Abstract
In newborn pigs, carbon monoxide (CO) contributes to regulation of cerebrovascular circulation. Results from isolated adult cerebral arteries suggest the CO may have less dilatory potential in mature animals. However, few data are available on direct effects of CO on cerebrovascular circulation in vivo except for those from newborn pigs. Therefore, we tested the hypothesis that 1) rat cerebral arterioles dilate to CO in vivo and 2) CO-induced cerebrovascular dilatory responses are age dependant in pigs. Also, we examined whether the permissive role of NO for CO-induced dilation observed in piglets is present in older pigs and rats. Experiments used anesthetized newborn, 7-week-old, and juvenile (3–4 month) pigs and rats with closed cranial windows and topical applications of CO and sodium nitroprusside (SNP). Dilations to SNP were not different at different ages in pigs or between pigs and rats. CO produced pial arteriolar dilations in all groups. Dilation to 10−5 M CO was reduced in juvenile pigs as compared to newborn and 7 week old pigs, and tended to less at 10−6 M CO. Dilations of rat pial arterioles to all concentrations were less than those of newborn and 7 week old pigs but not different from those of juvenile pig pial arterioles. In newborn and 7-week old pigs, L-nitro-arginine (LNA) inhibited the dilation to CO, an effect reversed by a constant background of SNP. In contrast, LNA did not reduce dilation to CO in juvenile pigs or rats. In conclusion, rat pial arterioles like those in piglets dilate to CO in vivo, but there are age and species differences with regard to reactivity and interaction with NO.
Keywords: postnatal development, cerebrovascular circulation, species dependence
INTRODUCTION
Carbon monoxide (CO) is an endogenous gaseous autocrine/paracrine messenger analogous to nitric oxide (NO) (1). CO is a major component in the regulation of cerebrovascular circulation in newborn pigs being involved in responses to excitatory amino acids, hypotension and hypoxia (1). Results from excised adult arteries suggest CO may have less dilatory potential in older animals when compared to newborns (1). However, while data are available from newborn pigs, there are few data regarding the effects of topical CO on the cerebral vasculature of older individuals of any species in vivo. Thus, the question remains open whether CO could be a functional dilator in the adult cerebral circulation, particularly in the rodent models.
Therefore, we test the hypothesis that CO dilates pial arterioles of rats in vivo. We also address the hypothesis that maturation reduces the cerebrovascular responsiveness to CO in pigs. In addition, we examined the question of whether the permissive enabling role of NO for CO-induced dilation observed in the newborn pig was present in older pigs and/or rats. These latter experiments were undertaken because all permissive interactions between NO and CO have been described in the newborn pig (1,2), but never examined at other ages or in other species, and contributions of NO to cerebrovascular control are age dependent (3,4,5,6).
METHODS
The animal protocols were reviewed and approved by the Animal Care and Use Committee of the University of Tennessee Health Science Center. Methods were as described in greater detail previously (e.g. 3,4,5,6). Newborn (1–3 days old, 2.0 ± 0.5kg), ~7 weeks old (13 ± 2 kg), and 3–4 month old (juvenile) (34 ± 1 kg) pigs, and male Sprague Dawley rats (~350 g, 3–4 month old) were used. Each animal was first anesthetized with ketamine (33 mg/kg, i.m.) and acepromazine (3.3 mg/kg, i.m.). Catheters were placed in a femoral artery and vein for blood pressure and blood gas monitoring and maintenance anesthesia (α-chloralose, 50mg/kg), respectively. Newborn and 7-week pigs as well as rats were ventilated using a neonatal ventilator through a tracheotomy. The older pigs breathed spontaneously. Core temperature was maintained at 37° ± 1°C.
A 2 cm-diameter hole was cut in the skull overlying the parietal cortex of the pigs. A smaller hole, approximately 1 cm-diameter, was used for rats. The dura was cut and reflected over the bone edges. A stainless steel and glass cranial window was placed in the hole and cemented in place with dental acrylic. The space under the window was filled with 37°C artificial cerebrospinal fluid (aCSF) (5,6) (pH 7.33, PCO2=46 mmHg, PO2=43 mmHg).
