Abstract
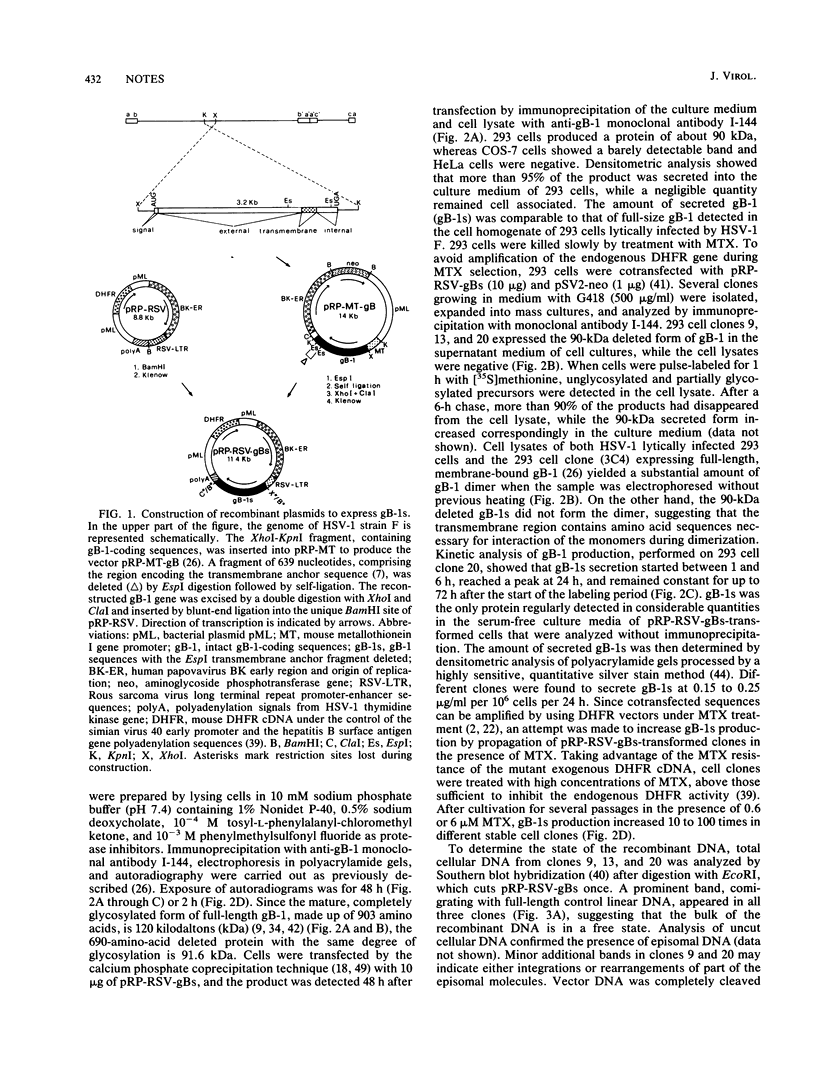
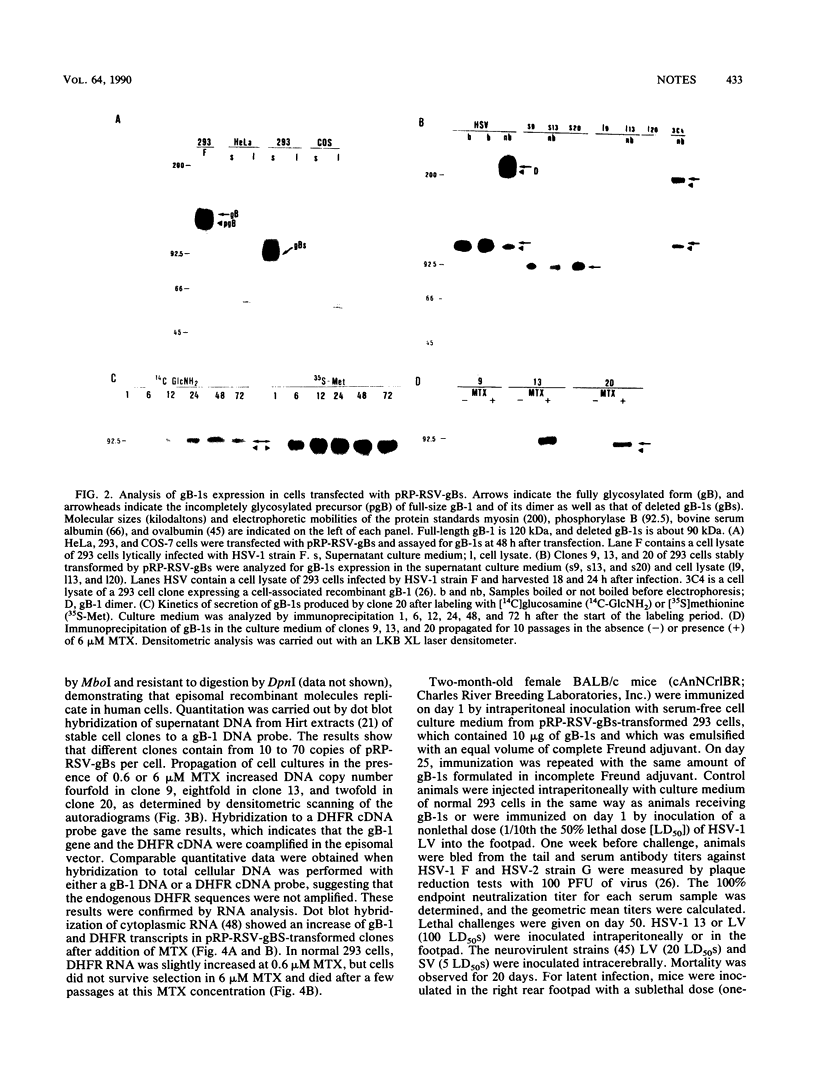
The herpes simplex virus type 1 (HSV-1) glycoprotein B (gB-1) gene, deleted of 639 nucleotides that encode the transmembrane anchor sequence and reconstructed with the extramembrane and intracytoplasmic domains, was cloned under control of the Rous sarcoma virus long terminal repeat in the episomal replicating vector pRP-RSV, which contains the origin of replication and early region of the human papovavirus BK as well as a cDNA for a mutant mouse dihydrofolate reductase that is resistant to methotrexate. gB-1 (0.15 to 0.25 pg per cell per 24 h) was constitutively secreted into the culture medium of pRP-RSV-gBs-transformed human 293 cells. Treatment of transformed cells with methotrexate at high concentrations (0.6 to 6 microM) increased gB-1 production 10- to 100-fold, because of an amplification of the episomal recombinant. Mice immunized with secreted gB-1 produced HSV-1- and HSV-2-neutralizing antibodies and were protected against HSV-1 lethal, latent, and recurrent infections. Constitutive expression of secreted gB-1 in human cells may establish a system to develop diagnostic material and a subunit vaccine for HSV infections.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali M. A., Butcher M., Ghosh H. P. Expression and nuclear envelope localization of biologically active fusion glycoprotein gB of herpes simplex virus in mammalian cells using cloned DNA. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5675–5679. doi: 10.1073/pnas.84.16.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt F. W., Kellems R. E., Bertino J. R., Schimke R. T. Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem. 1978 Mar 10;253(5):1357–1370. [PubMed] [Google Scholar]
- Arsenakis M., Hubenthal-Voss J., Campadelli-Fiume G., Pereira L., Roizman B. Construction and properties of a cell line constitutively expressing the herpes simplex virus glycoprotein B dependent on functional alpha 4 protein synthesis. J Virol. 1986 Nov;60(2):674–682. doi: 10.1128/jvi.60.2.674-682.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran N., Bacchetti S., Rawls W. E. Protection against lethal challenge of BALB/c mice by passive transfer of monoclonal antibodies to five glycoproteins of herpes simplex virus type 2. Infect Immun. 1982 Sep;37(3):1132–1137. doi: 10.1128/iai.37.3.1132-1137.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendig M. M. The production of foreign proteins in mammalian cells. Genet Eng. 1988;(7):91–127. [PubMed] [Google Scholar]
- Buckmaster E. A., Gompels U., Minson A. Characterisation and physical mapping of an HSV-1 glycoprotein of approximately 115 X 10(3) molecular weight. Virology. 1984 Dec;139(2):408–413. doi: 10.1016/0042-6822(84)90387-8. [DOI] [PubMed] [Google Scholar]
