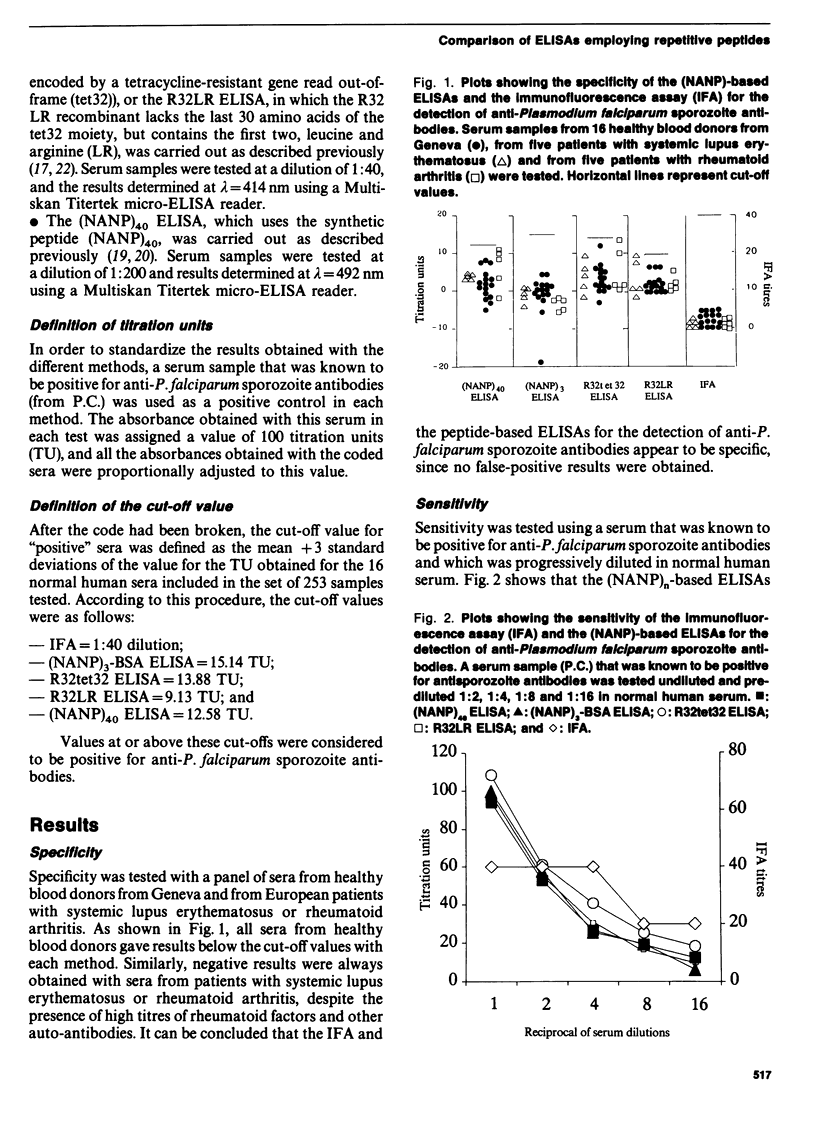
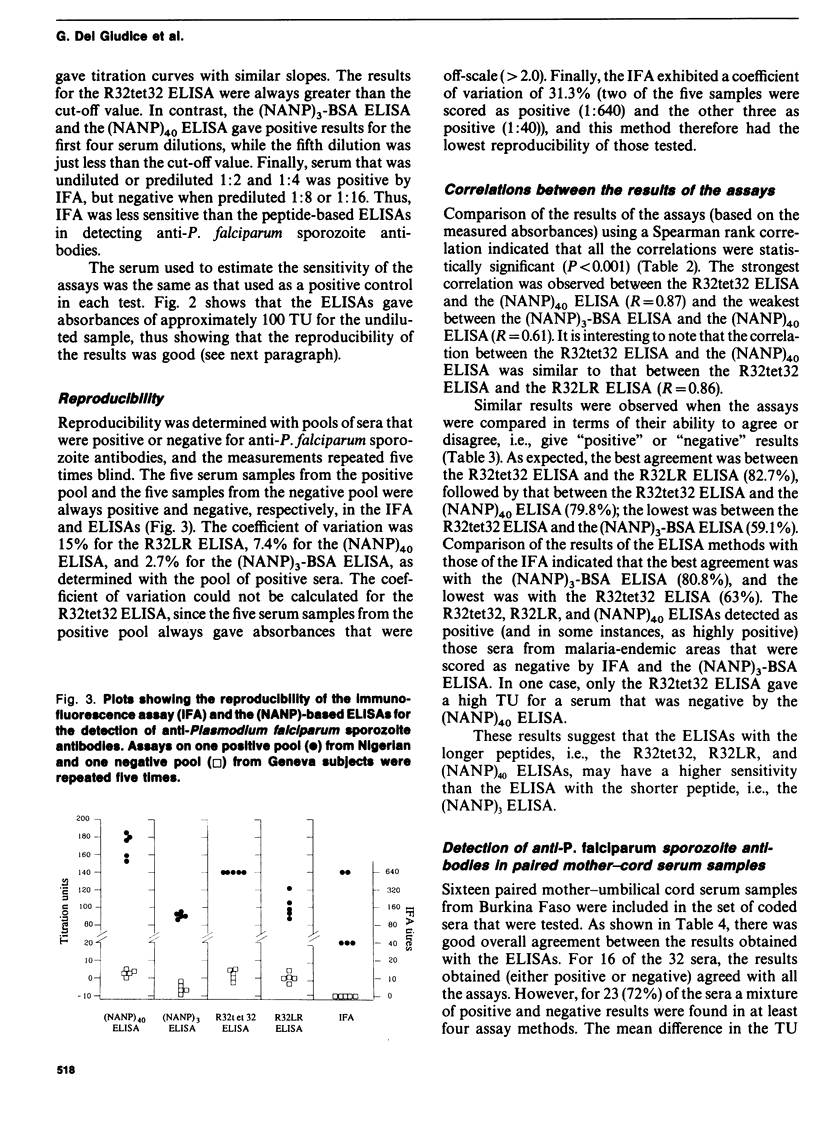
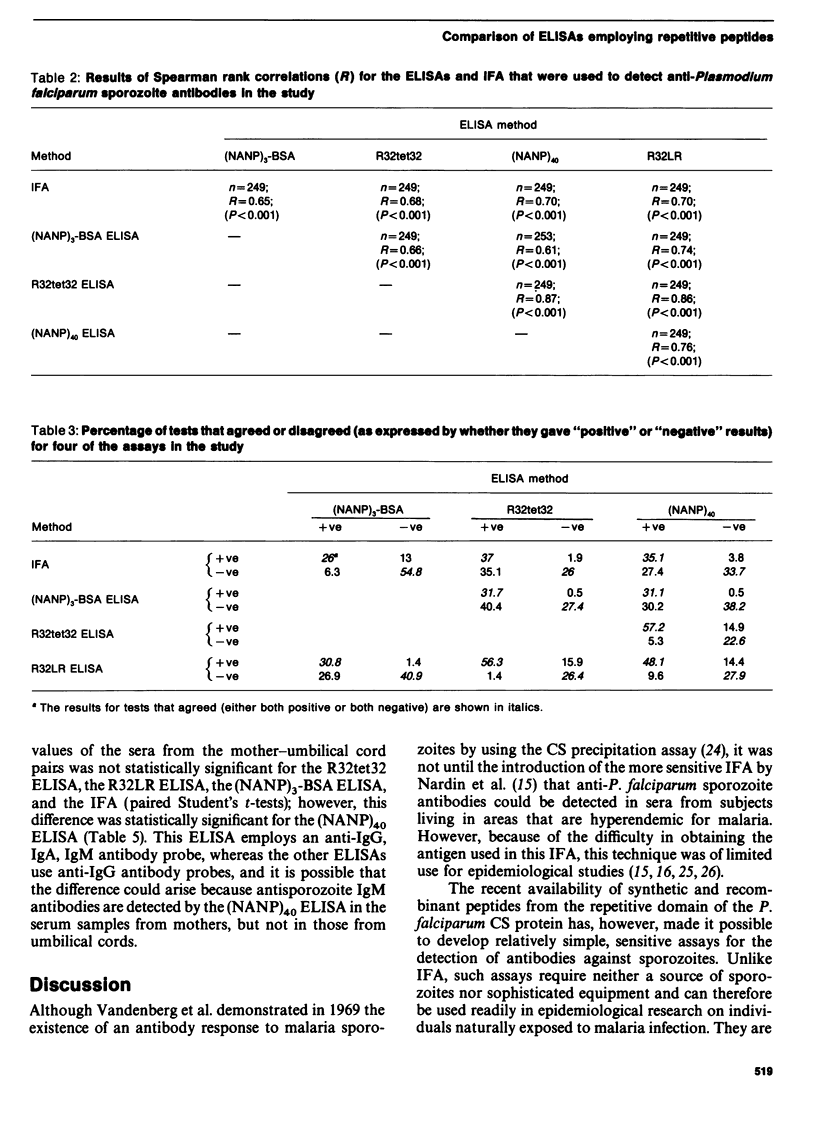
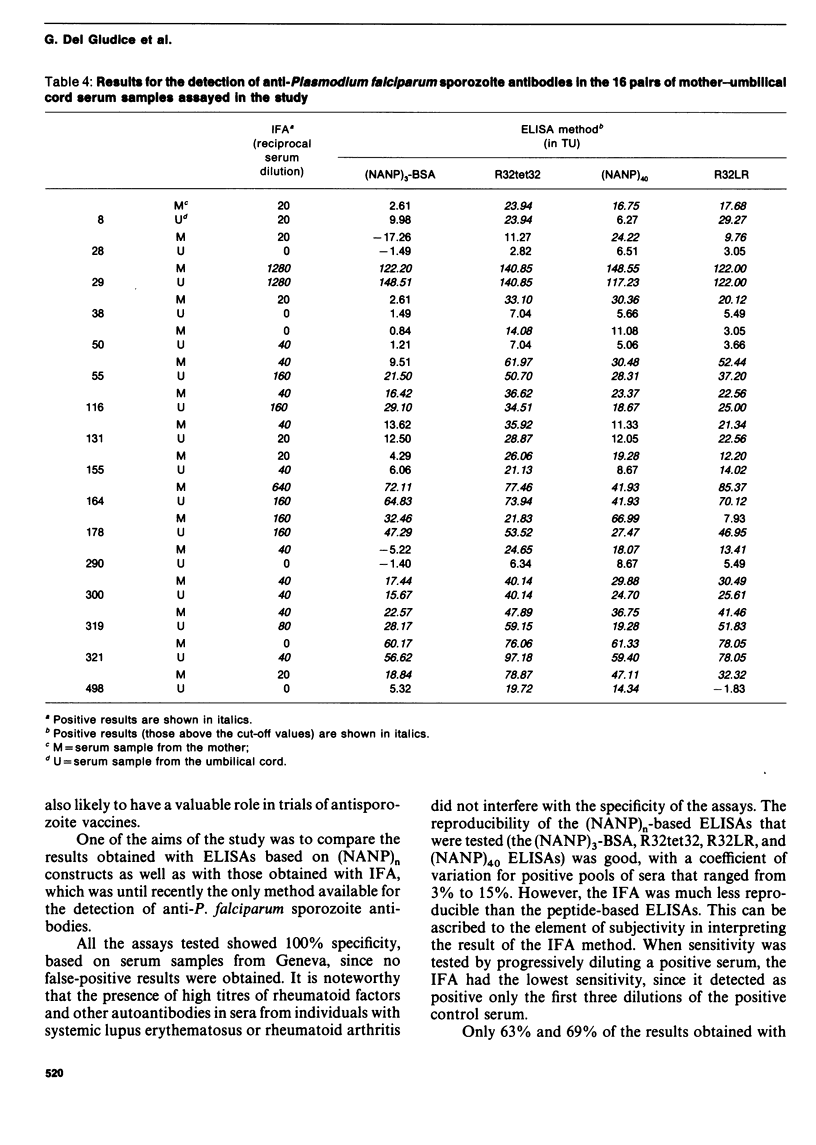
Abstract
In the last few years, a number of different recombinant and synthetic peptides consisting of the repetitive sequence of the Plasmodium falciparum circumsporozoite protein (NANP)n have been produced and used to develop immunoassays for the detection of antibodies against P. falciparum sporozoites in human sera. A comparative study of three enzyme-linked immunosorbent assays (ELISAs) that employed different (NANP)n peptides (the synthetic peptides (NANP)3 and (NANP)40 as well as the recombinant peptides R32tet32 and R32LR) was carried out using serum samples from individuals who were living in different malaria-endemic areas. The results obtained for these peptide-based ELISAs were compared with those obtained for an immunofluorescence assay (IFA) that used glutaraldehyde-fixed sporozoites. All the methods tested exhibited 100% specificity on sera from persons not exposed to malaria, good reproducibility (coefficients of variation ranged from 3% to 15% for peptide-based ELISAs), and good sensitivity. Reproducibility and sensitivity were lower for the IFA than for the peptide-based ELISAs, perhaps because of the subjective element in the interpretation of the results which is inherent in the IFA method. ELISAs based on peptides that