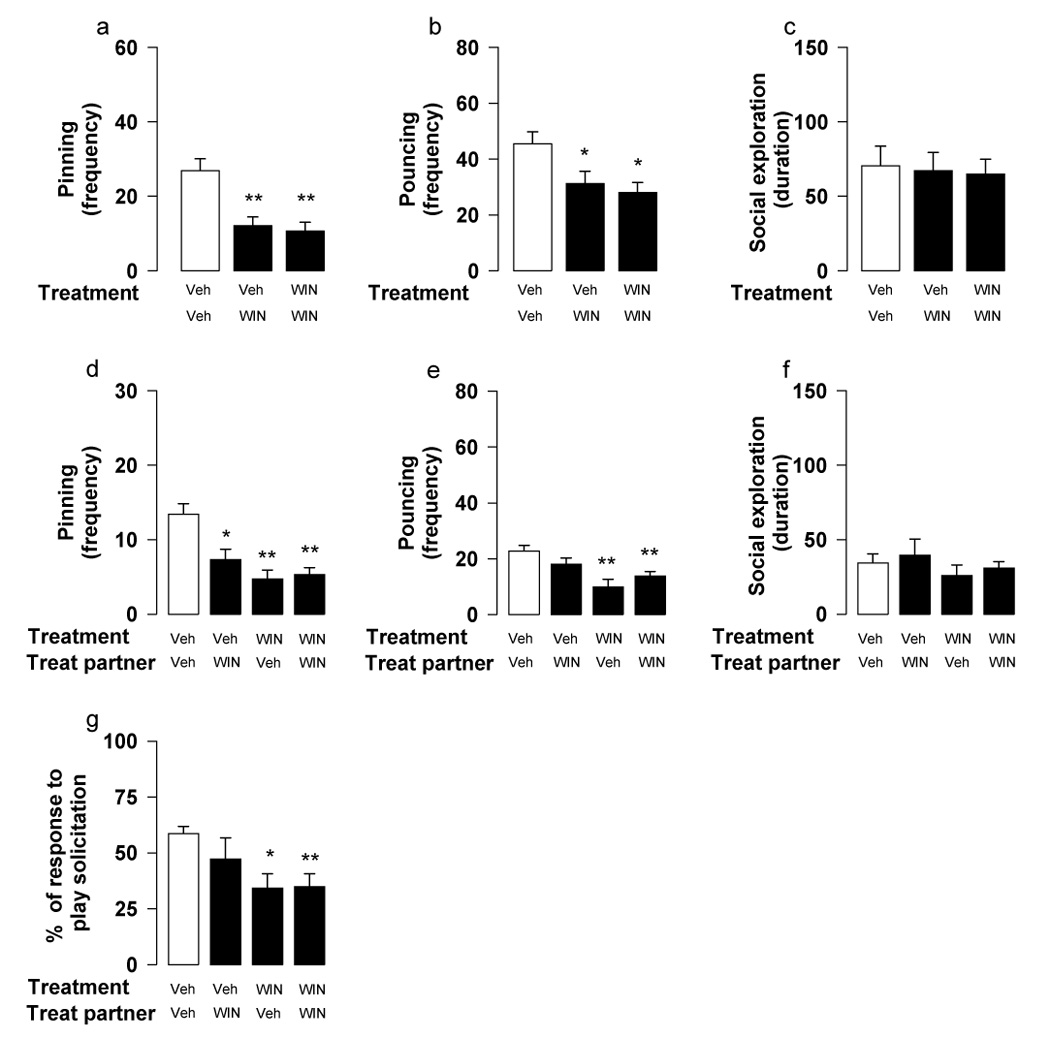
Effects of the CB1 receptor agonist WIN55,212-2 (WIN; 0.3 mg/kg, i.p., 30 min before test) on social play behavior. When social play behavior was assessed per pair of animals (a–c), there was a reduction in pinning (a) and pouncing (b) either when one or both rats in a pair were treated with WIN55,212-2, with no changes in social exploration (c). Similarly, when behavior of the members of a pair was scored separately (d–g), there was a reduction in pinning (d), pouncing (e) and percentage of responses to play solicitation (g) either when one or both rats in a pair were treated with WIN55,212-2. In addition, WIN55,212-2-treated rats affected behavior of their vehicle-treated partners: vehicle-treated rats interacting with WIN55,212-2-treated partners continued to solicit play (e) but, showed reduced pinning frequency (d). Social exploration was not affected by treatment with WIN55,212-2 (f). Data represent mean ± SEM frequency of pinning and pouncing, mean ± SEM duration of social exploration and mean ± SEM percentage of responses to play solicitation. *p<0.05, **p<0.01 vs. couples in which both rats were treated with vehicle (white bar; Tukey’s post hoc test, n = 12–24 per treatment group).
Fig. 3.
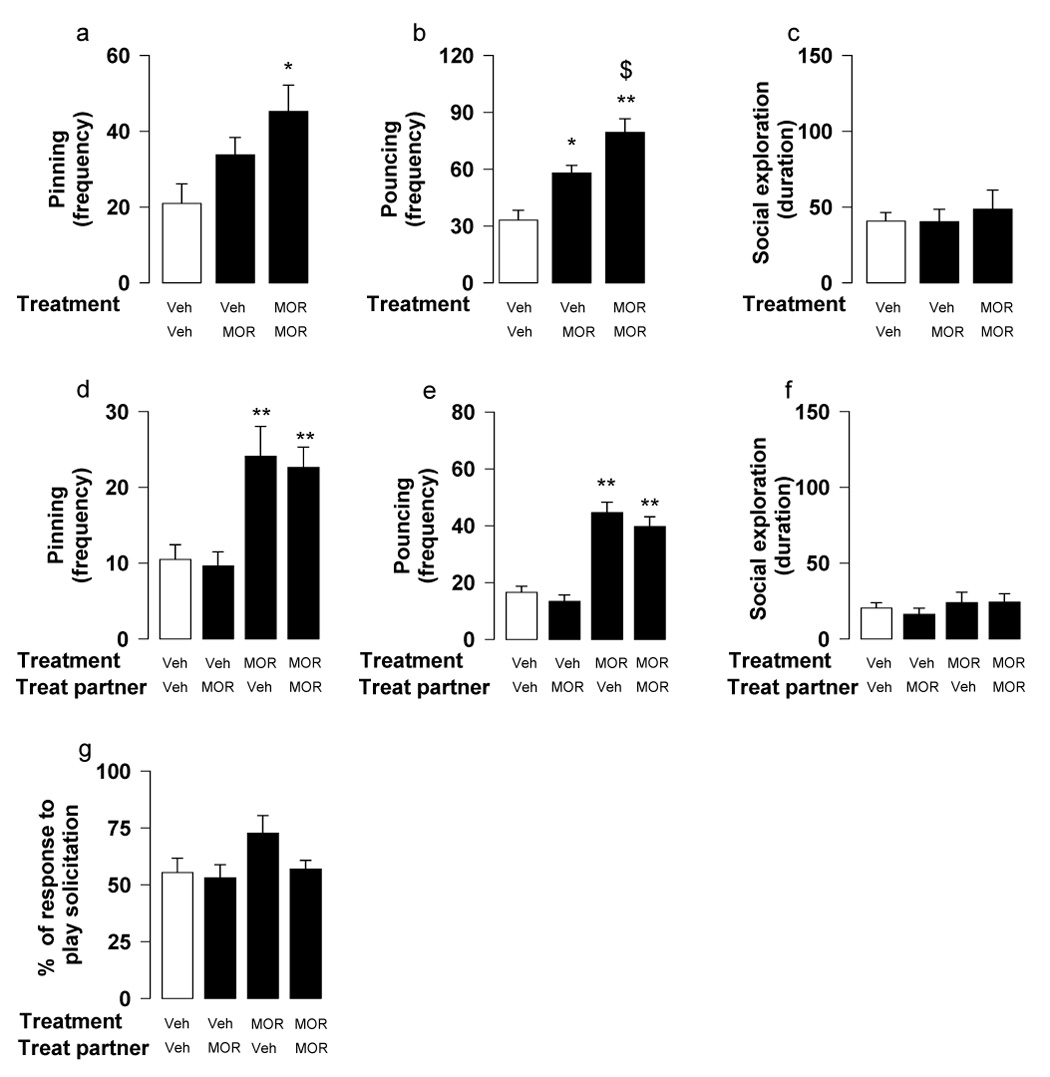
Effects of the opioid receptor agonist morphine (MOR; 1 mg/kg, s.c., 1 h before test) on social play behavior. When social play behavior was assessed per pair of animals (a–c), there was an increase in pinning only when both rats in a pair were treated with morphine (a); pouncing, however, was increased when either one or both rats in a pair were treated with morphine (b). Social exploration was not affected by morphine treatment (c). When behavior of the members of a pair was scored separately (d–g), pinning (d) and pouncing (e) were increased in all morphine-treated rats, irrespective of whether they were playing with vehicle- or morphine-treated partners. Social exploration (f) and percentage of responses to play solicitation (g) were unaffected. Data represent mean ± SEM frequency of pinning and pouncing, mean ± SEM duration of social exploration and mean ± SEM percentage of responses to play solicitation. *p<0.05, **p<0.01 vs. couples in which both rats were treated with vehicle (white bar); $p<0.05 vs. couples in which one animal was treated with morphine (Tukey’s post hoc test, n = 8–16 per treatment group).