Pial arterioles were observed via a dissecting microscope with a mounted video camera. Diameters were measured with a video micrometer. All measurement periods were 5 min in duration with the maximal diameter reported. Fresh aCSF, with or without experimental treatment, was placed beneath the window to begin the period. The responses to sodium nitroprusside (SNP) (10−6 M) were measured, the window was flushed and filled with aCSF and control pial arteriolar diameter reestablished, and then responses to ascending concentrations of CO (10−7, 10−6, 10−5 M) were measured. These concentrations cover a range from just above the threshold to near maximal. CO solutions were produced by saturation of water with CO (10−3M) with dilations made in gas tight containers without a gaseous interface.
L-nitro-arginine (LNA)(10−3 M) was added to the aCSF to inhibit NO synthase. Responses to SNP and CO, as above, were measured before and in the presence of LNA alone and then with the addition of 2×10−7 M SNP to the aCSF.
Values for each variable are presented as mean ± SEM. Comparisons among populations within each age/species group used ANOVA with repeated measures. For comparison among species and age, ANOVA without repeated measures was used. The Tukey-Kramer Multiple Comparisons test or the Bonferroni multiple comparison test for selected groups was used to isolate differences between groups as appropriate for the design structure.
RESULTS
Throughout the experiments, there were no significant changes in arterial blood gases, pH, or pressure when the values were compared at the beginning and end of the experiments. Combined control pial arteriole diameters for all experiments were: newborn pigs: 50±3 µm; 7-week old pigs: 52±5 µm; juvenile: 65±6 µm; and adult rats: 52±4 µm.
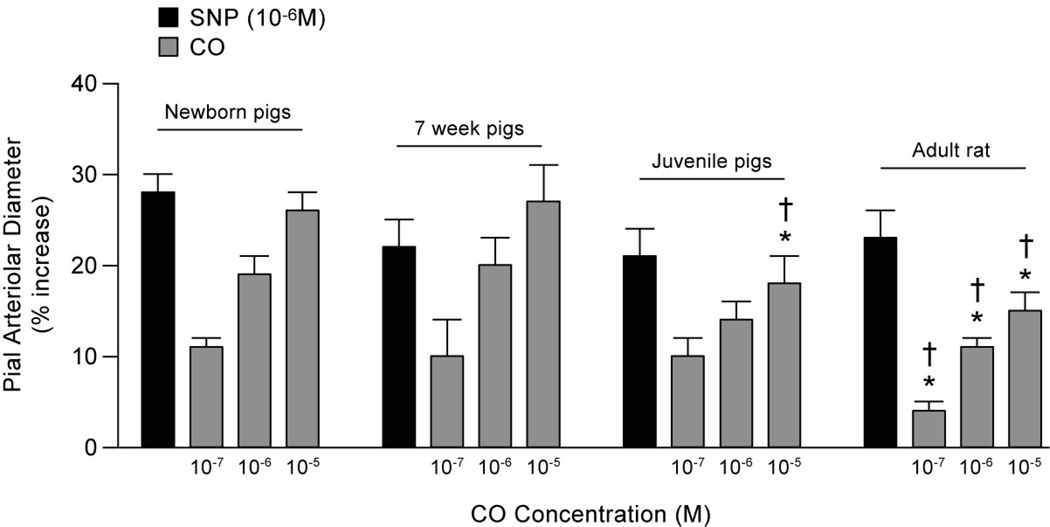
Figure 1 shows the effect of SNP (10−6M) and ascending doses of CO (10−7, 10−6, 10−5 M) on pial arteriolar diameter. The dilations to SNP were not different across ages or species. CO produced pial arteriolar dilations in newborn, 7-week old, and juvenile pigs, and in rats. In pigs, there was no difference in dilation in response to CO when comparing newborn and 7 week pigs. Pial arteriolar dilation to 10−5 M CO was less in juvenile pigs than newborn and 7 week old pigs and tended to be less to 10−6 M CO (P=0.057 compared to newborn). Pial arteriolar dilations in response to CO at all concentrations in rats were less than those of newborn and 7 week-old pigs, but not different from those of juvenile pigs. Comparisons between the responsiveness of pial arterioles to CO and NO should not be made from these experiments because the concentration of NO produced by the 10−6M SNP is not known.
