Abstract
Vitamin D-binding protein (DBP) and albumin (ALB) are abundant serum proteins and both possess high-affinity binding for saturated and unsaturated fatty acids. However, certain differences exist. We surmised that in cases where serum albumin level is low, DBP presumably can act as a transporter of fatty acids. To explore this possibility we synthesized several alkylating derivatives of 14C-palmitic acid to probe the fatty acid binding pockets of DBP and ALB. We observed that N-ethyl-5-phenylisooxazolium-3′-sulfonate-ester (WRK ester) of 14C-palmitic acid specifically labeled DBP; but p-nitrophenyl- and N-hydroxysuccinimidyl-esters failed to do so. However, p-nitrophenyl ester of 14C-palmitic acid specifically labeled bovine ALB, indicating that the micro-environment of the fatty acid-binding domains of DBP and ALB may be different; and DBP may not replace ALB as a transporter of fatty acids.
Keywords: Fatty acid-binding by vitamin D binding protein (DBP) and albumin (ALB), serum transport of fatty acids, affinity labeling analogs of palmitic acid, affinity labeling of fatty acid binding sites of DBP and ALB
Introduction
Group specific component (Gc) or vitamin D-binding protein (DBP) is a sparsely glycosylated and polymorphic serum protein. The two major phenotypes are Gc1 and Gc2, differing from each other by four (4) amino acids in the primary structure as well as structure of attached polysaccharide. Gc1 is further divided into two subtypes differing in primary structure as well as structure of the attached carbohydrates [1–3]
DBP is a multi-functional protein [4]. Its binding of vitamin D and its metabolites has been studied extensively leading to the understanding that DBP is responsible for the stepwise activation of vitamin D3 to 25-hydroxyvitamin D3 (25-OH-D3) and finally to its physiologically most active metabolite, 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3). It is also involved in the transportation of these small molecules to organs and cells wherever they are required. In addition DBP plays an integral role in the circulating actin-scavenging system in plasma. Plasma gelsolin severes filaments of F-actin, and DBP binds to actin monomer (G-actin) with high affinity, thus preventing G-actin to polymerize and clog arteries during cell-injury and lysis [5,6]. Presence of actin-DBP complex in the sera of human and animals sustaining injuries/inflammation, e.g. trophoblastic emboli, severe hepatitis, acute lung injury etc. positively implicates DBP in thrombosis and heart attack [7]. DBP also binds chemotactic agents such as C5a and C5a des Arg, thus enhancing complement activation on neutrophil chemotaxis [8,9]. Furthermore, a post-translationally modified form of DBP (DBP-macrophage activating factor, DBP-maf) has been shown to have strong macrophage- and osteoclast-activating [10–14] and anti-angiogenic and anti-tumor properties [15,16].
In addition to above properties of DBP and its derivative (DBP-maf), DBP binds saturated and unsaturated fatty acids with high affinity (Kd=105 − 106M−1), similar to plasma ALB [17,18]. However, certain differences do exist. For example, Ena et al. demonstrated that molar ratio of fatty acids, bound to human DBP to DBP is 0.4 compared with 1.8 for human ALB [19]. Furthermore, majority of DBP-bound fatty acids are mono-unsaturated or saturated, and abundance of poly-unsaturated fatty acids is less than 5% (of the total bound fatty acids) [19]. Another interesting observation includes competition between vitamin D sterols and fatty acids in terms of binding to DBP. For example, it was reported that poly-unsaturated fatty acids, such as arachidonic or linoleic acid, strongly compete with 25-OH-D3 and 1,25(OH)2D3 for binding to DBP, in sharp contrast with saturated fatty acids e.g. palmitic acid, which offer no significant competition [19,20]. Furthermore, Bouillon et al. observed that addition of human ALB in a physiological ALB:DBP ratio did not impair the inhibitory effect of linoleic acid towards DBP-25-OH-D3-binding [20].
We hypothesized that this apparent anomaly between DBP and ALB in terms of fatty acid binding might be related to the actual binding process between these proteins and fatty acids, which, in turn, might be related to the micro-environment of the fatty acid binding pockets of these proteins. In order to evaluate this possibility we synthesized several reactive esters of 14C-palmitic acid as potential affinity labeling reagents for DBP and ALB. Results of these studies and their probable physiological implications are discussed in this report.
