Abstract
The cytoplasmic C terminus of the β2-adrenergic receptor and many other G protein-coupled receptors contains a dileucine sequence that has been implicated in endosome/lysosome targeting of diverse proteins. In the present study, we provide evidence for an essential role of this motif in the agonist-induced internalization of the β2-adrenergic receptor. Mutation of Leu-339 and/or Leu-340 to Ala caused little changes in surface expression, ligand binding, G protein coupling, and signaling to adenylyl cyclase, when these receptors were transiently or stably expressed in CHO or HEK-293 cells. However, agonist-induced receptor internalization was markedly impaired in the L339,340A double mutant and reduced in the two single mutants. This impairment in receptor internalization was seen by using various approaches to determine internalization: binding of hydrophobic vs. hydrophilic ligands, loss of surface β2-adrenergic receptor immunoreactivity, and immunofluorescence microscopy. The selective effects of these mutations suggest that the C-terminal dileucine motif is involved in agonist-induced internalization of the β2-adrenergic receptor.
Keywords: adenylyl cyclase, receptor sequestration, Chinese hamster ovary cells
Many types of membrane-bound receptors undergo a dynamic trafficking between the cell surface and intracellular compartments. Such trafficking may be involved both in the transmission of receptor signals and in the termination of such signaling (1). For the β2-adrenergic receptor, a prototypical G protein-coupled receptor, the most remarkable trafficking process is agonist-induced receptor internalization. This internalization is part of a whole spectrum of adaptive processes triggered by agonist stimulation (2, 3).
Agonist-induced translocation of these receptors to an intracellular compartment has been established by several approaches: (i) After agonist stimulation, a certain (and variable) proportion of the receptors becomes inaccessible for hydrophilic ligands but remains accessible for hydrophobic ligands; this process is usually termed sequestration (4). (ii) Upon cell fractionation and sucrose density centrifugation, these receptors can be recovered in a fraction lighter than the plasma membrane fraction (5). (iii) Immunofluorescence studies show that agonist treatment results in the appearance of receptors in intracellular aggregates that might represent endosomes; these data thus provide evidence for true internalization (6–8).
Whereas earlier studies assumed that internalization of β2-adrenergic receptors was essentially a mechanism of receptor desensitization (9, 10), more recent studies have assigned it a recycling and sorting function. According to these models, receptor internalization may occur subsequent to receptor phosphorylation and desensitization and may serve to either dephosphorylate the receptors and recycle them back to the cell surface or—particularly in the case of prolonged receptor stimulation—to direct the receptors to lysosomes for degradation (8, 11–13).
The mechanism of β2-adrenergic receptor internalization is ill-defined at the cell biological and the structural levels. On the cell biological level, most data point toward an internalization via clathrin-coated pits (14–17), but also clathrin-independent internalization has been observed (6). Recent data suggest that the inhibitor protein β-arrestin might act as an adaptor between the receptors and clathrin. β-Arrestin binds to receptors after their phosphorylation by the β-adrenergic receptor kinases and, thereby, uncouples receptors from G proteins (18, 19). β-Arrestin has been shown to bind not only to β2-adrenergic receptors but also to clathrin in vitro, and overexpression of β-arrestin has been shown to promote agonist-induced internalization of these receptors (16, 17).
The receptor domains that might be involved in receptor sequestration are still unknown. Although the recent findings on β-arrestin involvement in this process suggest an essential role of the receptors’ C terminus, which is the target for β-adrenergic receptor kinase-initiated phosphorylation, Strader et al. (20) showed that this C terminus is not required for receptor sequestration. These data were supported by studies indicating that receptor phosphorylation and sequestration were independent events (21, 22). Similarly, Tsuga et al. (23) proposed that phosphorylation of m2-muscarinic receptors might be required for or at least facilitate their sequestration, and Pals-Rylaarsdam et al. (24) showed for the same receptors that sequestration could occur without receptor phosphorylation.
Cheung et al. (9) proposed that the regions involved in β2-adrenergic receptor sequestration might be the same ones as for G protein coupling, but later studies of receptor mutants with impaired G protein coupling but normal internalization suggested that the two functions occur via distinct domains (25, 26).
More recently, Barak et al. (27) showed that mutation of a highly conserved tyrosine residue (Tyr-326) localized at the cytoplasmic end of the seventh transmembrane α-helix to alanine resulted in markedly impaired receptor sequestration. However, later studies showed that multiple properties, including agonist binding, G protein coupling, and signaling, were impaired by this mutation, suggesting that this tyrosine was required for receptor activation rather than specifically for sequestration (28, 29).
