Abstract
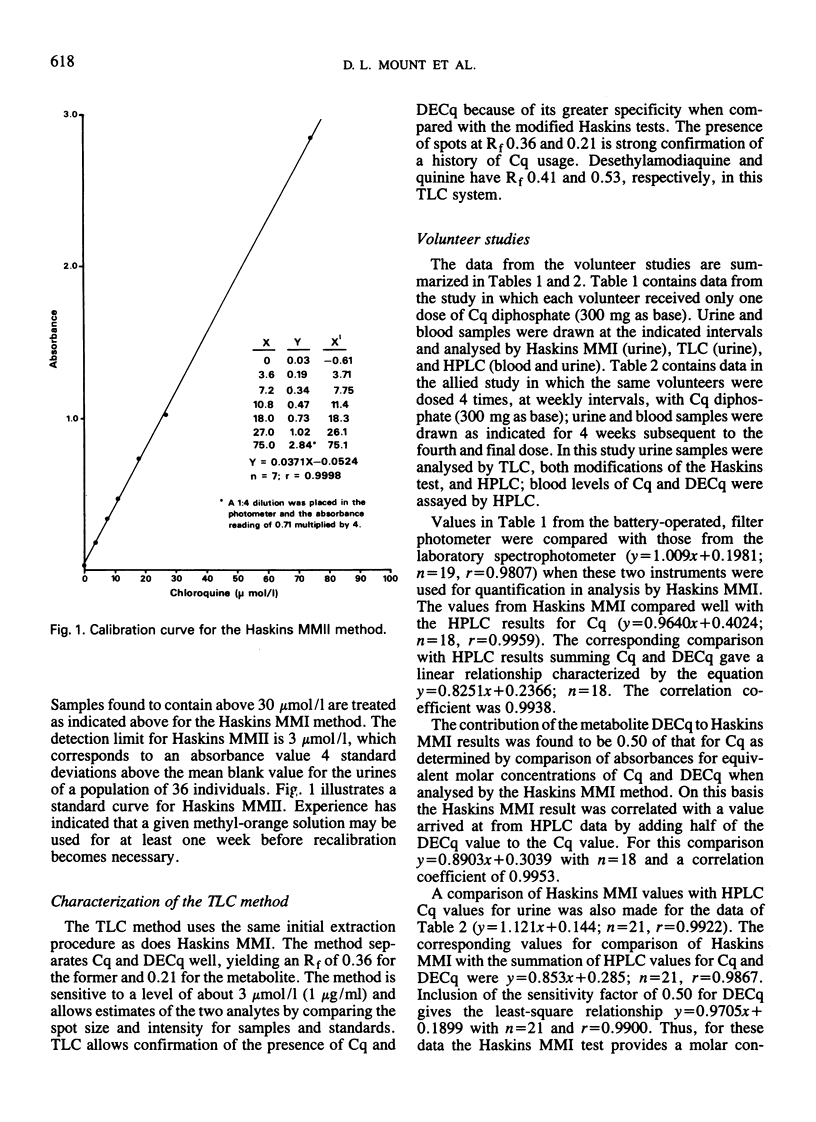
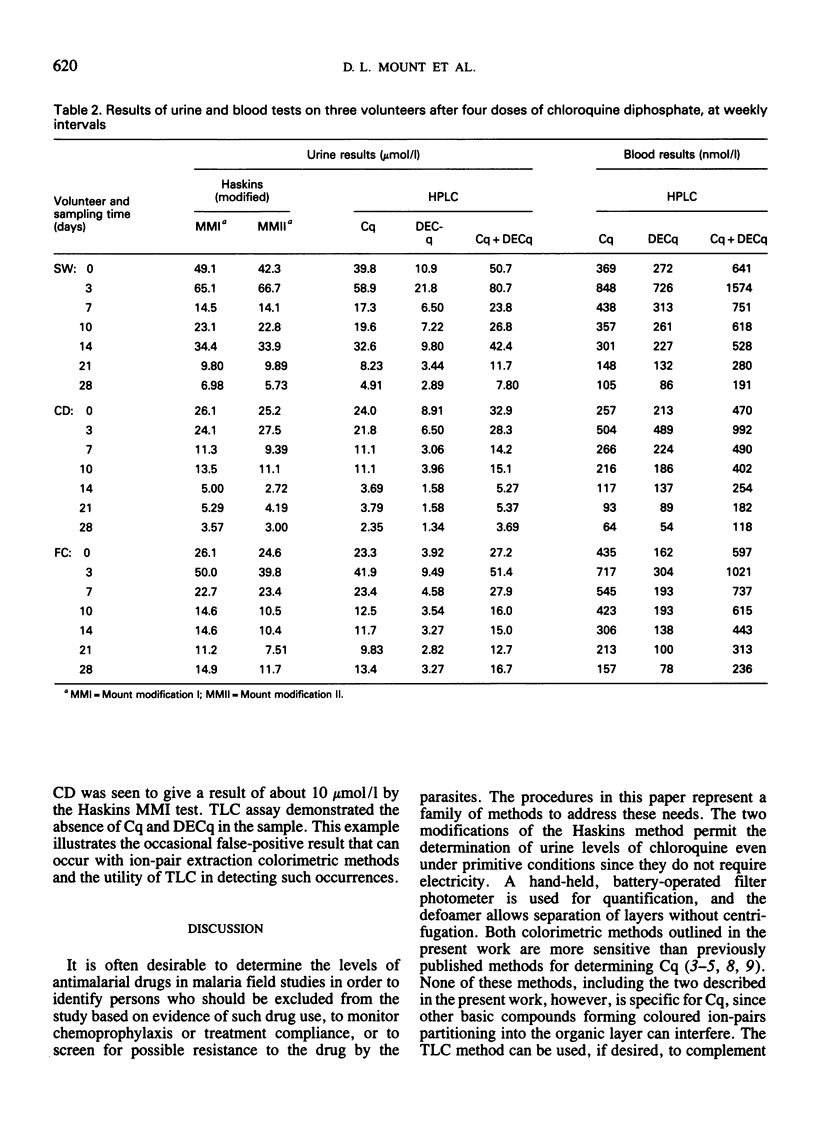
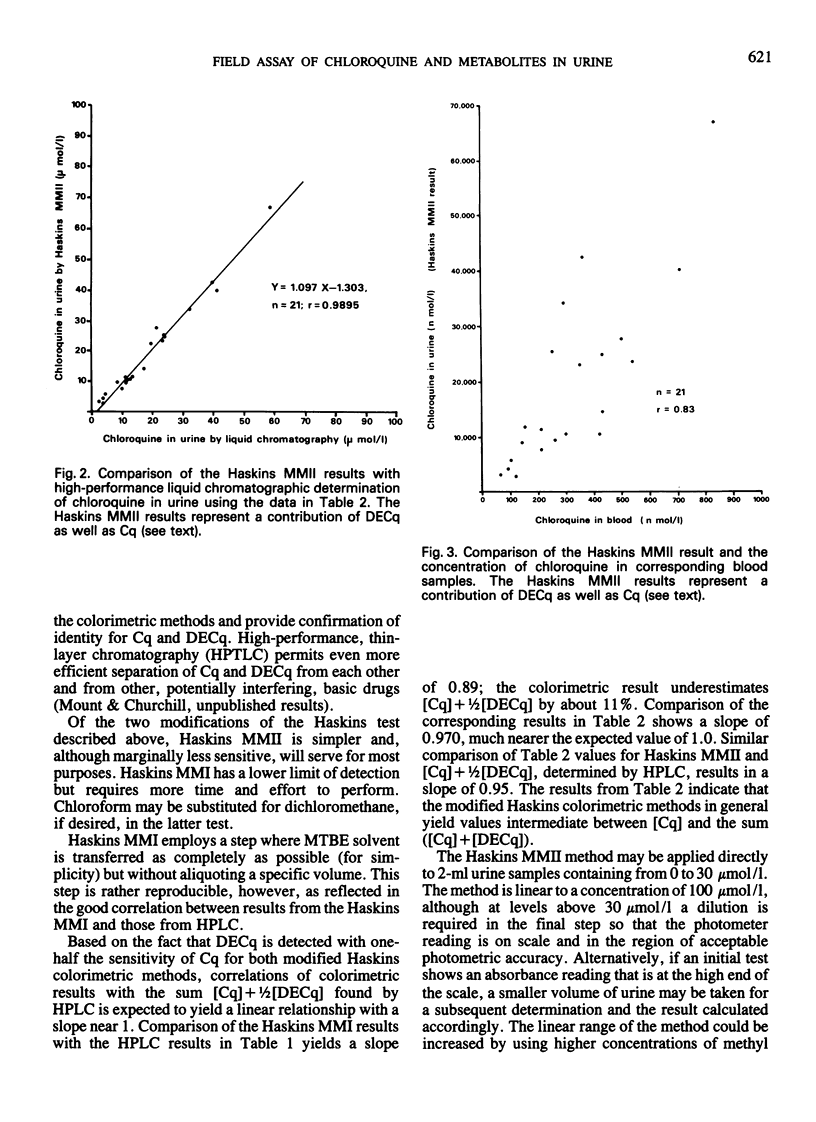
Three field-adapted methods for the quantification of the antimalarial drug chloroquine are described. Two of the methods are modifications of the Haskins test and are based on ion-pair formation between chloroquine and methyl orange in either dichloromethane or chloroform. Absorbance values measured at 420 nm with a hand-held, battery-operated filter photometer were linearly related to chloroquine concentrations in urine up to 100 μmol/l (32 μg/ml) for both methods. The contribution of the desethylchloroquine metabolite to the measured absorbance for both methods is less than that of chloroquine; the relative sensitivity for this metabolite is about 50% of that of chloroquine for both methods. The detection limit for modification I is 1 μmol/l (0.3 μg/ml), while that for modification II is 3 μmol/l (1 μg/ml). A single dose of chloroquine diphosphate (300 mg as base) administered