contain a higher number of (NANP) repeats, i.e., (NANP)40 and R32tet32 or R32LR, gave results which correlated better with each other than with those obtained with the ELISA that employed a shorter (NANP)3 peptide. (NANP)n-based ELISAs are relatively simple and inexpensive methods for the detection of anti-P. falciparum sporozoite antibodies and can readily be used in epidemiological research in the field. These assays could contribute to a better understanding of the natural history of the host-parasite relationship in malaria research.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballou W. R., Hoffman S. L., Sherwood J. A., Hollingdale M. R., Neva F. A., Hockmeyer W. T., Gordon D. M., Schneider I., Wirtz R. A., Young J. F. Safety and efficacy of a recombinant DNA Plasmodium falciparum sporozoite vaccine. Lancet. 1987 Jun 6;1(8545):1277–1281. doi: 10.1016/s0140-6736(87)90540-x. [DOI] [PubMed] [Google Scholar]
- Campbell G. H., Brandling-Bennett A. D., Roberts J. M., Collins F. H., Kaseje D. C., Barber A. M., Turner A. Detection of antibodies in human sera to the repeating epitope of the circumsporozoite protein of Plasmodium falciparum using the synthetic peptide (NANP)3 in an enzyme-linked immunosorbent assay (ELISA). Am J Trop Med Hyg. 1987 Jul;37(1):17–21. doi: 10.4269/ajtmh.1987.37.17. [DOI] [PubMed] [Google Scholar]