Another sequence motif involved in targeting of various proteins to endosomes and lysosomes is a dileucine sequence (30). Dileucine sequences have been proposed to act as binding sites for adaptor proteins required for intracellular protein trafficking (see Discussion). Such a dileucine sequence is also present in the C terminus of the β2-adrenergic receptor. Therefore, we set out to analyze the role of these two leucine residues by generating site-directed mutants. We report that the disruption of this dileucine results in inhibition of agonist-induced receptor internalization.
MATERIALS AND METHODS
Generation and Expression of Mutant β2-Adrenergic Receptors.
Oligonucleotide-directed mutagenesis (31) of the human β2-adrenergic receptor cDNA (32) was used to change Leu-339 and/or -340 to Ala (L339A mutant, L340A mutant, and L339,340A mutant). The identities of the mutations were confirmed by sequencing. Wild-type or mutant β2-adrenergic receptor cDNA were cloned into the plasmid pcDNA3. They were transfected (33) either transiently into human embryonic kidney (HEK)-293 cells or stably into HEK-293 or Chinese hamster ovary (CHO) cells. Stably transfected clones were selected with Geneticin (G418, Boehringer Mannheim; 1 mg/ml) and evaluated for receptor expression using 125I-labeled cyanopindolol (125I-CYP; Amersham) binding. Cells were maintained in monolayer culture in DMEM/F-12 medium (PanSystems) with 10% fetal calf serum. The cells were held in serum-free DMEM/F-12 overnight prior to all experiments.
Radioligand Binding Assays.
Cell membranes were prepared by homogenization of cells in ice-cold 5 mM Tris⋅HCl/5 mM MgCl2/1 mM EGTA, pH 7.4, with an UltraTurrax device, centrifugation at 1,000 × g for 10 min (4°C) and centrifugation of the supernatants at 50,000 × g for 15 min. The pellets were washed once and then resuspended in 50 mM Tris⋅HCl/10 mM MgCl2/1 mM EGTA, pH 7.4, at 200 μg of protein per ml.
For radioligand saturation assays, 0.2 ml of fresh membranes were incubated with 125I-CYP (5–400 pM) for 2 h at 37°C; 10 μM (−)-propranolol (Sigma) was used for nonspecific binding. Inhibition assays were done with 80 pM 125I-CYP with or without 100 μM guanosine 5′-[β,γ-imido]triphosphate [Gpp(NH)p; Sigma]. The incubations were terminated by filtration through Whatman GF/C filters. Nonlinear curve fitting with selection of the appropiate model was used to obtain binding parameters (34, 35).
Adenylyl Cyclase Assays.
Adenylyl cyclase activity was determined in freshly prepared membranes (70 μg of protein) in 100 μl of 50 mM Tris⋅HCl, pH 7.4/1 mM EDTA/100 μM cAMP/50 μM GTP/5 mM creatine phosphate/creatine kinase (0.4 mg/ml)/BSA (1 mg/ml)/100 μM [α-32P]ATP (0.2 μCi per tube; 1 Ci = 37 GBq; NEN/DuPont)/10−9–10−4 M (−)-isoproterenol. Incubations lasted for 30 min at 37°C, and the resulting [32P]cAMP was purified by precipitation and chromatography on alumina (36). Adenylyl cyclase activity was normalized to the protein content and concentration–response curves were analyzed (37) by curve fitting to the equation: E = E0 + EmaxA/(EC50 + A) with E denoting effect, E0 denoting basal activity, Emax denoting maximum effect, and A denoting agonist concentration.
Signaling efficiencies of different receptor mutants were determined by simultaneous curve fitting and calculation of the “transducer ratio” τ (38) by using the algorithm: E = E0 + Emax(τA)/[(KA + A) + τA], with Emax denoting the maximum possible effect (which was shared between all curves) and KA denoting the agonist dissociation constant. τ describes the signal transduction efficacy of the system and is estimated individually for each curve (39).
Receptor Sequestration Assays.
Cells were incubated in serum-free DMEM with or without 10 μM (−)-isoproterenol for the indicated times at 37°C. After the incubation, the cells were washed three times with ice-cold DMEM and resuspended in an appropriate volume of ice-cold DMEM for the binding assay. The percentage of sequestered receptors was determined in 0.2-ml aliquots of whole cells by binding of 35 pM 125I-CYP at 16°C for 4 h; 10 μM (−)-propranolol was used to define the total number of receptors and 0.6 μM CGP 12177 (Ciba Geigy) was used to define cell surface receptors (22).
Determination of Cell Surface β2-Adrenergic Receptors.
The loss of cell surface β2-adrenergic receptors after agonist exposure was also determined by using receptor-specific antibodies: after removal of (−)-isoproterenol by washing the cells were incubated in 1 ml of DMEM/F-12 medium with 0.4 μg of the antibody β2-N70 directed against the receptor’s N terminus (40) for 16 h at 4°C. Excess antibody was washed away by three centrifugation steps, and then the cells were incubated for 2 h at 20°C in 1 ml of DMEM/F-12 with a peroxidase-labeled secondary goat anti-rabbit IgG antibody (Boehringer Mannheim, 0.05 unit/ml). Excess antibody was washed away, and bound antibodies were quantified in an ELISA plate reader using o-phenylene diamine as the peroxidase substrate.
