Abstract
Current concepts of addiction focus on neuronal neurocircuitry and neurotransmitters and are largely based on animal model data, but the human brain is unique in its high myelin content and extended developmental (myelination) phase that continues until middle age. The biology of our exceptional myelination process and factors that influence it have been synthesized into a recently published myelin model of human brain evolution and normal development that cuts across the current symptom-based classification of neuropsychiatric disorders.
The developmental perspective of the model suggests that dysregulations in the myelination process contribute to prevalent early-life neuropsychiatric disorders, as well as to addictions. These disorders share deficits in inhibitory control functions that likely contribute to their high rates of comorbidity with addiction and other impulsive behaviors. The model posits that substances such as alcohol and psychostimulants are toxic to the extremely vulnerable myelination process and contribute to the poor outcomes of primary and comorbid addictive disorders in susceptible individuals.
By increasing the scientific focus on myelination, the model provides a rational biological framework for the development of novel, myelin-centered treatments that may have widespread efficacy across multiple disease states and could potentially be used in treating, delaying, or even preventing some of the most prevalent and devastating neuropsychiatric disorders.
The human brain is unique in its high myelin content and long developmental (myelination) phase that continues until approximately the age of 50 (Norton, 1981; Bartzokis et al., 2001; Semendeferi et al., 2002; reviewed in Bartzokis, 2004a). Before the advent of modern medicine, very few persons lived beyond age 50 and therefore, as a species, we evolved to continue myelinating over our entire natural life span. This extensive process of myelination markedly increases information-processing speed and underlies many of the brain’s unique capabilities such as language, inhibitory controls, and higher cognitive functions (reviewed in Bartzokis, 2004b). It is likely that the vulnerability of the myelination process to genetic and environmental insults underlies our brain’s unique susceptibility to developmental disorders such as autism, learning disabilities, attention-deficit/hyperactivity disorder (ADHD), schizophrenia, and addiction, as well as contributing to the male predominance of these disorders (Benes et al., 1994; Bartzokis, 2002; Bartzokis et al., 2002; Carper et al., 2002; Bartzokis, Neuchterlein, et al., 2003; Ho et al., 2003; for review, see Bartzokis, 2004a, b).
This chapter will focus on substance dependence and its relationship to inhibitory control of cognitive processes and behavioral impulses in the context of a recently published myelin model of human brain evolution and normal development (Bartzokis, 2002; 2004a, b). The model is useful in conceptualizing a wide range of age-related neuropsychiatric disorders over the entire human life span (Bartzokis, 2002; Bartzokis and Altshuler, 2003; Bartzokis, 2004a, b).
The focus on myelin and myelination is not intended to diminish the importance of other early brain developmental processes such as neurogenesis, which occurs primarily during the intrauterine period, and synaptic pruning and cell shrinkage, which occur after birth. Disruption of neurogenesis can cause catastrophic abnormalities that are usually evident at birth or in early infancy (Rakic, 2002). The later processes of pruning and elimination appear to reduce the connectivity of the infant’s and young child’s brain by as much as two-thirds (Huttenlocher and Dabholkar, 1997; Rakic, 2002). These processes of reduction, which have been hypothesized to affect several childhood disorders, are generally completed by puberty or mid-adolescence (Huttenlocher and Dabholkar, 1997; Rakic, 2002).
It is my intention, however, to point out that these reductive processes occur in concert with a precisely regulated but highly vulnerable and largely ignored developmental or growth process of myelination (Bartzokis et al., 2001; Bartzokis, 2002; Bartzokis et al., 2002; Bartzokis, Neuchterlein, et al., 2003; Bartzokis, 2004b). In fact, I argue that the regressive or pruning processes of childhood described in the prior paragraph occur in large part to provide the volume (space) and possibly other resources necessary to support the crucial process of myelination (Bartzokis et al., 2001; Bartzokis, 2004b). In humans, the regressive processes occur in a heterochronous pattern occurring in primary process areas (motor, sensory) before association areas (fontal, temporal, and parietal lobes) (Huttenlocher and Dabholkar, 1997), while in nonhuman primates these processes occur simultaneously in all cortical regions (Rakic et al., 1986). In humans, this pattern of the heterochronous volume-reducing regressive matches the pattern of heterochronous volume-increasing developmental process of myelination (Benes et al., 1994; Kemper, 1994; Bartzokis et al., 2001). The extra volume provided by synaptic, axonal, and dendritic pruning is necessary to accommodate the expanding volume of white matter as myelination continues throughout adolescence and adulthood in the fixed volume of the rigid human skull (Bartzokis et al., 2001).
The dramatic gains in processing speed, “bandwidth,” and reduced energy consumption provided by myelination more than compensate (functionally) for the regressive processes (Bartzokis, 2004a, b). Instead of reducing connectivity between different parts of the brain suggested by the regressive processes, the myelination process markedly increases the functional potential (connectivity) of the remaining circuits. It is thus possible to continue our cognitive development without the loss, and in fact with the addition, of higher level cognitive functions that eventually culminates in healthy middle-aged adults with better inhibitory controls underlying what is commonly referred to as wisdom (Bartzokis et al., 2001; Bartzokis, 2004b). Remarkably, and in the framework of the model not coincidentally, middle-aged adults have a markedly lower risk of addiction than they did in adolescence and young adulthood (Anthony and Helzer, 1991; Miller, 1991; Warner et al., 1995; Grant, 1997; Vega et al., 2002).
In this paper, I do not attempt a thorough review of other conceptual models of addiction. Many such paradigms have been published, some with complex schematics of brain circuitry and neurotransmitter systems. Most have focused on the impulsivity associated with drug abuse and the reward or motivation aspects of this impulsivity (Zuckerman, 1996; Evenden, 1999; Moeller et al., 2001; Chambers and Potenza, 2003; Chambers, Taylor, and Potenza, 2003; Deadwyler et al., 2004; Volkow et al., 2004). But no previous model has specifically focused on the central and unique role of continuing myelination to the developmental process, which contributes to the highly developed ability of humans to inhibit cognitive and behavioral impulses that trigger the compulsive drug use at the core of addictive behavior. Inhibition underlies an individual’s ability to discontinue addictive behaviors in the face of the negative consequences associated with them (Bartzokis et al., 2002; Bechara and Martin, 2004).
Our focus on the role of developing impulse inhibition functions (rather than impulsivity itself) is driven by the face validity and over-whelming scientific evidence that, unlike adulthood, impulsivity is essentially the default condition of childhood (Zuckerman, 1996; Cote et al., 2002). The long process of socializing children is inexorably intertwined with inhibiting impulsive behavior through development of inhibitory cognitive processes (for review, see Bjorklund and Harnishfeger, 1995; Harnishfeger, 1995). It is this developmental process that provides us with the ability to improve our performance when presented with the myriad of choices in everyday life. These choices are very frequently analogies of the often-used delayed discounting paradigm of childhood development experiments: you can have some candy now or wait (inhibit) and get a greater amount of candy later (Barkley et al., 2001; Alessi and Petry, 2003; Petry, 2003).
Inhibitory controls are necessary developments for expanding attention, language, cognition, emotional regulation, and socialization (Bjorklund and Harnishfeger, 1995; Dempster, 1995). In short, inhibitory controls are necessary for becoming a healthy human adult. Dysregulation of this developmental process likely contributes to many subsyndromal personality traits and neuropsychiatric disorders, including addiction (McElroy et al., 1992; Benes et al., 1994; Barkley et al., 2001; Bartzokis, 2002; Bartzokis et al., 2002; Carper et al., 2002; Alessi and Petry, 2003; Bartzokis, Neuchterlein, et al., 2003; Ho et al., 2003; Petry, 2003; for further review, see Bartzokis, 2004a, b). No matter how strong the reward, euphoria, or craving for drug-related experiences (or any other behavioral reinforcers, for that matter), if inhibitory control mechanisms are fast enough to interrupt the behaviors leading to drug use, the state of addiction can be interrupted and recovery (sobriety) can be achieved. The core focus of the current model is therefore the interactions between the biological developmental process of myelination, the physiological process of impulse inhibition, and genetic and environmental influences on these interactions. The model facilitates our understanding of the pathophysiological processes of multiple and often overlapping neuropsychiatric disorders that share deficits in inhibitory controls. Furthermore, it provides a biological basis for understanding the extensive comorbidity of these disorders (McElroy et al., 1992; Kessler et al., 1996; Keel and Mitchell, 1997; Merikangas et al., 1998; Alessi and Petry, 2003; Judd et al., 2003; Kaltiala-Heino et al., 2003; Petry, 2003; Stahlberg et al., 2004) by focusing on their shared deficits in the myelination process. This new focus cuts across our current symptom-based classification of such developmental disorders and could serve as the nidus of a more useful classification based on the biology and development of the human brain. The more immediate goal of presenting this myelin model of brain development, however, is to use its framework to suggest entirely novel treatment approaches of the developmental disorders we call addictions as well as the “comorbid” addictions that plague the successful treatment of most other neuropsychiatric disorders (Kessler et al., 1996; Merikangas et al., 1998; Swartz, Lurigio, and Goldstein, 2000; Kresina et al., 2004).
MYELIN, THE DEVELOPMENT OF THE HUMAN BRAIN’S “INTERNET,” AND AGE-RELATED CHANGES IN COGNITIVE PROCESSING
The importance of myelin in increasing axonal transmission speed, reducing action potential refractory time, and improving synchrony of brain function is well established (reviewed in Bartzokis, 2002, 2004a). The most important contributions of the model to the understanding of cognition and behavior is its focus on the high speed and frequency of action potentials needed for adequate information processing to be achieved in the human brain (Bartzokis, 2002, 2004a). The brain is organized in widely distributed neural networks, and its connectivity (as well as its speed of processing) is therefore paramount for higher cognitive functions (Salthouse and Kail, 1983; Verhaeghen and Salthouse, 1997). The impact of myelin on information processing speed makes the production and maintenance of myelin essential for normal brain function (Bartzokis et al., 2001; Bartzokis, 2002; Bartzokis et al., 2002; Bartzokis, Cummings, et al., 2003; Bartzokis, Neuchterlein, et al., 2003, Bartzokis, 2004b).
Myelination results in saltatory conduction of action potentials that increases signal transmission speed more than tenfold (Waxman, 1977). Speed makes it possible to integrate information across the highly spatially distributed neural networks that underlie higher cognitive functions (Fuster, 1999; Gould et al., 1999; Srinivasan, 1999; Mesulam, 2000; Bartzokis et al., 2001; Bartzokis, 2002). Axon myelination also decreases the refractory time (the interval needed for repolarization before a new action potential can be supported by the axon) to as little as 1/34th of its original value (Felts, Baker, and Smith, 1997). Thus, if the brain were compared to the Internet, we can say that myelination not only increases its speed (e.g., transforming it from a telephoneline-based system to a fiber optic system), but also its bandwidth, increasing the actual amount of information that can be transmitted per unit time (Bartzokis et al., 2001; Bartzokis, 2004a). This allows myelinated axons to support high frequency bursts of signals and thus facilitate information flow by allowing for precise temporal coding of these bursts of neuronal activity (Zhou, Abbas, and Assouline, 1995).
Until a circuit is fully myelinated, the network that it is part of will be unable to develop and maintain high frequency oscillations between regions on which many cognitive processes, including inhibitory processes, depend (Varela et al., 2001; for review, see Bartzokis, 2002, 2004b). In short, the circuit will not be on line to immediately influence cognition and behavior. Unfortunately, these crucial functional effects of myelination on cognitive processing speed have not been thoroughly integrated into neurophysiological and cognitive models of brain function (Bartzokis, 2003; Feinberg, 2003), partly because of an underappreciation of the lifelong nature of the human brain’s myelination (Bartzokis et al., 2001; Bartzokis, 2004b).
Over the last century, human life expectancy has doubled, from 40 to more than 80 years of age. The biology of myelination shows that this life span can be appropriately conceptualized as a roughly quadratic (inverted U) trajectory, as illustrated in Fig. 3.1 (Miller, Alston, and Corsellis, 1980; Benes et al., 1994; Kemper, 1994; Bartzokis et al., 2001; Ge et al., 2002; Bartzokis, Cummings, et al., 2003; Jernigan, 2003; Sowell et al., 2003; Bartzokis et al., 2004). The volume of myelinated white matter increases to a peak achieved at about age 50, followed by loss of myelinated white matter volume in older age (Kaes, 1907; Kemper, 1994; Bartzokis et al., 2001, 2004). This overall quadratic trajectory differs (is heterochronic) by brain region (Kaes, 1907; Kemper, 1994; Bartzokis et al., 2001, 2004) and function (Williams et al., 1999; Bartzokis et al., 2001, 2002; Bedard et al., 2002). Thus, association regions such as the frontal and temporal lobes reach peak myelinated white matter volumes decades after the peak volumes are reached in primary motor and sensory regions (Yakovlev and Lecours, 1967; Bartzokis et al., 2001, 2004). The view of development and aging as a continuum of interacting structural and functional developmental trajectories fosters the examination of multiple factors that promote or inhibit myelination throughout the protracted process of human brain development. Similar quadratic-like patterns of change over the lifespan have been demonstrated in neurophysiological and cognitive models of brain function (Salthouse and Kail, 1983; Dustman, Emmerson, and Shearer, 1996; Williams et al., 1999; Salthouse, 2000; Bedard et al., 2002) and are also observed in studies of clinical symptomatology in a variety of neuropsychiatric disorders (Cjte et al., 2002; Cote et al., 2002) including addiction (Warner et al., 1995; Grant, 1997; Vega et al., 2002).