Materials and methods
Purified human DBP was obtained from commercially available pooled human serum (American Red Cross, Dedham, MA) by a ligand affinity chromatographic method developed in our laboratory [21]. Defatted bovine serum ALB (BSA) and all chemicals were purchased from Sigma-Aldrich, Milwaukee, WI, except 1-14C-palmitic acid (specific activity 56 mCi/mmol) which was a product of NEN-DuPont, Boston, MA.
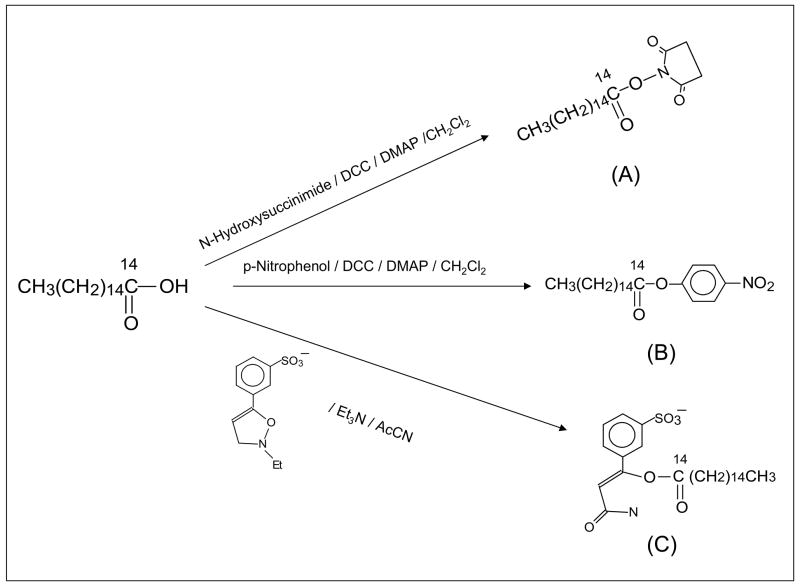
Synthesis (Figure 1)
Figure 1.
Scheme for the synthesis of N-hydroxy-succinimido-14C-palmitate (A), p-nitrophenyl-14C-palmitate (B), and WRK-14C-palmitate (C).
The N-hydroxysuccinimido- and p-nitrophenyl- esters of palmitic acid were synthesized by dicyclohexylcarbodiimide (DCC)-coupling of palmitic acid with N-hydroxysuccinimide, or p-nitrophenol in the presence of a catalytic amount of N,N′-dimethylaminopryridine (DMAP) in anhydrous dichloromethane. Synthesis of WRK-palmitate was carried out by treating palmitic acid with N-ethyl-5-phenyl-isooxazolium-3′-sulfonate (Woodward’s reagent K) and triethylamine in acetonitrile. Product from each reaction was purified by preparative chromatography on silica plates (Analtech, Vineland, NJ), and each product was characterized by NMR. Radioactive synthesis was carried out exactly the same way except palmitic acid was replaced with 14C-palmitic acid. Products from the radioactive reaction were isolated by TLC matching with corresponding unlabeled compounds.
Affinity labeling studies of bovine serum ALB and DBP with N-hydroxy-succinimido-14C-palmitate (A), p-nitrophenyl-14C-palmitate (B), and WRK-14C-palmitate (C)
20 μg Samples each of BSA and DBP in 20 μl of TEST buffer (50 mM Tris.HCL, 150 mM NaCl, 1.5 mM EDTA, 0.1%Triton X-100, pH 8.8) were treated with N-hydroxysuccinimdo-14C-palmitate (A), p-nitrophenyl-14C-palmitate (B), or WRK-14C-palmitate (C) (each 20,000 cpm) at 25°C for 20 hours. Parallel samples of BSA and DBP containing additional sodium palmitate (one μg in 10 μl of buffer) were also treated the same way. At the end of the experiment all the samples were analyzed on a 7.5% SDS-polycarylamide gel, followed by drying the gel and scanning of radioactivity in a Biosan phosphorimager.
Results and discussion
There is a remarkable structural homology among ALB, DBP, alpha-feto protein (AFP) and afamin, members of the albumin gene family. All these proteins have modular structures with three domains (domains I-III) and high cysteine-content [22]. In the case of DBP all the Cys residues (total 28) are oxidized to form 14 disulfide bonds. In contrast, ALB contains several free sulphydryl groups in its primary structure. Furthermore, DBP has a shorter domain III than ALB. These structural differences may explain gross functional differences between DBP and ALB. For instance, vitamin D sterols- and G-actin binding and related functions are unique to DBP. On the other hand, DBP possesses relatively weaker binding for fatty acids compared with ALB. Furthermore, DBP contains a single high affinity fatty acid-binding site compared to ALB which contains several low- and high-affinity binding sites [18]. In ALB these binding sites are distributed among various domains of the protein, although high affinity-binding sites are located in domain III [23]. Moreover, as described earlier, DBP, in contrast with ALB, discriminates between saturated and unsaturated fatty acids in terms of binding.
