Abstract
This review article describes several applications of the widely used enzyme immunoassay (EIA) procedure. EIA methods have been adapted to solve problems in diagnostic virology where sensitivity, specificity, or practicability is required. Concurrent developments in hybridoma and conjugation methods have increased significantly the use of these assays. A general overview of EIA methods is given together with typical examples of their use in diagnostic medical virology; attention is drawn to possible pitfalls. Recent advances in recombinant DNA technology have made it possible to produce highly specific nucleic acid probes that have a sensitivity approximately 100 times greater than that of EIA. Some applications of these probes are described. Although the non-labelled nucleic acid probes for use in the field are not as refined as non-labelled immunoassays, their range of applications is expected to expand rapidly in the near future.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Kaissi E. N., Mostratos A. Some reactions of influenza viruses adsorbed to polystyrene for enzyme immunoassay. J Virol Methods. 1982 Aug;4(6):353–358. doi: 10.1016/0166-0934(82)90060-x. [DOI] [PubMed] [Google Scholar]
- Brandsma J., Miller G. Nucleic acid spot hybridization: rapid quantitative screening of lymphoid cell lines for Epstein-Barr viral DNA. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6851–6855. doi: 10.1073/pnas.77.11.6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D. S., Nisalak A. Detection of Japanese encephalitis virus immunoglobulin M antibodies in serum by antibody capture radioimmunoassay. J Clin Microbiol. 1982 Mar;15(3):353–361. doi: 10.1128/jcm.15.3.353-361.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H. C., Takashima I., Arikawa J., Hashimoto N. Biotin-labeled protein-A enzyme-linked immunosorbent assay for the detection of Japanese encephalitis antibody in sera from humans, swine and several animal species. J Virol Methods. 1984 Oct;9(2):143–151. doi: 10.1016/0166-0934(84)90006-5. [DOI] [PubMed] [Google Scholar]
- Chou S., Merigan T. C. Rapid detection and quantitation of human cytomegalovirus in urine through DNA hybridization. N Engl J Med. 1983 Apr 21;308(16):921–925. doi: 10.1056/NEJM198304213081603. [DOI] [PubMed] [Google Scholar]
- Coulson B. S., Holmes I. H. An improved enzyme-linked immunosorbent assay for the detection of rotavirus in faeces of neonates. J Virol Methods. 1984 May;8(3):165–179. doi: 10.1016/0166-0934(84)90011-9. [DOI] [PubMed] [Google Scholar]