Figure 3.1.
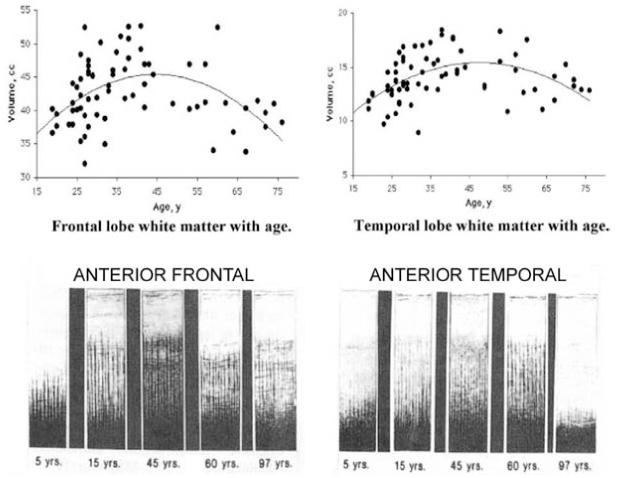
Quadratic trajectories of myelination of human brain over the life span. Myelination (Y axis) versus age (X axis) in frontal (left) and temporal (right) lobes of normal individuals. Top figures are in vivo data from Bartzokis et al. (2001). Lower figures show postmortem intracortical myelin stain data from Kaes (1907) adapted and reproduced in Kemper (1994). Used with permission.
The data were acquired 100 years apart, yet the two samples of normal individuals show remarkably similar myelination trajectories in the two regions. Note that different brain regions have significantly different myelination trajectories even when the regions are similar, as is the case with these two association regions. Peak myelination is reached in the frontal lobe at age 45 and even later in the temporal lobe.
Such quadratic-like life span trajectories have been clearly documented in age-related changes of cognitive speed that increase throughout childhood and early adulthood, then decrease with an accelerating rate in old age (Salthouse and Kail, 1983; Verhaeghen and Salthouse, 1997). Salthouse (1996) delineated the hypothesis that a basic parameter such as processing speed is directly related to biological factors and is essential for higher order cognitive processing. Similar patterns are apparent in paradigms that test inhibitory controls (Williams et al., 1999; Bedard et al., 2002), as well as some electroencephalographic parameters (Dustman et al., 1996). These similarities suggest that the principal underlying explanation for all these phenomena is the process of myelination (Bartzokis et al., 2001; Bartzokis, 2002; Peters and Sethares, 2002; Bartzokis, 2003, 2004a).
In the context of the myelin model (Bartzokis et al., 2001; Bartzokis, 2002, 2004a, b), the coordinated unfolding of many of the normal structural and functional brain changes listed in the previous paragraphs can be viewed as indirect evidence that, after infancy, myelination is the central biological process underlying human aging-related changes (Kemper, 1994; Bartzokis et al., 2001; Marner et al., 2003; Bartzokis, 2004a; Bartzokis et al., 2004). It is important to point out that the age-related cognitive improvements in processing speed and inhibitory control peak in adulthood (Dustman et al., 1996; Verhaeghen and Salthouse, 1997; Salthouse, 2000; Bedard et al., 2002). Thus, the development of inhibitory controls does not fit temporally (by more than a decade) with the regressive processes of neuronal, synaptic, axonal, and dendritic pruning and elimination that are basically complete before mid-adolescence (Huttenlocher and Dabholkar, 1997; Rakic, 2002).
MYELIN’S ROLE IN DEVELOPMENT OF BRAIN FUNCTIONS
Processing Speed and Language
In the first four decades of life, myelination is essential for establishing increasingly sophisticated psychological functional capacities such as inhibitory control, social behavior, and higher executive functions. The cognitive underpinnings of these functions depend on higher processing speeds (Salthouse and Kail, 1983; Verhaeghen and Salthouse, 1997) achieved through myelination (reviewed in Bartzokis, 2004a, b). Very high processing speed is also essential for a function that is essentially uniquely human: language (reviewed in Bartzokis, 2004a, b). Processing of speech sounds requires a very fast neuronal system capable of tracking rapid changes in acoustic input (Tallal, Miller, and Fitch, 1993; Belin et al., 1998; Zatorre, Belin, and Penhune, 2002). This functional necessity may have contributed to the development of human brain laterality (Zatorre et al., 2002), since localizing speech to one hemisphere would further increase the speed of processing by reducing the distances covered by this essential neural network. Postmortem and imaging evidence demonstrate increased white matter volume in the human speech region concerned with decoding the rapidly changing temporal information of speech (Penhune et al., 1996; Anderson, Southern, and Powers, 1999). This increase in white matter volume has been shown to be largely due to thicker axonal myelin sheaths (Anderson et al., 1999).
The special dependence of language on the process of myelination makes disturbances in language development a sensitive indicator of dysregulation of the myelination process in other structures and functions that are being actively myelinated concurrently. Thus in the context of the model, whose premise is that the quadratic trajectories of development differ by brain region (circuit) and function, it is not surprising that language impairments are highly comorbid in many earlier-onset psychiatric disorders (such as pervasive developmental disorders) whose pathophysiology is likely also influenced by dysregulation of the highly vulnerable myelination process (Carper et al., 2002; Bartzokis, 2004a, b). In the same vein of thought, a disturbance in the myelination process at a later stage of development, when more sophisticated attention and impulse inhibition functions are being brought on line, provides a similar explanation for the high rates of comorbid addiction in disorders that arise later in development such as ADHD, schizophrenia, and bipolar disorder. All of these disorders share deficits in cognitive and behavioral inhibitory functions. Imaging evidence suggests that these disorders are also likely affected by dysregulations in the process of myelination (de la Monte, 1988; Crosbie and Schachar, 2001; Fleck, Sax, and Strakowski, 2001; Bartzokis et al., 2002; Castellanos et al., 2002; Mostofsky et al., 2002; Bartzokis, Goldstein, et al., 2003; Ho et al., 2003; Kieseppa et al., 2003; Tkachev et al., 2003; Vinogradov et al., 2003; Uranova et al., 2004), especially the subpopulations that have a poor outcome and are considered treatment resistant (Bartzokis et al., 2002; Bartzokis, 2003; Bartzokis and Altshuler, 2003; Swann et al., 2004).
Processing Speed, Decision Making, and Inhibitory Controls
The frontal lobes undergo the greatest expansion in the course of both evolution and individual maturation. In adult humans, these lobes constitute as much as one-third of the total brain volume. As I described for speech in the prior section, in humans, the disproportional contributor to the enlargement of the late-maturing frontal lobes is the higher white matter and myelin content (Norton, 1981; Bartzokis et al., 2001; Semendeferi et al., 2002). Going against popular belief, a recent study shows that, in comparison with primates, adult humans do not have disproportionately enlarged frontal lobes for our overall brain size; rather, we have disproportionately more (20%) white matter in our frontal lobes (Semendeferi et al., 2002). This finding supports the overall evolutionary trend to have increasing cognitive abilities associated with disproportionate increases in glia rather than neuron numbers. During brain evolution, glial cell numbers have increased disproportionately more than neurons; from 10-20% of all brain cells in nematodes to 25% in flies, 65% in rodents, and 90% in humans (Pfrieger and Barres, 1995). This results in the disproportionately higher percentage (30%) of brain dry weight accounted for by myelin in humans than in rodents (Norton, 1981).
The frontal lobes are principally involved in higher executive functions such as the temporal organization of goal-directed actions in the domains of cognition, language, and behavior (Fuster, 2002). The second-by-second integration of neural inputs from the senses and the rest of the brain into coherent structures of action (as well as inhibition of those actions) is served by cognitive functions such as working memory, preparatory set, and inhibitory control (Fuster, 2002). The prefrontal cortex hosts multiple distinct mechanisms of decision making and inhibitory control (Fuster, 2002; Hinshaw, 2003; Bechara and Martin, 2004; Kamarajan et al., 2004), and persons with addictions may have impairments in any one or combination of them (Bartzokis et al., 2002; Bechara and Martin, 2004; Kamarajan et al., 2004). What is common to all these frontal lobe functions is the high degree of connectivity to other brain areas (Fuster, 2002). The speed and bandwidth provided by myelination, the last achievement in human brain development, is an essential aspect of this connectivity and makes the integrative function of the frontal lobes possible (Bartzokis et al., 2001; reviewed in Bartzokis, 2004a, b). Consistent with the multiple distinct mechanisms of decision making and inhibitory control, the process of myelination is regionally and temporally heterogeneous, or heterochronic (Kemper, 1994; Huttenlocher and Dabholkar, 1997; Bartzokis et al., 2001; Bartzokis, 2002; Bartzokis et al., 2004). Different neuropsychiatric phenomena could thus result, depending on the interaction of an individual’s genetic makeup and age when a dysregulation of this developmental process occurs (Bartzokis, 2002; Bartzokis, Neuchterlein, et al., 2003; reviewed in Bartzokis, 2004b).
The model suggests that dysregulations in this highly vulnerable process will contribute to the production of uniquely human diseases. At young ages (first decade of life), such dysregulations may manifest as disturbances (e.g., various degrees of autism, learning deficits, and severe ADHD) that involve language and processing-speed deficits (Rucklidge and Tannock, 2002; Wu, Anderson, and Castiello, 2002; Howlin, 2003; Jansson-Verkasalo et al., 2003). At older ages, they may manifest in disorders of higher level cognitive processes and inhibitory controls contributing to the development of ADHD, bipolar disorder, schizophrenia, and other forms of psychopathology (Crosbie and Schachar, 2001; Bartzokis, 2002; Wu et al., 2002; Bartzokis, Neuchterlein, et al., 2003; reviewed in Bartzokis, 2004b), including addictions and comorbid addictions (Barkley et al., 2001, 2002; Lim et al., 2002; Bjork, Grant, and Hommer, 2003; Bechara and Martin, 2004; Kamarajan et al., 2004).
Gender Effects on Myelination and Its Implications in Psychiatric Disease
Postmortem and imaging data show that human females reach high myelination levels earlier than males in multiple regions of the brain (Benes et al., 1994; Yurgelun-Todd, Killgore, and Young, 2002), and the association between processing speed and white matter volume is in part genetically determined (Posthuma et al., 2003). Not surprisingly, female gender is also associated with better language performance and faster trajectory of language acquisition (Karrass et al., 2002). In the context of the model, these observations suggest that gender-associated genes and hormonal influences may affect myelination of speech areas and may also increase the risk for a variety of developmental neuropsychiatric diseases in males (reviewed in Bartzokis, 2004b).
The model predicts that the promyelinating effects of female gender may protect from developmental vulnerabilities of the myelination process (reviewed in Bartzokis, 2004b). It thus suggests that the better outcomes in inhibitory control and hyperactivity ratings for girls during childhood (Cjte et al., 2002; Cote et al., 2002), as well as girls’ consistently lower prevalence of drug use at older ages (Hill and Chow, 2002; Vega et al., 2002), are due to the protective effect of faster myelination. In addition to addiction, a striking male predominance is also observed in many disorders such as learning disabilities, autism, ADHD, and schizophrenia (Silver, 1991; Volkmar, 1991; Weiss, 1991; Aleman, Kahn, and Selten, 2003; Constantino and Todd, 2003; reviewed in Bartzokis, 2004b). Protective effects of female gender and hormones, mediated by myelination, may explain gender discrepancies in these illnesses as well (Benes et al., 1994; Bartzokis, 2002, 2004b).
The impact of dysregulated myelination in these disorders should also have neuroimaging and cognitive correlates in common, such as abnormal development of white matter, decreased processing speed, and language abnormalities, even when the overall intellect of the patient appears intact (McAlonan et al., 2002; Howlin, 2003). Such observations have been reported in autism (Townsend et al., 2001; Carper et al., 2002; Herbert et al., 2002; McAlonan et al., 2002; Howlin, 2003; Jansson-Verkasalo et al., 2003), in ADHD (Barkley et al., 2001; Castellanos et al., 2002; Cjte et al., 2002; Cote et al., 2002; Mostofsky et al., 2002; Rucklidge and Tannock, 2002; Wu et al., 2002; McInnes et al., 2003), in addiction (de la Monte, 1988; Liu et al., 1998; Bartzokis et al., 2002; Mayfield et al., 2002; Bjork et al., 2003; Albertson et al., 2004), in schizophrenia (Fleck et al., 2001; Hof et al., 2002; Bartzokis, Neuchterlein, et al., 2003; Ho et al., 2003; Hof et al., 2003; Tkachev et al., 2003; Vinogradov et al., 2003; Uranova et al., 2004), and in bipolar disorder (Kieseppa et al., 2003; Tkachev et al., 2003; Adler et al., 2004; Uranova et al., 2004). These findings deserve further detailed investigation in a framework focused on age-related (developmental) changes in brain myelin content (Carper et al., 2002; Bartzokis, Neuchterlein, et al., 2003; Bartzokis, 2004b). In this myelin-based developmental framework, the shared deficits in inhibitory controls between all these disorders make the high rates of comorbid addiction (Kessler et al., 1996; Merikangas et al., 1998; Swartz et al., 2000; Bjork et al., 2003; Kresina et al., 2004) an expected functional epiphenomenon of shared myelination deficits.