Immunofluorescence Analysis.
Cells were grown on microscopy glass slides and exposed to (−)-isoproterenol as described above. Subsequently, the cells were fixed with 4% paraformaldehyde in phosphate-buffered saline, permeabilized with 0.2% Nonidet P-40, and incubated with β2-C814 antibody (1:300 dilution in 5% nonfat milk/50 mM Hepes) directed against the C terminus of the human β2-adrenergic receptor (40). Cy2-labeled secondary anti-rabbit IgG antibody (5 μg/ml, Biotrend, Köln) was used for visualization in a Zeiss Axiovert fluorescence microscope. In addition to photographic documentation of a few cells, a quantative assessment of the presence of internalized receptors was done by inspecting 100 cells and counting the percentage of cells with fluorescence signals in the cell interior.
RESULTS
Receptor Expression, Binding, and Signaling Properties.
Human wild-type β2-adrenergic receptors and the mutants produced by replacement of either one or both leucines at positions 339 and 340 were transfected and stably expressed in CHO cells. Saturation experiments with 5–400 pM 125I-CYP showed that the mutations caused no changes in the ligand affinity (Table 1). Clones with very similar receptor density were obtained for the wild-type and double mutants, whereas for the single mutants only clones with lower or higher expression levels were found.
Table 1.
Binding parameters for 125I-CYP in membranes from CHO cells expressing wild-type or mutant human β2-adrenergic receptors
| Receptor | K, pM | Bmax, fmol/mg protein |
|---|---|---|
| WT | 44.6 ± 2.5 | 282 ± 19 |
| L339,340A | 50.6 ± 6.5 | 286 ± 11 |
| L339A | 44.0 ± 5.1 | 512 ± 28 |
| L340A | 42.7 ± 4.0 | 121 ± 18 |
Binding parameters were determined from saturation experiments (5–400 pM) using the nonlinear regression program ligand. Each value represents the mean ± SEM of four experiments. WT, wild type.
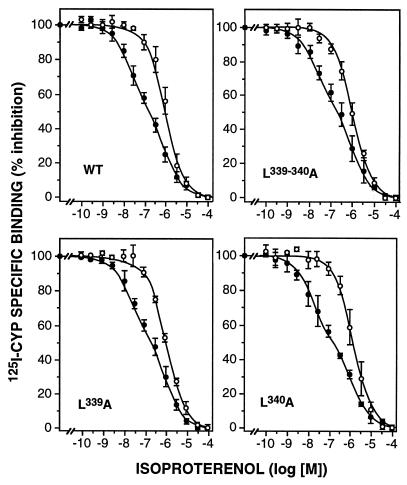
Coupling of the receptors to the G protein Gs was first studied in competition experiments with the agonist (−)-isoproterenol in the absence or presence of the stable GTP analog Gpp(NH)p (Fig. 1). In the absence of Gpp(NH)p, the competition curves were biphasic in all cases, indicating the presence of Gs coupled (high affinity) and uncoupled (low affinity) receptors. Gpp(NH)p (100 μM) shifted all receptors into the uncoupled low-affinity state, resulting in monophasic competition curves. Analysis of these data by curve fitting gave very similar affinities for wild-type and the three mutant receptors (Table 2). The proportion of coupled receptors was similar in all cases with the exception of the L339A mutant, where the higher expression level was presumably responsible for a slight reduction in the percentage of coupled receptors.
Figure 1.
Inhibition of 125I-CYP binding by (−)-isoproterenol in membranes from CHO cells stably expressing wild-type (WT) or mutant β2-adrenergic receptors. Binding was measured in the absence (•) or presence (○) of 100 μM Gpp(NH)p. Analysis of the experiments by nonlinear curve fitting gave the binding parameters shown in Table 2. Data are the mean ± SEM of four to six experiments.
Table 2.
Binding parameters for (−)-isoproterenol in membranes from CHO cells expressing wild-type or mutant human β2-adrenergic receptors
| Receptor | KiH, nM | KiL, nM | RH, % | Ki(+GppNHp), nM |
|---|---|---|---|---|
| WT | 7 ± 3 | 323 ± 111 | 48 ± 3 | 361 ± 88 |
| L339-340A | 6 ± 1 | 317 ± 119 | 44 ± 5 | 343 ± 42 |
| L339A | 7 ± 1 | 358 ± 78 | 30 ± 2 | 421 ± 98 |
| L340A | 5 ± 1 | 413 ± 31 | 52 ± 2 | 441 ± 61 |
Fitting of data to the appropriate binding model and estimation of binding parameters (Ki values for a single or a high (KiH) and a low (KiL) affinity state, respectively, and the percentage of receptors in the high-affinity state, RH) were done with the ebda-ligand programs (35). Selection between one-site or two-site model was made by using the extra sum of squares principle F test. Results are the mean ± SEM from four to six experiments. WT, wild type.