to each of three volunteers yielded detectable levels by modification I of chloroquine in the urine for 28 days after dosing. Results for the colorimetric methods correlated well with the liquid chromatographic reference method used. The related thin-layer chromatographic method confirmed the presence of chloroquine and desethylchloroquine in the urine and permitted independent estimation of the concentration of these two compounds if desired. The two colorimetric methods may be used in remote locations where no electricity is available.
Full text
PDF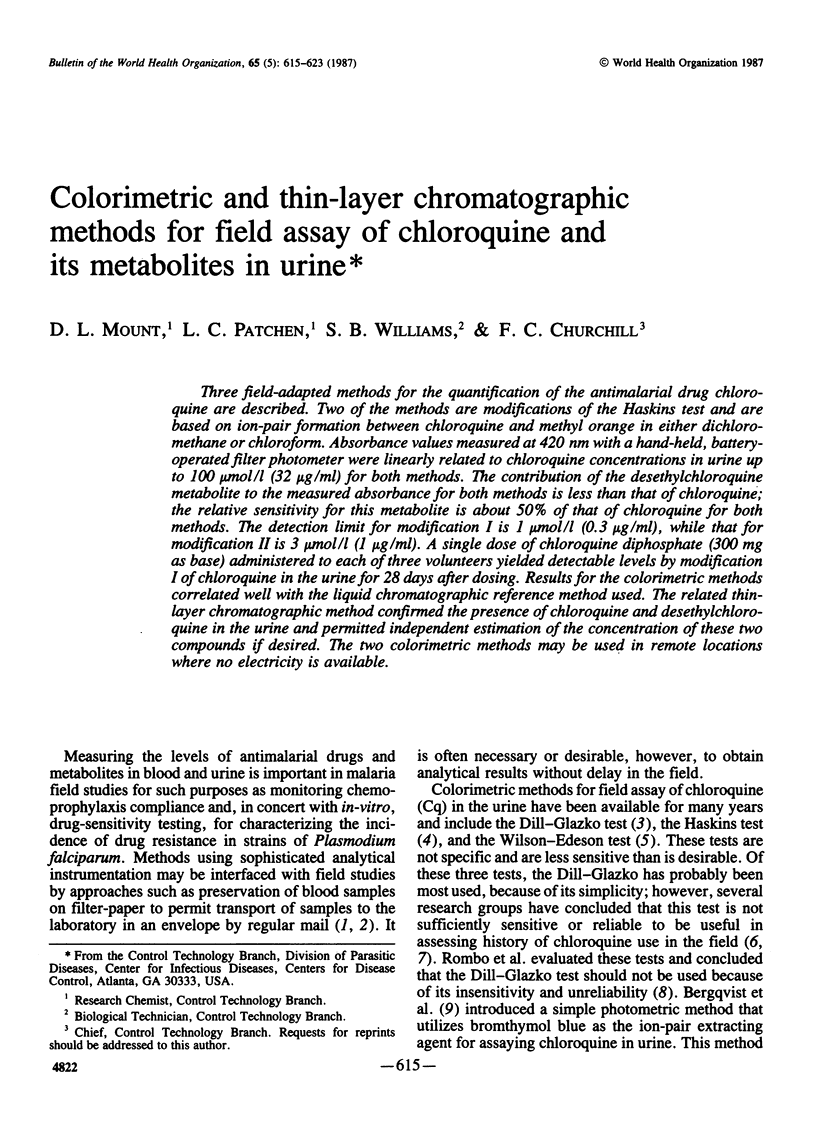





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergqvist Y., Hed C., Funding L., Suther A. Determination of chloroquine and its metabolites in urine: a field method based on ion-pair extraction. Bull World Health Organ. 1985;63(5):893–898. [PMC free article] [PubMed] [Google Scholar]
- Betschart B., Steiger S. Quantitative determination of chloroquine and desethylchloroquine in biological fluids by high performance thin layer chromatography. Acta Trop. 1986 Jun;43(2):125–130. [PubMed] [Google Scholar]