- Cradock-Watson J. E., Ridehalgh M. K. Specific immunoglobulins in infants with the congenital rubella syndrome. J Hyg (Lond) 1976 Feb;76(1):109–123. doi: 10.1017/s0022172400055005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranage M. P., Gardner P. S. Systemic cell-mediated and antibody responses in infants with respiratory syncytial virus infections. J Med Virol. 1980;5(2):161–170. doi: 10.1002/jmv.1890050210. [DOI] [PubMed] [Google Scholar]
- Cukor G., Perron D. M., Hudson R., Blacklow N. R. Detection of rotavirus in human stools by using monoclonal antibody. J Clin Microbiol. 1984 Jun;19(6):888–892. doi: 10.1128/jcm.19.6.888-892.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duermeyer W., van der Veen J. Specific detection of IgM-antibodies by ELISA, applied in hepatitis-A. Lancet. 1978 Sep 23;2(8091):684–685. doi: 10.1016/s0140-6736(78)92802-7. [DOI] [PubMed] [Google Scholar]
- Feinman S. V., Berris B., Guha A., Sooknanan R., Bradley D. W., Bond W. W., Maynard J. E. DNA: DNA hybridization method for the diagnosis of hepatitis B infection. J Virol Methods. 1984 May;8(3):199–206. doi: 10.1016/0166-0934(84)90014-4. [DOI] [PubMed] [Google Scholar]
- Forghani B., Myoraku C. K., Schmidt N. J. Production of monoclonal antibodies to human IgM for assay of viral IgM antibodies. J Virol Methods. 1982 Dec;5(5-6):317–327. doi: 10.1016/0166-0934(82)90023-4. [DOI] [PubMed] [Google Scholar]
- Gilljam G., Sundqvist V. A., Linde A., Pihlstedt P., Eklund A. E., Wahren B. Sensitive analytic ELISAs for subclass herpes virus IgG. J Virol Methods. 1985 Mar;10(3):203–214. doi: 10.1016/0166-0934(85)90061-8. [DOI] [PubMed] [Google Scholar]
- Grom J., Bernard S. Virus enzyme-linked cell immunoassay (VELCIA): detection and titration of rotavirus antigen and demonstration of rotavirus neutralizing and total antibodies. J Virol Methods. 1985 Feb;10(2):135–144. doi: 10.1016/0166-0934(85)90099-0. [DOI] [PubMed] [Google Scholar]
- Hogg R. J., Davidson G. P. Improved specificity of ELISA for rotavirus. Aust Paediatr J. 1982 Sep;18(3):184–185. doi: 10.1111/j.1440-1754.1982.tb02023.x. [DOI] [PubMed] [Google Scholar]
- Hornsleth A., Friis B., Grauballe P. C., Krasilnikof P. A. Detection by ELISA of IgA and IgM antibodies in secretion and IgM antibodies in serum in primary lower respiratory syncytial virus infection. J Med Virol. 1984;13(2):149–161. doi: 10.1002/jmv.1890130205. [DOI] [PubMed] [Google Scholar]
- Kalica A. R., Greenberg H. B., Wyatt R. G., Flores J., Sereno M. M., Kapikian A. Z., Chanock R. M. Genes of human (strain Wa) and bovine (strain UK) rotaviruses that code for neutralization and subgroup antigens. Virology. 1981 Jul 30;112(2):385–390. doi: 10.1016/0042-6822(81)90285-3. [DOI] [PubMed] [Google Scholar]
- Kramer S. M., Cremer N. E. Detection of IgG antibodies to measles virus using monoclonal antibodies for antigen-capture in enzyme immunoassay. J Virol Methods. 1984 May;8(3):255–263. doi: 10.1016/0166-0934(84)90020-x. [DOI] [PubMed] [Google Scholar]