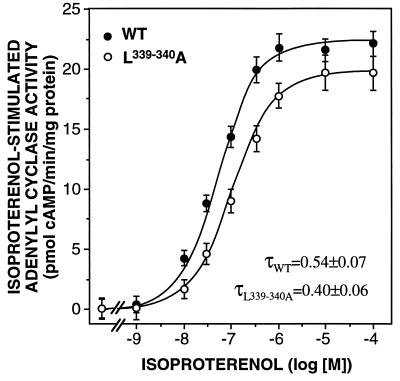
To further analyze the functionality of these mutant receptors, their ability to stimulate adenylyl cyclase activity in response to the agonist (−)-isoproterenol was tested (Fig. 2). (−)-Isoproterenol was able to promote a ≈6-fold stimulation of adenylyl cyclase activity via wild-type and the three mutant receptors. The curve of the double mutant was slightly shifted to the right and reduced in the maximal stimulation compared with the wild-type receptor (Fig. 2). Calculation of the transducer ratio τ indicated that the signaling efficiency of the double mutant was reduced by about 25%. The signaling efficiencies of the single mutants were even less impaired (data not shown). Thus, these data indicate very similar binding, G protein coupling, and signaling properties of the mutant and wild-type receptors.
Figure 2.
Adenylyl cyclase activity in membranes from CHO cells expressing wild-type (WT) or L339,340A mutant β2-adrenergic receptors. The activity was determined in the presence of increasing concentrations of (−)-isoprenaline. The data represent the mean ± SEM of 10 (wild type) or 6 (mutant) experiments. The signaling efficiencies of the wild-type and mutant receptors were quantified by calculating their transducer ratios τ.
Receptor Sequestration.
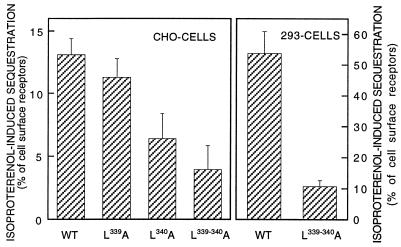
Incubation of CHO cells expressing the wild-type and mutant receptors with 10 μM (−)-isoproterenol for 30 min promoted various degrees of receptor sequestration, as defined by the receptors’ inaccessibility to the hydrophilic ligand CGP 12177 (Fig. 3). Agonist-induced sequestration was about 13% of cell surface receptors for the wild-type receptor but less than 4% for the L339,340A receptor (P < 0.01 by ANOVA). The single mutants showed an intermediate pattern, with the L340A mutant being more affected than the L339A mutant.
Figure 3.
Isoproterenol-induced sequestration of wild-type (WT) and mutant receptors stably expressed in CHO cells (Left) or HEK293 cells (Right). Sequestration was induced by incubating the cells in the presence of 10 μM (−)-isoproterenol for 30 min (CHO cells) or 5 min (HEK293 cells) and was determined by radioligand binding. Sequestration is defined as the percentage of cell surface receptors that became inaccessible for CGP 12177 after the incubation with isoproterenol. Data are the mean ± SEM of 15–20 determinations.
Because the extent of sequestration in the CHO cells was quite small, we also generated stably expressing clones of HEK293 cells for the wild-type and L339,340A double mutant receptors. In these cells, β2-adrenergic receptor sequestration is usually much more rapid and prominent. Cell clones with comparable receptor density (430 and 380 fmol/mg of membrane protein for wild-type and L339,340A mutant receptors, respectively) were used for this analysis. Their radioligand binding and signaling properties were similar to the results presented above for the CHO cell clones, although—as seen by others—Gs coupling was reduced (data not shown). Fig. 3 shows that in these HEK293 cells wild-type receptors were sequestered by more than 50%, whereas only about 10% of the L339,340A mutated receptor were sequestered upon exposure to isoproterenol.
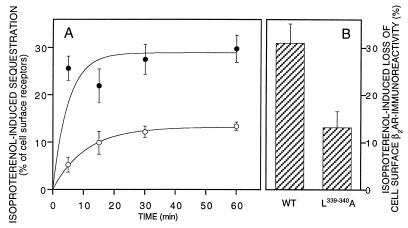
To exclude clonal artifacts, similar experiments were also done with transiently transfected HEK293 cells (Fig. 4A). Although the proportion of agonist-induced sequestration was somewhat smaller in these cells than in the stably transfected ones—probably due to the higher levels of receptor expression—the L339,340A mutation caused a very similar impairment of sequestration. The mutated receptors showed a decrease in the rate of sequestration and a reduction in the final level of sequestered receptors.