All the above observations point to difference in the nature of binding between ALB and DBP and fatty acids, which in turn may be related to the fatty acid-binding pocket structure of these proteins. Affinity and photoaffinity labeling techniques have been used widely to probe binding pockets and catalytic active sites of receptors and enzymes respectively [24]. Our laboratory has used these techniques, and others to probe the vitamin D and actin-binding domain structures of DBP, leading to crystal structure of the DBP-actin complex [25–35].
In the current study we synthesized radiolabeled versions of three reactive esters of palmitic acid to probe the fatty acid binding pockets of DBP and ALB. We chose palmitic acid, a saturated fatty acid as model because DBP has a propensity to bind saturated and mono-unsaturated fatty acids stronger that polyunsaturated fatty acids [19,20].
Reed employed WRK-14C-palmitate (C) to affinity label the fatty acid-binding pocket/s of bovine serum ALB [36]. In our case, incubation of a sample of human serum DBP (hDBP) with WRK-14C-palmitate (C) covalently labeled the protein as determined by autoradiography (Figure 2, Lane 1). When the incubation was carried out in the presence of an excess of sodium palmitate, labeling was completely obliterated (Figure 2, Lane 2). These results strongly indicated that WRK-14C-palmitate (C) specifically labeled the palmitic acid-binding pocket in hDBP. These results also suggested that structure and chemical environment of the fatty acid binding pockets of DBP and ALB are similar.
Figure 2.
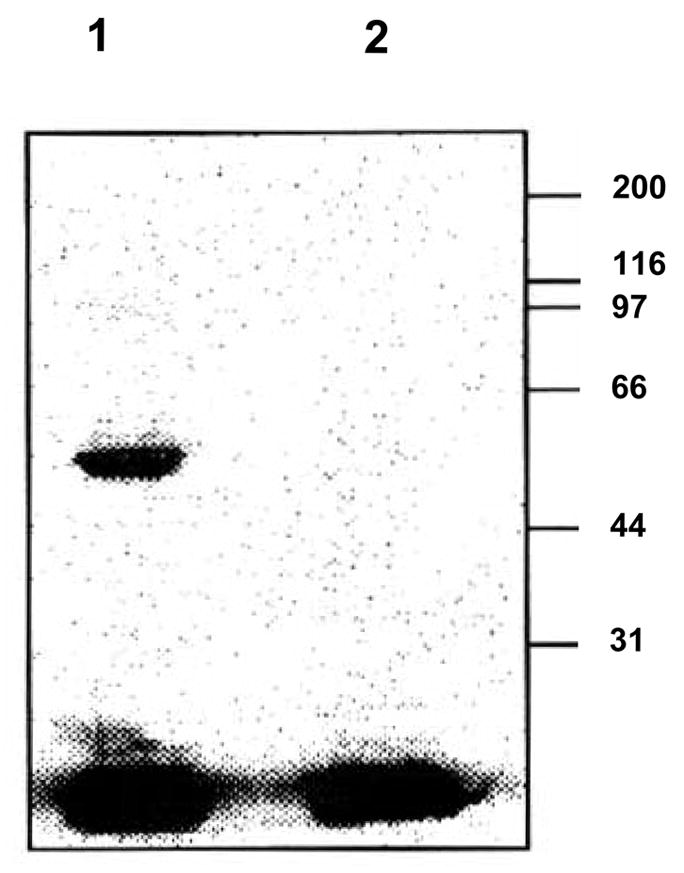
Affinity labeling of hDBP with WRK-14C-palmitate (C): samples of hDBP were incubated at 25°C with WRK-14C-palmitate (C) alone (Lane 1), or in the presence of an excess of sodium palmitate (Lane 2). The samples were electroporesed on a SDS gel and exposed to a phosphorimager. Positions of the standard molecular weight markers are denoted on the right.
Surprisingly other activated esters of palmitic acid i.e. N-hydroxysuccinimidyl-14C-palmitate (A) and p-nitrophenyl-14C-palmitate (B) failed to label DBP in the presence or in the absence of an excess of sodium palmitate. In the case of BSA, N-hydroxysuccinimidyl-14C-palmitate (A) failed to label this protein. But, p-nitrophenyl-14C-palmitate (B) labeled BSA, and labeling was significantly reduced in the presence of an excess of palmitic acid, denoting specific labeling of the fatty acid binding pocket (results not shown).