- Lelijveld J., Kortmann H. The eosin colour test of Dill and Glazko: a simple field test to detect chloroquine in urine. Bull World Health Organ. 1970;42(3):477–479. [PMC free article] [PubMed] [Google Scholar]
- Lindström B., Ericsson O., Alván G., Rombo L., Ekman L., Rais M., Sjöqvist F. Determination of chloroquine and its desethyl metabolite in whole blood: an application for samples collected in capillary tubes and dried on filter paper. Ther Drug Monit. 1985;7(2):207–210. doi: 10.1097/00007691-198506000-00012. [DOI] [PubMed] [Google Scholar]
- Patchen L. C., Mount D. L., Schwartz I. K., Churchill F. C. Analysis of filter-paper-absorbed, finger-stick blood samples for chloroquine and its major metabolite using high-performance liquid chromatography with fluorescence detection. J Chromatogr. 1983 Nov 11;278(1):81–89. doi: 10.1016/s0378-4347(00)84758-1. [DOI] [PubMed] [Google Scholar]
- Rombo L., Björkman A., Sego E., Lindström B., Ericsson O., Gustafsson L. L. Evaluation of three qualitative tests for detection of chloroquine in urine--agreement with plasma concentrations determined with liquid chromatography. Ann Trop Med Parasitol. 1986 Jun;80(3):293–298. doi: 10.1080/00034983.1986.11812019. [DOI] [PubMed] [Google Scholar]
- Rombo L., Björkman A., Sego E., Lindström B., Ericsson O., Gustafsson L. L. Reliability of Dill-Glazko test. Lancet. 1985 Jun 29;1(8444):1509–1509. doi: 10.1016/s0140-6736(85)92284-6. [DOI] [PubMed] [Google Scholar]
- Verdier F., Ramanamirija J. A., Pussard E., Clavier F., Biaud J. M., Coulanges P., Le Bras J. Unreliability of Dill Glazko test in detecting chloroquine in urine. Lancet. 1985 Jun 1;1(8440):1282–1283. doi: 10.1016/s0140-6736(85)92358-x. [DOI] [PubMed] [Google Scholar]
- WILSON T., EDESON J. F. Studies on the chemotherapy of malaria. III. The treatment of acute malaria with chloroquine. Med J Malaya. 1954 Dec;9(2):115–131. [PubMed] [Google Scholar]