Figure 4.
Isoproterenol-induced sequestration (A) and loss of cell surface β2-adrenergic receptor immunoreactivity (B) in transiently transfected HEK293 cells. Transiently expressed L339,340A mutant or wild-type receptors were stimulated with 10 μM (−)-isoproterenol for the indicated times (A) or for 30 min (B). Receptor sequestration (A) was determined by radioligand binding as in Fig. 3; cell surface β2-adrenergic receptor immunoreactivity (B) was determined with an antibody directed against the receptor’s N terminus and quantitation of antibodies bound to the cell surface with a peroxidase-labeled secondary antibody and quantification of the color reaction in an ELISA reader. Data are the mean ± SEM of six determinations.
By using the same model of transiently transfected HEK293 cells, we monitored the loss of cell surface receptors by determining the loss of receptor immunoreactivity caused by exposure to isoproterenol (Fig. 4B). In cells expressing wild-type β2-adrenergic receptors, isoproterenol caused a 31% loss of cell surface receptors, as detected by antibodies directed against the receptor’s N terminus. Again, the L339.340A mutation markedly reduced this loss of cell surface receptors.
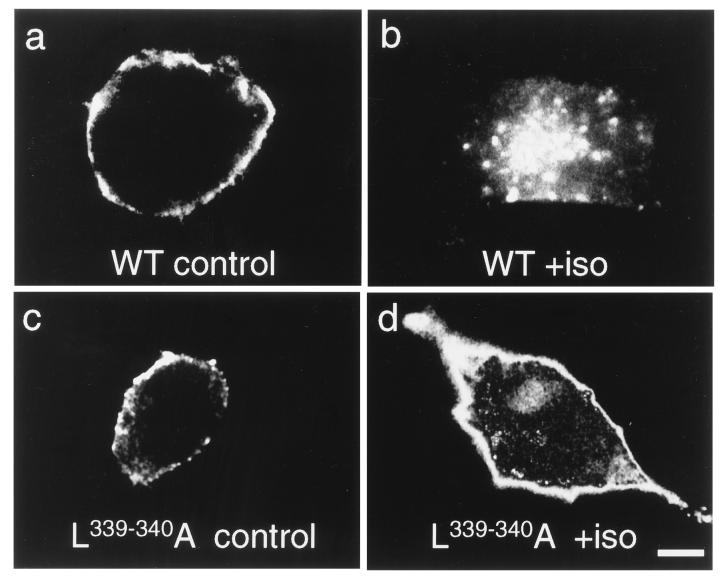
Immunofluorescence microscopy of the stably transfected HEK293 cells was then used to visualize true receptor internalization. Fig. 5 shows that wild-type receptors in these cells were mostly localized to the sharply defined plasma membrane. Upon stimulation with isoproterenol, a large proportion of the receptors became visible in discrete accumulations in the cell interior. Like the wild-type receptors, the L339,340A mutant receptors were localized in the plasma membrane under control conditions, indicating normal cell surface delivery. However, exposure to isoproterenol had very little effect on the subcellular distribution of the mutant receptors. For a more quantitative analysis, three samples of 100 randomly selected cells, as shown in Fig. 5, were screened for the presence (at any intensity) of intracellular vesicles containing β2-adrenergic receptors. Approximately 4 ± 3% of the wild-type receptor cells showed such receptor-containing vesicles under control conditions, but 90 ± 5% did so after exposure to isoproterenol. In contrast, for the cells expressing the L339,340A mutant receptors, these values were 5 ± 2% (control) but only 20 ± 6% (isoproterenol). These data are in line with those obtained by radioligand binding and surface immunoreactivity techniques and underline the major internalization impairment of the L339,340A mutant receptors.
Figure 5.
Subcellular distribution of wild-type and L339,340A mutant receptors stably expressed in HEK293-cells. Cells expressing wild-type (WT) or L339,340A mutant β2-adrenergic receptors were incubated without (control) or with 10 μM (−)-isoproterenol (+iso) for 5 min, fixed, and permeabilized, and β2-adrenergic receptors were visualized with specific antibodies. (Bar = 7 μm.)
DISCUSSION
Our data point to a critical role of the dileucine motif in the C terminus of the β2-adrenergic receptor in receptor internalization. Replacement of both leucines by alanines results in a very marked reduction of internalization, whether measured by radioligand binding with hydrophobic/hydrophilic ligands (“sequestration”), by loss of cell surface receptor immunoreactivity, or by receptor immunofluorescence. In contrast to mutations of Tyr326, which produced marked alterations in internalization but also in G protein coupling, signaling, and cell surface delivery (28, 29), all other functional properties of the mutant receptors were little affected. The internalization of these receptors as monitored by radioligand binding was slowed in its rate and in its extent at steady state. This pattern would be expected if the mutation affected the internalization but not the recycling of internalized receptors (13). Substitution of only one of the leucines by alanine caused intermediate effects, with Leu340 apparently being more critical.
