Abstract
Common variable immunodeficiency (CVID) is characterized by low levels of immune globulins and lack of antibody. Mutations in transmembrane activator and calcium-modulating cyclophilin ligand (TACI), are found in 8-10%, associated with autoimmunity and splenomegaly. Some patients with mutations had increased serum levels of TACI. Because of this, and the prevalence of autoimmunity, splenomegaly, and lymphadenopathy, we quantitated levels of TACI ligands, a proliferation inducing ligand (APRIL) and B cell activating factor (BAFF) and TACI in serum of 77 patients. CVID subjects had markedly increased serum levels of BAFF, (p<0.0001) APRIL (p<0.0001) and TACI (p=0.001) but there was no relationship between levels and autoimmunity, lymphadenopathy, splenomegaly, B cell numbers, or mutations in TACI. Peripheral blood mononuclear cells of CVID subjects had increased levels of BAFF mRNA. We conclude that increased constitutive production and/or underlying immuno-regulatory or inflammatory conditions leads to enhanced release of ligands, however the biological result remains unclear.
Introduction
Common variable immunodeficiency (CVID) is a primary immunodeficiency disease characterized by reduced to absent B cell function, with low levels of serum IgG and IgA and/or IgM, antibody deficiency, and lack of plasma cells 1,2. While B cell maturation is abnormal, and there is a lack of isotype switched memory B cells and impaired somatic hypermutation 3-5, the clinical phenotype is heterogeneous, with an onset of symptoms in early to middle adult life in the majority of patients. Upper respiratory tract infections, especially pneumonias, are common, but for unknown reasons, about 20 to 25% of subjects have at the onset or later, autoimmunity, lymphoid hypertrophy with splenomegally, granulomatous disease, or gastrointestinal tract inflammatory disease 6-8. Since many patients also have reduced T cell activation and cell proliferation, cytokine and dendritic cell defects 9-14, the CVID syndrome has been assumed to be caused by mutations in a number of genes. The discovery that mutations of ICOS, a T cell activation antigen in one kindred, or CD19 in two families, agree with this suggestion 15,16. Recently, mutations in receptors of the tumor necrosis factor (TNF) family members, the B cell-activating factor (BAFF, also called Blys/zTNF4/TALL) or transmembrane activator and calcium-modulating cyclophilin ligand interactor (TACI, CD267), have been identified in some patients with autosomal recessive or dominant B cell defects of the CVID phenotype 17-19. TACI binds two TNF family members, BAFF and another soluble ligand, a proliferation inducing ligand (APRIL) 20,21. BAFF also binds with high affinity to the BAFF receptor (BAFF-R, CD268) but less well to a third receptor, B cell maturation antigen (BCMA, CD269). TACI is expressed mostly on mature B cells and to some extent on activated T cells. Activation of B cell TACI by its ligands, leads to T cell independent responses and isotype switch 22,23. On the other hand, TACI deficient mice have B cell hyperplasia, splenomegaly, B cell lymphoma, increased immune globulin production and autoimmunity, all of which suggest that in mice, some components of TACI signaling play an inhibitory role 24.
BAFF is produced by cytokine-stimulated cells of myeloid origin 25,26 and also constitutively by radiation-resistant cells resulting in levels of BAFF which control the size of the B cell pool 27-29. APRIL is similarly expressed and produced by cells of myeloid origin, also by T cells, and several types of tumor cells21. Dysregulated production of BAFF and APRIL and increased ligand release by proteolytic enzymes, leads to increased levels of both factors, possibly in hetero-trimeric or other multimeric and biologically active forms in serum30. Much of the information about the function of these ligands has been deduced from knockout and transgenic mice. BAFF and its receptor are important or essential for the survival and maturation of B cells, but over-expression of BAFF in mice leads to B cell hyperplasia, hyperglobulinemia, splenomegaly and autoimmunity 31-33. Mammalian cells transfected with BAFF cDNA secrete active forms of BAFF, and over expression of BAFF in the liver causes distant effects in the B cell compartment, including B cell expansion, autoimmunity and immune complex deposition in tissues34. A substantial literature demonstrates that both BAFF and APRIL are present in excess amounts in the sera of patients with systemic autoimmune disease such as rheumatoid arthritis, systemic lupus erythematosus, and systemic sclerosis 33,35-39,40,41. Since some of the clinically problematic hallmarks of CVID are autoimmune disease, striking lymphadenopathy and splenomegaly, we quantitated APRIL and BAFF levels in the serum of patients to determine if there were any correlations between the levels of these cytokines, autoimmunity, lymphadenopathy, and/or splenomegaly. Second, since subjects with CVID may have almost absent to normal number of B cells, and mice with targeted disruption of μ heavy chain and no B cells have very high levels of BAFF, we investigated if BAFF was related to B cell numbers, B cell phenotype or initial serum immune globulins.42 Our results show that serum BAFF, APRIL and TACI are all markedly increased in CVID but with no relation to these clinical or immunologic parameters. BAFF mRNA was also greatly elevated in peripheral blood mononuclear cells. Although TACI was previously reported elevated in the sera of subjects with TACI mutations 18, we found that TACI is increased in the sera of many patients with CVID, but not related to the immunologic or clinical phenotype, or the presence of mutations.