- Kronvall G., Williams R. C., Jr Differences in anti-protein A activity among IgG subgroups. J Immunol. 1969 Oct;103(4):828–833. [PubMed] [Google Scholar]
- Kryger P. Significance of anti-HBc IgM in the differential diagnosis of viral hepatitis. J Virol Methods. 1985 Apr;10(4):283–289. doi: 10.1016/0166-0934(85)90043-6. [DOI] [PubMed] [Google Scholar]
- Kunz C., Hofmann H. Due Frühdiagnose der Früsommer-meningoenzephalitis (FSME) im Hämaggflutinationshemmungstest durch Behandlung des Serums mit 2-Mercaptoäthanol. Zentralbl Bakteriol Orig A. 1971 Nov;218(3):273–279. [PubMed] [Google Scholar]
- Kurstak E. Progress in enzyme immunoassays: production of reagents, experimental design, and interpretation. Bull World Health Organ. 1985;63(4):793–811. [PMC free article] [PubMed] [Google Scholar]
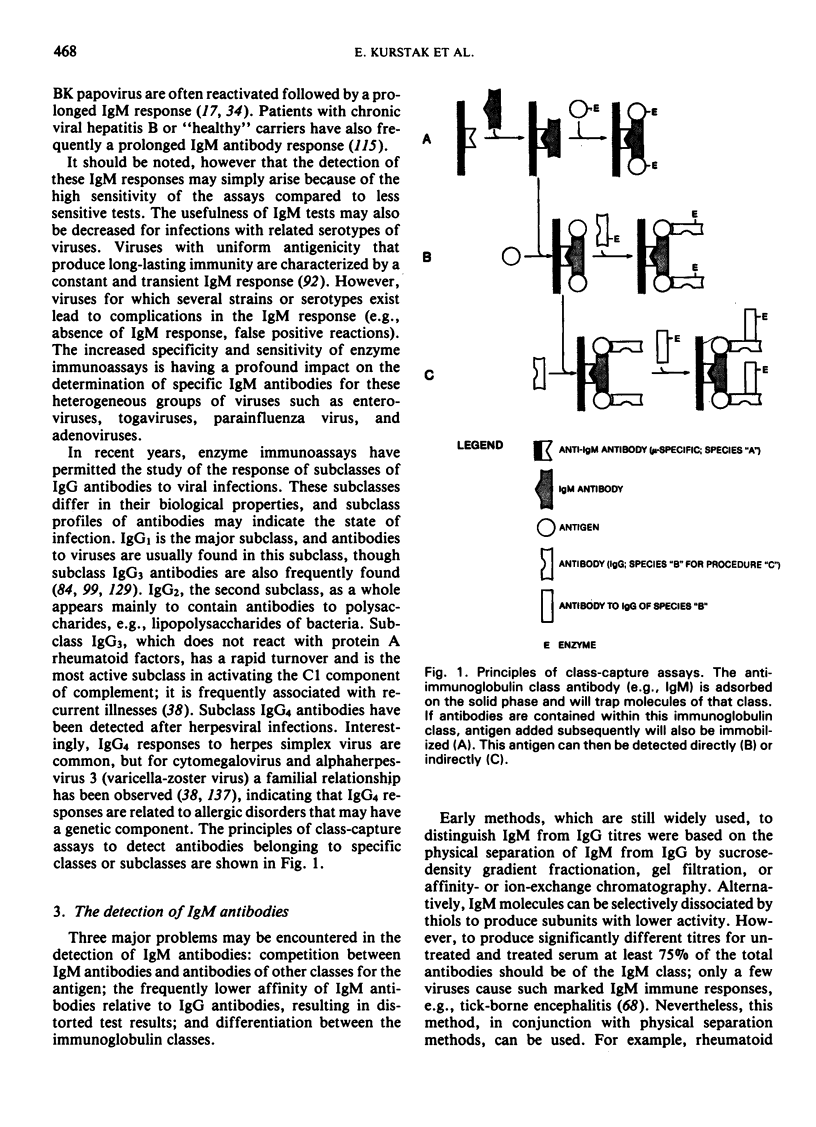
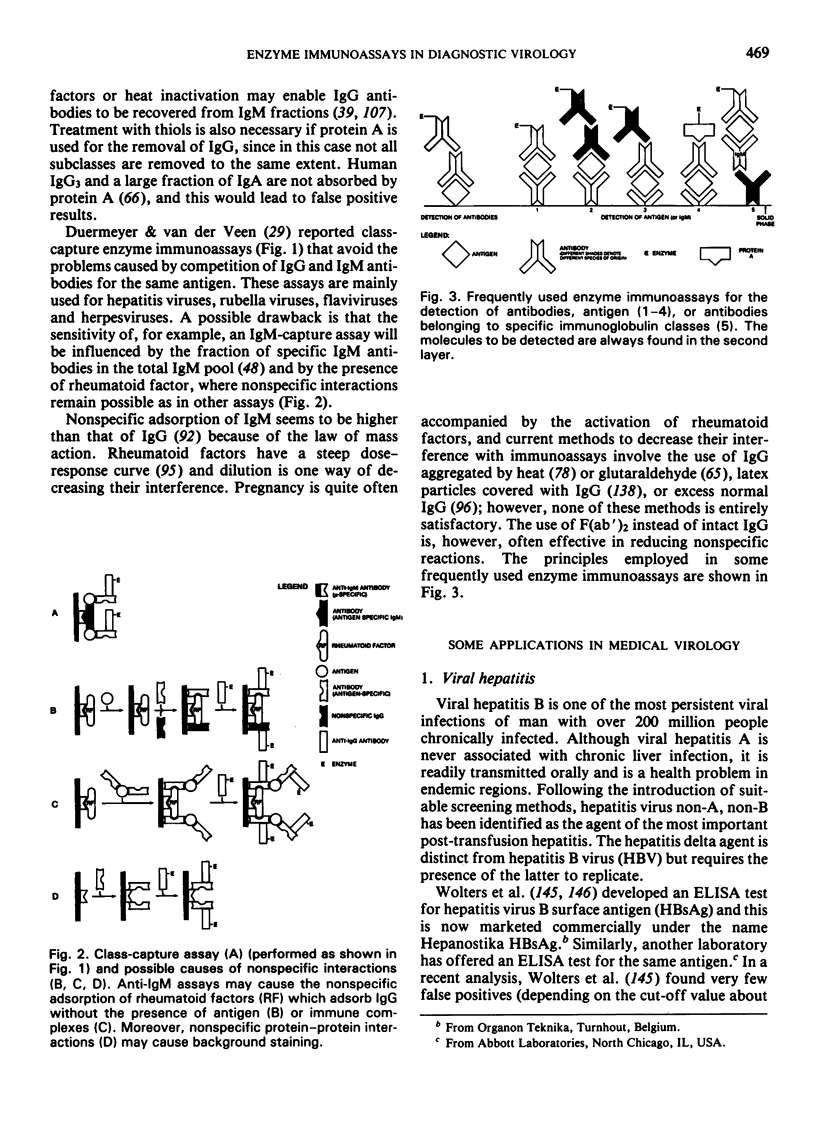
- Meurman O. H., Ziola B. R. IgM-class rheumatoid factor interference in the solid-phase radioimmunoassay of rubella-specific IgM antibodies. J Clin Pathol. 1978 May;31(5):483–487. doi: 10.1136/jcp.31.5.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millán J. L., Stigbrand T. "Sandwich" enzyme immunoassay for placental alkaline phosphatase. Clin Chem. 1981 Dec;27(12):2014–2018. [PubMed] [Google Scholar]
- Morgan-Capner P., Tedder R. S., Mace J. E. Rubella-specific IgM reactivity in sera from cases of infectious mononucleosis. J Hyg (Lond) 1983 Jun;90(3):407–413. doi: 10.1017/s0022172400029041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan E. M., Rapp F. Measles virus and its associated diseases. Bacteriol Rev. 1977 Sep;41(3):636–666. doi: 10.1128/br.41.3.636-666.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauls F. P., Dowdle W. R. A serologic study of herpesvirus hominis strains by microneutralization tests. J Immunol. 1967 May;98(5):941–947. [PubMed] [Google Scholar]
- Rapicetta M., D'Ambrosio E., Proietti E., Morace G., Donatelli I., Macchia T. Antibody assay for measles virus: comparisons of immune-adherence haemagglutination, single radial haemolysis, enzyme immunoassay and haemagglutination inhibition. J Virol Methods. 1983 Jun;6(6):303–310. doi: 10.1016/0166-0934(83)90052-6. [DOI] [PubMed] [Google Scholar]
- Rawls W. E., Iwamoto K., Adam E., Melnick J. L. Measurement of antibodies to herpesvirus types 1 and 2 in human sera. J Immunol. 1970 Mar;104(3):599–606. [PubMed] [Google Scholar]
- Recchia S., Rizzi R., Acquaviva F., Rizzetto M., Tison V., Bonino F., Verme G. Immunoperoxidase staining of the HBV-associated delta antigen in paraffinated liver specimens. Pathologica. 1981 Sep-Oct;73(1027):773–777. [PubMed] [Google Scholar]