INHIBITORY CONTROLS AND THE ADDICTIONS
Gibson (1991) has argued, on the basis of neuropsychological and linguistic data, that the cognitive development of the child is dependent on the development of cortical myelin. I have argued that the cognitive development of the adolescent and adult is also dependent on cortical myelin and that our extensive myelination process underlies the functions that make us human as well as vulnerable to human neuropsychiatric disorders (Bartzokis et al., 2001; Bartzokis, 2002; Bartzokis et al., 2002; Bartzokis, 2004a, b).
Although the reaction time needed to act on a stimulus is shortest from the end of adolescence through early adulthood (18-29 years) and then lengthens in older age (Williams et al., 1999; Bedard et al., 2002), the reaction time needed to inhibit such psychomotor responses continues improving and, for more complex inhibitory responses, does not peak until middle age (30-59 years), after which it also declines (Bedard et al., 2002). Although both the action and inhibitory processes have a roughly quadratic lifetime trajectory, these trajectories are different from each other, with a later peak for more sophisticated and processing-intensive inhibitory responses (Bedard et al., 2002) that would be expected to involve the later myelinating frontal lobe functions (Bartzokis et al., 2001; Fuster, 2002; Bartzokis, Cummings, et al., 2003; Bartzokis et al., 2004). The quadratic relationship of the inhibitory responses is strikingly similar to the quadratic trajectory of myelination, in which peak myelination in the frontal lobe is not achieved until middle age (Kemper, 1994; Bartzokis et al., 2001) and is followed by myelin breakdown in older age (Kemper, 1994; Bartzokis et al., 2001; Bartzokis, Cummings, et al., 2003; for review, see Bartzokis, 2004a, b). Consistent with the myelination model, in childhood (ages 6-12), girls develop impulse inhibition and control of hyperactive behavior at a strikingly faster rate than boys, resulting in much lower rates of ADHD and conduct disorder in adolescence (Cjte et al., 2002). Furthermore, in the context of the model, the late development of these inhibitory functions suggests that they are especially vulnerable to dysregulation from a variety of influences (Bartzokis, 2004b).
The everyday environment necessitates a continual series of appropriate responses to constantly changing conditions (Fuster, 2002; Bechara and Martin, 2004). In this fast-moving setting, inhibitory control is not just the result of knowing that a response should be inhibited or even of the inhibition process itself, but results instead from its timing—how quickly and precisely one can inhibit the upcoming inappropriate or impulsive action. For inhibitory mechanisms to be practically useful, they must be precisely timed in the second-by-second continuously unfolding and ongoing environment of the everyday world. To continue the Internet metaphor, the inhibitory processing circuits must be on line and wired with fast and wide bandwidth connections to the rest of the brain’s Internet. Thus, only myelination can make it possible for inhibitory circuits to achieve the precise and reliable timing needed to influence ongoing cognition and behavior. Individuals with poor inhibitory controls have difficulty navigating the instantaneously unfolding world even if—given enough time (e.g., in the setting of a therapist’s office or alcoholics anonymous meeting)—they are able to discern the course of action that is most advantageous and appropriate. More bluntly put, having knowledge of right and wrong does not always guarantee that the right choice will be made consistently, in the everyday world.
The evolutionary necessity of inhibitory cognitive and behavioral functions, in order for human societies to develop, is established (reviewed in Bjorklund and Harnishfeger, 1995). This necessity is clinically evident in the case of recovering addicts, who can usually clearly express their desire to achieve sobriety and can easily describe the negative effects of drug use in their lives. When such individuals are faced with an environment conducive to drug use combined with distractive stimuli (such as those produced by craving for the drug), however, their inhibitory controls often fail; their inhibitory responses are not fast or well timed enough. This leads to a series of poor choices that allow addictive behavior to unfold and ultimately to result in drug use and relapse.
The relapse into addictive behavior may take place over minutes, hours, or even days. When inhibitory controls are not fast or precise enough, an addict will miss myriad sequential opportunities for inhibitory control mechanisms to prevent the next behavior in the chain comprising the addictive behavior pattern. Going down the “slippery slope” of relapse captures the essence of this lack of control. Thus, addicts often experience their relapse as occurring in an instant, as if there were not enough time to contemplate and process the pros and cons of their actions. Conversely, recovering addicts with better inhibitory controls will have myriad opportunities to inhibit their impulses precisely and for long enough to interrupt this chain of events at many points. In such persons, inhibitory control may become so fast and precise that they would not even allow the conscious concept of the first action, such as walking into a bar, to develop.
Going beyond addiction to psychoactive chemicals, it is not surprising that many reinforcing activities that do not depend on the ingestion of such substances (e.g., gambling, sex, shopping, food) sometimes become compulsive and are experienced as being out of control. They are in fact often referred to as addictions (McElroy et al., 1992; Alessi and Petry, 2003; Petry, 2003). The same inhibitory functions must develop in order to control all such reinforcing activities when they become disadvantageous to the individual. The means to engage in these reinforcing activities (drugs and nondrug) outside parental control become available in adolescence. It is therefore to be expected that, since in adolescence the more sophisticated impulse control mechanisms are underdeveloped or in the midst of developing, many such behaviors begin and become habitual during adolescence and young adulthood (Pietrzak, Ladd, and Petry, 2003; Vitaro et al., 2004).
Like the addicted individuals just described, adolescents most often have knowledge of the correct or appropriate actions in high-risk situations involving driving, sexual, criminal, and drug use behaviors (to name a few), but their ability to process this information fast and precisely enough to inhibit inappropriate action is less well developed than the same ability in adults (Bedard et al., 2002). It is thus not surprising that many socially inappropriate actions, from crime and violence to drug use, are much more prevalent in adolescence and young adulthood (Aarons et al., 2001; Lacourse et al., 2003; Pietrzak et al., 2003; Gjeruldsen, Myrvang, and Opjordsmoen, 2004). These age-related influences on the ability to inhibit inappropriate behaviors and actions have been known for many generations, and I argue that their face validity is even reflected in the Constitution of the United States of America, which requires a U.S. president to be at least 35 years old.
Myelin Vulnerability Facilitates the Co-occurrence of Neuropsychiatric Disorders and Comorbid Addiction
Oligodendrocytes, the CNS cells that produce myelin and underlie the protracted course of human brain development, are unique in multiple ways that make them the most vulnerable cells in the brain, its Achilles’ heel (for review, see Bartzokis, 2004a, b). First, oligodendrocytes have a unique relationship to brain cholesterol. Cholesterol is highly concentrated in myelin membranes and, in effect, the brain supply of this molecule—which is essential to all membranes—is entirely synthesized by oligodendrocytes with a great expenditure of energy. The brain’s dependence on oligodendrocyte-produced cholesterol has implications for CNS development and its continual functional plasticity. In gray matter, cholesterol deficits can directly affect neuronal plasticity, because CNS synaptogenesis and dendritic outgrowth are promoted by oligodendrocyte-derived cholesterol, and impairment of these remodeling processes may interfere with new learning (Mauch et al., 2001; Fan et al., 2002; Walsh et al., 2002; for review see Bartzokis, 2004a, b). Second, oligodendrocytes also have a unique relationship to brain iron that is primarily stored in them and their myelin sheaths (Connor and Menzies, 1996; Erb, Osterbur, and LeVine, 1996; de los Monteros et al., 2000). Finally, oligodendrocytes and especially their precursors have energy requirements two or three times greater than those of other brain cells (Connor and Menzies, 1996; for review, see Bartzokis, 2004a, b).
The unique functions, structure, and biochemistry of these cells may all contribute to their high and region-specific vulnerability to a multitude of insults (Bauer et al., 2002; for review, see Bartzokis, 2004a, b). Oligodendrocytes are more susceptible than neurons and astrocytes to chronic hypoperfusion (Juurlink, 1997; Pantoni and Garcia, 1997; Kurumatani et al., 1998; Petty and Wettstein, 1999), toxic products of activated microglia such as nitric oxide (Merrill et al., 1993; Mitrovic et al., 1995; Sloane et al., 1999), iron toxicity (Kress, Dineley, and Reynolds, 2002), and excitotoxicity (Matute et al., 1997; McDonald et al., 1998; Alonso, 2000; Hopkins, Wang, and Schmued, 2000). In addition, later myelinating oligodendrocytes (which are increasingly complex as they myelinate increasing numbers of axons) and oligodendrocyte precursors (which are beginning to produce vast amounts of membranes in the process of differentiation) are especially vulnerable to oxidative damage, making intracortical and subcortical regions that are actively myelinating or later myelinating especially vulnerable (Husain and Juurlink, 1995; Back et al., 1998; Cheepsunthorn et al., 2001; for review, see Bartzokis, 2004a, b). The heightened vulnerability of developing oligodendrocytes is manifested in the wide variety of insults such as exogenous glucocorticoids (Deng, McKinnon, and Poretz, 2001; Huang et al., 2001); excitotoxicity (Rosenberg et al., 2003); hypothyroidism (Rodriguez-Pena, 1999); other heavy metal toxicity (Myers and Davidson, 1998; Deng et al., 2001; Kress et al., 2002); deficiencies in necessary nutrients such as iron (Wiggins, 1982; Connor and Menzies, 1996; Roncagliolo et al., 1998); brain trauma (Stone et al., 2002; Uryu et al., 2002); and abuse of drugs such as alcohol (de la Monte, 1988; Liu et al., 1998; Harris, Wilce, and Bedi, 2000; Mayfield et al., 2002), cocaine (Bartzokis, Beckson, et al., 1999; Bartzokis et al., 2002; Niess et al., 2002; Albertson et al., 2004), and heroin (Schiffer et al., 1985; Rizzuto et al., 1997; Koussa, Tamraz, and Nasnas, 2001). Drug abuse can result in myelination arrests or decrements during the long developmental trajectory of human brain myelination (Yakovlev and Lecours, 1967; Benes et al., 1994; Kemper, 1994; Bartzokis et al., 2001).
Given the recent evolution of extensive myelination and its singular role in human brain function (Bartzokis, 2002, 2004a), the myelination model predicts that humans will have a high prevalence of early-life neuropsychiatric disorders such as autism, ADHD, conduct disorder, bipolar disorder, and schizophrenia, as well as addictions involving this very vulnerable myelination process (Bartzokis, 2002; Bartzokis et al., 2002; Bartzokis, 2004b; Bartzokis & Altshuler, 2003). All these disorders share deficits in various levels of inhibitory control functions that likely contribute to their high rates of comorbidity with addiction and other impulsive behaviors. The relatively later and protracted development of our inhibitory functions suggest that these functions are especially vulnerable to dysregulation from a variety of influences (Bartzokis et al., 2002; Bartzokis, 2004b). Substances frequently abused, such as alcohol and psychostimulants, are toxic to the extremely vulnerable myelination process (Liu et al., 1998; Bartzokis et al., 2002; Bjork et al., 2003). By damaging or interfering with the myelination process, such substances can exacerbate the problem of impulse control and contribute to the poor outcomes of primary and comorbid addictive disorders in susceptible individuals (Kessler et al., 1996; Merikangas et al., 1998; Swartz et al., 2000; Kresina et al., 2004; Swann et al., 2004). The implications of these interactive effects for normal development, addiction, and comorbid addictions will be elaborated in the sections below.
Myelin Vulnerability, Drug Toxicity, and the Hardcore (Poor Outcome) Addict
The heterochronic myelination of different brain regions is well demonstrated in human postmortem studies, revealing that primary motor and sensory pathways fully, heavily, and completely myelinate in childhood (Yakovlev and Lecours, 1967; Kemper, 1994). The association regions (such as the frontal and temporal lobes) involved in inhibitory controls, however, myelinate much later and do not reach full myelination until middle age (Yakovlev and Lecours, 1967; Benes et al., 1994; Kemper, 1994; Bartzokis et al., 2001, 2004). The temporal discrepancy of functional maturity between primary regions concerned with action and association regions concerned with higher level cognitive processing is greatest in adolescence and early adulthood, resulting in a high-risk period for many problem behaviors, including drug use (Grant, 1997; Hill and Chow, 2002; Vega et al., 2002; Pietrzak et al., 2003; Kresina et al., 2004). This same period is one of high vulnerability during the development of higher level cognitive processes involved in inhibitory controls (Bartzokis et al., 2001, 2002; Bedard et al., 2002).