- Bzik D. J., Fox B. A., DeLuca N. A., Person S. Nucleotide sequence specifying the glycoprotein gene, gB, of herpes simplex virus type 1. Virology. 1984 Mar;133(2):301–314. doi: 10.1016/0042-6822(84)90397-0. [DOI] [PubMed] [Google Scholar]
- Campadelli-Fiume G., Lombardo M. T., Foà-Tomasi L., Avitabile E., Serafini-Cessi F. Individual herpes simplex virus 1 glycoproteins display characteristic rates of maturation from precursor to mature form both in infected cells and in cells that constitutively express the glycoproteins. Virus Res. 1988 Apr;10(1):29–40. doi: 10.1016/0168-1702(88)90055-x. [DOI] [PubMed] [Google Scholar]
- Cantin E. M., Eberle R., Baldick J. L., Moss B., Willey D. E., Notkins A. L., Openshaw H. Expression of herpes simplex virus 1 glycoprotein B by a recombinant vaccinia virus and protection of mice against lethal herpes simplex virus 1 infection. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5908–5912. doi: 10.1073/pnas.84.16.5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer K. J., Mackett M., Wohlenberg C., Notkins A. L., Moss B. Vaccinia virus recombinant expressing herpes simplex virus type 1 glycoprotein D prevents latent herpes in mice. Science. 1985 May 10;228(4700):737–740. doi: 10.1126/science.2986288. [DOI] [PubMed] [Google Scholar]
- Dix R. D., Mills J. Acute and latent herpes simplex virus neurological disease in mice immunized with purified virus-specific glycoproteins gB or gD. J Med Virol. 1985 Sep;17(1):9–18. doi: 10.1002/jmv.1890170103. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Glorioso J., Schröder C. H., Kumel G., Szczesiul M., Levine M. Immunogenicity of herpes simplex virus glycoproteins gC and gB and their role in protective immunity. J Virol. 1984 Jun;50(3):805–812. doi: 10.1128/jvi.50.3.805-812.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Gompels U., Minson A. The properties and sequence of glycoprotein H of herpes simplex virus type 1. Virology. 1986 Sep;153(2):230–247. doi: 10.1016/0042-6822(86)90026-7. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Green M. R., Treisman R., Maniatis T. Transcriptional activation of cloned human beta-globin genes by viral immediate-early gene products. Cell. 1983 Nov;35(1):137–148. doi: 10.1016/0092-8674(83)90216-7. [DOI] [PubMed] [Google Scholar]
- Hill T. J., Field H. J., Blyth W. A. Acute and recurrent infection with herpes simplex virus in the mouse: a model for studying latency and recurrent disease. J Gen Virol. 1975 Sep;28(3):341–353. doi: 10.1099/0022-1317-28-3-341. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kaufman R. J., Sharp P. A. Amplification and expression of sequences cotransfected with a modular dihydrofolate reductase complementary dna gene. J Mol Biol. 1982 Aug 25;159(4):601–621. doi: 10.1016/0022-2836(82)90103-6. [DOI] [PubMed] [Google Scholar]