Figure 1.
Dilation of pial arterioles in response to sodium nitroprusside (SNP, 10−6 M) and CO. Values are mean ± SE. * and + are P<0.05 compared to newborn and 7-week pig, respectively. Increases in diameters at all CO concentrations in pigs of all ages and rats are significant at P<0.05. Dilations of juvenile pig and rat pial arterioles to CO at each concentration are not different (P>0.05). N= 28, 10, 15, and 13, newborn, 7-week, juvenile pigs and rats, respectively.
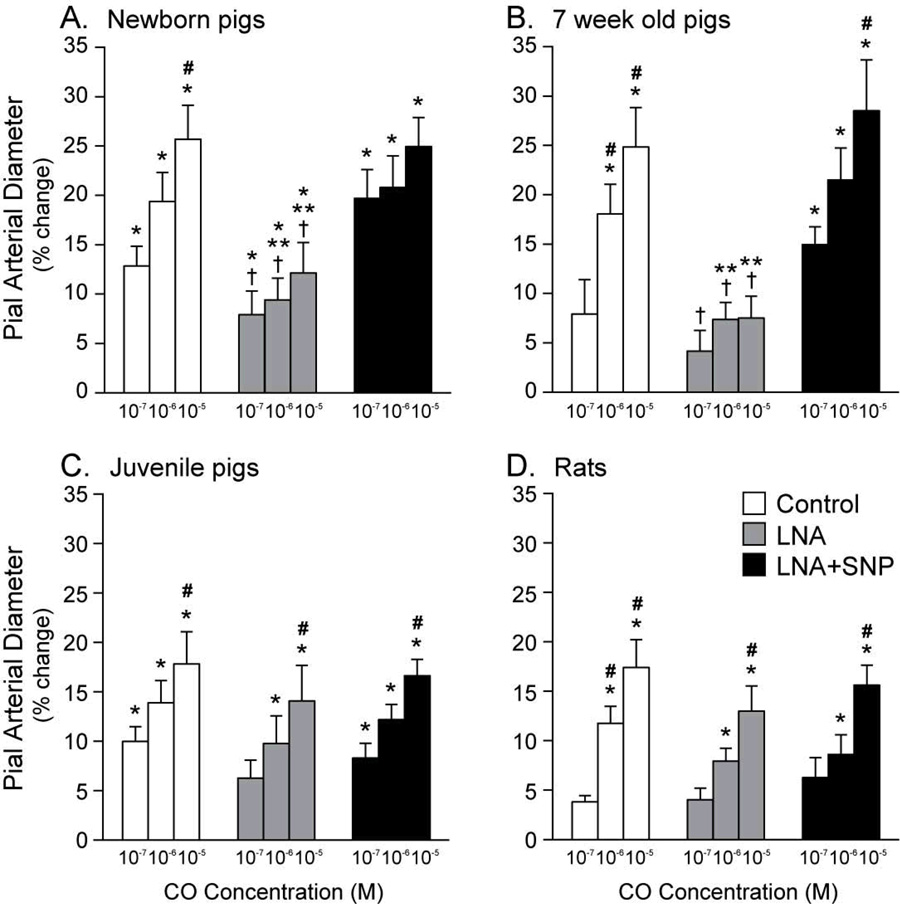
L-nitro-arginine (LNA)(10−3 M) was used to inhibit NO synthase, thereby blocking NO production. In both newborn and 7-week old pigs, LNA inhibited the dilation to CO (Fig. 2). In contrast, LNA did not significantly reduce dilation to CO in juvenile pigs or adult rats. Addition of a constant background concentration of SNP restored dilation to CO in newborn and 7-week old pigs, but had no effect on the dilation to CO in either juvenile pigs or rats.
Figure 2.