Materials and Methods
Patients and Blood samples Patients and Controls
A group of 77 patients with CVID (ages 17-71) were studied. Subjects who had other causes of congenital or acquired hypogammaglobulinemia were excluded; two had relatives known to have either CVID or IgA deficiency. All had reduced serum IgG, IgA, and/or IgM two or more confidence intervals below the normal ranges for age. All were being given intravenous immune globulin. Antibody deficiency was verified by reduced levels of protective antibody to tetanus, diphtheria, measles, mumps, rubella, and to pneumococcal serotypes after vaccination. Sixteen (21%) of the 77 subjects had a history of autoimmunity (either ITP, or hemolytic anemia), 19 (25%) had enlarged spleens with or without lymphadenopathy or lymphocytic organ infiltrates, and 9 (12%) had known granulomatous disease. None had either ITP or hemolytic anemia, or had any acute infection at the time of study. Several blood samples at different time points were obtained prior to an infusion of immune globulin. The controls were 21 healthy adult volunteers and normal blood bank donors.
Serum APRIL, BAFF and TACI levels
Peripheral blood was obtained by venipuncture from patients and healthy adult controls using an Institutional Review Board approved protocol and informed consent. Soluble BAFF was quantitated in serum diluted 1:2 by ELISA using a commercial kit (Apotech/Axorra, San Diego). APRIL was determined in the same serum, using a kit from Bender MedSystems (Vienna, Austria.) (The limits of detection of both assays was 30 pg/ml.) Serum TACI was quantitated in serum by ELISA using 96 well plates (Maxisorb, Nalge Nunc, Rochester NY) coated with 1:500 PBS diluted goat antibody to human TACI (PeproTech Inc. Rocky Hill NJ) as a capture antibody; wells were blocked with 10% FBS for 1 hour. The same antibody was used in the biotinylated form diluted 1:250, and then using streptavidin-horseradish peroxidase (BD-Pharmingen) and TMB-reagents (BD Biosciences) as developers. Recombinant human TACI (PeproTech) was used as a standard; the lowest detectable amount was 40 pg/ml. Sera were obtained prior to an infusion of immune globulin and were tested in duplicate.
B cells, memory B cells, and BAFF and APRIL by FACS analysis
Numbers of B cells, memory and switched memory B cells were determined for CVID subjects and controls using freshly isolated peripheral blood mononuclear cells (PBMC) (2.5 × 106/50 μL in RPMI 1640 medium (GIBCO, Carlsbad, CA) plus 10% fetal calf serum) as previously described 43.
Cell Surface Expression of BAFF and APRIL and Cell Cultures
Cell surface expression of BAFF on CD14+ monocytes was examined by FASCan, using 5μg/ml rat monoclonal anti-BAFF (Abcam, Cambridge MA), a 1:25 diluted goat anti rat CY5 conjugate (Abcam), and appropriate IgG2a isotype controls. Data acquisition was performed with a FACSCalibur (BD-Biosciences) and data analysis by forward versus side scatter, gating on viable cells(CellQuest). Cytokine stimulation, especially INF-γ, was previously shown to enhance the surface expression and production of BAFF by mononuclear cells 25. To test this, PBMC of CVID or control subjects were incubated with 5,10 or 20 ng/ml INF-γ (PeproTech, Inc) for 48 and 72 hours in RPMI 1640 (GIBCO Life Technologies, Grand Island NY) supplemented with 1% human serum (Cellgro, Herndon, VA), 0.3 mg/mL L-glutamine, 10,000 units/ml penicillin, 10 ng/ml streptomycin sulfate, and 0.025 μg/ml amphotericin B. Cells were cultured at 37°C in 5% CO2 at a cell density of 2×105/200μl in 96 well plates. As additional stimulators in other experiments, cells were also cultured with 100 ng/ml lipopolysaccharide (LPS) (Sigma, St Louis MO) or 300 ng/ml soluble CD40L (sCD40L) fusion protein, (Alexis, Axxora LLC, San Diego, CA.) Cell culture supernatants from these cultures were also harvested and kept at −70°C until testing for soluble BAFF and APRIL, using non stimulated cells as controls.
BAFF and APRIL mRNA; treatment with INFγ
To test if the addition of INF-γ increased BAFF mRNA in PBMCs, cultures of these cells from CVID subjects with a range of serum BAFF and APRIL levels, were compared to normal controls, after incubation with or without an optimum amount (5 ng/ml) INF-γ for 24 hours using real time PCR. Primers for BAFF were: forward: 5’-A T G C A G A A A G G C A G A A A G G A-3’, and reverse: 5’-A G G C A A G A A G T A A G G C G T G A-3’. Primers for APRIL were: forward: 5’- G A G G G A C T G G A A C C T A A T T C T C -3’, and reverse: 5’-A G G G T A C T G T T A G T G C T C C T G G -3’. After culture, PBMCs were snap frozen and stored at −80°C. Total RNA was isolated using RNeasy Mini Kit (Qiagen). First-strand cDNA was synthesized from 0.1μg RNA using random primers and the SuperScript III First-Strand Synthesis System for RT-PCR (InVitrogen). Two microliters of cDNA was used per 25-μL reverse transcriptase (RT) reaction. The reaction was carried out using the LightCycler FastStart DNA Master SYBR Green I kit (Roche) according to manufacturer’s instructions. Each sample was measured in triplicate. A range of primer concentrations was tested to identify to the optimum amplification efficiency. The amplification was determined by plotting against the threshold concentration. The BAFF or APRIL copy number was standardized relative to β-actin, and expressed for each sample as a relative amount of mRNA as an n=fold difference. The specificity of the amplification was controlled by analysis of the melting curve analysis and crossing point. No amplification of non-specific products was observed.