- Dame J. B., Williams J. L., McCutchan T. F., Weber J. L., Wirtz R. A., Hockmeyer W. T., Maloy W. L., Haynes J. D., Schneider I., Roberts D. Structure of the gene encoding the immunodominant surface antigen on the sporozoite of the human malaria parasite Plasmodium falciparum. Science. 1984 Aug 10;225(4662):593–599. doi: 10.1126/science.6204383. [DOI] [PubMed] [Google Scholar]
- Del Giudice G., Engers H. D., Tougne C., Biro S. S., Weiss N., Verdini A. S., Pessi A., Degremont A. A., Freyvogel T. A., Lambert P. H. Antibodies to the repetitive epitope of Plasmodium falciparum circumsporozoite protein in a rural Tanzanian community: a longitudinal study of 132 children. Am J Trop Med Hyg. 1987 Mar;36(2):203–212. doi: 10.4269/ajtmh.1987.36.203. [DOI] [PubMed] [Google Scholar]
- Enea V., Ellis J., Zavala F., Arnot D. E., Asavanich A., Masuda A., Quakyi I., Nussenzweig R. S. DNA cloning of Plasmodium falciparum circumsporozoite gene: amino acid sequence of repetitive epitope. Science. 1984 Aug 10;225(4662):628–630. doi: 10.1126/science.6204384. [DOI] [PubMed] [Google Scholar]
- Hoffman S. L., Oster C. N., Plowe C. V., Woollett G. R., Beier J. C., Chulay J. D., Wirtz R. A., Hollingdale M. R., Mugambi M. Naturally acquired antibodies to sporozoites do not prevent malaria: vaccine development implications. Science. 1987 Aug 7;237(4815):639–642. doi: 10.1126/science.3299709. [DOI] [PubMed] [Google Scholar]