Various sequences have been assigned an essential function for internalization in other G protein-coupled receptors, such as the receptors for thyrotropin-releasing hormone (41), gastrin-releasing peptide (42), neurotensin (43), parathyroid hormone (44), and the AT1 angiotensin (45), and m3 muscarinic (46) receptors. However, there is no detectable consensus among these sequences, and therefore, it appears unlikely that they serve a general and common function. In contrast, the dileucine motif is highly conserved among G protein-coupled receptors, where it is predicted to be in the C terminus close to the plasma membrane.
Such dileucine motifs are present in multiple membrane-bound proteins and have been shown to play a role in protein trafficking (30, 47–49). As in the present case, there are examples such as the interleukin 6 signal transducer where one of the two leucines is more resilient to substitution than the other (50). Dileucine sequences have been shown to bind to AP1 and AP2 clathrin adaptor protein complexes (47), and this process is thought to be responsible for their assembly in clathrin-coated pits and subsequent trafficking in clathrin-coated vesicles. These sequences have been proposed to exist in two forms: membrane-distal accessible dileucines mediate direct transport from the trans Golgi network to endosomes, whereas membrane-proximal nonaccessible dileucines are involved in regulated trafficking from the plasma membrane to endosomes (48). The latter motifs have been suggested to become accessible by phosphorylation of an adjacent Ser/Thr residue (48), although such phosphoserine-dependent AP1 binding has recently also been shown for the membrane-distal dileucine motif in the mannose-6-phosphate receptor (49).
Such regulated accessibility of the dileucine motif in the β2-adrenergic receptor might be achieved via two processes: (i) This motif is immediately adjacent to the palmitoylated cysteine that causes formation of the C-terminal fourth intracellular “loop” of the receptor; this cysteine has been shown to become depalmitoylated upon agonist stimulation of the receptor (51), presumably increasing the accessability of the dileucine. (ii) A phosphorylation site for protein kinase A (PKA) immediately downstream from the palmitoylated cysteine has been shown to become phosphorylated after receptor stimulation, possibly because it becomes accessible after depalmitoylation of the cysteine (52). Although a role for such PKA-mediated phosphorylation would be in line with the phosphoserine-dependent regulation of dileucines, the PKA phosphorylation sites are not essential for receptor sequestration (21), suggesting that depalmitoylation would be the more likely mechanism to make the dileucine motif accessible. On the other hand, a replacement of the palmitoylated Cys-341 by glycine has been reported to result in at least as much agonist-induced sequestration (26). Whether—in line with this hypothesis—this receptor shows enhanced basal sequestration has to our knowledge not been investigated. In the V2-vasopressin receptor a similar mutant results in significant loss of the percentage of receptors at the cell surface (53).
The nature of the adaptor protein for this dileucine motif remains to be elucidated. Such dileucine motifs are usually thought to be bound by the AP1 and AP2 adaptor protein complexes, but binding of these proteins to β2-adrenergic receptor has never been shown, and there may well be other adaptor proteins that bind to these motifs. β-Arrestin has recently been proposed as a receptor/clathrin adaptor (16, 17) and might bind not only to phosphoserines and phosphothreonines in the receptors’ C terminus but also to the dileucine motif. However, the desensitization behavior of our mutant receptors was normal (data not shown), suggesting that β-arrestin is not involved in dileucine-mediated internalization.
These data suggest that both a dileucine-dependent and a phosphorylation/β-arrestin-dependent pathway may exist for the internalization of β2-adrenergic receptors. Such a dual pathway for internalization would be reminiscent of the many other often overlapping mechanisms that control receptor function.
Acknowledgments
These studies were supported by grants from the Deutsche Forschungsgemeinschaft (SFB 355) and the Fonds der Chemischen Industrie. A.M.G. was recipient of a fellowship by the Humboldt Foundation.