TACI: DNA and cDNA sequencing
Peripheral blood cells or B cells from B cell lines were lysed, genomic DNA was isolated using Puregene DNA Purification Kits (Gentra Systems, Inc. Minneapolis, MN) and then treated with RNAase, and cDNA was prepared by reverse transcription. Five exons of TACI were PCR amplified from cDNA using HotStarTaq DNA Polymerase (Qiagen) using primers hybridized to intronic sequences that flanked the five exons17,18. PCR products were visualized and isolated from 1% agarose gels using 0.8-4.0% ethidium bromide under ultraviolet transillumination. DNA amplicons were sequenced on an ABI PRISM® 377 DNA Sequencer. Sequences were aligned to the wild-type TACI sequence 44 using the DNAStar software.
Statistical Analysis
Differences between levels of BAFF, APRIL and TACI in the sera of CVID patients and controls were examined using the Mann Whitney test. Relationships between the amounts of serum BAFF, APRIL and TACI and the presence of autoimmunity, granulomatous disease, radiographically demonstrated splenomegaly or lymphadenopathy were also tested using the Mann Whitney test. Correlations of serum BAFF, APRIL and TACI with the number of circulating B cells, CD27+B cells, or switched memory B cells (CD27+ IgM-, IgD-) and correlations between amounts of BAFF or APRIL mRNA and serum BAFF and APRIL were tested by Spearman r test. Statistical analyses were performed using GraphPad Prism 3.03 (GraphPad Software Inc, San Diego, CA)
Results
Serum levels of APRIL, BAFF and TACI and relation to mutations in TACI
Patients with CVID had markedly increased serum levels of APRIL (p<0.0001), BAFF (p<0.0001), and TACI (p=0.001) as compared to control sera (Figure 1.) The median serum APRIL for CVID = 34.5 ng/ml, controls = 2.0 ng/ml; median serum BAFF for CVID =3.3 ng/ml, controls= 0.8 ng/ml; median TACI for CVID =77.8 ng/ml, controls = 0.01 ng/ml. Seven of the 77 subjects tested (10%) had one of the described heterozygous mutations in TACI, including C104R (2 subjects), A181E(1), R72H(1), C172Y (1), and V220A(2) mutations. However, the amounts of BAFF, APRIL or TACI in the sera of the subjects with mutations in TACI were not significantly different as compared to 70 patients with no mutations.
Figure 1. High serum levels of APRIL, BAFF and TACI.
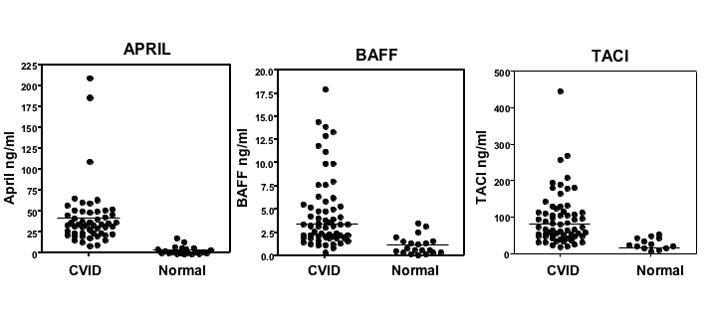
The 3 panels show that the amounts of APRIL, BAFF and TACI (ng/ml) in the serum of CVID subjects are very much higher than sera of controls tested at the same time. For APRIL, sera of 53 patients were compared to 21 controls; for BAFF, 65 patients to 20 controls and for TACI, 66 patients to 20 control sera. The median serum APRIL for CVID =34.5 ng/ml, controls = 2.0 ng/ml; median serum BAFF for CVID =3.3 ng/ml, controls=0.8 ng/ml; median TACI for CVID =77.8 ng/ml, controls = 0.01 ng/ml. The limits of detection of BAFF, APRIL and TACI in these assays were 30 pg/ml for BAFF and APRIL and 50 pg/ml for TACI.
Immunologic and Clinical Correlates
The levels of serum BAFF, APRIL or TACI were not associated with the number or percentages of peripheral B cells, CD27+ memory B cells, or CD27+ IgM-IgD- switched memory B cells, or pretreatment levels of serum IgG. The levels of these ligands were also not associated with the presence or history of autoimmune disease, lymphadenopathy, splenomegaly, lymphoid infiltrates, or granulomatous disease in these subjects.