In rats, a bout of social play behavior starts with one rat soliciting (‘pouncing’) another animal, by attempting to nose or rub the nape of its neck. The animal that is pounced upon can respond in different ways: if the animal fully rotates to its dorsal surface, ‘pinning’ is the result, i.e. one animal lying with its dorsal surface on the floor with the other animal standing over it. From this position, the supine animal can easily initiate another play bout, by trying to gain access to the other animal’s neck. Thus, during social play, pinning, which is considered to be the most obvious posture in social play behavior in rats, is not an endpoint, but rather functions as a releaser of a prolonged play bout (Pellis and Pellis, 1987). If the animal that is pounced upon responds by evading, the soliciting rat may start to chase it, thus making another attempt to launch a play bout (Vanderschuren et al., 1997).
The following behaviors were scored per 15 min: frequency of pinning, frequency of pouncing, and time spent in social exploration, i.e. sniffing any part of the body of the test partner, including the anogenital area. Play responsiveness was calculated as the probability of an animal of being pinned in response to play solicitation (pouncing) by the test partner (Pellis and Pellis, 1990; 1991).
Effects of the CB1 cannabinoid receptor agonist WIN55,212-2, the FAAH inhibitor URB597 and the opioid receptor agonist morphine on social play behavior in adolescent rats tested in a familiar or unfamiliar environment
Since environmental factors induce changes in social play behavior of adolescent rats (Vanderschuren et al., 1995b), WIN55,212-2, URB597 and morphine were tested in animals which were either habituated or not habituated to the test cage prior to the experiment, to assess whether non-familiarity to the test cage modulates the effects of these drugs on social play behavior. To this aim, the effects of WIN55,212-2, URB597 and morphine on social play behavior in familiar and unfamiliar environments were tested in four separate experiments. At 26–28 days of age, rats to be tested in a familiar environment were individually habituated to the test cage for 10 min on two days prior to testing (effects of WIN55,212-2 in the first experiment, effects of URB597 and morphine in the second experiment). Animals to be tested in an unfamiliar environment were left undisturbed in their home cage at 26–28 days of age (effects of WIN55,212-2 in the third experiment, effects of URB597 and morphine in the fourth experiment). In all the experiments, on the test day, the animals were socially isolated for 3.5 h prior to the experiment. At the appropriate time before testing, both animals of a pair were treated with a drug (WIN55,212-2, URB597 or morphine) or their corresponding vehicle. Each pair was then placed into the test cage for 15 min.
Since the social behaviors scored are not evenly distributed over the 15 min test period (Vanderschuren et al., 1995a), the behavior displayed by the animals (pinning and pouncing frequencies and time spent in social exploration) was analyzed in 5 min intervals as well as for the entire 15 min test period.
Statistical analysis
Pinning and pouncing frequencies, time spent in social exploration and play responsiveness (percentage of responses to play solicitation) were expressed as mean ± SEM.
One or both rats treated
The behavior of a pair was analyzed using one-way analysis of variance (ANOVA), followed by Tukey's post hoc test where appropriate. To evaluate whether drug-treated animals affected behavior of their vehicle-treated partners (and vice-versa), the behavior of individual animals in a pair and the percentage of response to play solicitation were analyzed using two-way ANOVA, followed by Tukey's post hoc test where appropriate.
Drug effects in a familiar and unfamiliar environment
To assess the overall effects of WIN55,212-2, URB597 and morphine on social play behavior in a familiar or unfamiliar test cage, data were analyzed using one-way ANOVA, followed by Tukey's post hoc test where appropriate. To assess whether the effects of WIN55,212-2, URB597 and morphine changed over time in animals tested in a familiar or unfamiliar environment, the 15 min session was divided in three blocks of 5 min, which were analyzed using one-way ANOVA for repeated measures, followed by Tukey's post hoc test where appropriate.
RESULTS
Effects of the CB1 cannabinoid receptor agonist WIN55,212-2 on social play behavior
When behavior was assessed per pair of animals, the direct CB1 cannabinoid receptor agonist WIN55,212-2 (0.3 mg/kg, i.p.) reduced pinning [F2,35=11.24, p<0.001] (Figure 1a) and pouncing [F2,35=5.29, p=0.01] (Figure 1b) when either one or both rats in a test pair were treated; social exploration was unaffected [F2,35=0.05, n.s.] (Figure 1c).
When behavior of the individual members of a test pair was scored, there was a reduction in pinning [F(treatment subject)1,66=15.41, p<0.001; F(treatment partner)1,66=4.40, p<0.05; F(treatment subject × treatment partner)1,66=9.55, p<0.01] (Figure 1d) and pouncing [F(treatment subject)1,66 =16.85, p<0.001; F(treatment partner)1,66=0.05, n.s.; F(treatment subject × treatment partner)1,66=4.50, p<0.05] (Figure 1e), with no changes in social exploration [F(treatment subject)1,66=1.57, n.s.; F(treatment partner)1,66=0.68, n.s.; F(treatment subject × treatment partner)1,66=0.01, n.s.] (Figure 1f) when both rats in a pair were treated with WIN55,212-2. Play responsiveness, expressed as the probability of being pinned following solicitation by the test partner, was reduced in all WIN55,212-2-treated rats, independent of whether they were playing with vehicle- or WIN55,212-2-treated partners [F(treatment subject)1,66=6.85, p=0.01; F(treatment partner)1,66=1.43, n.s.; F(treatment subject × treatment partner)1,66=0.4, n.s.] (Figure 1g). In couples where only one animal was treated, WIN55,212-2 reduced pouncing in rats paired with vehicle-treated partners, but there was no difference in pouncing between vehicle-treated rats interacting with vehicle- or with WIN55,212-2-treated-partners. Thus, vehicle-treated rats playing with WIN55,212-2-treated partners continued to solicit upon their test partners (Figure 1e); the latter, however, were unresponsive to play solicitation (Figure 1g). As a result, pinning frequency was reduced in both vehicle-treated rats (playing with WIN55,212-2-treated partners) and in WIN55,212-2-treated rats (playing with vehicle-treated partners) (Figure 1d). These results suggest that WIN55,212-2-treated rats displayed both reduced initiation to play and responsiveness to play solicitation, indirectly decreasing playfulness in their vehicle-treated partners as well.