Collectively the above results suggest that chemical/electronic environments of the fatty acid binding pockets of DBP and ALB are different, so that ALB can tolerate a hydrophobic (p-nitrophenyl) as well as a hydrophilic (Woodward K reagent) head group at the carboxy terminal of palmitic acid. But, fatty acid binding site of DBP can only accommodate a polar and Zwitterionic carboxy head group (Woodward K reagent).
Analbuminemia is a rare hereditary disease in which the afflicted individuals have very low or negligible amount of circulating serum ALB [37–39]. We surmised that since both ALB and DBP bind fatty acids with high affinity, DBP may replace ALB in carrying fatty acids, particularly saturated and mono-unsaturated fatty acids in the cases of low or negligible amount of circulating ALB. However, results of the study delineated in this communication suggest that chemical and electronic environment of the fatty acid binding pockets of DBP and ALB might be different. As a result binding and transportation of various fatty acids might be different. Thus, DBP may not replace ALB in terms of fatty acid scavenging and transportation.
Acknowledgments
This work was supported in part by a grant from National Institute of Diabetes, Digestive and Kidney Diseases (RO1 DK 44337).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lichenstein HS, Lyons DE, Wurfel MM, Johnson DA, McGinley MD, Leidli JC, Trollinger DB, Mayer JP, Wright SD, Zukowski MMSD. Afamin is a new member of the albumin, alpha-fetoprotein, and vitamin D-binding protein gene family. J Biol Chem. 1994;269:18149–18154. [PubMed] [Google Scholar]
- 2.Ray R. Molecular recognition in vitamin D-binding protein. Proc Soc Exp Biol Med. 1996;212:305–312. doi: 10.3181/00379727-212-44020. [DOI] [PubMed] [Google Scholar]
- 3.Cooke NE, David EV. Serum vitamin D-binding protein is a third member of the albumin and alpha-fetoprotein gene family. J Clin Invest. 1985;76:2420–2424. doi: 10.1172/JCI112256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haddad JG JG. Plasma vitamin D-binding protein (Gc-globulin): multiple tasks. J Steroid Biochem Mol Biol. 1995;53:579–582. doi: 10.1016/0960-0760(95)00104-8. [DOI] [PubMed] [Google Scholar]
- 5.Goldschmidtt-Clermont PJ, van Baelen H, Bouillon R, Shook TE, Williams MH, Nel AE, Galbraith RM. Role of group-specific component (vitamin D binding protein) in clearance of actin from the circulation in rabbit. J Clin Invest. 1988;81:1519–1527. doi: 10.1172/JCI113484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee WM, Galbraith RM. The extracellular actin-scavenger system and actin toxicity. N Engl J Med. 1992;326:1335–1341. doi: 10.1056/NEJM199205143262006. [DOI] [PubMed] [Google Scholar]
- 7.Lind SE, Smith DB, Janmey PA, Stossel TP. Role of plasma gelsolin and vitamin D binding protein in clearing actin from circulation. J Clin Invest. 1986;78:736–742. doi: 10.1172/JCI112634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah AB, DiMartino SJ, Trujillo G, Kew RR. Selective inhibition of the C5a chemotactic cofactor function of the vitamin D binding protein by 1,25(OH)2vitamin D3. Mol Immunol. 2006;43:1109–1115. doi: 10.1016/j.molimm.2005.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J, Kew RR. Identification of a region in the vitamin D binding protein that mediates its C5a chemotactic function. J Biol Chem. 2004;279:53282–53287. doi: 10.1074/jbc.M411462200. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto N, Homma S. Vitamin D3 binding protein (group-specific component) is a precursor for the macrophage-activating signal factor from lysophosphatidylcholine-treated lymphocytes. Proc Natl Acad Sci. 1991;88:8539–8543. doi: 10.1073/pnas.88.19.8539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto N, Kumashiro R. Conversion of vitamin D3 binding protein (group-specific component) to a macrophage activating factor by the stepwise action of beta-galactosidase of B cells and sialidase of T cells. J Immunol. 1993;151:2794–2802. [PubMed] [Google Scholar]