Cell surface, intracellular and secreted BAFF
Since cells of myeloid origin are main producers of BAFF, we examined peripheral monocytes for surface BAFF expression. Both patients and controls had detectable BAFF on monocytes, but cells of CVID subjects did not appear to have more BAFF expressed than controls. Figure 2a illustrates the BAFF expression on monocytes of two representative CVID subjects in comparison to a control tested at the same time. Since cytokines, particularly INF-γ, may increase surface BAFF expression, CD14+ monocytes were also tested after 2–3 day culture with a range of concentrations of INF-γ. CD14+ monocytes of both CVID subjects and controls were found to have detectable increases in the amounts of surface BAFF, but again there was no apparent difference in BAFF expression (mean fluorescence intensity) for cells of patients in comparison to controls (Figure 2b.) After culturing PBMCs with either LPS or anti-CD40 ligand, we saw no consistent increase in CD14+ cell surface expression of BAFF for cells of patients as compared to controls (data not shown.) Using these methods, we also could not detect BAFF or APRIL in the PBMC cell culture supernatants after a 72 hour incubation with INF-γ, ◻◻◻, or CD40-ligand, confirming a previous report 25.
Figure 2a. BAFF expression on CD14+ monocytes of CVID subjects and controls.
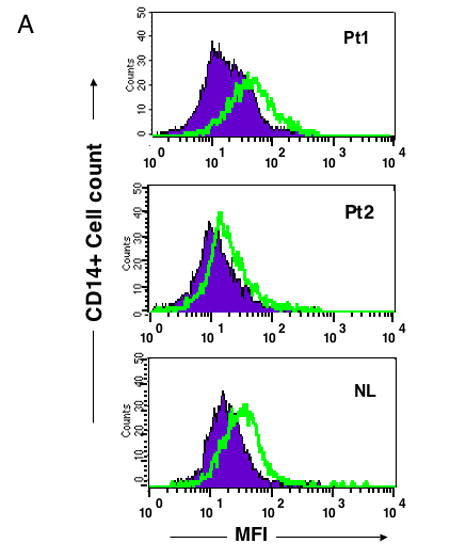
BAFF expression was demonstrated by mean fluorescence intensity (MFI) as detected by FACScan gating on CD14+ peripheral blood monocytes from freshly isolated PBMC from two representative CVID subjects shown here (of 10 tested) in comparison to a normal control (of 10) done at the same time. CD14+ cells were identified by FITC labeled anti-CD14 antibody; BAFF was detected by a rat monoclonal anti-BAFF followed by a goat anti rat CY5 conjugate, using appropriate rat IgG2a isotype controls. The closed areas indicate isotype controls and open areas, BAFF staining.
Figure 2b. Increased surface BAFF with INF-γ.
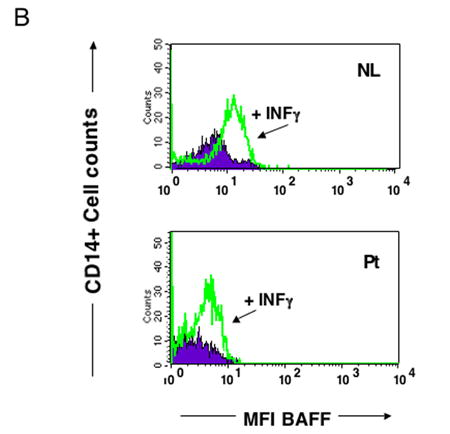
Both CVID and control CD14+ monocytes had increased expression of surface BAFF after culture for 72 hours with an optimum amount (5 ng/ml) of INF-γ. PBMCs of CVID subjects and controls were examined by FASCan as above, to assess surface BAFF on CD14+ cells, expressed as mean fluorescence intensity (MFI), in this case, before and after incubation with INFγ. Results for one of five CVID subjects in comparison to a control tested at the same time are shown. Untreated cells are indicated in the closed area, INF-γ treated cells are shown in open areas.
BAFF and APRIL mRNA levels
To determine whether mRNA levels of BAFF or APRIL were up-regulated in PBMCs either before or after exposure to INF-γ, real time PCR was used for mRNA quantitation. Compared to control cells, PBMCs of CVID subjects had markedly increased expression of BAFF mRNA (p=0.01) (Figure 3a.) While these subjects had a range of serum BAFF levels, BAFF mRNA was not related to serum levels of BAFF (p=.672.) After INF-γ exposure, there was a modest reduction in BAFF mRNA for CVID cells, but no change in BAFF mRNA for controls. Levels of PBMC APRIL mRNA, before and after INF-γ, were variable for CVID subjects, but not significantly different from the controls.
Figure 3. Increased BAFF mRNA in CVID PBMCs.
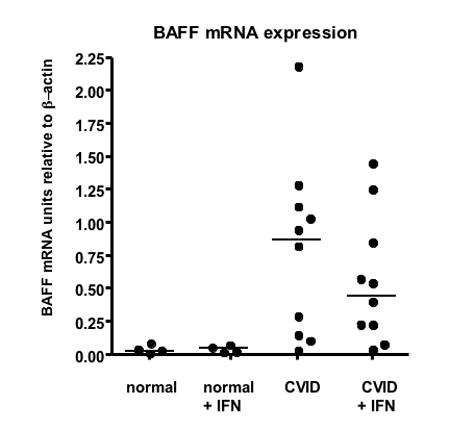
Real time PCR was used to quantitate BAFF mRNA for cells of 10 CVID subjects in comparison to 4 normal controls before or after 24 hours of culture with 5 ng/ml INF-γ. In comparison to controls, the CVID subjects had greatly enhanced amounts of BAFF mRNA (p=0.001). (median, 0.89 for CVID and 0.03 for controls.) After culture with INF-γ there was no increase in mRNA for controls but a somewhat lower amount of BAFF mRNA (but still enhanced) amount in CVID cells (median, 0.48 for CVID and 0.040 for controls.) There was no relationship between serum BAFF levels and BAFF mRNA for CVID subjects.