- Hoffman S. L., Wistar R., Jr, Ballou W. R., Hollingdale M. R., Wirtz R. A., Schneider I., Marwoto H. A., Hockmeyer W. T. Immunity to malaria and naturally acquired antibodies to the circumsporozoite protein of Plasmodium falciparum. N Engl J Med. 1986 Sep 4;315(10):601–606. doi: 10.1056/NEJM198609043151001. [DOI] [PubMed] [Google Scholar]
- Lockyer M. J., Schwarz R. T. Strain variation in the circumsporozoite protein gene of Plasmodium falciparum. Mol Biochem Parasitol. 1987 Jan 2;22(1):101–108. doi: 10.1016/0166-6851(87)90073-9. [DOI] [PubMed] [Google Scholar]
- Nardin E. H., Nussenzweig R. S., McGregor I. A., Bryan J. H. Antibodies to sporozoites: their frequent occurrence in individuals living in an area of hyperendemic malaria. Science. 1979 Nov 2;206(4418):597–599. doi: 10.1126/science.386511. [DOI] [PubMed] [Google Scholar]
- Webster H. K., Boudreau E. F., Pang L. W., Permpanich B., Sookto P., Wirtz R. A. Development of immunity in natural Plasmodium falciparum malaria: antibodies to the falciparum sporozoite vaccine 1 antigen (R32tet32). J Clin Microbiol. 1987 Jun;25(6):1002–1008. doi: 10.1128/jcm.25.6.1002-1008.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. F., Hockmeyer W. T., Gross M., Ballou W. R., Wirtz R. A., Trosper J. H., Beaudoin R. L., Hollingdale M. R., Miller L. H., Diggs C. L. Expression of Plasmodium falciparum circumsporozoite proteins in Escherichia coli for potential use in a human malaria vaccine. Science. 1985 May 24;228(4702):958–962. doi: 10.1126/science.2988125. [DOI] [PubMed] [Google Scholar]