- 12.Yamamoto N, Lindsay DD, Naraparaju VR, Ireland RA, Popoff SN. A defect in the inflammation-primed macrophage-activation cascade in osteopetrotic rats. J Immunol. 1994;152:5100–5107. [PubMed] [Google Scholar]
- 13.Schneider GB, Benis KA, Flay NW, Ireland RA, Popoff SN. Effects of vitamin D binding protein-macrophage activating factor (DBP-MAF) infusion on bone resorption in two osteopetrotic mutations. Bone. 1995;16:657–662. doi: 10.1016/8756-3282(95)00118-w. [DOI] [PubMed] [Google Scholar]
- 14.Swamy N, Ghosh S, Schneider GB, Ray R. Baculovirus-expressed vitamin D-binding protein-macrophage activating factor (DBP-maf) activates osteoclasts, and binding of 25-hydroxyvitamin D3 does not influence this activity. J Cell Biochem. 2001;81:535–546. doi: 10.1002/1097-4644(20010601)81:3<535::aid-jcb1067>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 15.Koga Y, Naraparaju VR, Yamamoto N. Antitumor effect of vitamin D-binding protein-derived macrophage activating factor on Ehrlich ascites tumor-bearing mice. Proc Soc Exp Biol Med. 999;220:20–26. doi: 10.1046/j.1525-1373.1999.d01-3.x. [DOI] [PubMed] [Google Scholar]
- 16.Kisker O, Onizuka S, Becker CM, Fannon M, Flynn E, D’Amato R, Zetter B, Folkman J, Ray R, Swamy N, Pirie-Shepherd S. Vitamin D binding protein macrophage activating factor (DBP-maf) inhibits angiogenesis and tumor growth in mice. Neoplasia. 2003;5:32–40. doi: 10.1016/s1476-5586(03)80015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams MH, Van Alstyne EL, Galbraith RM. Evidence of a novel association of unsaturated fatty acids with Gc (vitamin D-binding) protein. Biochem Biophys Res Comm. 1988;153:1019–1024. doi: 10.1016/s0006-291x(88)81330-5. [DOI] [PubMed] [Google Scholar]
- 18.Calvo M, Ena JM. Relations between vitamin D and fatty acid binding properties of vitamin D-binding protein. Biophys Res Comm. 1989;163:14–17. doi: 10.1016/0006-291x(89)92091-3. [DOI] [PubMed] [Google Scholar]
- 19.Ena JM, Esteban C, Perez MD, Uriel J, Calvo M. Fatty acids bound to vitamin D-binding protein (DBP) from human and bovine sera. Biochem Intl. 1989;19:1–7. [PubMed] [Google Scholar]
- 20.Bouillon R, Xiang DZ, Convents R, Van Baelen H. Polyunstaurated fatty acids decrease the apparent affinity of vitamin D metabolites for human vitamin D-binding protein. J Steroid Biochem Mol Biol. 1992;42:855–861. doi: 10.1016/0960-0760(92)90094-y. [DOI] [PubMed] [Google Scholar]
- 21.Swamy N, Roy A, Chang R, Brisson M, Ray R. Affinity purification of human plasma vitamin D-binding protein. Protn Expressn Purifn. 1995;6:185–188. doi: 10.1006/prep.1995.1023. [DOI] [PubMed] [Google Scholar]
- 22.Svasti J, Kurosky A, Bennett A, Bowman BH. Molecular basis for the three major forms of human serum vitamin D binding protein (group specific component) Biochemistry. 1979;18:1611–1617. doi: 10.1021/bi00575a036. [DOI] [PubMed] [Google Scholar]
- 23.Spector AA. In: Biochemistry ad biology of plasma lipoproteins. Scanu AM, Spector AA, editors. Marcel Dekkar Inc; NY: 1986. pp. 247–279. [Google Scholar]
- 24.Sweet FW, Murdock GL. Affinity labeling of hormone-specific proteins. Endo Rev. 1987;8:154–184. doi: 10.1210/edrv-8-2-154. [DOI] [PubMed] [Google Scholar]
- 25.Ray R, Holick SA, Hanafin N, Holick MF. Photoaffinity labeling of the rat plasma vitamin D binding protein with [26,27-3H]-25-hydroxyvitamin D3-3-[N-(4-amido-2-nitro phenyl) glycinate] Biochemistry. 1986;25:4729–4733. doi: 10.1021/bi00365a001. [DOI] [PubMed] [Google Scholar]