- Kino Y., Eto T., Ohtomo N., Hayashi Y., Yamamoto M., Mori R. Passive immunization of mice with monoclonal antibodies to glycoprotein gB of herpes simplex virus. Microbiol Immunol. 1985;29(2):143–149. doi: 10.1111/j.1348-0421.1985.tb00812.x. [DOI] [PubMed] [Google Scholar]
- Kousoulas K. G., Huo B., Pereira L. Antibody-resistant mutations in cross-reactive and type-specific epitopes of herpes simplex virus 1 glycoprotein B map in separate domains. Virology. 1988 Oct;166(2):423–431. doi: 10.1016/0042-6822(88)90513-2. [DOI] [PubMed] [Google Scholar]
- Manservigi R., Gualandri R., Negrini M., Albonici L., Milanesi G., Cassai E., Barbanti-Brodano G. Constitutive expression in human cells of herpes simplex virus type 1 glycoprotein B gene cloned in an episomal eukaryotic vector. Virology. 1988 Nov;167(1):284–288. doi: 10.1016/0042-6822(88)90080-3. [DOI] [PubMed] [Google Scholar]
- Milanesi G., Barbanti-Brodano G., Negrini M., Lee D., Corallini A., Caputo A., Grossi M. P., Ricciardi R. P. BK virus-plasmid expression vector that persists episomally in human cells and shuttles into Escherichia coli. Mol Cell Biol. 1984 Aug;4(8):1551–1560. doi: 10.1128/mcb.4.8.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R. Induction of the synthesis of a 70,000 dalton mammalian heat shock protein by the adenovirus E1A gene product. Cell. 1982 Jul;29(3):913–919. doi: 10.1016/0092-8674(82)90453-6. [DOI] [PubMed] [Google Scholar]
- Norrby E. Toward new viral vaccines for man. Adv Virus Res. 1987;32:1–34. doi: 10.1016/s0065-3527(08)60473-x. [DOI] [PubMed] [Google Scholar]
- Norrild B., Shore S. L., Nahmias A. J. Herpes simplex virus glycoproteins: participation of individual herpes simplex virus type 1 glycoprotein antigens in immunocytolysis and their correlation with previously identified glycopolypeptides. J Virol. 1979 Dec;32(3):741–748. doi: 10.1128/jvi.32.3.741-748.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki C., Makizumi K., Kino Y., Nakatake H., Eto T., Mizuno K., Hamada F., Ohtomo N. Expression of herpes simplex virus glycoprotein B gene in yeast. Virus Res. 1985 Dec;4(1):107–113. doi: 10.1016/0168-1702(85)90024-3. [DOI] [PubMed] [Google Scholar]