Dilation of pial arterioles in newborn pigs (n=9) (A), 7-week old pigs (n=6) (B), juvenile pigs (n=7) (C) and adult rats (n=10) (D) to ascending doses of CO alone, with L-nitro arginine (LNA, 10−3 M), and with LNA plus sodium nitroprusside (SNP, 2×10−7 M). Values are mean ± SE and *,#,**, and t are P<0.05 compared to zero % change, 10−7M CO, without LNA, and LNA+SNP, respectively.
DISCUSSION
Summarized findings are 1) CO dilates pial arterioles of baby and juvenile pigs and young adult rats in vivo, 2) responsiveness of cerebral arterioles to CO is greater in baby pigs than in older pigs, 3) pial arterioles of rats and pigs of all ages studied dilate similarly to topical NO, 4) rat arterioles show a reduced dilator response to CO when compared to newborn and baby, but not juvenile pigs, and 5) a basal level of NO is necessary to permit dilation in response to CO in newborn and baby pigs, but this role for NO is not seen in older pigs or rats.
Pial arterioles of both rats and pigs dilated to topical CO, in vivo. In contrast, most prior studies, all performed in vitro on adults, have been unable to detect CO-induced dilation of cerebral arteries. For example, CO did not alter the tone of excised adult white rabbit cerebral arteries (7). Additionally, Andresen et al (8) concluded that CO, at physiological concentrations, is not a dilator of rat or mice pressurized and perfused cerebral arteries. However, dog basilar artery segments did dilate to CO at about the highest concentration used in the present study (9). The dog arteries were denuded of endothelium suggesting dog cerebral arteries, like those of rats and juvenile pigs in the present study, do not require the permissive signal from the endothelium necessary in the newborn pig (2). Pressurized pial arterioles of newborn pigs in vitro do dilate to a light-activated CO releasing molecule in the superfusion solution (2). The present data show rat arterioles in vivo can dilate to CO. Therefore, endogenously produced CO could contribute to cerebrovascular dilatory responses in adult rats. It is uncertain why rat cerebral vessels do not dilate to CO in vitro but do show a dilatory response in vivo. Since CO is applied to the intact cortical surface in vivo, contributions of other cell types not present with isolated arteries and arterioles including neurons, astrocytes and even blood cells to the arteriolar responses could result in differences between in situ and ex vivo data. Furthermore, such variables as vessel size and tone must be considered.
Prior to the present study, all in vivo data available on immediate cerebrovascular responses to direct application of CO were from newborn pigs. In the rat pial circulation, in vivo, endogenous CO has a tonic inhibitory effect on NO production (10). Thus, the heme oxygenase (HO) inhibitor, zinc protoporphrin (ZnPP) progressively dilated pial arterioles over 60 min. This dilation was blocked by L-NAME and by addition of CO to the superfusion solution. Further, ZnPP enhanced cerebral NO production as estimated using diaminofluorescein-2. No direct effect was detected following 60 min superfusion with CO (10−5 M). These results coupled with the present report suggest that CO may have time-dependent actions, CO produced by acute activation of HO functioning as a dilatory gasotransmitter while basal CO production and prolonged elevation can produce tonic inhibition of NO production and increase cerebrovascular tone. Our previous results in piglets indicate endogenously produced CO stimulated by heme and inhibited by HO blockers causes dilation (reviewed in reference 1). However, affects of prolonged application of heme to the brain surface are unknown in piglets, pigs, or rats.
Postnatal development appears to alter CO-induced dilation of pial arterioles. We found a reduction of the dilatory response to CO in the oldest pigs studied when compared to newborn and baby pigs. The juvenile pigs, while large animals and the same absolute age as the rats, are immature as compared to the rats. Whether mature adult pigs (6 months) that weigh more than 100 kg or even older larger pigs might show further reduced responses to CO or whether newborn rats may show increased responses are not known. However, regarding pigs our previous work found marked shifts in cerebrocirculatory control mechanisms over the developmental period represented in the present study. For example, acetylcholine produces pial arteriolar dilation in 3–4 week old pigs (4), similarly to adults of various species (11), but not in newborn pigs (4). In addition, cerebrovascular dilation to hypercapnia in newborn pigs is largely NO independent, while NO assumes an increasing role with maturation within the age range used in the present study (3,6).