- 26.Ray R, Bouillon R, Van Baelen HG, Holick MF. Photoaffinity labeling of rat plasma vitamin D binding protein with a second generation photoaffinity analog of 25-hydroxyvitamin D3. Biochemistry. 1991;36:4809–4813. doi: 10.1021/bi00233a024. [DOI] [PubMed] [Google Scholar]
- 27.Ray R, Bouillon R, Van Baelen HG, Holick MF. Photoaffinity labeling of human serum vitamin D binding protein, and chemical cleavages of the labeled protein: Identification of a 11.5 KDa peptide, containing the putative 25-hydroxyvitamin D3-binding site. Biochemistry. 1991;30:7638–7642. doi: 10.1021/bi00244a036. [DOI] [PubMed] [Google Scholar]
- 28.Swamy N, Ray R. 25-Hydroxy[26,27-methyl-3H]vitamin D3-3-(1,2- epoxypropyl)ether: an affinity labeling reagent for human vitamin D-binding protein. Arch Biochem Biophys. 1995;319:504–507. doi: 10.1006/abbi.1995.1323. [DOI] [PubMed] [Google Scholar]
- 29.Swamy N, Ray R. Affinity labeling of rat serum vitamin D binding protein. Arch Biochem Biophys. 1996;333:139–144. doi: 10.1006/abbi.1996.0374. [DOI] [PubMed] [Google Scholar]
- 30.Addo JK, Ray R. Synthesis and binding analysis of 5E-[19-(2-bromoacetoxy)methyl]25-hydroxyvitamin D3 and 5E-25-hydroxyvitamin D3-19-methyl[(4-azido-2-nitro)phenyl]glycinate: novel C19-modified affinity and photoaffinity analogs of 25hydroxyvitamin D3. Steroids. 1998;63:218–223. doi: 10.1016/s0039-128x(98)00009-9. [DOI] [PubMed] [Google Scholar]
- 31.Addo JK, Swamy N, Ray R. C-6 functionalized analogs of 25-hydroxyvitamin D3 and 1α,25-dihydroxyvitamin D3: synthesis and binding analysis with vitamin D-binding protein and vitamin D receptor. Steroids. 1999;64:273–282. doi: 10.1016/s0039-128x(99)00009-4. [DOI] [PubMed] [Google Scholar]
- 32.Swamy N, Addo J, Uskokovic MR, Ray R. Probing the vitamin D sterol-binding pocket of human vitamin D-binding protein with affinity labeling reagents with the bromoacetate affinity probe at C-3, C-6, C-11 and C-19 positions of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3. Arch Biochem Biophys. 2000;373:471–478. doi: 10.1006/abbi.1999.1537. [DOI] [PubMed] [Google Scholar]
- 33.Ray A, Swamy N, Ray R. Cross-talk among structural domains of human DBP upon binding 25-hydroxyvitamin D3. Biochem Biophys Res Comm. 2007;365:746–750. doi: 10.1016/j.bbrc.2007.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swamy N, Head JF, Weitz D, Ray R. Biochemical and preliminary crystallographic characterization of the vitamin d sterol- and actin-binding by human vitamin d-binding protein. Arch Biochem Biophys. 2002;402:14–23. doi: 10.1016/S0003-9861(02)00033-4. [DOI] [PubMed] [Google Scholar]
- 35.Head JF, Swamy N, Ray R. Crystal structure of the complex between actin and human vitamin D-binding protein at 2.5A resolution. Biochemistry. 2002;41:9015–9020. doi: 10.1021/bi026054y. [DOI] [PubMed] [Google Scholar]
- 36.Reed RG. Location of long chain fatty acid-binding sites of bovine serum albumin by affinity labeling. J Biol Chem. 1986;261:15619–15624. [PubMed] [Google Scholar]
- 37.Watkins S, Madison J, Galliano M, Minchiotti L, Putnam FW. Analbuminemia: three cases resulting from different point mutations in the albumin gene. Proc Natl Acad Sci USA. 1994;91:9417–9421. doi: 10.1073/pnas.91.20.9417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campagna F, Fioretti F, Burattin M, Romeo S, Sentinelli F, Bifolco M, Sirinian MI, Del Ben M, Angelico F, Arca M. Congenital analbuminemia attributable to compound heterozygosity for novel mutations in the albumin gene. Clin Chem. 2005;51:1256–1258. doi: 10.1373/clinchem.2005.048561. [DOI] [PubMed] [Google Scholar]
- 39.Docini L, Caridi G, Dagnino M, Sala A, Gokce G, Sokucu S, Campagnoli M, Galliano M, Minchiotti L. Analbuminemia produced by a novel splicing mutation. Clin Chem. 2007;53:1549–1552. doi: 10.1373/clinchem.2007.089748. [DOI] [PubMed] [Google Scholar]