- Zavala F., Cochrane A. H., Nardin E. H., Nussenzweig R. S., Nussenzweig V. Circumsporozoite proteins of malaria parasites contain a single immunodominant region with two or more identical epitopes. J Exp Med. 1983 Jun 1;157(6):1947–1957. doi: 10.1084/jem.157.6.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala F., Tam J. P., Hollingdale M. R., Cochrane A. H., Quakyi I., Nussenzweig R. S., Nussenzweig V. Rationale for development of a synthetic vaccine against Plasmodium falciparum malaria. Science. 1985 Jun 21;228(4706):1436–1440. doi: 10.1126/science.2409595. [DOI] [PubMed] [Google Scholar]
- Zavala F., Tam J. P., Masuda A. Synthetic peptides as antigens for the detection of humoral immunity to Plasmodium falciparum sporozoites. J Immunol Methods. 1986 Oct 23;93(1):55–61. doi: 10.1016/0022-1759(86)90432-1. [DOI] [PubMed] [Google Scholar]
- de la Cruz V. F., Lal A. A., McCutchan T. F. Sequence variation in putative functional domains of the circumsporozoite protein of Plasmodium falciparum. Implications for vaccine development. J Biol Chem. 1987 Sep 5;262(25):11935–11939. [PubMed] [Google Scholar]
- del Portillo H. A., Nussenzweig R. S., Enea V. Circumsporozoite gene of a Plasmodium falciparum strain from Thailand. Mol Biochem Parasitol. 1987 Jul;24(3):289–294. doi: 10.1016/0166-6851(87)90161-7. [DOI] [PubMed] [Google Scholar]