Discussion
Although BAFF and APRIL are produced by many cells of the hematopoietic system, it is unclear how the expression and production of these cytokines are regulated. Both are type II transmembrane proteins, proteolytically cleaved to generate active forms; homotrimers are the predominant active forms, but BAFF and APRIL can also form biologically active heterotrimers 30. Either increased production or release from cells by these enzymes could lead to high levels of biologically active forms in serum. Increased levels of mRNA in monocytes, and elevated serum levels of BAFF and APRIL have been found for patients with a number of autoimmune syndromes, including rheumatoid arthritis, multiple sclerosis, Sjogren’s syndrome and systemic lupus erythematosus, suggesting that these TNF family member cytokines drive autoimmunity and play a role in these immune deviation states. 33,38,40,45. Several polymorphisms in the BAFF promoter site are associated with higher serum BAFF levels and several autoimmune diseases linking BAFF and a genetic predisposition to autoimmunity 46,47. On the other hand, ligand specific blocking of BAFF in animal models of autoimmune disease reduces autoimmunity 48 and BAFF-targeting therapies are being explored for potential use in human disease 49-51.
Autoimmunity, especially immune thrombocytopenia and hemolytic anemia are common in CVID, as well as lymphadenopathy, splenomegaly, lymphoid hyperplasia and organ infiltrates 8,52,6,7. While for at least 90% of patients the molecular cause is unknown, recent work has demonstrated that heterozygous and homogeneous mutations of TACI are found in some patients with CVID who have varying degrees of hypogammaglobulinemia, lack of antibody and a possible increase in autoimmunity 17,18. The extracellular mutation of TACI reported in CVID (C104R) leads to a disruption of the cystine-rich domain in the second exon, a region important for binding BAFF and APRIL53. Other transmembrane or intracytoplasmic TACI mutations found presumably lead to impaired BAFF and APRIL signaling and possibly impaired class switch, although the molecular pathways disrupted, especially in the heterozygous state, are not clarified. In one study, elevated serum TACI was noted in subjects with TACI mutations18. Because of this, and the prevalence of autoimmunity and the known complications of splenomegaly, lymphadenopathy in CVID 6,8,54, we measured serum APRIL, BAFF and TACI in a large group of well-characterized subjects with CVID. Here we show that the sera of subjects with CVID contain large amounts of BAFF and APRIL, but there was no relationship between serum levels of BAFF, APRIL or TACI and autoimmunity, lymphoid hyperplasia or splenomegally. Autoimmunity is poorly understood in CVID, but it is more likely in subjects with accompanying granulomatous disease55, fewer circulating switched memory B cells 56 and possibly those with mutations in TACI 17. Autoantigen engaged B cells have been shown to have as increased dependence on BAFF 32, and can be rescued from elimination by high levels of BAFF42. While we did not find that higher levels of serum BAFF or APRIL were related to existing or previous autoimmunity in CVID, these substances may still permit the survival of autoimmune B cell clones which are blocked by other existing intrinsic B cell defects, from becoming clinically important. In this regard, B cells of CVID subjects appear capable of responding to BAFF in vitro, resulting in both proliferation and IgM secretion57, suggesting that the endogenously produced BAFF could produce a biologic effect.
BAFF and APRIL sustain B cell and plasma cell survival, CD40 ligand independent isotype switch, maintenance of germinal centers, and T cell dependent and independent antibody production21,26,58,59,26. In the mouse, BAFF is constitutively produced by cells of myeloid origin 21,25,60, as well as by radiation-resistant cells in amounts which control the size of the B cell pool, as BAFF production by bone marrow derived cells appears insufficient to support normal B cells numbers 21,27-29. Mice with targeted disruption of μ heavy chain which excludes B cell development, have very high levels of BAFF 42. Similarly, BAFF elimination, either by gene deletion or blocking by soluble receptors such as BAFF receptor or BCMA-Fc61, markedly reduces the numbers of both transitional and mature B cells. In agreement with this, rituximab treatment, which deletes B cells in systemic lupus erythematosus and rheumatoid arthritis, increases levels of serum BAFF, which reverses on re-population 62,63. However, in CVID we did not find any relationship between the levels of BAFF or APRIL and numbers of peripheral B cells, memory B cells, or isotype-switched memory B cells, showing that in this circumstance, the size of the B cell pool or its phenotype, does not appear to regulate the production BAFF or APRIL. Inflammation in general, and cytokines such as INF-γ or G-CSF in particular, enhance BAFF production 25,60. Here, INF-γ stimulation resulted in similarly enhanced surface monocyte BAFF expression for both CVID and normal subjects. However, freshly isolated peripheral blood mononuclear cells of CVID subjects had much larger amounts of BAFF mRNA as compared to normal controls, suggesting that either underlying endogenous inflammatory conditions in CVID, or heightened constitutive production (or both), lead to high levels of BAFF and APRIL in serum. Levels of TACI in serum were also elevated in many subjects with CVID, although whether this is present in an active form or not is unknown. Chronic exposure to TACI-Ig is associated with reduced circulating B cells in both mouse and non-human primates and results in a decrease in serum immunoglobulins48; here, higher levels of TACI were not related to either reduced numbers of B cells or lower serum IgG levels. Although it was suggested that subjects with mutations in TACI may have higher levels of serum TACI 18, the serum levels of subjects with mutations tested here were similar to those without.