- Pachl C., Burke R. L., Stuve L. L., Sanchez-Pescador L., Van Nest G., Masiarz F., Dina D. Expression of cell-associated and secreted forms of herpes simplex virus type 1 glycoprotein gB in mammalian cells. J Virol. 1987 Feb;61(2):315–325. doi: 10.1128/jvi.61.2.315-325.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rector J. T., Lausch R. N., Oakes J. E. Use of monoclonal antibodies for analysis of antibody-dependent immunity to ocular herpes simplex virus type 1 infection. Infect Immun. 1982 Oct;38(1):168–174. doi: 10.1128/iai.38.1.168-174.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney J. F., Wohlenberg C., Cremer K. J., Moss B., Notkins A. L. Immunization with a vaccinia virus recombinant expressing herpes simplex virus type 1 glycoprotein D: long-term protection and effect of revaccination. J Virol. 1988 May;62(5):1530–1534. doi: 10.1128/jvi.62.5.1530-1534.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotula A., Di Luca D., Gerna G., Manservigi R., Tognon M., Cassai E. Herpes simplex virus latency in immunosuppressed mice. Microbiologica. 1984 Jul;7(3):219–227. [PubMed] [Google Scholar]
- Schröder C. H., Kümel G., Glorioso J., Kirchner H., Kaerner H. C. Neuropathogenicity of herpes simplex virus in mice: protection against lethal encephalitis by co-infection with a non-encephalitogenic strain. J Gen Virol. 1983 Sep;64(Pt 9):1973–1982. doi: 10.1099/0022-1317-64-9-1973. [DOI] [PubMed] [Google Scholar]
- Sheares B. T., Robbins P. W. Glycosylation of ovalbumin in a heterologous cell: analysis of oligosaccharide chains of the cloned glycoprotein in mouse L cells. Proc Natl Acad Sci U S A. 1986 Apr;83(7):1993–1997. doi: 10.1073/pnas.83.7.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen C. C., Levinson A. D. Isolation and expression of an altered mouse dihydrofolate reductase cDNA. Proc Natl Acad Sci U S A. 1983 May;80(9):2495–2499. doi: 10.1073/pnas.80.9.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Spear P. G. Membrane proteins specified by herpes simplex viruses. I. Identification of four glycoprotein precursors and their products in type 1-infected cells. J Virol. 1976 Mar;17(3):991–1008. doi: 10.1128/jvi.17.3.991-1008.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer R. C., 3rd, Merril C. R., Shifrin S. A highly sensitive silver stain for detecting proteins and peptides in polyacrylamide gels. Anal Biochem. 1979 Sep 15;98(1):231–237. doi: 10.1016/0003-2697(79)90732-2. [DOI] [PubMed] [Google Scholar]
- Tognon M., Manservigi R., Sebastiani A., Bragliani G., Busin M., Cassai E. Analysis of HSV isolated from patients with unilateral and bilateral herpetic keratitis. Int Ophthalmol. 1985 Apr;8(1):13–18. doi: 10.1007/BF00136456. [DOI] [PubMed] [Google Scholar]
- Treisman R., Green M. R., Maniatis T. cis and trans activation of globin gene transcription in transient assays. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7428–7432. doi: 10.1073/pnas.80.24.7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]