Effects of the FAAH inhibitor URB597 on social play behavior
When behavior was assessed per pair of animals, the FAAH inhibitor URB597 (0.1 mg/kg, i.p.) increased pinning [F2,41=18.44, p<0.001] (Figure 2a) and pouncing [F2,41=12.59, p<0.001] (Figure 2b) only when both members of a test pair were treated. URB597 reduced social exploration (F2,41=8.67, p<0.001) in all groups (Figure 2c).
Fig. 2.
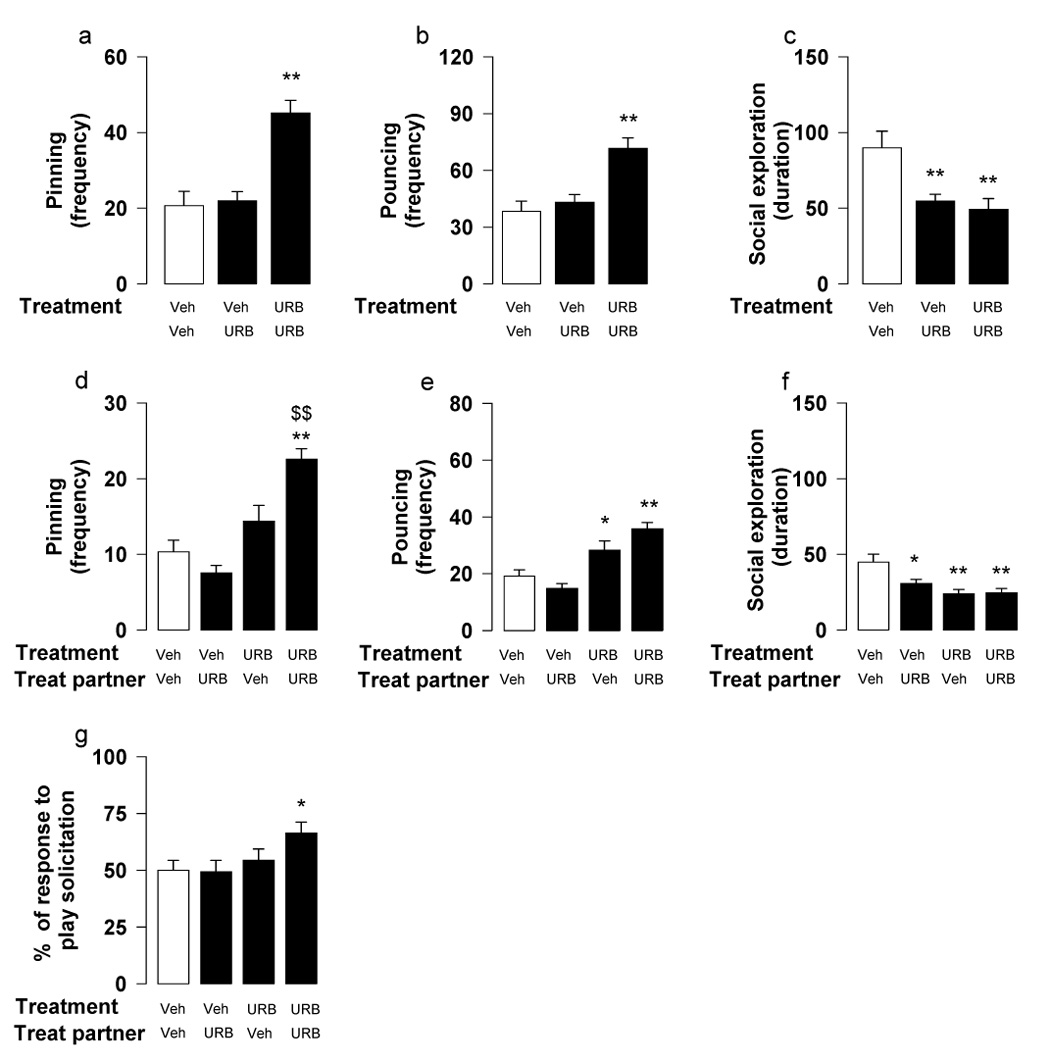
Effects of the FAAH inhibitor URB597 (URB; 0.1 mg/kg, i.p., 2 h before test) on social play behavior. When social play behavior was assessed per pair of animals (a–c), URB597 increased pinning (a) and pouncing (b) only when both rats of a pair were treated with the drug; URB597 reduced social exploration (c) in all experimental groups. When behavior of the members of a pair was scored separately (d–g), pinning (d) and percentage of responses to play solicitation (g) were increased when both members of a pair were injected with URB597; pouncing, however, was increased in all URB597-treated rats, irrespective of whether they were playing with URB597- or vehicle-treated partners (e). URB597 reduced social exploration in all experimental groups (f). Data represent mean ± SEM frequency of pinning and pouncing, mean ± SEM duration of social exploration and mean ± SEM percentage of responses to play solicitation. *p<0.05, **p<0.01 vs. couples in which both rats were treated with vehicle (white bar); $$p<0.01 vs. couples in which one animal was treated with URB597 (Tukey’s post hoc test, n = 18–24 per treatment group).
The analysis of individual animals confirmed that pinning was increased [F(treatment subject)1,80=37.91, p<0.001; F(treatment partner)1,80=3.05, n.s.; F(treatment subject × treatment partner)1,80=12.53, p<0.001] only when both rats in a pair were treated with URB597 (Figure 2d). Conversely, pouncing was increased [F(treatment subject)1,80=40.48, p<0.001; F(treatment partner)1,80=0.46, n.s.; F(treatment subject × treatment partner)1,80=6.31, p<0.05] in URB597-treated rats playing either with URB597- or vehicle-treated partners (Figure 2e). In other words, all URB597-treated rats solicited their partners more. URB597-treated partners, however, were more responsive to play solicitation than vehicle-treated rats [F(treatment subject)1,80=5.02, p<0.05; F(treatment partner)1,80 =1.04, n.s.; F(treatment subject × treatment partner)1,80=1.75, n.s.] (Figure 2g), so that pinning frequency was significantly increased only when both rats in a pair were injected with URB597. Thus, URB597 increased both the initiation to play and the responsiveness to play initiation. URB597 reduced social exploration [F(treatment subject)1,80=13.16, p<0.001; F(treatment partner)1,80=3.32, n.s.; F(treatment subject × treatment partner)1,80=3.92, p=0.051] in all groups (Figure 2f).