We conclude that in contrast to both animal and human models, in which high levels of BAFF and APRIL are related to B cell numbers, sponsor lymphoid growth and may direct the development of autoimmunity, patients with CVID have greatly enhanced production and release of BAFF, and APRIL and the receptor TACI, with as yet unclear biological consequences. However, elevated BAFF levels have been found in serum of patients with chronic lymphocytic leukemia 64,65 multiple myeloma32,66,67 and non Hodgkin’s lymphoma, where higher levels are correlated with poorer clinical outcome66. Malignant Hodgkin and Reed Sternberg cells express TACI and BCMA receptors, and engagement of BAFF and APRIL on these cells results in both cell survival and growth 68. Since patients with CVID are known to have increased risk for non Hodgkin’s lymphoma, especially of B cell type, and perhaps other cancers 54,69-72, excess endogenous production of BAFF and APRIL, could facilitate or enhance this predilection.
Acknowledgments
This work was supported by grants from the National Institutes of Health, AI 101093, AI-467320, AI-48693 and NIAID Contract 03-22.
A. K. Knight: concepts/ statistics/real time PCR/ ELISA assays/editing MS
L. Radigan: ELISA, serum collection, cell isolation/flow cytometry
T. Marron: Monocyte isolation/culture/ real time PCR
A. Langs: cell isolation /real time PCR
L Zhang: DNA sequence TACI and RT PCR
C. Cunningham-Rundles: design/ concepts/ writing and editing MS
We thank Dr Bodo Grimbacher, Dr Ulrich Salzar and Dr. Timothy Behrens for TACI DNA sequencing information.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cunningham-Rundles C. Common variable immunodeficiency. Curr Allergy Asthma Rep. 2001;1:421–429. doi: 10.1007/s11882-001-0027-1. [DOI] [PubMed] [Google Scholar]
- 2.Chapel H, Geha R, Rosen F. Primary immunodeficiency diseases: an update. Clin Exp Immunol. 2003;132:9–15. doi: 10.1046/j.1365-2249.2003.02110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levy Y, Gupta N, Le Deist F, et al. Defect in IgV gene somatic hypermutation in common variable immuno-deficiency syndrome. Proc Natl Acad Sci U S A. 1998;95:13135–13140. doi: 10.1073/pnas.95.22.13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonhomme D, Hammarstrom L, Webster D, et al. Impaired antibody affinity maturation process characterizes a subset of patients with common variable immunodeficiency. J Immunol. 2000;165:4725–4730. doi: 10.4049/jimmunol.165.8.4725. [DOI] [PubMed] [Google Scholar]
- 5.Andersen P, Permin H, Andersen V, et al. Deficiency of somatic hypermutation of the antibody light chain is associated with increased frequency of severe respiratory tract infection in common variable immunodeficiency. Blood. 2004 doi: 10.1182/blood-2003-12-4359. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol. 1999;92:34–48. doi: 10.1006/clim.1999.4725. [DOI] [PubMed] [Google Scholar]
- 7.Hammarstrom L, Vorechovsky I, Webster D. Selective IgA deficiency (SIgAD) and common variable immunodeficiency (CVID) Clin Exp Immunol. 2000;120:225–231. doi: 10.1046/j.1365-2249.2000.01131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunningham-Rundles C. Hematologic complications of primary immune deficiencies. Blood Rev. 2002;16:61–64. doi: 10.1054/blre.2001.0185. [DOI] [PubMed] [Google Scholar]
- 9.North ME, Spickett GP, Allsop J, Webster AD, Farrant J. Defective DNA synthesis by T cells in acquired ‘common-variable’ hypogammaglobulinaemia on stimulation with mitogens. Clin Exp Immunol. 1989;76:19–23. [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer MB, Hauber I, Eggenbauer H, et al. A defect in the early phase of T-cell receptor-mediated T-cell activation in patients with common variable immunodeficiency. Blood. 1994;84:4234–4241. [PubMed] [Google Scholar]
- 11.Kondratenko I, Amlot PL, Webster AD, Farrant J. Lack of specific antibody response in common variable immunodeficiency (CVID) associated with failure in production of antigen-specific memory T cells. MRC Immunodeficiency Group. Clin Exp Immunol. 1997;108:9–13. doi: 10.1046/j.1365-2249.1997.d01-993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aukrust P, Aandahl EM, Skalhegg BS, et al. Increased activation of protein kinase A type I contributes to the T cell deficiency in common variable immunodeficiency. J Immunol. 1999;162:1178–1185. [PubMed] [Google Scholar]