Chronic drug use can reduce or arrest the normal process of continued development (myelination) of the frontal and temporal lobes (Bartzokis et al., 2002). Imaging studies have demonstrated that both cocaine and alcohol dependence dysregulate white matter development (Liu et al., 1998; Bartzokis et al., 2002; Bjork et al., 2003), and similar white matter toxicity has been reported with other frequently abused drugs such as heroin (Schiffer et al., 1985; Rizzuto et al., 1997; Koussa et al., 2001). Studies that examined a wide age span detect a pattern of increasing myelination deficits in those who remain addicted as they reach middle age as compared with healthy controls, who continue to myelinate into middle age (Bartzokis et al., 2002; Bjork et al., 2003). This suggests that over time, in susceptible persons, the toxic effects of the drugs themselves produce a recidivistic, hard-core, or poor-outcome group (Swartz et al., 2000; Kresina et al., 2004) whose myelin content in middle age remains the same as it was in teenage years (Bartzokis et al., 2002). Their underdevelopment of inhibitory controls impairs their ability to say no and is likely a reflection of this biological myelination deficit (Bartzokis et al., 2002). The common experience that addicts describe as having their brains hijacked by drugs may be most appropriately attributable to the toxic effects of the drugs on inhibitory control mechanisms and the consequences of this toxicity on their cognitive functions and subsequent behavior.
The damaging effects of drug abuse on myelination have been further confirmed through gene expression studies that demonstrate a striking reduction of myelin gene expression in alcohol-dependent and cocaine-dependent persons (Mayfield et al., 2002; Albertson et al., 2004). Similar declines in expression of myelin genes have been observed in those with bipolar disorder and schizophrenia (Hof et al., 2002; Tkachev et al., 2003). The vulnerability of oligodendrocytes to developmental abnormalities has also been confirmed by recent studies assessing oligodendrocyte numbers, that found reduced or abnormal oligodendrocytes in subjects with bipolar disorder, schizophrenia, and major depression (Hof et al., 2002, 2003; Uranova et al., 2004)—disorders that are plagued by high rates of comorbid addiction, which often contributes to poor outcomes (Kessler et al., 1996; Merikangas et al., 1998; Swartz et al., 2000; Kresina et al., 2004; Swann et al., 2004).
The vulnerable process of myelination and brain development can be negatively influenced during development in many other ways. For example, persons with neuropsychiatric disorders such as schizophrenia have abnormalities in peripheral lipid metabolism (Horrobin and Bennett, 1999; Assies et al., 2001; Arvindakshan et al., 2003). Abnormalities that affect building blocks of myelin membranes such as essential fatty acids (which must be obtained from the diet) can affect oligodendrocyte development (reviewed in Bartzokis, 2002; Middleton et al., 2002). Similarly, dysregulation of endogenously produced building blocks (e.g., “nonessential” lipids such as cholesterol) that depend on adequate oligodendrocyte synthesis can also be crucially important (reviewed in Bartzokis, 2004a, b). Given the poor eating habits of many adolescents, such dietary deficits may affect them, especially during their growth spurts (Bowman et al., 2004).
This brief discussion of such environmental factors is intended to emphasize that persons with neuropsychiatric disorders associated with poor inhibitory controls (e.g., ADHD, bipolar disorder, schizophrenia), who also abuse drugs, could further jeopardize their brain development through several other interactive mechanisms. In addition to use of toxic drugs and inadequate diet, many other detrimental environmental factors such as head trauma and stress could similarly interfere with the vulnerable process of myelination (reviewed in Bartzokis, 2004a, b). On the other hand, even in the absence of severe neuropsychiatric disorders (e.g., schizophrenia, bipolar disorder), environmental and genetic insults could contribute to poor or delayed development of inhibitory control functions that may result in comorbid impulsive behavioral profiles and addictions (Byrne, Byrne, and Reinhart, 1995; Cjte et al., 2002; Cote et al., 2002). During high-risk periods of brain development (adolescence and young adulthood), any combination of factors—genetic factors; toxic damage such as that produced by abuse of certain drugs (alcohol, cocaine, and heroin for example); and environmental insults such as stress, head trauma, and inadequate diet—that impact the extremely vulnerable myelination process (reviewed in Bartzokis, 2004a, b) may interact and contribute to deficits in myelination and inhibitory controls. This developmental dysregulation manifests clinically in progression of symptoms and poor outcomes (Swartz et al., 2000; Bartzokis et al., 2002; Bartzokis and Altshuler, 2003; Kresina et al., 2004; Medina et al., 2004; Swann et al., 2004).
The toxicity of drugs of abuse to the process of myelination is of central concern for addiction research, because the onset of drug use occurs in adolescence and early adulthood, the critical period when more sophisticated inhibitory control functions are being established (myelinated). Thus, since the default program of “go” (motor/impulsivity) myelinates in childhood, before sophisticated inhibitory controls (Bedard et al., 2002), the bulk of the damage of drug use in adolescence will impact the actively developing circuits involved in inhibitory control, as opposed to the earlier and fully myelinated default action or go circuits. Adolescence and young adulthood are the periods of greatest disparity between these processes and therefore are the times of greatest risk from direct toxic effects of drugs of abuse (Bartzokis et al., 2001, 2002).
It is important to note that drugs of addiction that are associated with the most severe functional and psychosocial disruption (such as cocaine, alcohol, and heroin) have been shown to have detrimental effects on human myelin as well as cognition (Schiffer et al., 1985; Rizzuto et al., 1997; Liu et al., 1998; Koussa et al., 2001; Bartzokis et al., 2002; Block, Erwin, and Ghoneim, 2002; Mayfield et al., 2002; Bjork et al., 2003; Albertson et al., 2004; Bechara and Martin, 2004; Kamarajan et al., 2004; Medina et al., 2004). Other addictive substances such as nicotine are much less psychosocially impairing (Cavedini et al., 2002). In fact, nicotine has long been hypothesized to have potentially beneficial effects (Jarvik, 1991), especially when disengaged from the toxicity associated with smoking, as has been accomplished through the development of alternative delivery mechanisms such as nicotine patches (Jarvik, 1991). Epidemiological evidence suggests that smoking (and presumably nicotine) is associated with reduced risk of developing neuropsychiatric disorders with myelination deficits such as schizophrenia (Bartzokis, Neuchterlein, et al., 2003; Ho et al., 2003; Zammit et al., 2003; Bartzokis, 2004a) and also with reduced risk of developing disorders involving premature myelin degeneration, such as Alzheimer’s disease (Bartzokis, Cummings, et al., 2003; for review, see Bartzokis, 2004a; White and Levin, 2004). Furthermore, nicotine treatment has been shown to improve cognitive function in these as well as other developmental and degenerative disorders in which myelin deficits have been demonstrated (Newhouse, Potter, and Singh, 2004), and it has been suggested that this compound may have promyelinating and myelin protective effects (Costa, Abin-Carriquiry, and Dajas, 2001; Rogers et al., 2001; Shytle et al., 2002; Myers et al., 2004; White and Levin, 2004).
Thus, in the framework of the model, substances of abuse may be advantageously separated into those that impair myelin development and those that do not. This segregation could be helpful in focusing research efforts toward developing alternative strategies for treatment (Levin and Rezvani, 2002; Newhouse et al., 2004; Rueter et al., 2004). It may also provide a better understanding of the clinical impression that smoking may be an attempt at self-medication by patients suffering from neuropsychiatric disorders associated with high rates of smoking (Dalack, Healy, and Meador-Woodruff, 1998; de Leon, Diaz, et al., 2002; de Leon, Tracy, et al., 2002; De Luca et al., 2004; Potter and Newhouse, 2004).
Myelination and the Epidemiology of Addiction
In the previous sections, the implications of the myelin model for normal and dysregulated brain development are reviewed. The implications of the model for addiction are now further elaborated by using the model to explain observed epidemiological and clinical phenomena as well as the associated implications for current and future treatments.
Any model of disease, including the addictions, must provide the framework to explain the observed phenomena associated with the disease. One of the most striking yet largely ignored phenomena of addiction is the age-dependent decline in the risk of becoming addicted and, in addicted persons, the opposite age-dependent increase in the likelihood of achieving long-term abstinence. Epidemiological studies have repeatedly demonstrated that, in general, adolescents and young adults exhibit higher rates of experimental drug use and drug use disorders than do older adults (Anthony and Helzer, 1991; Miller, 1991; Warner et al., 1995; Grant, 1997; Hill and Chow, 2002; Vega et al., 2002). Even though middle-aged adults have much better financial resources than adolescents to engage in and maintain addictions, the epidemiology is strikingly different from what this financial disparity suggests. Adolescents and young adults, despite much more limited resources, have the highest rates of drug abuse and dependence. The data have repeatedly and consistently shown that rates of illicit drug dependence are markedly skewed toward younger persons, with those younger than 29 demonstrating the highest rate of dependence (17%). The rate drops to 4% for ages 30-59 and virtually disappears in those more than 60 years old (Miller, 1991). Younger persons are more likely to become addicted, with approximately 80% of all cases of alcoholism, for example, beginning before age 30 (Helzer, Burnham, and McEvoy, 1991).
Brain myelination (or the lack thereof) is likely involved in both the higher addictability of the younger brain and the increasing likelihood of achieving sobriety with increasing age (Bartzokis et al., 2001, 2002). As described previously, however, the direct toxic effects of some drugs may be involved in altering these general developmental trends. Thus, persons who are especially susceptible to the myelotoxic effects of drugs (or myelotoxic effects associated with their lifestyle) continue their addiction through middle age despite increasingly severe health and psychosocial consequences (Bartzokis, Goldstein, et al., 1999; Bartzokis et al., 2002; Bartzokis, 2004b). Persons in this subgroup (of those who do not achieve sobriety) are predominantly male. As previously described, this male predominance may be due to lower myelination of males during development, possibly interacting with higher rates of neuropsychiatric disorders in males. Additional physiological gender differences, such as higher vasoconstrictive effects of cocaine in males, may further increase the susceptibility of males to toxic drug effects that impair the development of inhibitory controls (Bartzokis, Goldstein, et al., 1999; Kaufman et al., 2001; Bartzokis et al., 2002).
In the framework of the model, the striking epidemiological phenomena of addiction are consistent with the normal age-dependent process of myelination that, in most (less-susceptible) persons, overcomes the myelotoxic risk factors described previously and results in a relatively healthier adult function. Such adults achieve improved executive functioning, including increased inhibitory controls of cognitive and behavioral processes that are destructive (e.g., drug use).1
IMPLICATIONS FOR FUTURE DIRECTIONS
The model just presented proposes that the unique vulnerabilities of myelin and the highly protracted and extensive process of human brain myelination make myelin’s lifelong developmental trajectory directly pertinent to many neuropsychiatric diseases (Bartzokis et al., 2001), including primary and comorbid addictive disorders (Bartzokis, 2002, 2004a). The model delineates a myelin hypothesis of human addiction based on a normal adolescent and young-adult developmental phase when underdevelopment of adequate inhibitory control functions result in a period of high risk for initiating drug use and developing addictions and other hazardous behaviors and neuropsychiatric disorders. On the basis of vulnerabilities of the myelination process, the model explains the male gender prevalence of frequency and severity of addictive disorders, the high rates of comorbid addiction with other developmental neuropsychiatric disorders, and the epidemiology of addiction consisting of high prevalence and low rates of successful abstinence in youth, which reverse by middle age (Bartzokis, 2002, 2004b). The model assigns a central role to the myelotoxic properties of drugs in generating a subpopulation of highly recidivistic individuals with poor outcomes, whether suffering from primary or comorbid addiction (Bartzokis, Goldstein, et al., 1999; Bartzokis, 2002; Bartzokis and Altshuler, 2003; Swann et al., 2004).
The myelination model brings into focus multiple factors that can contribute to deviations from the normal myelination trajectory. This developmental perspective can thus help to clarify the effects of environmental and genetic perturbations of this process, which may ultimately result in divergent-appearing outcomes such as recovery (for most addicts) versus poor-outcome recidivism (Bartzokis, 2002; Bartzokis and Altshuler, 2003; Bartzokis, 2004a). This framework makes the explicit assumption that, all other factors being equal (e.g., adequate intellect), strong enough inhibitory controls can override or inhibit all other drug-related behavioral and psychological phenomena, including craving for drugs. The model therefore suggests that treatments focused on improving myelination could have an important role in allowing individuals to succeed in their efforts to say no to drug use in the naturalistic setting of their lives (Bartzokis, 2002; Bartzokis and Altshuler, 2003; Bartzokis, 2004a).
The greatest promise of the focus that the model brings to myelination is the possibility of providing a conceptual framework for considering and eventually developing novel myelin-centered treatments with wide spectra of efficacy that likely will extend beyond the treatment of addiction (Bartzokis and Altshuler, 2003). The model suggests that the current focus on neuronal neurotransmitter imbalances, which much of neuropsychopharmacology is attempting to correct, may be too narrow (Bartzokis, 2002; Bartzokis and Altshuler, 2003; Bartzokis, 2004a). It suggests that expanding the research focus to include interventions that affect vulnerable structural developmental processes, and specifically the process of myelination, may provide opportunities for novel and powerful interventions (Bartzokis and Altshuler, 2003; Bartzokis, 2004a). Interventions that affect myelination may be effective in strengthening inhibitory controls of cognition and behavior in addiction, as well as enhancing the treatment outcomes of other neurodevelopmental disorders such as schizophrenia, bipolar disorders, and ADHD (Bartzokis et al., 2001; Bartzokis, 2002; Bartzokis and Altshuler, 2003; Bartzokis, 2004a). The tools (imaging, genetic, molecular, clinical, etc.) to perform such medication-development work directly in humans are either already available or are being developed at a rapid pace (Bartzokis, 2004a). For example, the development and breakdown of myelin and important risk factors such as iron levels can be indirectly measured in vivo with increasing specificity using noninvasive imaging methods (reviewed in Bartzokis, 2004a).