Effects of the opioid receptor agonist morphine on social play behavior
When behavior was assessed per pair of animals, the opioid receptor agonist morphine (1 mg/kg, s.c.) increased pinning [F2,23=4.69, p<0.05] (Figure 3a) only when both rats in a pair were treated. Morphine increased pouncing when either one or both rats of a pair were treated [F2,23=17.24, p<0.001] (Figure 3b); social exploration was unaffected by treatment with morphine [F2,23=0.26, n.s.] (Figure 3c).
When behavior of individual members of a test pair was scored separately, all morphine-treated rats displayed increased pinning [F(treatment subject)1,44=22.70, p<0.001; F(treatment partner)1,44=0.18, n.s.; F(treatment subject × treatment partner)1,44=0.01, n.s.] (Figure 3d) and pouncing [F(treatment subject)1,44 =70.01, p<0.001; F(treatment partner)1,44=1.56, n.s.; F(treatment subject × treatment partner)1,44=0.07, n.s.] (Figure 3e), independent of whether they were playing with vehicle- or morphine-treated partners. Pinning and pouncing frequencies of the vehicle-treated animals, however, were not influenced by the drug treatment of their test partner (Figure 3d–e). This suggests that treatment with morphine not only resulted in an increase in playfulness, but also caused the behavior of the morphine-treated rats to become relatively insensitive to the level of playfulness of their test partners. Indeed, responsiveness to play solicitation was not affected by treatment with morphine [F(treatment subject)1,44=2.97, p=0.09; F(treatment partner)1,44=2.19, n.s.; F(treatment subject × treatment partner)1,44=1.21, n.s.] (Figure 3g). Morphine did not affect social exploration [F(treatment subject)1,44=1.17, n.s.; F(treatment partner)1,44=0.11, n.s.; F(treatment subject × treatment partner)1,44=0.15, n.s.] (Figure 3f).
Effects of the CB1 cannabinoid receptor agonist WIN55,212-2, the FAAH inhibitor URB597 and the opioid receptor agonist morphine on social play behavior in adolescent rats tested in a familiar or an unfamiliar environment
When rats were tested in a familiar environment, WIN55,212-2 (0.3 mg/kg, i.p.) reduced pinning [F1,26=12.76, p=0.001] (Figure 4a) and pouncing [F1,26=9.55, p<0.01] (Figure 4b), without affecting social exploration [F1,26=0.095, n.s.] (Figure 4c). When behaviors were analyzed per 5-min interval, WIN55,212-2 reduced pinning in the first and second 5 min periods of the test [F(treatment)1,50=12.86, p<0.01; F(interval)1,50=5.71, p<0.01; F(treatment × interval)1,50=1.62, n.s.] (Figure 4d), reduced pouncing in the second 5 min interval [F(treatment)1,50=9.02, p<0.01; F(interval)1,50=14.27, p<0.001; F(treatment × interval)1,50=1.42, n.s.] (Figure 4e), and did not affect social exploration [F(treatment)1,50=0.004, n.s.; F(interval)1,50=2.2, n.s.; F(treatment × interval)1,50=0.52, n.s.] (Figure 4f). In an unfamiliar environment, WIN55,212-2 reduced pinning [F1,15=15.62, p=0.001] (Figure 4g) and pouncing [F1,15=4.36, p<0.05] (Figure 4h), without affecting social exploration [F1,15=2.77, n.s.] (Figure 4i). When behaviors were analyzed per 5 min interval, there was an effect of WIN55,212-2 on pinning [F(treatment)1,28=15.62, p<0.01; F(interval)1,28=2.38, n.s.; F(treatment × interval)1,28=0.1, n.s.] (Figure 4l) and pouncing [F(treatment)1,28=4.36, p<0.05; F(interval)1,28=3.36, p<0.05; F(treatment × interval)1,28=0.16, n.s.] (Figure 4m), and no effect on social exploration [F(treatment)1,28=2.77, n.s.; F(interval)1,28=2.49, n.s.; F(treatment × interval)1,28=0.87, n.s.] (Figure 4n). However, post hoc comparisons did not reveal statistically significant differences during any of the 5 min blocks.