- Reiner M., Wecker E. A modified absorption-reduction method to detect virus-specific hemagglutination inhibiting and neutralizing IgM antibodies. Med Microbiol Immunol. 1981;169(4):237–245. doi: 10.1007/BF02125523. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Scotto J., Hadchouel M., Hery C., Alvarez F., Yvart J., Tiollais P., Bernard O., Brechot C. Hepatitis B virus DNA in children's liver diseases: detection by blot hybridisation in liver and serum. Gut. 1983 Jul;24(7):618–624. doi: 10.1136/gut.24.7.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequiera L. W., Jennings L. C., Carrasco L. H., Lord M. A., Curry A., Sutton R. N. Detection of herpes-simplex viral genome in brain tissue. Lancet. 1979 Sep 22;2(8143):609–612. doi: 10.1016/s0140-6736(79)91667-2. [DOI] [PubMed] [Google Scholar]
- Spector S. A. Transmission of cytomegalovirus among infants in hospital documented by restriction-endonuclease-digestion analyses. Lancet. 1983 Feb 19;1(8321):378–381. doi: 10.1016/s0140-6736(83)91499-x. [DOI] [PubMed] [Google Scholar]
- Stålhandske P., Pettersson U. Identification of DNA viruses by membrane filter hybridization. J Clin Microbiol. 1982 Apr;15(4):744–747. doi: 10.1128/jcm.15.4.744-747.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas H. C., Karayiannis P., Fowler M. J., Monjardino J. Clinical uses of HBV-DNA assays. J Virol Methods. 1985 Apr;10(4):291–294. doi: 10.1016/0166-0934(85)90044-8. [DOI] [PubMed] [Google Scholar]
- Whelchel J. D., Pass R. F., Diethelm A. G., Whitley R. J., Alford C. A., Jr Effect of primary and recurrent cytomegalovirus infections upon graft and patient survival after renal transplantation. Transplantation. 1979 Dec;28(6):443–446. [PubMed] [Google Scholar]
- Wolters G., Kuijpers L., Kacaki J., Schuurs A. Solid-phase enzyme-immunoassay for detection of hepatitis B surface antigen. J Clin Pathol. 1976 Oct;29(10):873–879. doi: 10.1136/jcp.29.10.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters G., Nelissen P., Kuijpers L. Improved ELISA for the detection of HBsAg. J Virol Methods. 1985 Apr;10(4):299–305. doi: 10.1016/0166-0934(85)90046-1. [DOI] [PubMed] [Google Scholar]
- van Loon A. M., Heessen F. W., van der Logt J. T., van der Veen J. Direct enzyme-linked immunosorbent assay that uses peroxidase-labeled antigen for determination of immunoglobulin M antibody to cytomegalovirus. J Clin Microbiol. 1981 Mar;13(3):416–422. doi: 10.1128/jcm.13.3.416-422.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon A. M., van der Logt J. T., Heessen F. W., van der Veen J. Quantitation of immunoglobulin E antibody to cytomegalovirus by antibody capture enzyme-linked immunosorbent assay. J Clin Microbiol. 1985 Apr;21(4):558–561. doi: 10.1128/jcm.21.4.558-561.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]