The importance of human brain myelination is generally underappreciated and there is a paucity of detailed postmortem or in vivo maps of myelination (Yakovlev and Lecours, 1967; Meyer, 1981; Brody et al., 1987; Kemper, 1994; Bartzokis et al., 2001). The advent of in vivo neuroimaging methods that can assess myelination on a regional basis is beginning to correct this gap in our knowledge and should provide an impetus to gather additional region-, network-, neurotransmitter-, function-specific information (reviewed in Bartzokis, 2004a; Bartzokis et al., 2004). These methods will produce the age-specific three-dimensional maps of normal myelination that are needed for a better understanding of multiple disease processes and development of therapeutic interventions that affect myelination (Bartzokis et al., 2002; Carper et al., 2002; Bartzokis, Neuchterlein, et al., 2003; Ho et al., 2003; Bartzokis, 2004a; Bartzokis et al., 2005). Such maps could alter our very classification of diseases into biologically based disorders of different circuits (such as those involved in inhibitory controls) on whose adequate function normal cognition and behavior likely depend and whose dysfunction results in neuropsychiatric disorders. It is currently feasible to track pharmacological and other (dietary, psychosocial) myelin-centered interventions in vivo through imaging markers (Bartzokis, 2004a; Bartzokis et al., unpublished data). The model suggests that interceding early in dysregulated developmental trajectories may increase the effectiveness of treatments, decrease the need for more aggressive interventions later, and thus be accomplished with reduced risk to patients (Bartzokis, 2002; 2004a).
Acknowledgments
This work was supported in part by NIMH grant (MH 0266029); Research and Psychiatry Services of the Department of Veterans Affairs; by NIA Alzheimer’s Disease Center Grant (AG 16570); by an Alzheimer’s Disease Research Center of California grant; The author is grateful to Mace Beckson, M.D., C. Kelly Phelan, M.D., and Po H. Lu, Psy.D., for their careful review of the manuscript.
Footnotes
Physiological withdrawal, such as that seen in addictions to alcohol and heroin, would not be affected directly by such improved inhibitory control, however. This final point is made to explicitly clarify that detoxification from substances that produce physiological withdrawal must remain an important safety concern of early treatment interventions.
REFERENCES
- Aarons GA, Brown SA, Hough RL, Garland AF, Wood PA. Prevalence of adolescent substance use disorders across five sectors of care. J. Amer. Acad. Child Adolesc. Psychiat. 2001;40:419–426. doi: 10.1097/00004583-200104000-00010. [DOI] [PubMed] [Google Scholar]
- Adler CM, Holland SK, Schmithorst V, Wilke M, Weiss KL, Pan H, Strakowski SM. Abnormal frontal white matter tracts in bipolar disorder: A diffusion tensor imaging study. Bipolar Disord. 2004;6:197–203. doi: 10.1111/j.1399-5618.2004.00108.x. [DOI] [PubMed] [Google Scholar]
- Albertson DN, Pruetz B, Schmidt CJ, Kuhn DM, Kapatos G, Bannon MJ. Gene expression profile of the nucleus accumbens of human evidence for dysregulation of myelin. J. Neurochem. 2004;88:1211–1219. doi: 10.1046/j.1471-4159.2003.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleman A, Kahn RS, Selten JP. Sex differences in the risk of schizophrenia: Evidence from meta-analysis. Arch. Gen. Psychiat. 2003;60:565–571. doi: 10.1001/archpsyc.60.6.565. [DOI] [PubMed] [Google Scholar]
- Alessi SM, Petry NM. Pathological gambling severity is associated with impulsivity in a delay discounting procedure. Behav. Processes. 2003;64:345–354. doi: 10.1016/s0376-6357(03)00150-5. [DOI] [PubMed] [Google Scholar]
- Alonso G. Prolonged corticosterone treatment of adult rats inhibits the proliferation of oligodendrocyte progenitors present throughout white and gray matter regions of the brain. Glia. 2000;31:219–231. doi: 10.1002/1098-1136(200009)31:3<219::aid-glia30>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Anderson B, Southern BD, Powers RE. Anatomic asymmetries of the posterior superior temporal lobes: A postmortem study. Neuropsychiat. Neuropsychol. Behav. Neurol. 1999;12:247–254. [PubMed] [Google Scholar]
- Anthony J, Helzer JE. Syndromes of drug abuse and dependence. In: Robins LN, Regier DA, editors. Psychiatric Disorders in America: The Epidemiologic Catchment Area Study. Free Press; New York: 1991. pp. 116–154. [Google Scholar]
- Arvindakshan M, Ghate M, Ranjekar PK, Evans DR, Mahadik SP. Supplementation with a combination of omega-3 fatty acids and antioxidants (vitamins E and C) improves the outcome of schizophrenia. Schizophr. Res. 2003;62:195–204. doi: 10.1016/s0920-9964(02)00284-0. [DOI] [PubMed] [Google Scholar]
- Assies J, Lieverse R, Vreken P, Wanders RJ, Dingemans PM, Linszen DH. Significantly reduced docosahexaenoic and docosapentaenoic acid concentrations in erythrocyte membranes from schizophrenic patients compared with a carefully matched control group. Biol. Psychiat. 2001;49:510–522. doi: 10.1016/s0006-3223(00)00986-0. [DOI] [PubMed] [Google Scholar]
- Back SA, Gan X, Li Y, Rosenberg PA, Volpe JJ. Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced death caused by glutathione depletion. J. Neurosci. 1998;18:6241–6253. doi: 10.1523/JNEUROSCI.18-16-06241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA, Edwards G, Laneri M, Fletcher K, Metevia L. Executive functioning, temporal discounting, and sense of time in adolescents with attention-deficit/hyperactivity disorder (ADHD) and oppositional defiant disorder (ODD) J. Abnorm. Child Psychol. 2001;29:541–556. doi: 10.1023/a:1012233310098. [DOI] [PubMed] [Google Scholar]
- Bartzokis G. Schizophrenia: Breakdown in the well-regulated lifelong process of brain development and maturation. Neuropsychopharmacol. 2002a;27:672–683. doi: 10.1016/S0893-133X(02)00364-0. [DOI] [PubMed] [Google Scholar]
- Bartzokis G. Myelination and brain electrophysiology in healthy and schizophrenic individuals. Neuropsychopharmacol. 2003;28:1217–1218. doi: 10.1038/sj.npp.1300180. [DOI] [PubMed] [Google Scholar]
- Bartzokis G. Age-related myelin breakdown: A developmental model of cognitive decline and Alzheimer’s disease. Neurobiol. Aging. 2004a;25:5–18. doi: 10.1016/j.neurobiolaging.2003.03.001. [DOI] [PubMed] [Google Scholar]
- Bartzokis G. Quadratic trajectories of brain myelin content: Unifying construct for neuropsychiatric disorders. Neurobiol. Aging. 2004b;25:49–62. [Google Scholar]
- Bartzokis G, Altshuler LL. Biological underpinnings of treatment resistance in schizophrenia: An hypothesis. Psychopharmacol. Bull. 2003;37:5–7. [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Hance DB, Lu PH, Foster JA, Mintz J, Ling W, Bridge PT. Magnetic resonance imaging evidence of “silent” cerebrovascular toxicity in cocaine dependence. Biol. Psychiat. 1999;45:1203–1211. doi: 10.1016/s0006-3223(98)00228-5. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Edwards N, Bridge P, Mintz J. Brain maturation may be arrested in chronic cocaine addicts. Biol. Psychiat. 2002;51:605–611. doi: 10.1016/s0006-3223(02)01315-x. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal lobe volumes in men: A magnetic resonance imaging study. Arch. Gen. Psychiat. 2001;58:461–465. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Cummings JL, Sultzer D, Henderson VW, Nuechterlein KH, Mintz J. White matter structural integrity in healthy aging adults and patients with Alzheimer disease: A magnetic resonance imaging study. Arch. Neurol. 2003;60:393–398. doi: 10.1001/archneur.60.3.393. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Goldstein IB, Hance DB, Beckson M, Shapiro D, Lu PH, Edwards N, Mintz J, Bridge P. The incidence of T2-weighted MR imaging signal abnormalities in the brain of cocaine-dependent patients is age-related and region-specific. Amer. J. Neuroradiol. 1999;20:1628–1635. [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Nuechterlein KH, Lu, Gitlin M, Doi C, Oh L, Edwards N, Arzoian R, Mintz J. In-vivo evidence of differential impact of typical and atypical antipsychotics on frontal lobe maturation of adults with schizophrenia; Presented at The Society of Biological Psychiatry’s 60th Annual Meeting; Atlanta. 2005.May, [Google Scholar]
- Bartzokis G, Nuechterlein KH, Lu PH, Gitlin M, Rogers S, Mintz J. Dysregulated brain development in adult men with schizophrenia: A magnetic resonance imaging study. Biol. Psychiat. 2003;53:412–421. doi: 10.1016/s0006-3223(02)01835-8. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Sultzer D, Lu PH, Nuechterlein KH, Mintz J, Cummings J. Heterogeneous age-related breakdown of white matter structural integrity: Implications for cortical “disconnection” in aging and Alzheimer’s disease. Neurobiol. Aging. 2004;25:843–851. doi: 10.1016/j.neurobiolaging.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Bauer J, Bradl M, Klein M, Leisser M, Deckwerth TL, Wekerle H, Lassmann H. Endoplasmic reticulum stress in PLP-overexpressing transgenic rats: Gray matter oligodendrocytes are more vulnerable than white matter oligodendrocytes. J. Neuropathol. Exp. Neurol. 2002;61:12–22. doi: 10.1093/jnen/61.1.12. [DOI] [PubMed] [Google Scholar]
- Bechara A, Martin EM. Impaired decision making related to working memory deficits in individuals with substance addictions. Neuropsychol. 2004;18:152–162. doi: 10.1037/0894-4105.18.1.152. [DOI] [PubMed] [Google Scholar]
- Bedard AC, Nichols S, Barbosa JA, Schachar R, Logan GD, Tannock R. The development of selective inhibitory control across the life span. Devel. Neuropsychol. 2002;21:93–111. doi: 10.1207/S15326942DN2101_5. [DOI] [PubMed] [Google Scholar]
- Belin P, Zilbovicius M, Crozier S, Thivard L, Fontaine A, Masure MC, Samson Y. Lateralization of speech and auditory temporal processing. J. Cogn. Neurosci. 1998;10:536–540. doi: 10.1162/089892998562834. [DOI] [PubMed] [Google Scholar]
- Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Arch. Gen. Psychiat. 1994;51:477–484. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Grant SJ, Hommer DW. Cross-sectional volumetric analysis of brain atrophy in alcohol dependence: Effects of drinking history and comorbid substance use disorder. Amer. J. Psychiatry. 2003;160:2038–2045. doi: 10.1176/appi.ajp.160.11.2038. [DOI] [PubMed] [Google Scholar]
- Bjorklund DF, Harnishfeger KK. The evolution of inhibition mechanisms and their role in human cognition and behavior. In: Dempster FN, Brainerd CJ, editors. Interference and Inhibition in Cognition. Academic Press; San Diego: 1995. pp. 142–169. [Google Scholar]
- Block RI, Erwin WJ, Ghoneim MM. Chronic drug use and cognitive impairments. Pharmacol. Biochem. Behav. 2002;73:491–504. doi: 10.1016/s0091-3057(02)00816-x. [DOI] [PubMed] [Google Scholar]
- Bowman SA, Gortmaker SL, Ebbeling CB, Pereira MA, Ludwig DS. Effects of fast-food consumption on energy intake and diet quality among children in a national household survey. Pediatrics. 2004;113:112–118. doi: 10.1542/peds.113.1.112. [DOI] [PubMed] [Google Scholar]
- Brody BA, Kinney HC, Kloman AS, Gilles FH. Sequence of central nervous system myelination in human infancy: I. An autopsy study of myelination. J. Neuropathol. Exp. Neurol. 1987;46:283–301. doi: 10.1097/00005072-198705000-00005. [DOI] [PubMed] [Google Scholar]
- Byrne DG, Byrne AE, Reinhart MI. Personality, stress, and the decision to commence cigarette smoking in adolescence. J. Psychosom. Res. 1995;39:53–62. doi: 10.1016/0022-3999(94)00074-f. [DOI] [PubMed] [Google Scholar]