Fig. 4.
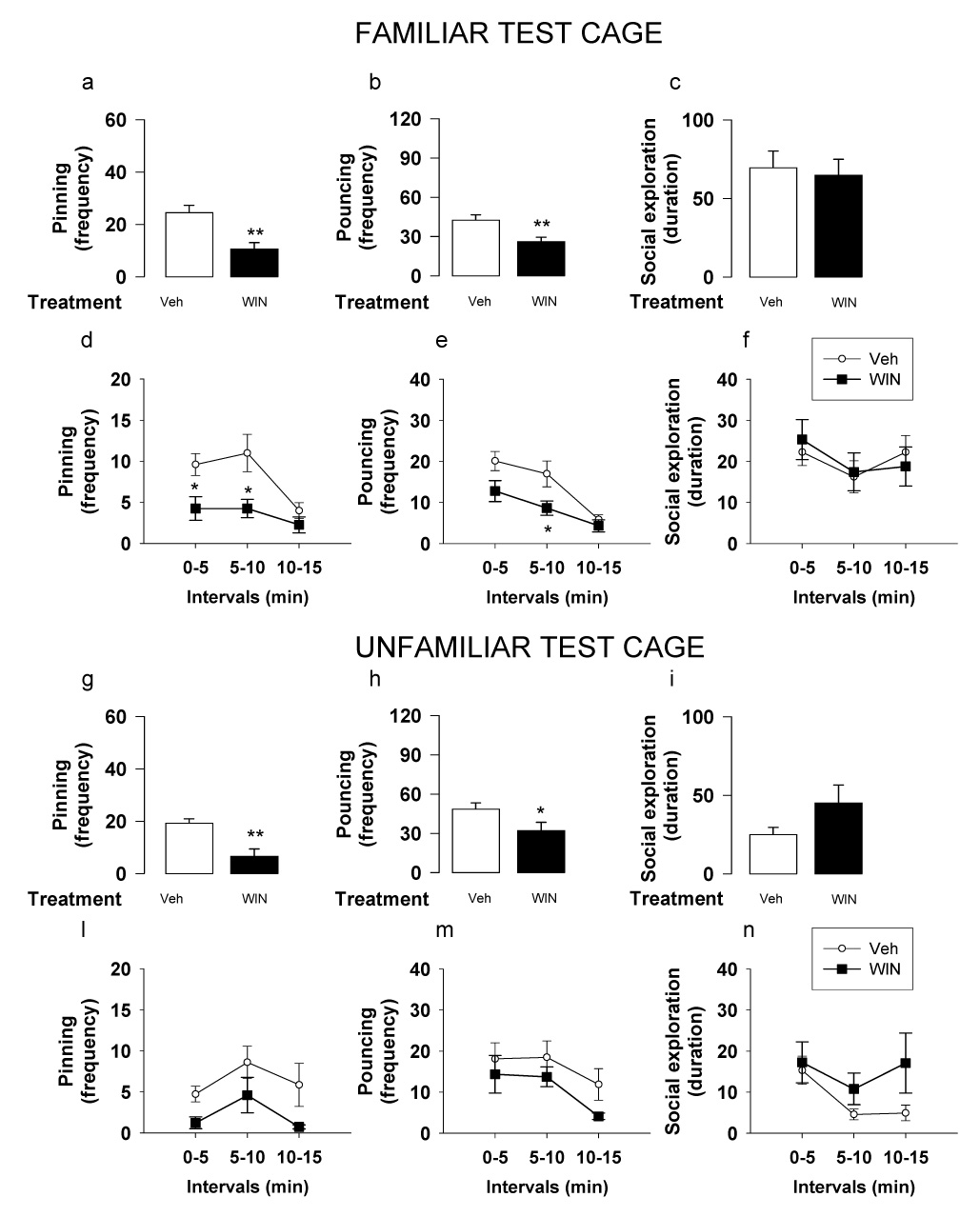
Effects of the CB1 cannabinoid receptor agonist WIN55,212-2 (WIN; 0.3 mg/kg, i.p., 30 min before test) on social play behavior in adolescent rats tested in a familiar (a–f) or an unfamiliar environment (g–n). In a familiar environment, when social play behavior was assessed per 15 min, WIN55,212-2 reduced pinning (a) and pouncing (b), without affecting social exploration (c). WIN55,212-2 reduced pinning in the first and second 5 min intervals (d), reduced pouncing in the second 5 min interval (e) and did not affect social exploration during any of the 5 min blocks (f). In an unfamiliar environment, when social play behavior was assessed per 15 min, WIN55,212-2 reduced pinning (g) and pouncing (h), without affecting social exploration (i). When behavior was analyzed per 5 min intervals, post hoc comparisons for pinning (l), pouncing (m) and social exploration (n) failed to reach statistical significance during any of the 5 min blocks. Data represent mean ± SEM frequency of pinning and pouncing and mean ± SEM duration of social exploration. *p<0.05, **p<0.01 vs. vehicle treatment (Tukey’s post hoc test, n = 12 per treatment group)
URB597 (0.1 mg/kg, i.p.) and morphine (1 mg/kg, s.c.) increased pinning [F2,34=10.14, p<0.001] (Figure 5a) and pouncing [F2,34=14.76, p<0.001] (Figure 5b), without affecting social exploration [F2,34=1.48, n.s.] (Figure 5c) when rats were tested in a familiar environment. Both URB597 and morphine increased pinning in the first and last 5 min periods of the test [F(treatment)2,64=11.64, p<0.001; F(interval)2,64=4.64, p<0.05; F(treatment × interval)2,64=1.62, n.s.] (Figure 5d), increased pouncing in the first 5 min period [F(treatment)2,64=15.87, p<0.001; F(interval)2,64=33.4, p<0.001; F(treatment × interval)2,64=2.67, p<0.05] (Figure 5e) and did not affect social exploration [F(treatment)2,64=0.7, n.s.; F(interval)2,64=0.85, n.s.; F(treatment × interval)2,64=0.36, n.s.] (Figure 5f) during any of the 5 min blocks.
Fig. 5.
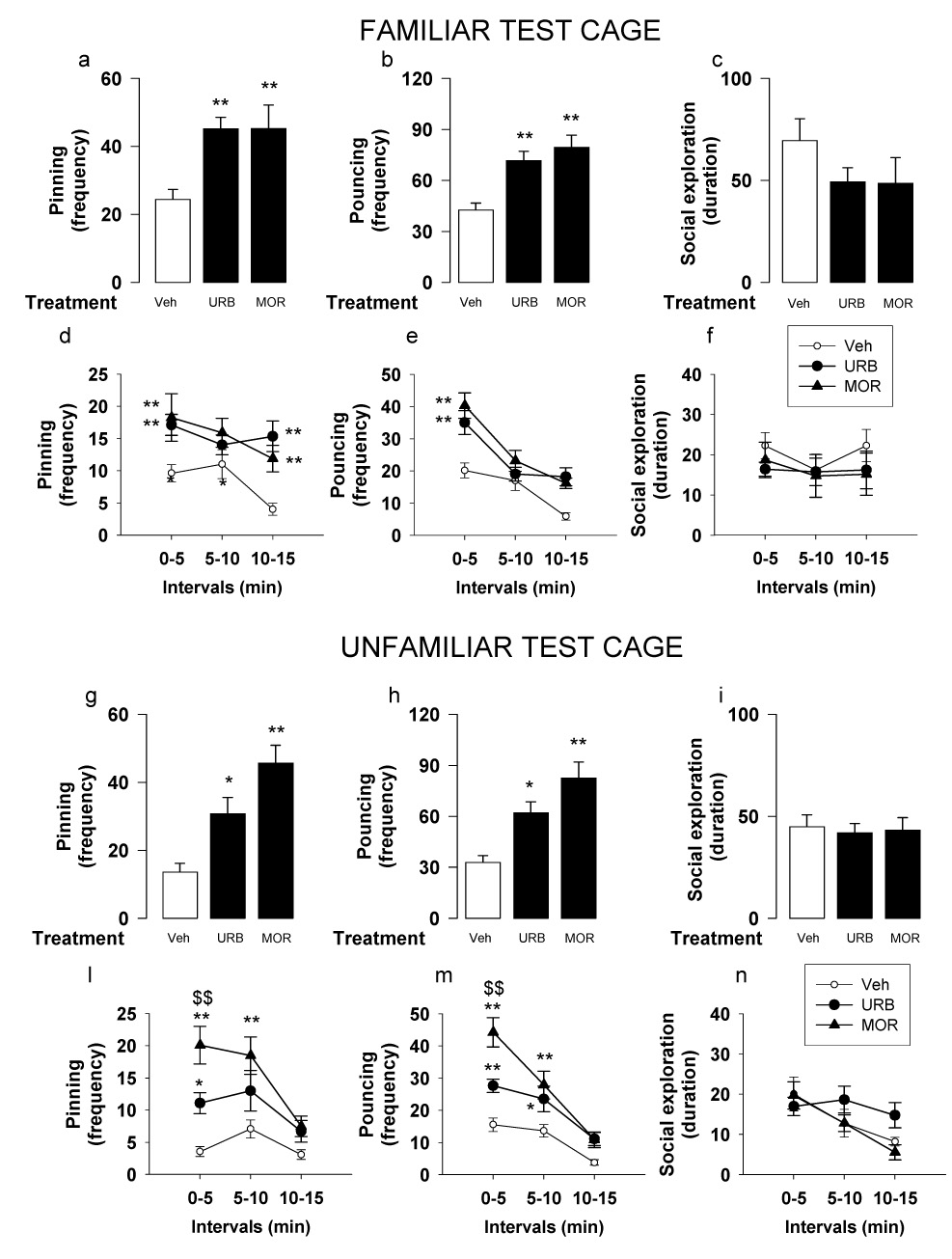
Effects of the FAAH inhibitor URB597 (URB; 0.1 mg/kg, i.p., 2 h before test) and the opioid receptor agonist morphine (MOR; 1 mg/kg, s.c., 1 h before test) on social play behavior in adolescent rats tested in a familiar (a–f) or an unfamiliar environment (g–n). In a familiar environment, when social play behavior was assessed per 15 min, both URB597 and morphine increased pinning (a) and pouncing (b), without affecting social exploration (c). Both URB597 and morphine increased pinning in the first and last 5 min periods of the test (d), increased pouncing in the first 5 min period (e) and did not affect social exploration (f). In an unfamiliar environment, when social play behavior was assessed per 15 min, both URB597 and morphine increased pinning (g) and pouncing (h), without affecting social exploration (i). When behavior was analyzed per 5 min intervals, pinning (l) and pouncing (m) were significantly increased during the first 5 min of the test in both URB597- and morphine-treated rats. During the second 5 min period, morphine increased both pinning (l) and pouncing (m), whereas URB597 significantly increased pouncing only (m). Data represent mean ± SEM frequency of pinning and pouncing and mean ± SEM duration of social exploration. *p<0.05, **p<0.01 vs. vehicle treatment, $$p<0.01 vs. URB597 treatment (Tukey’s post hoc test, n = 13–14 per treatment group).
