Abstract
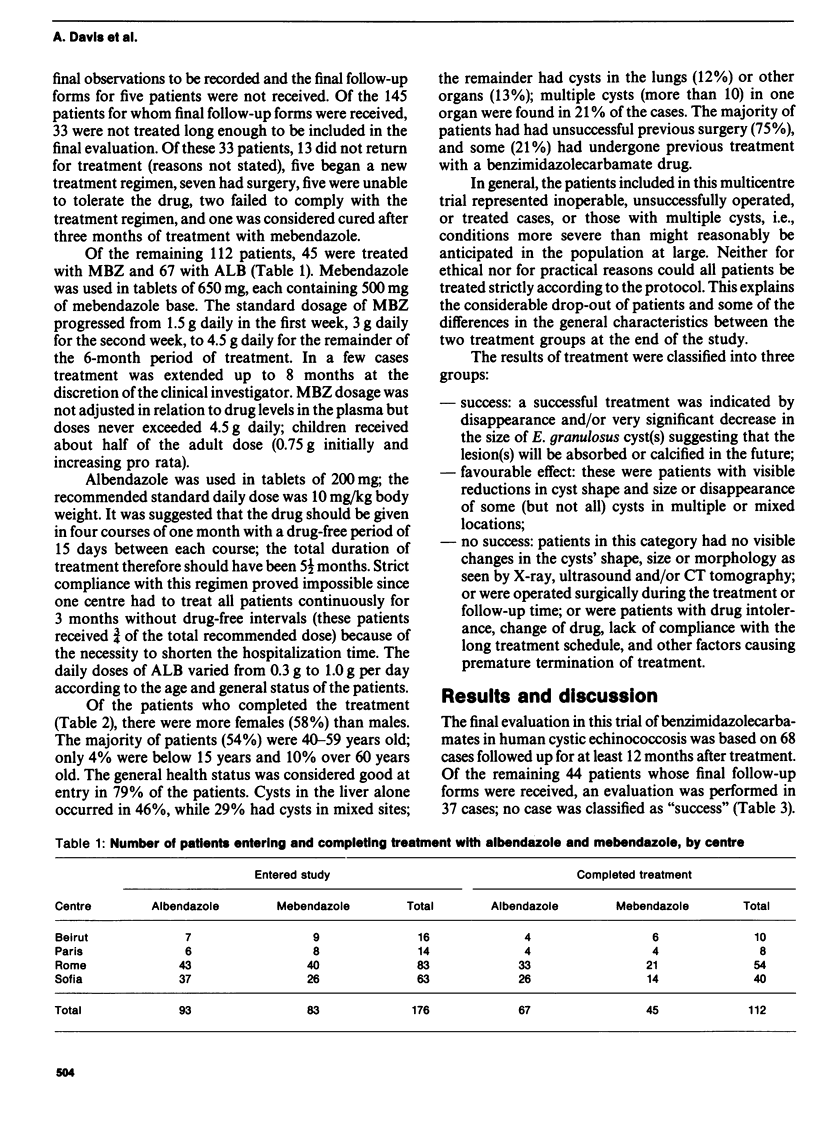
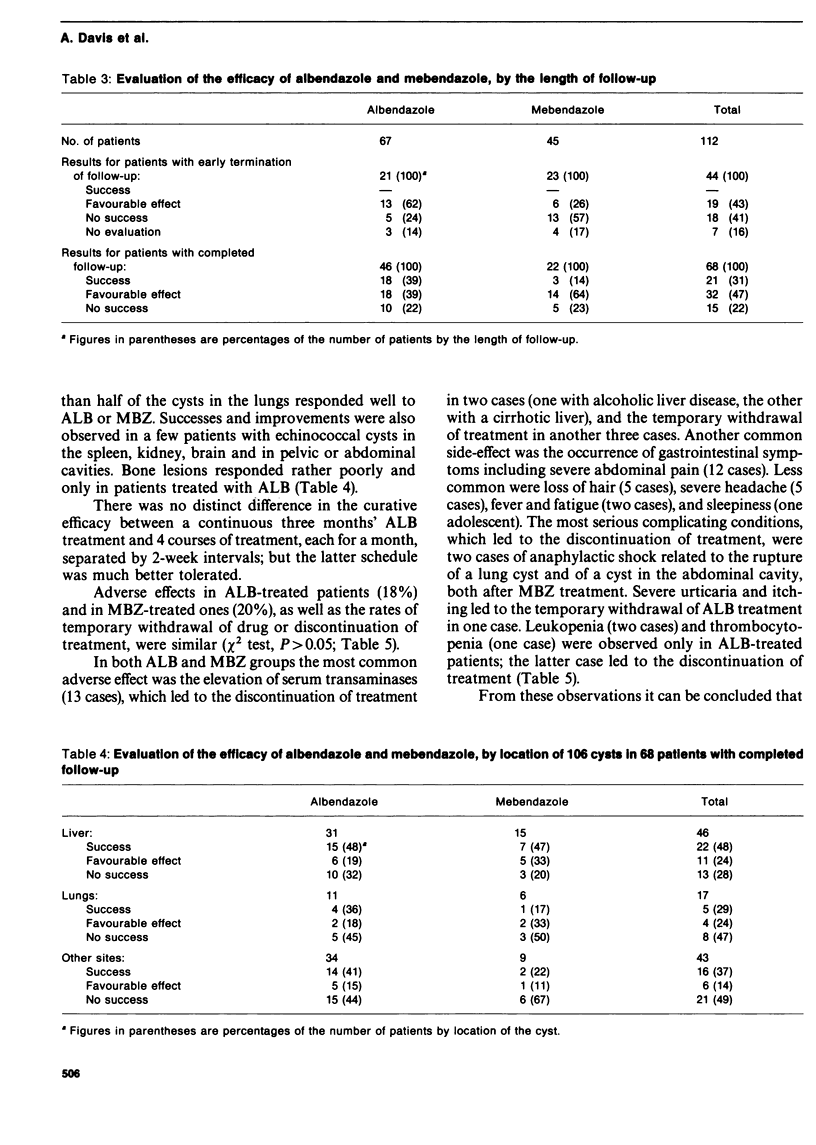
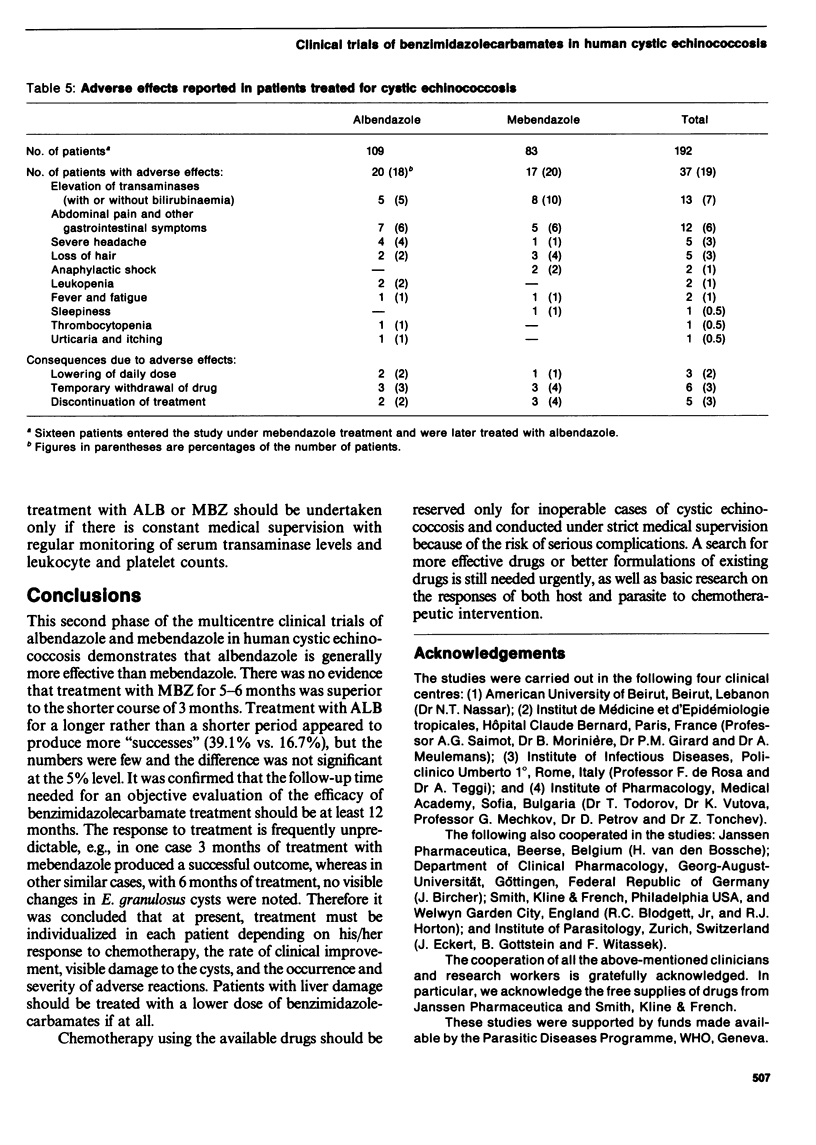
A multicentre study which constituted the second phase of trials of the efficacy of albendazole and mebendazole in human cystic echinococcosis was coordinated by WHO. A total of 112 patients from four clinical centres in Beirut, Paris, Rome and Sofia completed standardized dosage of regimens of each drug and 68 patients were followed up for at least 12 months after treatment. Albendazole was more effective than mebendazole and adverse reactions were comparable with both treatment regimens. At least 12 months is needed after treatment for an objective evaluation of the efficacy of benzimidazoles. At present, treatment with albendazole or mebendazole should be reserved for inoperable cases of cystic echinococcosis (under strict medical supervision) and individualized according to the patient's response and the occurrence and severity of adverse reactions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davis A., Pawlowski Z. S., Dixon H. Multicentre clinical trials of benzimidazolecarbamates in human echinococcosis. Bull World Health Organ. 1986;64(3):383–388. [PMC free article] [PubMed] [Google Scholar]