- 13.Bayry J, Lacroix-Desmazes S, Kazatchkine MD, et al. Common variable immunodeficiency is associated with defective functions of dendritic cells. Blood. 2004;104:2441–2443. doi: 10.1182/blood-2004-04-1325. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham-Rundles C, Radigan L. Deficient IL-12 and dendritic cell function in common variable immune deficiency. Clin Immunol. 2005;115:147–153. doi: 10.1016/j.clim.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Grimbacher B, Hutloff A, Schlesier M, et al. Homozygous loss of ICOS is associated with adult-onset common variable immunodeficiency. Nat Immunol. 2003;4:261–268. doi: 10.1038/ni902. [DOI] [PubMed] [Google Scholar]
- 16.van Zelm MC, Reisli I, van der Burg M, et al. An antibody-deficiency syndrome due to mutations in the CD19 gene. N Engl J Med. 2006;354:1901–1912. doi: 10.1056/NEJMoa051568. [DOI] [PubMed] [Google Scholar]
- 17.Salzer U, Chapel HM, Webster AD, et al. Mutations in TNFRSF13B encoding TACI are associated with common variable immunodeficiency in humans. Nat Genet. 2005;37:820–828. doi: 10.1038/ng1600. [DOI] [PubMed] [Google Scholar]
- 18.Castigli E, Wilson SA, Garibyan L, et al. TACI is mutant in common variable immunodeficiency and IgA deficiency. Nat Genet. 2005;37:829–834. doi: 10.1038/ng1601. [DOI] [PubMed] [Google Scholar]
- 19.Warnatz K, Salzer U, Gutenberg S, et al. Finally Found: Human BAFF-R deficency causes hypogammaglobulinemia. Clincal Immunology. 2005;(Supplement 1):20. [Google Scholar]
- 20.Yan M, Marsters SA, Grewal IS, Wang H, Ashkenazi A, Dixit VM. Identification of a receptor for BLyS demonstrates a crucial role in humoral immunity. Nat Immunol. 2000;1:37–41. doi: 10.1038/76889. [DOI] [PubMed] [Google Scholar]
- 21.Schneider P. The role of APRIL and BAFF in lymphocyte activation. Curr Opin Immunol. 2005;17:282–289. doi: 10.1016/j.coi.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 22.von Bulow GU, van Deursen JM, Bram RJ. Regulation of the T-independent humoral response by TACI. Immunity. 2001;14:573–582. doi: 10.1016/s1074-7613(01)00130-3. [DOI] [PubMed] [Google Scholar]
- 23.Castigli E, Scott S, Dedeoglu F, et al. Impaired IgA class switching in APRIL-deficient mice. Proc Natl Acad Sci U S A. 2004;101:3903–3908. doi: 10.1073/pnas.0307348101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seshasayee D, Valdez P, Yan M, Dixit VM, Tumas D, Grewal IS. Loss of TACI causes fatal lymphoproliferation and autoimmunity, establishing TACI as an inhibitory BLyS receptor. Immunity. 2003;18:279–288. doi: 10.1016/s1074-7613(03)00025-6. [DOI] [PubMed] [Google Scholar]
- 25.Nardelli B, Belvedere O, Roschke V, et al. Synthesis and release of B-lymphocyte stimulator from myeloid cells. Blood. 2001;97:198–204. doi: 10.1182/blood.v97.1.198. [DOI] [PubMed] [Google Scholar]
- 26.Sutherland AP, Ng LG, Fletcher CA, et al. BAFF augments certain Th1-associated inflammatory responses. J Immunol. 2005;174:5537–5544. doi: 10.4049/jimmunol.174.9.5537. [DOI] [PubMed] [Google Scholar]
- 27.Khare SD, Sarosi I, Xia XZ, et al. Severe B cell hyperplasia and autoimmune disease in TALL-1 transgenic mice. Proc Natl Acad Sci U S A. 2000;97:3370–3375. doi: 10.1073/pnas.050580697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khare SD, Hsu H. The role of TALL-1 and APRIL in immune regulation. Trends Immunol. 2001;22:61–63. doi: 10.1016/s1471-4906(00)01843-3. [DOI] [PubMed] [Google Scholar]
- 29.Gorelik L, Gilbride K, Dobles M, Kalled SL, Zandman D, Scott ML. Normal B cell homeostasis requires B cell activation factor production by radiation-resistant cells. J Exp Med. 2003;198:937–945. doi: 10.1084/jem.20030789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roschke V, Sosnovtseva S, Ward CD, et al. BLyS and APRIL form biologically active heterotrimers that are expressed in patients with systemic immune-based rheumatic diseases. J Immunol. 2002;169:4314–4321. doi: 10.4049/jimmunol.169.8.4314. [DOI] [PubMed] [Google Scholar]
- 31.Melchers F. Actions of BAFF in B cell maturation and its effects on the development of autoimmune disease. Ann Rheum Dis. 2003;62(Suppl 2):ii25–27. doi: 10.1136/ard.62.suppl_2.ii25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thien M, Phan TG, Gardam S, et al. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20:785–798. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 33.Stohl W, Xu D, Kim KS, et al. BAFF overexpression and accelerated glomerular disease in mice with an incomplete genetic predisposition to systemic lupus erythematosus. Arthritis Rheum. 2005;52:2080–2091. doi: 10.1002/art.21138. [DOI] [PubMed] [Google Scholar]