In an unfamiliar environment, both URB597 and morphine increased pinning [F2,39=13.15, p<0.001] (Figure 5g) and pouncing [F2,39=12.79, p<0.001] (Figure 5h), without affecting social exploration [F2,39=0.079, n.s.] (Figure 5i). However, pinning and pouncing were higher in morphine- than in URB597-treated rats (p=0.053). When behaviors were analyzed per 5 min interval, both URB597 and morphine increased pinning [F(treatment)2,74=13.33, p<0.001; F(interval)2,74=13.31, p<0.001; F(treatment × interval)2,74=2.94, p<0.05] and pouncing [F(treatment)2,74=12.84, p<0.001; F(interval)2,74=65.1, p<0.001; F(treatment × interval)2,74=6.63, p<0.001], with no effects on social exploration [F(treatment)2,74=0.73, n.s.; F(interval)2,74=8.37, p<0.001; F(treatment × interval)2,74=1.70, n.s.]. During the first 5 min of the test, pinning (Figure 5l) and pouncing (Figure 5m) were significantly increased in both URB597- and morphine- treated rats. Pinning and pouncing frequencies, however, were twice as high in morphine-treated rats as in URB597-treated animals. During the second 5 min period, morphine increased both pinning (Figure 5l) and pouncing (Figure 5m), whereas URB597 significantly increased pouncing only (Figure 4e). Thus, both URB597 and morphine increased social play both a familiar and an unfamiliar test cage. However, in an unfamiliar environment, morphine enhanced social play to a greater extent than URB597.
DISCUSSION
The present data extend our previous findings, showing that cannabinoid and opioid systems modulate social play behavior through overlapping, but partially distinct mechanisms in adolescent rats. More precisely, whereas we have previously shown that the pharmacological mechanisms through which WIN55,212-2, URB597 and morphine influence social play can be partially dissociated (Trezza and Vanderschuren 2007), here we show that there are also differences in how these drugs affect social play on a behavioral level.
The present study, where one or both partners of the test dyad were treated with drugs, allowed us to dissect the effects of cannabinoid and opioid drugs on the motivation to play and the responsiveness to play solicitation. Both these components were significantly reduced by the CB1 cannabinoid receptor agonist WIN55,212-2. That is, rats treated with WIN55,212-2 were not only less likely to initiate a play bout, but were also less likely to respond when solicited by their test partners. The altered behavior of WIN55,22-2-treated rats also affected the behavior of vehicle-treated partners. They continued to solicit play but, since they were not reciprocated, pinning frequency in vehicle-treated rats interacting with WIN55,212-2-treated partners was reduced to the same extent as in WIN55,212-2-treated rats. This result is consistent with findings showing that social play behavior is influenced by the level of social activity of the partner (Pellis and McKenna, 1995; Pellis and McKenna, 1992; Varlinskaya et al., 1999). Consistent with our previous data (Trezza and Vanderschuren, 2007), the FAAH inhibitor URB597 enhanced social play. Pouncing was increased in all URB597-treated rats, independent of whether they were interacting with vehicle- or URB597-treated partners. Pinning, however, was significantly increased only when both rats in a pair were treated with URB597. In other words, URB597-treated rats were more responsive to play solicitation, but only when reciprocated by an equally motivated partner, such as another URB597-treated animal. This suggests that the increase in social play induced by URB597 is influenced by the level of social activity of the test partner: both pinning and being pinned, pouncing and being pounced, seem to be important for URB597-treated rats to maintain their high levels of playfulness, likely because they experience social play as more pleasant. URB597 reduced social exploration in this experiment. We have previously found that this dose of URB597 did not alter social exploration (Trezza and Vanderschuren, 2007), which is consistent with the second set of experiments. One possible explanation for this effect of URB597 is the high level of social exploration in the control group, which may cause slight changes in social exploration in animals treated with URB597, as well as their vehicle-treated partners, to reach statistical significance in this particular experiment.
Like URB597, morphine increases social play behavior in adolescent rats (Vanderschuren et al., 1995a), but unlike URB597, the effect of morphine does not depend upon dopaminergic neurotransmission (Trezza and Vanderschuren, 2007). Here, we show that the effects of morphine and URB597 on social play can also be dissociated on the behavioral level. In contrast to URB597, the increase in social play induced by morphine is not dependent on the level of social activity exhibited by the test partner. Pinning and pouncing were increased not only when both members of a pair were treated with morphine, but also in morphine-treated rats interacting with vehicle-treated partners. Interestingly, in couples where only one animal was treated with morphine, vehicle-treated rats were not different from vehicle-treated rats interacting with vehicle-treated partners. This suggests that the social motivation displayed by morphine-treated rats was such that it induced an increase in pinning, even when test partners exhibited normal levels of playfulness. Thus, elevated levels of play behavior may be imposed on vehicle-treated rats by the morphine-treated partners. Indeed, responsiveness to play solicitation was not altered by morphine treatment. Thus, behavioral reciprocity, which plays a critical role in the effects of URB597 on social play, is much less important for morphine-treated rats.
Although social play behavior has a major role in social and cognitive development, it is not displayed unconditionally, but only when primary physiological needs have been met and in the absence of immediate threats. Thus, social play is reduced by hunger (Siviy and Panksepp, 1985) and is suppressed under intense light conditions (Vanderschuren et al., 1995b) or when rats are exposed to predator odour (Siviy et al., 2006). When rats are tested for social play in an unfamiliar test cage, two different stimuli compete for their attention: a novel test cage and an unfamiliar test partner. For this reason, rats will explore the unfamiliar test cage before engaging in social play, which leads to a temporary suppression of pinning in the first 5 min of the test, while net levels of social play as measured over 15 min are not affected (Vanderschuren et al., 1995b).
In the present study, WIN55,212-2 reduced social play in animals which were either familiar or unfamiliar to the test cage. Interestingly, the effects of WIN55,212-2 were specific for play-associated behaviors; social exploration was unaffected by WIN55,212-2. It is therefore unlikely that the reduction in social play induced by WIN55,212-2 is secondary to any anxiogenic effect of the drug.
In a familiar environment, the effects of URB597 and morphine on social play were of the same magnitude. However, in a novel environment, morphine stimulated social play to a greater extent than URB597. This suggests that, in a novel setting, URB597- and morphine-treated rats paid more attention to novel social than to novel environmental stimuli. In particular, morphine-treated rats displayed maximal levels of play behavior immediately, as if they were familiar to the test cage.
Previously, it has been shown that treatment with a dose of morphine 10-fold lower than the dose used in the present study (Vanderschuren et al., 1995a), as well as with the κ-opioid receptor antagonist nor-binaltorphimine (Vanderschuren et al., 1995c) attenuated the effects of a novel environment on social play, without influencing total levels of play. It was suggested that these effects are not a consequence of opioid modulation of the rewarding properties of social play. Rather, these effects may be due to an opioid-induced shift in selective attention due to an altered integration of sensory information (Vanderschuren et al., 1995a; Vanderschuren et al., 1995c; Vanderschuren et al., 1997). Thus, we cannot exclude that the dose of morphine used in the present study counteracted the initial suppression of social play induced by an unfamiliar test cage not only by increasing the rewarding value of play, but also by altering the attentional processes which are normally recruited in response to a novel environment.