The mechanism(s) involved in the diminished responses of older pigs and rat pial arterioles as compared to newborn and baby pigs require further investigation. It does not appear that a reduction in vasoreactivity in general is involved because pial arterioles of rat and pigs of all ages responded similarly to the NO source, SNP, that increases cyclic GMP (11). CO dilates via activation of large conductance Ca2+ activated K+ channels (KCa channels)(1,12). Ca2+ transients termed Ca2+ sparks activate adjacent KCa channels (13), leading to a transient outward K+ current. In adult rats, virtually all Ca2+ sparks produce transient K+ currents in cerebral vascular smooth muscle cells (14). However, in newborn pigs only about 60% of sparks cause KCa channel currents (15). CO increases Ca2+ sparks and increases coupling of Ca2+sparks to KCa channels (16,17). It is possible that the ability of CO to dilate rat arterioles is less than in piglets because rat basal KCa channel activity is higher and all Ca2+ sparks induce KCa channel currents in the basal state.
NO provides a permissive signal for CO-induced vasodilation in newborn and baby pigs. As shown in newborn pigs before, LNA blocks dilation to CO and that dilation is restored by a constant, minimally vasodilator concentration of NO (1). In contrast, in the older pigs and rats, the dilation to CO was not diminished by the addition of LNA, nor increased by the addition of SNP, suggesting absence of this signal with advancing age. Similarly, in the rat hypothalamus, the contribution of CO to the regulation of vascular tone became more evident in the absence of NO (18). Of note, the similarity of responses to CO of LNA-treated baby pigs, juvenile pigs and rats suggests the permissive NO signal that markedly accentuates the response of the baby cerebral microvasculature to CO is absent in the older animals. This absence could contribute to the reduced response of the intact pial arterioles compared those of babies.
Overall, we find that the gasotransmitter, CO, dilates arterioles on the cerebral cortical surface of both pigs and rats. However, pial arteriolar dilator reactivity to CO in older pigs and adult rats, is less than that of newborn and baby pigs. In addition, postnatal maturation appears to be accompanied by alteration in interactions between CO and NO, in the control of cerebral arteriolar tone.
ACKNOWLEDGEMENTS
Research was supported by NHLBI/NIH HL34059 and HL42851. We thank G. Short for assistance with preparation of figures. D. Holt was supported by a medical student summer internship from NIDDK/NIH (DK007405). A. Vaughn was supported by a Memphis Rotary Foundation Medical Internship.
REFERENCES
- 1.Leffler CW, Parfenova H, Jaggar JH, Wang R. Carbon Monoxide and hydrogen sulfide: gaseous messengers in cerebrovascular circulation. J Appl Physiol. 2006;100:1065–1076. doi: 10.1152/japplphysiol.00793.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barkoudah E, Jaggar JH, Leffler CW. The permissive role of endothelial NO in CO-induced cerebrovascular dilatation. Am J Physiol. 2004;287:H1459–H1465. doi: 10.1152/ajpheart.00369.2004. [DOI] [PubMed] [Google Scholar]
- 3.Willis AP, Leffler CW. NO and prostanoids: age dependence of hypercapnia and histamine-induced dilations of pig pial arterioles. Am J Physiol. 1999;277:H299–H307. doi: 10.1152/ajpheart.1999.277.1.H299. [DOI] [PubMed] [Google Scholar]
- 4.Armstead WM, Zuckerman SL, Shibata M, Parfenova H, Leffler CW. Different pial arteriolar responses to acetylcholine in the newborn and juvenile pig. J Cereb Blood Flow Metab. 1994;14:1088–1095. doi: 10.1038/jcbfm.1994.142. [DOI] [PubMed] [Google Scholar]