ABBREVIATIONS
- CYP
cyanopindolol
- Gpp(NH)p
guanosine 5′-[β,γ-imido]triphosphate
References
- 1.Vieira A V, Lamaze C, Schmid S L. Science. 1996;274:2086–2089. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- 2.Hausdorff W P, Caron M G, Lefkowitz R J. FASEB J. 1990;4:2881–2889. [PubMed] [Google Scholar]
- 3.Lohse M J. Biochim Biophys Acta. 1993;1179:171–188. doi: 10.1016/0167-4889(93)90139-g. [DOI] [PubMed] [Google Scholar]
- 4.Staehelin M, Simons P. EMBO J. 1982;1:187–190. doi: 10.1002/j.1460-2075.1982.tb01145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harden T K, Cotton C V, Waldo G L, Lutton J K, Perkins J P. Science. 1980;210:441–443. doi: 10.1126/science.6254143. [DOI] [PubMed] [Google Scholar]
- 6.Raposo G, Dunia I, Delavier-Klutchko C, Kaveri S, Strosberg A D, Benedetti E L. Eur J Cell Biol. 1989;50:340–352. [PubMed] [Google Scholar]
- 7.Wang H, Lipfert L, Malbon C C, Bahouth S. J Biol Chem. 1989;264:14424–14431. [PubMed] [Google Scholar]
- 8.Von Zastrow M, Kobilka B K. J Biol Chem. 1992;267:3530–3538. [PubMed] [Google Scholar]
- 9.Cheung A H, Sigal I S, Dixon R A F, Strader C D. Mol Pharmacol. 1989;34:132–138. [PubMed] [Google Scholar]
- 10.Hertel C, Nunnally M, Wong S K-F, Murphy E A, Ross E M, Perkins J P. J Biol Chem. 1990;265:17988–17994. [PubMed] [Google Scholar]
- 11.Kurz J B, Perkins J P. Mol Pharmacol. 1992;41:375–381. [PubMed] [Google Scholar]
- 12.Yu S S, Lefkowitz R J, Hausdorff W P. J Biol Chem. 1993;268:337–341. [PubMed] [Google Scholar]
- 13.Pippig S, Andexinger S, Lohse M J. Mol Pharm. 1995;47:666–676. [PubMed] [Google Scholar]
- 14.Wakshull E, Hertel C, O’Keefe E J, Perkins J P. J Cell Biol. 1985;29:127–141. doi: 10.1002/jcb.240290208. [DOI] [PubMed] [Google Scholar]
- 15.Hertel C, Coulter S J, Perkins J P. J Biol Chem. 1986;261:5974–5980. [PubMed] [Google Scholar]
- 16.Goodman O B, Jr, Krupnick J G, Santini F, Gurevich V V, Penn R B, Gagnon A W, Keen J H, Benovic J L. Nature (London) 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- 17.Ferguson S S, Downey W E, 3rd, Colapietro A M, Barak L S, Menard L, Caron M G. Science. 1996;271:363–366. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- 18.Lohse M J, Benovic J L, Codina J, Caron M G, Lefkowitz R J. Science. 1990;248:1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- 19.Lohse M J, Andexinger S, Pitcher J, Trukawinski S, Codina J, Faure J-P, Caron M G, Lefkowitz R J. J Biol Chem. 1992;267:8558–8564. [PubMed] [Google Scholar]
- 20.Strader C D, Sigal I S, Blake A D, Cheung A H, Register R B, Rands E, Zemcik B A, Candelore M R, Dixon R A F. Cell. 1987;49:855–863. doi: 10.1016/0092-8674(87)90623-4. [DOI] [PubMed] [Google Scholar]
- 21.Hausdorff W P, Bouvier M, O’Dowd B F, Irons G P, Caron M G, Lefkowitz R J. J Biol Chem. 1989;264:12657–12665. [PubMed] [Google Scholar]
- 22.Lohse M J, Benovic J L, Caron M G, Lefkowitz R J. J Biol Chem. 1990;265:3202–3209. [PubMed] [Google Scholar]
- 23.Tsuga H, Kameyama K, Haga T, Kurose H, Nagao T. J Biol Chem. 1994;269:32522–32527. [PubMed] [Google Scholar]
- 24.Pals-Rylaarsdam R, Xu Y, Witt-Enderby P, Benovic J L, Hosey M M. J Biol Chem. 1995;270:29004–29011. doi: 10.1074/jbc.270.48.29004. [DOI] [PubMed] [Google Scholar]
- 25.Cheung A H, Dixon R A F, Hill W S, Sigal I S, Strader C D. Mol Pharmacol. 1990;37:775–779. [PubMed] [Google Scholar]
- 26.Campbell P T, Hnatowich M, O’Dowd B F, Caron M G, Lefkowitz R J, Hausdorff W P. Mol Pharmacol. 1991;39:192–198. [PubMed] [Google Scholar]
- 27.Barak L, Tiberi M, Freedman N J, Kwatra M M, Lefkowitz R J, Caron M G. J Biol Chem. 1994;269:2790–2795. [PubMed] [Google Scholar]