URB597 has been shown to have positive effects in rodent models used to screen anxiolytic and antidepressant drugs (Gobbi et al., 2005; Hill et al., 2007; Kathuria et al., 2003; Moreira et al., 2007; Patel and Hillard, 2006; Naidu et al., 2007). Therefore, the increase in play behavior during the first 5 min of the test displayed by URB597-treated rats in an unfamiliar environment may be the consequence of anxiolytic or antidepressant effects of this drug. However, several findings argue against this possibility. Unfamiliarity to the test cage reduces social play behavior, but not social exploration, in the first 5 min of the test only, leaving total levels of social play unaffected (Vanderschuren et al. 1995b). This suggests that an unfamiliar environment is not an anxiogenic condition for adolescent rats. In other words, the initial reduction of social play in an unfamiliar environment is likely the result of increased exploration of the environment, rather than any anxiogenic properties of the unfamiliar test cage resulting in a general suppression of social behavior. In addition, anxiolytic and antidepressant drugs do not necessarily enhance social play behavior. Thus, the anxiolytic chlordiazepoxide only enhances play when it is suppressed in a conditioned emotional response paradigm (Vanderschuren et al., 1997), whereas alpha-2 adrenoceptor antagonists, that have anxiogenic effects, do not affect or even enhance play (Siviy and Baliko, 2000; Siviy et al., 1994). In addition, we have recently found that the typical antidepressant drug fluoxetine potently reduces play (Homberg et al., 2007). Furthermore, in the present study, the magnitude of the effects of URB597 on social play was highly comparable in familiar and unfamiliar environments. Together, these data suggest that the effects of URB597 on social play are not secondary to its anxiolytic or antidepressant properties. Rather, we suggest that URB597 increases the motivational and rewarding properties of social play so that, compared to vehicle-treated rats, URB597-treated adolescents prefer to engage immediately in playful interactions with their partners instead of first investigating the new environment.
In summary, the following conclusions can be drawn from the present study. First, the the direct cannabinoid agonist WIN55,212-2 reduced both play solicitation and responsiveness to play solicitation, and also reduced social play in undrugged test partners. Second, endocannabinoid and opioid neurotransmission differently modulate social play behavior in adolescent rats: the increase in social play induced by the FAAH inhibitor URB597 was influenced by the level of social activity exhibited by the partner, whereas that induced by the opioid agonist morphine was not. Furthermore, the effects of WIN55,212-2, URB597 and morphine were highly comparable in rats tested in a familiar or in an unfamiliar environment, making it unlikely that anxiogenic or anxiolytic effects of these drugs cause the changes in social play behavior observed.
Our results highlight how exposure to cannabinoids and opioids during adolescence can induce changes in social behavior. Since social experiences early in life shape brain development and adult behavior (Champagne and Curley, 2005; Hol et al., 1999; Potegal and Einon, 1989; van den Berg et al., 1999a; van den Berg et al., 1999b), changes in the social repertoire caused by drug use during adolescence may have profound, long-lasting behavioral effects. Our results also suggest a role for anandamide in psychopathological disorders associated with social dysfunctions, such as autism and schizophrenia, and extend the potential therapeutic utility of indirect cannabinoid agonists, such as URB597, to these disorders. Particularly relevant is our present finding that URB597 enhanced playfulness, but, unlike morphine, left the animals’ sensitivity to the social activity of their conspecifics intact.
ACKNOWLEDGEMENTS
Supported by National Institute on Drug Abuse Grant R01 DA022628-01 (L.J.M.J.V.). This research was performed within the framework of project T5-107 of the Dutch Top Institute Pharma. V. Trezza was a visiting scientist from the Dept. of Human Physiology and Pharmacology, University of Rome 'Sapienza', Rome, Italy, supported by a fellowship from “Fondazione Enrico ed Enrica Sovena”, Rome.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Beatty WW, Costello KB. Naloxone and play fighting in juvenile rats. Pharmacol Biochem Behav. 1982;17:905–907. doi: 10.1016/0091-3057(82)90470-1. [DOI] [PubMed] [Google Scholar]
- Calcagnetti DJ, Schechter MD. Place conditioning reveals the rewarding aspect of social interaction in juvenile rats. Physiol Behav. 1992;51:667–672. doi: 10.1016/0031-9384(92)90101-7. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Curley JP. How social experiences influence the brain. Curr Opin Neurobiol. 2005;15:704–709. doi: 10.1016/j.conb.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Fagen R. Animal Play Behavior. Oxford: Oxford University Press; 1981. [Google Scholar]
- Fattore L, Deiana S, Spano SM, Cossu G, Fadda P, Scherma M, Fratta W. Endocannabinoid system and opioid addiction: behavioural aspects. Pharmacol Biochem Behav. 2005;81:343–359. doi: 10.1016/j.pbb.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, Cassano T, Morgese MG, Debonnel G, Duranti A, Tontini A, Tarzia G, Mor M, Trezza V, Goldberg SR, Cuomo V, Piomelli D. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci U S A. 2005;102:18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Karacabeyli ES, Gorzalka BB. Estrogen recruits the endocannabinoid system to modulate emotionality. Psychoneuroendocrinology. 2007;32:350–357. doi: 10.1016/j.psyneuen.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Hol T, Van den Berg CL, Van Ree JM, Spruijt BM. Isolation during the play period in infancy decreases adult social interactions in rats. Behav Brain Res. 1999;100:91–97. doi: 10.1016/s0166-4328(98)00116-8. [DOI] [PubMed] [Google Scholar]
- Homberg JR, Schiepers OJ, Schoffelmeer AN, Cuppen E, Vanderschuren LJMJ. Acute and constitutive increases in central serotonin levels reduce social play behaviour in peri-adolescent rats. Psychopharmacology. 2007;195:175–182. doi: 10.1007/s00213-007-0895-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys AP, Einon DF. Play as a reinforcer for maze-learning in juvenile rats. Anim Behav. 1981;29:259–270. [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Valverde O, Berrendero F. Involvement of the endocannabinoid system in drug addiction. Trends Neurosci. 2006;29:225–232. doi: 10.1016/j.tins.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Kaiser N, Monory K, Lutz B. Reduced anxiety-like behaviour induced by genetic and pharmacological inhibition of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (FAAH) is mediated by CB1 receptors. Neuropharmacology. 2007 doi: 10.1016/j.neuropharm.2007.07.005. in press. [DOI] [PubMed] [Google Scholar]
- Naidu PS, Varvel SA, Ahn K, Cravatt BF, Martin BR, Lichtman AH. Evaluation of fatty acid amide hydrolase inhibition in murine models of emotionality. Psychopharmacology. 2007;192:61–70. doi: 10.1007/s00213-006-0689-4. [DOI] [PubMed] [Google Scholar]