- 5.Willis AP, Leffler CW. Endothelial NO and prostanoid involvement in newborn and juvenile pig pial arteriolar vasomotor responses. Am J Physiol. 2001;281:H2366–H2377. doi: 10.1152/ajpheart.2001.281.6.H2366. [DOI] [PubMed] [Google Scholar]
- 6.Zuckerman SL, Armstead WM, Hsu P, Shibata M, Leffler CW. Age dependence of cerebrovascular response mechanisms in domestic pigs. Am J Physiol. 1996;271:H535–H540. doi: 10.1152/ajpheart.1996.271.2.H535. [DOI] [PubMed] [Google Scholar]
- 7.Brian JE, Jr, Heistad DD, Faraci FM. Effect of carbon monoxide on rabbit cerebral arteries. Stroke. 1994;25:639–643. doi: 10.1161/01.str.25.3.639. [DOI] [PubMed] [Google Scholar]
- 8.Andresen JJ, Shafi NI, Durante W, Bryan RM., Jr Effects of carbon monoxide and heme oxygenase inhibitors in cerebral vessels of rats and mice. Am J Physiol. 2006;291:H223–H230. doi: 10.1152/ajpheart.00058.2006. [DOI] [PubMed] [Google Scholar]
- 9.Komuro T, Borsody MK, Onon S, Marton LS, Weir BK, Zhang ZD, Paik E, MacDonald RL. The vasorelaxation of cerebral arteries by carbon monoxide. Exp Biol Med. 2001;226:860–865. doi: 10.1177/153537020122600909. [DOI] [PubMed] [Google Scholar]
- 10.Ishikawa M, Kajimura M, Adachi T, Maruyama K, Makino N, Yamaguchi T, Sekizuka E, Suematsu M. Carbon Monoxide from heme oxygenase-2 is a vasodilation in the adult rat cerebral microcirculation. Circ Res. 2005;97:e104–e114. doi: 10.1161/01.RES.0000196681.34485.ec. [DOI] [PubMed] [Google Scholar]
- 11.Andresen J, Shafi NI, Bryan RM., Jr Endothelial influence on cerebrovascular tone. J Appl Physiol. 2006;100:318–327. doi: 10.1152/japplphysiol.00937.2005. [DOI] [PubMed] [Google Scholar]
- 12.Wang R, Wang Z, Wu L. Carbon monoxide-induced vasorelaxation and underlying mechanisms. Br J Pharmacol. 1997;121:927–934. doi: 10.1038/sj.bjp.0701222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaggar JH, Porter VA, Lederer WJ, Nelson MT. Calcium sparks in smooth muscle. Am J Physiol. 2000;278:C235–C256. doi: 10.1152/ajpcell.2000.278.2.C235. [DOI] [PubMed] [Google Scholar]
- 14.Perez GJ, Bonev AD, Patlak JB, Nelson MT. Functional coupling of ryanodine receptors to KCa channels in smooth muscle cells from rat cerebral arteries. J Gen Physiol. 1999;113:229–238. doi: 10.1085/jgp.113.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaggar JH, Leffler CW, Cheranov SY, Tcheranova DES, Cheng X. Carbon monoxide dilates cerebral arterioles by enhancing the coupling of Ca2+ sparks to Ca2+-activated K+ channels. Circ Res. 2002;91:610–617. doi: 10.1161/01.res.0000036900.76780.95. [DOI] [PubMed] [Google Scholar]
- 16.Jaggar JH, Li A, Parfenova H, Liu J, Umstot ES, Dopico AM, Leffler CW. Heme is a carbon monoxide receptor for large-conductance Ca2+-activated K+ channels. Circ Res. 2005;97:805–812. doi: 10.1161/01.RES.0000186180.47148.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xi Q, Tcheranova D, Parfenova H, Horowitz B, Leffler CW, Jaggar JH. Carbon monoxide activates KCa channels in newborn arteriole smooth muscle cells by increasing the apparent Ca2+ sensitivity of the a-subunit. Am J Physiol. 2004;286:H610–H618. doi: 10.1152/ajpheart.00782.2003. [DOI] [PubMed] [Google Scholar]
- 18.Horvath B, Hrabac A, Kaldi K, Sandor P, Benyo Z. Contribution of the heme oxygenase pathway to the maintenance of hypothalamic blood flow during diminished nitric oxide synthesis. J Cerebral Blood Flow Metab. 2003;23:653–657. doi: 10.1097/01.WCB.0000071890.63724.C9. [DOI] [PubMed] [Google Scholar]