- Carper RA, Moses P, Tigue ZD, Courchesne E. Cerebral lobes in autism: Early hyperplasia and abnormal age effects. Neuroimage. 2002;16:1038–1051. doi: 10.1006/nimg.2002.1099. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, Blumenthal JD, James RS, Ebens CL, Walter JM, Zijdenbos A, Evans AC, Giedd JN, Rapoport JL. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. J. Amer. Med. Assn. 2002;288:1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- Cavedini P, Riboldi G, Keller R, D’Annucci A, Bellodi L. Frontal lobe dysfunction in pathological gambling patients. Biol. Psychiat. 2002;51:334–341. doi: 10.1016/s0006-3223(01)01227-6. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Potenza MN. Neurodevelopment, impulsivity, and adolescent gambling. J. Gambl. Stud. 2003;19:53–84. doi: 10.1023/a:1021275130071. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: A critical period of addiction vulnerability. Amer. J. Psychiat. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheepsunthorn P, Palmer C, Menzies S, Roberts RL, Connor JR. Hypoxic/ischemic insult alters ferritin expression and myelination in neonatal rat brains. J. Comp. Neurol. 2001;431:382–396. doi: 10.1002/1096-9861(20010319)431:4<382::aid-cne1077>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Cjte S, Tremblay RE, Nagin D, Zoccolillo M, Vitaro F. The development of impulsivity, fearfulness, and helpfulness during childhood: Patterns of consistency and change in the trajectories of boys and girls. J. Child Psychol. Psychiat. 2002;43:609–618. doi: 10.1111/1469-7610.00050. [DOI] [PubMed] [Google Scholar]
- Connor JR, Menzies SL. Relationship of iron to oligodendrocytes and myelination. Glia. 1996;17:83–93. doi: 10.1002/(SICI)1098-1136(199606)17:2<83::AID-GLIA1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Todd RD. Autistic traits in the general population: A twin study. Arch. Gen. Psychiat. 2003;60:524–530. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- Costa G, Abin-Carriquiry JA, Dajas F. Nicotine prevents striatal dopamine loss produced by 6-hydroxydopamine lesion in the substantia nigra. Brain Res. 2001;888:336–342. doi: 10.1016/s0006-8993(00)03087-0. [DOI] [PubMed] [Google Scholar]
- Cote S, Tremblay RE, Nagin DS, Zoccolillo M, Vitaro F. Childhood behavioral profiles leading to adolescent conduct disorder: Risk trajectories for boys and girls. J. Amer. Acad. Child Adolesc. Psychiat. 2002;41:1086–1094. doi: 10.1097/00004583-200209000-00009. [DOI] [PubMed] [Google Scholar]
- Crosbie J, Schachar R. Deficient inhibition as a marker for familial ADHD. Amer. J. Psychiat. 2001;158:1884–1890. doi: 10.1176/appi.ajp.158.11.1884. [DOI] [PubMed] [Google Scholar]
- Dalack GW, Healy DJ, Meador-Woodruff JH. Nicotine dependence in schizophrenia: Clinical phenomena and laboratory findings. Amer. J. Psychiat. 1998;155:1490–1501. doi: 10.1176/ajp.155.11.1490. [DOI] [PubMed] [Google Scholar]
- Deadwyler SA, Hayashizaki S, Cheer J, Hampson RE. Reward, memory, and substance abuse: Functional neuronal circuits in the nucleus accumbens. Neurosci. Biobehav. Rev. 2004;27:703–711. doi: 10.1016/j.neubiorev.2003.11.011. [DOI] [PubMed] [Google Scholar]
- de la Monte SM. Disproportionate atrophy of cerebral white matter in chronic alcoholics. Arch. Neurol. 1988;45:990–992. doi: 10.1001/archneur.1988.00520330076013. [DOI] [PubMed] [Google Scholar]
- de Leon J, Diaz FJ, Rogers T, Browne D, Dinsmore L. Initiation of daily smoking and nicotine dependence in schizophrenia and mood disorders. Schizophr. Res. 2002;56:47–54. doi: 10.1016/s0920-9964(01)00217-1. [DOI] [PubMed] [Google Scholar]
- de Leon J, Tracy J, McCann E, McGrory A, Diaz FJ. Schizophrenia and tobacco smoking: A replication study in another U.S. psychiatric hospital. Schizophr. Res. 2002;56:55–65. doi: 10.1016/s0920-9964(01)00192-x. [DOI] [PubMed] [Google Scholar]
- de los Monteros AE, Korsak RA, Tran T, Vu D, de Vellis J, Edmond J. Dietary iron and the integrity of the developing rat brain: A study with the artificially reared rat pup. Cell Mol. Biol. (Noisy-le-grand) 2000;46:501–515. [PubMed] [Google Scholar]
- De Luca V, Wong AH, Muller DJ, Wong GW, Tyndale RF, Kennedy JL. Evidence of association between smoking and alpha7 nicotinic receptor subunit gene in schizophrenia patients. Neuropsychopharmacol. 2004;29:1522–1526. doi: 10.1038/sj.npp.1300466. [DOI] [PubMed] [Google Scholar]
- Dempster F. Interference and inhibition in cognition: An historical perspective. In: Dempster FN, Brainerd CJ, editors. Interference and Inhibition in Cognition. Academic Press; San Diego: 1995. pp. 3–22. [Google Scholar]
- Deng W, McKinnon RD, Poretz RD. Lead exposure delays the differentiation of oligodendroglial progenitors in vitro. Toxicol. Appl. Pharmacol. 2001;174:235–244. doi: 10.1006/taap.2001.9219. [DOI] [PubMed] [Google Scholar]
- Dustman RE, Emmerson RY, Shearer DE. Life span changes in electrophysiological measures of inhibition. Brain Cogn. 1996;30:109–126. doi: 10.1006/brcg.1996.0007. [DOI] [PubMed] [Google Scholar]
- Erb GL, Osterbur DL, LeVine SM. The distribution of iron in the brain: A phylogenetic analysis using iron histochemistry. Brain Res. Dev. Brain Res. 1996;93:120–128. doi: 10.1016/0165-3806(96)00020-x. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacol. (Berlin) 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Fan QW, Yu W, Gong JS, Zou K, Sawamura N, Senda T, Yanagisawa K, Michikawa M. Cholesterol-dependent modulation of dendrite outgrowth and microtubule stability in cultured neurons. J. Neurochem. 2002;80:178–190. doi: 10.1046/j.0022-3042.2001.00686.x. [DOI] [PubMed] [Google Scholar]
- Feinberg I. Physiological evidence for lifelong brain development: A comment on Bartzokis. Neuropsychopharmacol. 2003;28:1215–1216. doi: 10.1038/sj.npp.1300179. [DOI] [PubMed] [Google Scholar]
- Felts PA, Baker TA, Smith KJ. Conduction in segmentally demyelinated mammalian central axons. J. Neurosci. 1997;17:7267–7277. doi: 10.1523/JNEUROSCI.17-19-07267.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleck DE, Sax KW, Strakowski SM. Reaction time measures of sustained attention differentiate bipolar disorder from schizophrenia. Schizophr. Res. 2001;52:251–259. doi: 10.1016/s0920-9964(01)00170-0. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Synopsis of function and dysfunction of the frontal lobe. Acta Psychiatr. Scand. Suppl. 1999;395:51–57. doi: 10.1111/j.1600-0447.1999.tb05983.x. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Frontal lobe and cognitive development. J. Neurocytol. 2002;31:373–385. doi: 10.1023/a:1024190429920. [DOI] [PubMed] [Google Scholar]
- Ge Y, Grossman RI, Babb JS, Rabin ML, Mannon LJ, Kolson DL. Age-related total gray matter and white matter changes in normal adult brain. Part II: Quantitative magnetization transfer ratio histogram analysis. Amer. J. Neuroradiol. 2002;23:1334–1341. [PMC free article] [PubMed] [Google Scholar]
- Gibson KR. Myelination and behavioral development: A comparative perspective on questions of neoteny, altriciality, and intelligence. In: Gibson KR, Peterson AC, editors. Brain Maturation and Cognitive Development. Aldine de Gruyter; New York: 1991. pp. 29–63. [Google Scholar]
- Gjeruldsen S, Myrvang G, Opjordsmoen S. Criminality in drug addicts: A follow-up study over 25 years. Eur. Addict. Res. 2004;10:49–55. doi: 10.1159/000076113. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat. Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Grant BF. Prevalence and correlates of alcohol use and DSM-IV alcohol dependence in the United States: Results of the National Longitudinal Alcohol Epidemiologic Survey. J. Stud. Alcohol. 1997;58:464–473. doi: 10.15288/jsa.1997.58.464. [DOI] [PubMed] [Google Scholar]
- Harnishfeger KK. The development of cognitive inhibition: Theories, definitions, and research evidence. In: Dempster FN, Brainerd CJ, editors. Interference and Inhibition in Cognition. Academic Press; San Diego: 1995. pp. 175–204. [Google Scholar]
- Harris SJ, Wilce P, Bedi KS. Exposure of rats to a high but not low dose of ethanol during early postnatal life increases the rate of loss of optic nerve axons and decreases the rate of myelination. J. Anat. 2000;197:477–485. doi: 10.1046/j.1469-7580.2000.19730477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helzer JE, Burnam MA, McEvoy LT. Alcohol abuse and dependence. In: Robins LN, Regier DA, editors. Psychiatric Disorders in America: The Epidemiologic Catchment Area Study. Free Press; New York: 1991. pp. 81–115. [Google Scholar]
- Herbert MR, Harris GJ, Adrien KT, Ziegler DA, Makris N, Kennedy DN, Lange NT, Chabris CF, Bakardjiev A, Hodgson J, Takeoka M, Tager-Flusberg H, Caviness VS., Jr. Abnormal asymmetry in language association cortex in autism. Ann. Neurol. 2002;52:588–596. doi: 10.1002/ana.10349. [DOI] [PubMed] [Google Scholar]
- Hill EM, Chow K. Life-history theory and risky drinking. Addiction. 2002;97:401–413. doi: 10.1046/j.1360-0443.2002.00020.x. [DOI] [PubMed] [Google Scholar]
- Hinshaw SP. Impulsivity, emotion regulation, and developmental psychopathology: Specificity versus generality of linkages. Ann. NY Acad. Sci. 2003;1008:149–159. doi: 10.1196/annals.1301.016. [DOI] [PubMed] [Google Scholar]
- Ho BC, Andreasen NC, Nopoulos P, Arndt S, Magnotta V, Flaum M. Progressive structural brain abnormalities and their relationship to clinical outcome: A longitudinal magnetic resonance imaging schizophrenia. Arch. Gen. Psychiat. 2003;60:585–594. doi: 10.1001/archpsyc.60.6.585. [DOI] [PubMed] [Google Scholar]
- Hof PR, Haroutunian V, Copland C, Davis KL, Buxbaum JD. Molecular and cellular evidence for an oligodendrocyte abnormality in schizophrenia. Neurochem. Res. 2002;27:1193–1200. doi: 10.1023/a:1020981510759. [DOI] [PubMed] [Google Scholar]
- Hof PR, Haroutunian V, Friedrich VL, Jr., Byne W, Buitron C, Perl DP, Davis KL. Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biol. Psychiat. 2003;53:1075–1085. doi: 10.1016/s0006-3223(03)00237-3. [DOI] [PubMed] [Google Scholar]
- Hopkins KJ, Wang G, Schmued LC. Temporal progression of kainic acid induced neuronal and myelin degeneration in the rat forebrain. Brain Res. 2000;864:69–80. doi: 10.1016/s0006-8993(00)02137-5. [DOI] [PubMed] [Google Scholar]
- Horrobin DF, Bennett CN. New gene targets related to schizophrenia and other psychiatric disorders: Enzymes, binding proteins and transport proteins involved in phospholipid and fatty acid metabolism. Prostagland. Leukot. Essent. Fatty Acids. 1999;60:141–167. doi: 10.1054/plef.1999.0027. [DOI] [PubMed] [Google Scholar]
- Howlin P. Outcome in high-functioning adults with autism with and without early language delays: Implications for the differentiation between autism and Asperger syndrome. J. Autism Devel. Disord. 2003;33:3–13. doi: 10.1023/a:1022270118899. [DOI] [PubMed] [Google Scholar]
- Huang WL, Harper CG, Evans SF, Newnham JP, Dunlop SA. Repeated prenatal corticosteroid administration delays myelination of the corpus callosum in fetal sheep. Internat. J. Devel. Neurosci. 2001;19:415–425. doi: 10.1016/s0736-5748(01)00026-0. [DOI] [PubMed] [Google Scholar]
- Husain J, Juurlink BH. Oligodendroglial precursor cell susceptibility to hypoxia is related to poor ability to cope with reactive oxygen species. Brain Res. 1995;698:86–94. doi: 10.1016/0006-8993(95)00832-b. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J. Comp. Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Jansson-Verkasalo E, Ceponiene R, Kielinen M, Suominen K, Jantti V, Linna SL, Moilanen I, Naatanen R. Deficient auditory processing in children with Asperger syndrome, as indexed by event-related potentials. Neurosci. Lett. 2003;338:197–200. doi: 10.1016/s0304-3940(02)01405-2. [DOI] [PubMed] [Google Scholar]