- Niesink RJ, Van Ree JM. Involvement of opioid and dopaminergic systems in isolation-induced pinning and social grooming of young rats. Neuropharmacology. 1989;28:411–418. doi: 10.1016/0028-3908(89)90038-5. [DOI] [PubMed] [Google Scholar]
- Normansell L, Panksepp J. Effects of morphine and naloxone on play-rewarded spatial discrimination in juvenile rats. Dev Psychobiol. 1990;23:75–83. doi: 10.1002/dev.420230108. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Siviy SM, Normansell L. The psychobiology of play: theoretical and methodological perspectives. Neurosci Biobehav Rev. 1984;8:465–492. doi: 10.1016/0149-7634(84)90005-8. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Jalowiec J, DeEskinazi FG, Bishop P. Opiates and play dominance in juvenile rats. Behav Neurosci. 1985;99:441–453. doi: 10.1037//0735-7044.99.3.441. [DOI] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. J Pharmacol Exp Ther. 2006;318:304–311. doi: 10.1124/jpet.106.101287. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Pellis VC. Play-fighting differs from serious fighting in both target of attack and tactics of fighting in the laboratory rat Rattus norvegicus. Aggr Behav. 1987;13:227–242. [Google Scholar]
- Pellis SM, Pellis VC. Differential rates of attack, defense, and counterattack during the developmental decrease in play fighting by male and female rats. Dev Psychobiol. 1990;23:215–231. doi: 10.1002/dev.420230303. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Pellis VC. Attack and defense during play fighting appear to be motivationally independent behaviors in muroid rodents. Psychol Rec. 1991;41:184–191. [Google Scholar]
- Pellis SM, McKenna MM. What do rats find rewarding in play fighting?--an analysis using drug-induced non-playful partners. Behav Brain Res. 1995;68:65–73. doi: 10.1016/0166-4328(94)00161-8. [DOI] [PubMed] [Google Scholar]
- Pellis SM, McKenna MM. Intrinsic and extrinsic influences on play fighting in rats: effects of dominance, partner's playfulness, temperament and neonatal exposure to testosterone propionate. Behav Brain Res. 1992;50:135–145. doi: 10.1016/s0166-4328(05)80295-5. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Pellis VC. Play fighting of rats in comparative perspective: a schema for neurobehavioral analyses. Neurosci Biobehav Rev. 1998;23:87–101. doi: 10.1016/s0149-7634(97)00071-7. [DOI] [PubMed] [Google Scholar]
- Potegal M, Einon D. Aggressive behaviors in adult rats deprived of playfighting experience as juveniles. Dev Psychobiol. 1989;22:159–172. doi: 10.1002/dev.420220206. [DOI] [PubMed] [Google Scholar]
- Siegel MA, Jensen RA. The effects of naloxone and cage size on social play and activity in isolated young rats. Behav Neural Biol. 1986;45:155–168. doi: 10.1016/s0163-1047(86)90739-9. [DOI] [PubMed] [Google Scholar]
- Siegel MA, Jensen RA, Panksepp J. The prolonged effects of naloxone on play behavior and feeding in the rat. Behav Neural Biol. 1985;44:509–514. doi: 10.1016/s0163-1047(85)91024-6. [DOI] [PubMed] [Google Scholar]
- Siviy SM. Neurobiological substrates of play behavior: glimpses into the structure and function of mammalian playfulness. In: Bekoff M, Byers JA, editors. Animal play. Cambridge: Cambridge University Press; 1998. [Google Scholar]
- Siviy SM, Baliko CN. A further characterization of alpha-2 adrenoceptor involvement in the rough-and-tumble play of juvenile rats. Dev Psychobiol. 2000;37:25–34. doi: 10.1002/1098-2302(200007)37:1<25::aid-dev4>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Siviy SM, Fleischhauer AE, Kerrigan LA, Kuhlman SJ. D2 dopamine receptor involvement in the rough-and-tumble play behavior of juvenile rats. Behav Neurosci. 1996;110:1168–1176. doi: 10.1037//0735-7044.110.5.1168. [DOI] [PubMed] [Google Scholar]
- Siviy SM, Fleischhauer AE, Kuhlman SJ, Atrens DM. Effects of alpha-2 adrenoceptor antagonists on rough-and-tumble play in juvenile rats: evidence for a site of action independent of non-adrenoceptor imidazoline binding sites. Psychopharmacology. 1994;113:493–499. doi: 10.1007/BF02245229. [DOI] [PubMed] [Google Scholar]
- Siviy SM, Harrison KA, McGregor IS. Fear, risk assessment, and playfulness in the juvenile rat. Behav Neurosci. 2006;120:49–59. doi: 10.1037/0735-7044.120.1.49. [DOI] [PubMed] [Google Scholar]
- Siviy SM, Panksepp J. Energy balance and play in juvenile rats. Physiol Behav. 1985;35:435–441. doi: 10.1016/0031-9384(85)90320-8. [DOI] [PubMed] [Google Scholar]
- Siviy SM, Panksepp J. Sensory modulation of juvenile play in rats. Dev Psychobiol. 1987;20:39–55. doi: 10.1002/dev.420200108. [DOI] [PubMed] [Google Scholar]
- Solinas M, Goldberg SR. Motivational effects of cannabinoids and opioids on food reinforcement depend on simultaneous activation of cannabinoid and opioid systems. Neuropsychopharmacology. 2005;30:2035–2045. doi: 10.1038/sj.npp.1300720. [DOI] [PubMed] [Google Scholar]
- Špinka M, Newberry RC, Bekoff M. Mammalian play: training for the unexpected. Q Rev Biol. 2001;76:141–168. doi: 10.1086/393866. [DOI] [PubMed] [Google Scholar]
- Trezza V, Vanderschuren LJMJ. Bidirectional cannabinoid modulation of social behavior in adolescent rats. Psychopharmacology. 2007 doi: 10.1007/s00213-007-1025-3. [DOI] [PubMed] [Google Scholar]
- van den Berg CL, Hol T, Van Ree JM, Spruijt BM, Everts H, Koolhaas JM. Play is indispensable for an adequate development of coping with social challenges in the rat. Dev Psychobiol. 1999a;34:129–138. [PubMed] [Google Scholar]
- van den Berg CL, Pijlman FT, Koning HA, Diergaarde L, Van Ree JM, Spruijt BM. Isolation changes the incentive value of sucrose and social behaviour in juvenile and adult rats. Behav Brain Res. 1999b;106:133–142. doi: 10.1016/s0166-4328(99)00099-6. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Niesink RJM, Spruijt BM, Van Ree JM. Effects of morphine on different aspects of social play in juvenile rats. Psychopharmacology. 1995a;117:225–231. doi: 10.1007/BF02245191. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Niesink RJM, Spruijt BM, Van Ree JM. Influence of environmental factors on social play behavior of juvenile rats. Physiol Behav. 1995b;58:119–123. doi: 10.1016/0031-9384(94)00385-i. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Niesink RJM, Spruijt BM, Van Ree JM. Mu- and kappa-opioid receptor-mediated opioid effects on social play in juvenile rats. Eur J Pharmacol. 1995c;276:257–266. doi: 10.1016/0014-2999(95)00040-r. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Niesink RJM, Van Ree JM. The neurobiology of social play behavior in rats. Neurosci Biobehav Rev. 1997;21:309–326. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Trezza V, Griffioen-Roose S, Schiepers OJG, Van Leeuwen N, De Vries TJ, Schoffelmeer ANM. Methylphenidate disrupts social play behavior in adolescent rats. Neuropsychopharmacology. 2008 doi: 10.1038/npp.2008.10. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP, Spear NE. Social behavior and social motivation in adolescent rats: role of housing conditions and partner's activity. Physiol Behav. 1999;67:475–482. doi: 10.1016/s0031-9384(98)00285-6. [DOI] [PubMed] [Google Scholar]