- 28.Barak L S, Ménard L, Ferguson S S G, Colapietro A-M, Caron M G. Biochemistry. 1995;34:15407–15414. doi: 10.1021/bi00047a003. [DOI] [PubMed] [Google Scholar]
- 29.Gabilondo A M, Krasel C, Lohse M J. Eur J Pharmacol. 1996;307:243–250. doi: 10.1016/0014-2999(96)00247-6. [DOI] [PubMed] [Google Scholar]
- 30.Letourneur F, Klausner R D. Cell. 1992;69:1143–1157. doi: 10.1016/0092-8674(92)90636-q. [DOI] [PubMed] [Google Scholar]
- 31.Kunkel T A. Proc Natl Acad Sci USA. 1985;82:488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kobilka B K, Dixon R A F, Frielle T, Dohlman H G, Bolanowski M A, Sigal I S, Yang-Feng T L, Francke U, Caron M G, Lefkowitz R J. Proc Natl Acad Sci USA. 1987;84:46–50. doi: 10.1073/pnas.84.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen C, Okayama H. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lohse M J, Lenschow V, Schwabe U. Mol Pharmacol. 1984;26:1–9. [PubMed] [Google Scholar]
- 35.McPherson G A. J Pharmacol Meth. 1985;14:213–228. doi: 10.1016/0160-5402(85)90034-8. [DOI] [PubMed] [Google Scholar]
- 36.Pippig S, Andexinger S, Daniel K, Puzicha M, Caron M G, Lefkowitz R J, Lohse M J. J Biol Chem. 1993;268:3201–3208. [PubMed] [Google Scholar]
- 37.Lohse M J, Klotz K-N, Schwabe U. Mol Pharmacol. 1986;30:403–409. [PubMed] [Google Scholar]
- 38.Black J W, Leff P, Shankley N P. Br J Pharmacol. 1985;84:561–571. doi: 10.1111/j.1476-5381.1985.tb12941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lohse M J. J Biol Chem. 1990;265:3210–3211. [PubMed] [Google Scholar]
- 40.Jahns R, Siegmund C, Jahns V, Reiländer H, Maidhof A, Müller-Esterl W, Lohse M J, Boege F. Eur J Pharmacol. 1996;316:111–121. doi: 10.1016/s0014-2999(96)00654-1. [DOI] [PubMed] [Google Scholar]
- 41.Nussenzveig D R, Heinflink M, Gershengorn M C. J Biol Chem. 1993;268:2389–2392. [PubMed] [Google Scholar]
- 42.Benya R, Fathi Z, Batti J F, Jensen R T. J Biol Chem. 1993;268:20285–20290. [PubMed] [Google Scholar]
- 43.Chabry J, Botto J M, Nouel D, Beaudet A, Vincent J P, Mazella J. J Biol Chem. 1995;270:2439–2442. doi: 10.1074/jbc.270.6.2439. [DOI] [PubMed] [Google Scholar]
- 44.Huang Z, Chen Y, Nissenson R A. J Biol Chem. 1995;270:151–156. doi: 10.1074/jbc.270.1.151. [DOI] [PubMed] [Google Scholar]
- 45.Thomas W G, Baker K M, Motel T J, Thekkumkara T J. J Biol Chem. 1995;270:22153–22159. doi: 10.1074/jbc.270.38.22153. [DOI] [PubMed] [Google Scholar]
- 46.Yang J, Williams J A, Yule D I, Logsdon C D. Mol Pharmacol. 1995;48:477–485. [PubMed] [Google Scholar]
- 47.Heilker R, Manning-Krieg U, Zuber J-F, Spiess M. EMBO J. 1996;15:2893–2899. [PMC free article] [PubMed] [Google Scholar]
- 48.Dietrich J, Hou X, Wegener A-M K, Geisler C. EMBO J. 1994;13:2156–2166. doi: 10.1002/j.1460-2075.1994.tb06492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mauxion F, Le Borgne R, Munier-Lehmann H, Hoflack B. J Biol Chem. 1996;271:2171–2178. doi: 10.1074/jbc.271.4.2171. [DOI] [PubMed] [Google Scholar]
- 50.Dittrich E, Haft C R, Muys L, Heinrich P C, Graeve L. J Biol Chem. 1996;271:5487–5494. doi: 10.1074/jbc.271.10.5487. [DOI] [PubMed] [Google Scholar]
- 51.Loisel T P, Adam L, Hebert T, Bouvier M. Biochemistry. 1996;35:15923–15932. doi: 10.1021/bi9611321. [DOI] [PubMed] [Google Scholar]
- 52.Moffet S, Adam L, Bonin H, Loisel T P, Bouvier M, Mouillac B. J Biol Chem. 1996;271:21490–21497. doi: 10.1074/jbc.271.35.21490. [DOI] [PubMed] [Google Scholar]
- 53.Schülein R, Liebenhoff U, Müller H, Birnbaumer M, Rosenthal W. Biochem J. 1996;313:611–616. doi: 10.1042/bj3130611. [DOI] [PMC free article] [PubMed] [Google Scholar]