- Jarvik ME. Beneficial effects of nicotine. Br. J. Addict. 1991;86:571–575. doi: 10.1111/j.1360-0443.1991.tb01810.x. [DOI] [PubMed] [Google Scholar]
- Jernigan TL. White matter mapping is needed. Neurobiol. Aging. 2003;25:37–39. doi: 10.1016/j.neurobiolaging.2003.06.002. [DOI] [PubMed] [Google Scholar]
- Judd LL, Schettler PJ, Akiskal HS, Maser J, Coryell W, Solomon D, Endicott J, Keller M. Long-term symptomatic status of bipolar I vs. bipolar II disorders. Internat. J. Neuropsychopharmacol. 2003;6:127–137. doi: 10.1017/S1461145703003341. [DOI] [PubMed] [Google Scholar]
- Juurlink BH. Response of glial cells to ischemia: Roles of reactive oxygen species and glutathione. Neurosci. Biobehav. Rev. 1997;21:151–166. doi: 10.1016/s0149-7634(96)00005-x. [DOI] [PubMed] [Google Scholar]
- Kaes T. Die Grosshirnrinde des Menschen in ihren Massen und in inhrem Fasergehalt. Gustav Fischer; Jena, Germany: 1907. [Google Scholar]
- Kaltiala-Heino R, Rissanen A, Rimpela M, Rantanen P. Bulimia and impulsive behaviour in middle adolescence. Psychother. Psychosom. 2003;72:26–33. doi: 10.1159/000067187. [DOI] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, Choi K, Chorlian DB, Padmanabhapillai A, Rangaswamy M, Stimus AT, Begleiter H. The role of brain oscillations as functional correlates of cognitive systems: A study of frontal inhibitory control in alcoholism. Internat. J. Psychophysiol. 2004;51:155–180. doi: 10.1016/j.ijpsycho.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrass J, Braungart-Rieker JM, Mullins J, Lefever JB. Processes in language acquisition: The roles of gender, attention, and maternal encouragement of attention over time. J. Child Lang. 2002;29:519–543. doi: 10.1017/s0305000902005196. [DOI] [PubMed] [Google Scholar]
- Kaufman MJ, Levin JM, Maas LC, Kukes TJ, Villafuerte RA, Dostal K, Lukas SE, Mendelson JH, Cohen BM, Renshaw PF. Cocaine-induced cerebral vasoconstriction differs as a function of sex and menstrual cycle phase. Biol. Psychiat. 2001;49:774–781. doi: 10.1016/s0006-3223(00)01091-x. [DOI] [PubMed] [Google Scholar]
- Keel PK, Mitchell JE. Outcome in bulimia nervosa. Amer. J. Psychiat. 1997;154:313–321. doi: 10.1176/ajp.154.3.313. [DOI] [PubMed] [Google Scholar]
- Kemper T. Neuroanatomical and neuropathological changes during aging and dementia. 2nd ed. Oxford University Press; New York: 1994. [Google Scholar]
- Kessler RC, Nelson CB, McGonagle KA, Edlund MJ, Frank RG, Leaf PJ. The epidemiology of co-occurring addictive and mental disorders: Implications for prevention and service utilization. Amer. J. Orthopsychiat. 1996;66:17–31. doi: 10.1037/h0080151. [DOI] [PubMed] [Google Scholar]
- Kieseppa T, van Erp TG, Haukka J, Partonen T, Cannon TD, Poutanen VP, Kaprio J, Lonnqvist J. Reduced left hemispheric white matter volume in twins with bipolar I disorder. Biol. Psychiat. 2003;54:896–905. doi: 10.1016/s0006-3223(03)00373-1. [DOI] [PubMed] [Google Scholar]
- Koussa S, Tamraz J, Nasnas R. Leucoencephalopathy after heroin inhalation. A case with partial regression of MRI lesions. J. Neuroradiol. 2001;28:268–271. [PubMed] [Google Scholar]
- Kresina TF, Normand J, Khalsa J, Mitty J, Flanigan T, Francis H. Addressing the need for treatment paradigms for drug-abusing patients with multiple morbidities. Clin. Infect. Dis. 2004;38(Suppl 5):S398–S401. doi: 10.1086/421403. [DOI] [PubMed] [Google Scholar]
- Kress GJ, Dineley KE, Reynolds IJ. The relationship between intracellular free iron and cell injury in cultured neurons, astrocytes, and oligodendrocytes. J. Neurosci. 2002;22:5848–5855. doi: 10.1523/JNEUROSCI.22-14-05848.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurumatani T, Kudo T, Ikura Y, Takeda M. White matter changes in the gerbil brain under chronic cerebral hypoperfusion. Stroke. 1998;29:1058–1062. doi: 10.1161/01.str.29.5.1058. [DOI] [PubMed] [Google Scholar]
- Lacourse E, Nagin D, Tremblay RE, Vitaro F, Claes M. Developmental trajectories of boys’ delinquent group membership and facilitation of violent behaviors during adolescence. Devel. Psychopathol. 2003;15:183–197. doi: 10.1017/s0954579403000105. [DOI] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH. Nicotinic treatment for cognitive dysfunction. Curr. Drug Targets CNS Neurol. Disord. 2002;1:423–431. doi: 10.2174/1568007023339102. [DOI] [PubMed] [Google Scholar]
- Lim KO, Choi SJ, Pomara N, Wolkin A, Rotrosen JP. Reduced frontal white matter integrity in cocaine dependence: A controlled diffusion tensor imaging study. Biol. Psychiat. 2002;51:890–895. doi: 10.1016/s0006-3223(01)01355-5. [DOI] [PubMed] [Google Scholar]
- Liu X, Matochik JA, Cadet JL, London ED. Smaller volume of prefrontal lobe in polysubstance abusers: A magnetic resonance imaging study. Neuropsychopharmacol. 1998;18:243–252. doi: 10.1016/S0893-133X(97)00143-7. [DOI] [PubMed] [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. J. Comp. Neurol. 2003;462:144–152. doi: 10.1002/cne.10714. [DOI] [PubMed] [Google Scholar]
- Matute C, Sanchez-Gomez MV, Martinez-Millan L, Miled R. Glutamate receptor-mediated toxicity in optic nerve oligodendrocytes. Neurobiol. 1997;94:8830–8835. doi: 10.1073/pnas.94.16.8830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch DH, Nagler K, Schumacher S, Goritz C, Muller EC, Otto A, Pfrieger FW. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- Mayfield RD, Lewohl JM, Dodd PR, Herlihy A, Liu J, Harris RA. Patterns of gene expression are altered in the frontal and motor cortices of human alcoholics. J. Neurochem. 2002;81:802–813. doi: 10.1046/j.1471-4159.2002.00860.x. [DOI] [PubMed] [Google Scholar]
- McAlonan GM, Daly E, Kumari V, Critchley HD, van Amelsvoort T, Suckling J, Simmons A, Sigmundsson T, Greenwood K, Russell A, Schmitz N, Happe F, Howlin P, Murphy DG. Brain anatomy and sensorimotor gating in Asperger’s syndrome. Brain. 2002;125:1594–1606. doi: 10.1093/brain/awf150. [DOI] [PubMed] [Google Scholar]
- McDonald JW, Althomsons SP, Hyrc KL, Choi DW, Goldberg MP. Oligodendrocytes from forebrain are highly vulnerable to AMPA/kainate receptor-mediated excitotoxicity. Nat. Med. 1998;4:291–297. doi: 10.1038/nm0398-291. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Hudson JI, Pope H, Jr., Keck PE, Jr., Aizley HG. The DSM-III-R impulse control disorders not elsewhere classified: Clinical characteristics and relationship to other psychiatric disorders. Amer. J. Psychiat. 1992;149:318–327. doi: 10.1176/ajp.149.3.318. [DOI] [PubMed] [Google Scholar]
- McInnes A, Humphries T, Hogg-Johnson S, Tannock R. Listening comprehension and working memory are impaired in attention-deficit/hyperactivity disorder irrespective of language impairment. J. Abnorm. Child Psychol. 2003;31:427–443. doi: 10.1023/a:1023895602957. [DOI] [PubMed] [Google Scholar]
- Medina KL, Shear PK, Schafer J, Armstrong TG, Dyer P. Cognitive functioning and length of abstinence in polysubstance dependent men. Arch. Clin. Neuropsychol. 2004;19:245–258. doi: 10.1016/S0887-6177(03)00043-X. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Mehta RL, Molnar BE, Walters EE, Swendsen JD, Aguilar-Gaziola S, Bijl R, Borges G, Caraveo-Anduaga JJ, DeWit DJ, Kolody B, Vega WA, Wittchen HU, Kessler RC. Comorbidity of substance use disorders with mood and anxiety disorders: Results of the International Consortium in Psychiatric Epidemiology. Addict. Behav. 1998;23:893–907. doi: 10.1016/s0306-4603(98)00076-8. [DOI] [PubMed] [Google Scholar]
- Merrill JE, Ignarro LJ, Sherman MP, Melinek J, Lane TE. Microglial cell cytotoxicity of oligodendrocytes is mediated through nitric oxide. J. Immunol. 1993;151:2132–2141. [PubMed] [Google Scholar]
- Mesulam MM. A plasticity-based theory of the pathogenesis of Alzheimer’s disease. Ann. NY Acad. Sci. 2000;924:42–52. doi: 10.1111/j.1749-6632.2000.tb05559.x. [DOI] [PubMed] [Google Scholar]
- Meyer A. Paul Flechsig’s system of myelogenetic cortical localization in the light of recent research in neuroanatomy and neurophysiology, Part II. Can. J. Neurol. Sci. 1981;8:95–104. doi: 10.1017/s0317167100042980. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Mirnics K, Pierri JN, Lewis DA, Levitt P. Gene expression profiling reveals alterations of specific metabolic pathways in schizophrenia. J. Neurosci. 2002;22:2718–2729. doi: 10.1523/JNEUROSCI.22-07-02718.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AK, Alston RL, Corsellis JA. Variation with age in the volumes of grey and white matter in the cerebral hemispheres of man: Measurements with an image analyser. Neuropathol. Appl. Neurobiol. 1980;6:119–132. doi: 10.1111/j.1365-2990.1980.tb00283.x. [DOI] [PubMed] [Google Scholar]
- Miller NS. Alcohol and drug dependence. In: Sadavoy J, Lazarus LW, Jarvik LF, editors. Comprehensive Review of Geriatric Psychiatry. American Psychiatric Press; Washington, DC: 1991. pp. 387–401. [Google Scholar]
- Mitrovic B, Ignarro LJ, Vinters HV, Akers MA, Schmid I, Uittenbogaart C, Merrill JE. Nitric oxide induces necrotic but not apoptotic cell death in oligodendrocytes. Neurosci. 1995;65:531–539. doi: 10.1016/0306-4522(94)00491-m. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. Amer. J. Psychiat. 2001;158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Cooper KL, Kates WR, Denckla MB, Kaufmann WE. Smaller prefrontal and premotor volumes in boys with attention-deficit/hyperactivity disorder. Biol. Psychiat. 2002;52:785–794. doi: 10.1016/s0006-3223(02)01412-9. [DOI] [PubMed] [Google Scholar]
- Myers CS, Robles O, Kakoyannis AN, Sherr JD, Avila MT, Blaxton TA, Thaker GK. Nicotine improves delayed recognition in schizophrenic patients. Psychopharmacol. (Berlin) 2004;174:334–340. doi: 10.1007/s00213-003-1764-8. [DOI] [PubMed] [Google Scholar]
- Myers GJ, Davidson PW. Prenatal methylmercury exposure and children: Neurologic, developmental, and behavioral research. Environ. Health Perspect. 1998;106:841–847. doi: 10.1289/ehp.98106841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhouse PA, Potter A, Singh A. Effects of nicotinic stimulation on cognitive performance. Curr. Opin. Pharmacol. 2004;4:36–46. doi: 10.1016/j.coph.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Niess C, Grauel U, Toennes SW, Bratzke H. Incidence of axonal injury in human brain tissue. Acta Neuropathol. (Berlin) 2002;104:79–84. doi: 10.1007/s00401-002-0525-9. [DOI] [PubMed] [Google Scholar]
- Norton WT. Formation, structure, and biochemistry of myelin. In: Siegel GJ, Albers RW, Agranoff BW, Katzman R, editors. Basic Neurochemistry. Little, Brown; Boston: 1981. pp. 63–92. [Google Scholar]
- Pantoni L, Garcia JH. Pathogenesis of luekoaraiosis. Stroke. 1997;28:652–659. doi: 10.1161/01.str.28.3.652. [DOI] [PubMed] [Google Scholar]
- Penhune VB, Zatorre RJ, MacDonald JD, Evans AC. Interhemispheric anatomical differences in human primary auditory cortex: Probabilistic mapping and volume measurement from magnetic resonance scans. Cereb. Cortex. 1996;6:661–672. doi: 10.1093/cercor/6.5.661. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C. Aging and the myelinated fibers in prefrontal cortex and corpus callosum of the monkey. J. Comp. Neurol. 2002;442:277–291. doi: 10.1002/cne.10099. [DOI] [PubMed] [Google Scholar]
- Petry NM. Discounting of money, health, and freedom in substance abusers and controls. Drug Alcohol Depend. 2003;71:133–141. doi: 10.1016/s0376-8716(03)00090-5. [DOI] [PubMed] [Google Scholar]
- Petty MA, Wettstein JG. White matter ischaemia. Brain Res. Rev. 1999;31:58–64. doi: 10.1016/s0165-0173(99)00025-9. [DOI] [PubMed] [Google Scholar]
- Pfrieger FW, Barres BA. What the fly’s glia tell the fly’s brain. Cell. 1995;83:671–674. doi: 10.1016/0092-8674(95)90178-7. [DOI] [PubMed] [Google Scholar]
- Pietrzak RH, Ladd GT, Petry NM. Disordered gambling in adolescents: Epidemiology, diagnosis, and treatment. Paediatr. Drugs. 2003;5:583–595. doi: 10.2165/00148581-200305090-00002. [DOI] [PubMed] [Google Scholar]
- Posthuma D, Baare WF, Hulshoff Pol HE, Kahn RS, Boomsma DI, De Geus EJ. Genetic correlations between brain volumes and the WAIS-III dimensions of verbal comprehension, working memory, perceptual organization, and processing speed. Twin Res. 2003;6:131–139. doi: 10.1375/136905203321536254. [DOI] [PubMed] [Google Scholar]
- Potter AS, Newhouse PA. Effects of acute nicotine administration on behavioral inhibition in adolescents with attention-deficit/hyperactivity disorder. Psychopharmacol. (Berlin) 2004;176:183–194. doi: 10.1007/s00213-004-1874-y. [DOI] [PubMed] [Google Scholar]
- Rakic P. Genesis of neocortex in human and nonhuman primates. In: Lewis M, editor. Child and Adolescent Psychiatry: A Comprehensive Textbook. Lippincott Williams & Wilkins; Philadelphia: 2002. pp. 22–46. [Google Scholar]
- Rakic P, Bourgeois JP, Eckenhoff MF, Zecevic N, Goldman-Rakic PS. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986;232:232–235. doi: 10.1126/science.3952506. [DOI] [PubMed] [Google Scholar]
- Rizzuto N, Morbin M, Ferrari S, Cavallaro T, Sparaco M, Boso G, Gaetti L. Delayed spongiform leukoencephalopathy after heroin abuse. Acta Neuropathol. (Berlin) 1997;94:87–90. doi: 10.1007/s004010050676. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pena A. Oligodendrocyte development and thyroid hormone. J. Neurobiol. 1999;40:497–512. doi: 10.1002/(sici)1097-4695(19990915)40:4<497::aid-neu7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Rogers SW, Gregori NZ, Carlson N, Gahring LC, Noble M. Neuronal nicotinic acetylcholine receptor expression by O2A/oligodendrocyte progenitor cells. Glia. 2001;33:306–313. [PubMed] [Google Scholar]
- Roncagliolo M, Garrido M, Walter T, Peirano P, Lozoff B. Evidence of altered central nervous system development in infants with iron deficiency anemia at six months: Delayed maturation of auditory brainstem responses. Amer. J. Clin. Nutr. 1998;68:683–690. doi: 10.1093/ajcn/68.3.683. [DOI] [PubMed] [Google Scholar]
- Rosenberg PA, Dai W, Gan XD, Ali S, Fu J, Back SA, Sanchez RM, Segal MM, Follett PL, Jensen FE, Volpe JJ. Mature myelin basic protein-expressing oligodendrocytes are insensitive to kainate toxicity. J. Neurosci. Res. 2003;71:237–245. doi: 10.1002/jnr.10472. [DOI] [PubMed] [Google Scholar]
- Rucklidge JJ, Tannock R. Neuropsychological profiles of adolescents with ADHD: Effects of reading difficulties and gender. J. Child Psychol. Psychiat. 2002;43:988–1003. doi: 10.1111/1469-7610.00227. [DOI] [PubMed] [Google Scholar]
- Rueter LE, Anderson DJ, Briggs CA, Donnelly-Roberts DL, Gintant GA, Gopalakrishnan M, Lin NH, Osinski MA, Reinhart GA, Buckley MJ, Martin RL, McDermott JS, Preusser LC, Seifert TR, Su Z, Cox BF, Decker MW, Sullivan JP. ABT-089: Pharmacological properties of a neuronal nicotinic acetylcholine receptor agonist for the potential treatment of cognitive disorders. CNS Drug Rev. 2004;10:167–182. doi: 10.1111/j.1527-3458.2004.tb00011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol. Rev. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Aging and measures of processing speed. Biol. Psychol. 2000;54:35–54. doi: 10.1016/s0301-0511(00)00052-1. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Kail R. Life-Span Development and Behavior. Vol. 5. Academic Press; New York: 1983. Memory development through the lifespan: The role of processing rate; pp. 99–116. [Google Scholar]
- Schiffer D, Brignolio F, Giordana MT, Mongini T, Migheli A, Palmucci L. Spongiform encephalopathy in addicts inhaling preheated heroin. Clin. Neuropathol. 1985;4:174–180. [PubMed] [Google Scholar]
- Semendeferi K, Lu A, Schenker N, Damasio H. Humans and great apes share a large frontal cortex. Nat. Neurosci. 2002;5:272–276. doi: 10.1038/nn814. [DOI] [PubMed] [Google Scholar]
- Shytle RD, Silver AA, Wilkinson BJ, Sanberg PR. A pilot controlled trial of transdermal nicotine in the treatment of attention-deficit/hyperactivity disorder. World J. Biol. Psychiat. 2002;3:150–155. doi: 10.3109/15622970209150616. [DOI] [PubMed] [Google Scholar]
- Silver LB. Developmental learning disorders. In: Fisher MG, editor. Child and Adolescent Psychiatry. Williams & Wilkins; Baltimore: 1991. pp. 522–529. [Google Scholar]
- Sloane JA, Hollander W, Moss MB, Rosene DL, Abraham CR. Increased microglial activation and protein nitration in white matter of the aging monkey. Neurobiol. Aging. 1999;20:395–405. doi: 10.1016/s0197-4580(99)00066-4. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat. Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Srinivasan R. Spatial structure of the human alpha rhythm: Global correlation in adults and local correlation in children. Clin. Neurophysiol. 1999;1999:1351–1362. doi: 10.1016/s1388-2457(99)00080-2. [DOI] [PubMed] [Google Scholar]
- Stahlberg O, Soderstrom H, Rastam M, Gillberg C. Bipolar disorder, schizophrenia, and other psychotic disorders in adults with childhood onset ADHD and/or autism spectrum disorders. J. Neural Transm. 2004;111:891–902. doi: 10.1007/s00702-004-0115-1. [DOI] [PubMed] [Google Scholar]
- Stone JR, Okonkwo DO, Singleton RH, Mutlu LK, Helm GA, Povlishock JT. Caspase-3-mediated cleavage of amyloid precursor protein and formation of amyloid Beta peptide in traumatic axonal injury. J. Neurotrauma. 2002;19:601–614. doi: 10.1089/089771502753754073. [DOI] [PubMed] [Google Scholar]
- Swann AC, Dougherty DM, Pazzaglia PJ, Pham M, Moeller FG. Impulsivity: A link between bipolar disorder and substance abuse. Bipolar Disord. 2004;6:204–212. doi: 10.1111/j.1399-5618.2004.00110.x. [DOI] [PubMed] [Google Scholar]
- Swartz JA, Lurigio AJ, Goldstein P. Severe mental illness and substance use disorders among former Supplemental Security Income beneficiaries for drug addiction and alcoholism. Arch. Gen. Psychiat. 2000;57:701–707. doi: 10.1001/archpsyc.57.7.701. [DOI] [PubMed] [Google Scholar]
- Tallal P, Miller S, Fitch RH. Neurobiological basis of speech: A case for the preeminence of temporal processing. Ann. NY Acad. Sci. 1993;682:27–47. doi: 10.1111/j.1749-6632.1993.tb22957.x. [DOI] [PubMed] [Google Scholar]
- Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, Jones PB, Starkey M, Webster MJ, Yolken RH, Bahn S. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- Townsend J, Westerfield M, Leaver E, Makeig S, Jung T, Pierce K, Courchesne E. Event-related brain response abnormalities in autism: Evidence for impaired cerebello-frontal spatial attention networks. Brain Res. Cogn. Brain Res. 2001;11:127–145. doi: 10.1016/s0926-6410(00)00072-0. [DOI] [PubMed] [Google Scholar]
- Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: A study from the Stanley Neuropathology Consortium. Schizophr. Res. 2004;67:269–275. doi: 10.1016/S0920-9964(03)00181-6. [DOI] [PubMed] [Google Scholar]
- Uryu K, Laurer H, McIntosh T, Pratico D, Martinez D, Leight S, Lee VM, Trojanowski JQ. Repetitive mild brain trauma accelerates Abeta deposition, lipid peroxidation, and cognitive impairment in a transgenic mouse model of Alzheimer amyloidosis. J. Neurosci. 2002;22:446–454. doi: 10.1523/JNEUROSCI.22-02-00446.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: Phase synchronization and large-scale integration. Nat. Rev. Neurosci. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- Vega WA, Aguilar-Gaxiola S, Andrade L, Bijl R, Borges G, Caraveo-Anduaga JJ, DeWit DJ, Heeringa SG, Kessler RC, Kolody B, Merikangas KR, Molnar BE, Walters EE, Warner LA, Wittchen HU. Prevalence and age of onset for drug use in seven international sites: Results from the international consortium of psychiatric epidemiology. Drug Alcohol Depend. 2002;68:285–297. doi: 10.1016/s0376-8716(02)00224-7. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Salthouse TA. Meta-analyses of age-cognition relations in adulthood: Estimates of linear and nonlinear age effects and structural models. Psychol. Bull. 1997;122:231–249. doi: 10.1037/0033-2909.122.3.231. [DOI] [PubMed] [Google Scholar]
- Vinogradov S, Kirkland J, Poole JH, Drexler M, Ober BA, Shenaut GK. Both processing speed and semantic memory organization predict verbal fluency in schizophrenia. Schizophr. Res. 2003;59:269–275. doi: 10.1016/s0920-9964(02)00200-1. [DOI] [PubMed] [Google Scholar]
- Vitaro F, Wanner B, Ladouceur R, Brendgen M, Tremblay RE. Trajectories of gambling during adolescence. J. Gambl. Stud. 2004;20:47–69. doi: 10.1023/B:JOGS.0000016703.84727.d3. [DOI] [PubMed] [Google Scholar]
- Volkmar FR. Autism and the pervasive developmental disorders. In: Fisher MG, editor. Child and Adolescent Psychiatry. Williams & Wilkins; Baltimore: 1991. pp. 499–508. [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: Results from imaging studies and treatment implications. Molec. Psychiat. 2004;9:557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Warner LA, Kessler RC, Hughes M, Anthony JC, Nelson CB. Prevalence and correlates of drug use and dependence in the United States: Results from the National Comorbidity Survey. Arch. Gen. Psychiat. 1995;52:219–229. doi: 10.1001/archpsyc.1995.03950150051010. [DOI] [PubMed] [Google Scholar]
- Waxman SG. Conduction in myelinated, unmyelinated, and demyelinated fibers. Arch. Neurol. 1977;34:585–589. doi: 10.1001/archneur.1977.00500220019003. [DOI] [PubMed] [Google Scholar]
- Weiss G. Attention-deficit/hyperactivity disorder. In: Fisher MG, editor. Child and Adolescent Psychiatry. Williams & Wilkins; Baltimore: 1991. pp. 544–561. [Google Scholar]
- White HK, Levin ED. Chronic transdermal nicotine patch treatment effects on cognitive performance in age-associated memory impairment. Psychopharmacol. (Berlin) 2004;171:465–471. doi: 10.1007/s00213-003-1614-8. [DOI] [PubMed] [Google Scholar]
- Wiggins RC. Myelin development and nutritional insufficiency. Brain Res. 1982;257:151–175. doi: 10.1016/0165-0173(82)90016-9. [DOI] [PubMed] [Google Scholar]
- Williams BR, Ponesse JS, Schachar RJ, Logan GD, Tannock R. Development of inhibitory control across the life span. Devel. Psychol. 1999;35:205–213. doi: 10.1037//0012-1649.35.1.205. [DOI] [PubMed] [Google Scholar]
- Wu KK, Anderson V, Castiello U. Neuropsychological evaluation of deficits in executive functioning for ADHD children with or without learning disabilities. Devel. Neuropsychol. 2002;22:501–531. doi: 10.1207/S15326942DN2202_5. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR. Regional Development of the Brain in Early Life. Blackwell Scientific Publications; Boston: 1967. [Google Scholar]
- Yurgelun-Todd DA, Killgore WD, Young AD. Sex differences in cerebral tissue volume and cognitive performance during adolescence. Psychol. Rep. 2002;91:743–757. doi: 10.2466/pr0.2002.91.3.743. [DOI] [PubMed] [Google Scholar]
- Zammit S, Allebeck P, Dalman C, Lundberg I, Hemmingsson T, Lewis G. Investigating the association between cigarette smoking and schizophrenia in a cohort study. Amer. J. Psychiat. 2003;160:2216–2221. doi: 10.1176/appi.ajp.160.12.2216. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Belin P, Penhune VB. Structure and function of auditory cortex: Music and speech. Trends Cogn. Sci. 2002;6:37–46. doi: 10.1016/s1364-6613(00)01816-7. [DOI] [PubMed] [Google Scholar]
- Zhou R, Abbas PJ, Assouline JG. Electrically evoked auditory brainstem response in peripherally myelin-deficient mice. Hear. Res. 1995;88:98–106. doi: 10.1016/0378-5955(95)00105-d. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. The psychobiological model for impulsive unsocialized sensation seeking: A comparative approach. Neuropsychobiol. 1996;34:125–129. doi: 10.1159/000119303. [DOI] [PubMed] [Google Scholar]