- 34.Mackay F, Woodcock SA, Lawton P, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheema GS, Roschke V, Hilbert DM, Stohl W. Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthritis Rheum. 2001;44:1313–1319. doi: 10.1002/1529-0131(200106)44:6<1313::AID-ART223>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 36.Stohl W. SLE--systemic lupus erythematosus: a BLySful, yet BAFFling, disorder. Arthritis Res Ther. 2003;5:136–138. doi: 10.1186/ar755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szodoray P, Jonsson R. The BAFF/APRIL system in systemic autoimmune diseases with a special emphasis on Sjogren’s syndrome. Scand J Immunol. 2005;62:421–428. doi: 10.1111/j.1365-3083.2005.01688.x. [DOI] [PubMed] [Google Scholar]
- 38.Mackay F, Sierro F, Grey ST, Gordon TP. The BAFF/APRIL system: an important player in systemic rheumatic diseases. Curr Dir Autoimmun. 2005;8:243–265. doi: 10.1159/000082106. [DOI] [PubMed] [Google Scholar]
- 39.Seyler TM, Park YW, Takemura S, et al. BLyS and APRIL in rheumatoid arthritis. J Clin Invest. 2005;115:3083–3092. doi: 10.1172/JCI25265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsushita T, Hasegawa M, Yanaba K, Kodera M, Takehara K, Sato S. Elevated serum BAFF levels in patients with systemic sclerosis: enhanced BAFF signaling in systemic sclerosis B lymphocytes. Arthritis Rheum. 2006;54:192–201. doi: 10.1002/art.21526. [DOI] [PubMed] [Google Scholar]
- 41.Becker-Merok A, Nikolaisen C, Nossent HC. B-lymphocyte activating factor in systemic lupus erythematosus and rheumatoid arthritis in relation to autoantibody levels, disease measures and time. Lupus. 2006;15:570–576. doi: 10.1177/0961203306071871. [DOI] [PubMed] [Google Scholar]
- 42.Lesley R, Xu Y, Kalled SL, et al. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity. 2004;20:441–453. doi: 10.1016/s1074-7613(04)00079-2. [DOI] [PubMed] [Google Scholar]
- 43.Cunningham-Rundles C, Radigan L, Knight AK, Zhang L, Bauer L, Nakazawa A. TLR9 activation is defective in common variable immune deficiency. J Immunol. 2006;176:1978–1987. doi: 10.4049/jimmunol.176.3.1978. [DOI] [PubMed] [Google Scholar]
- 44.Du X, Poltorak A, Wei Y, Beutler B. Three novel mammalian toll-like receptors: gene structure, expression, and evolution. Eur Cytokine Netw. 2000;11:362–371. [PubMed] [Google Scholar]
- 45.Groom J, Kalled SL, Cutler AH, et al. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjogren’s syndrome. J Clin Invest. 2002;109:59–68. doi: 10.1172/JCI14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawasaki A, Tsuchiya N, Fukazawa T, Hashimoto H, Tokunaga K. Analysis on the association of human BLYS (BAFF, TNFSF13B) polymorphisms with systemic lupus erythematosus and rheumatoid arthritis. Genes Immun. 2002;3:424–429. doi: 10.1038/sj.gene.6363923. [DOI] [PubMed] [Google Scholar]
- 47.Emmerich F, Bal G, Barakat A, et al. High-level serum B-cell activating factor and promoter polymorphisms in patients with idiopathic thrombocytopenic purpura. Br J Haematol. 2006 doi: 10.1111/j.1365-2141.2006.06431.x. [DOI] [PubMed] [Google Scholar]
- 48.Gross JA, Dillon SR, Mudri S, et al. TACI-Ig neutralizes molecules critical for B cell development and autoimmune disease. impaired B cell maturation in mice lacking BLyS. Immunity. 2001;15:289–302. doi: 10.1016/s1074-7613(01)00183-2. [DOI] [PubMed] [Google Scholar]
- 49.Kalled SL. BAFF: a novel therapeutic target for autoimmunity. Curr Opin Investig Drugs. 2002;3:1005–1010. [PubMed] [Google Scholar]
- 50.Sabahi R, Anolik JH. B-cell-targeted therapy for systemic lupus erythematosus. Drugs. 2006;66:1933–1948. doi: 10.2165/00003495-200666150-00004. [DOI] [PubMed] [Google Scholar]
- 51.Tangye SG, Bryant VL, Cuss AK, Good KL. BAFF, APRIL and human B cell disorders. Semin Immunol. 2006;18:305–317. doi: 10.1016/j.smim.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 52.Wang J, Rodriguez-Davalos M, Levi G, Sauter B, Gondolesi GE, Cunningham-Rundles C. Common variable immunodeficiency presenting with a large abdominal mass. J Allergy Clin Immunol. 2005;115:1318–1320. doi: 10.1016/j.jaci.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 53.Hymowitz SG, Patel DR, Wallweber HJ, et al. Structures of APRIL-receptor complexes: like BCMA, TACI employs only a single cysteine-rich domain for high affinity ligand binding. J Biol Chem. 2005;280:7218–7227. doi: 10.1074/jbc.M411714200. [DOI] [PubMed] [Google Scholar]
- 54.Cunningham-Rundles C, Cooper DL, Duffy TP, Strauchen J. Lymphomas of mucosal-associated lymphoid tissue in common variable immunodeficiency. Am J Hematol. 2002;69:171–178. doi: 10.1002/ajh.10050. [DOI] [PubMed] [Google Scholar]
- 55.Mechanic LJ, Dikman S, Cunningham-Rundles C. Granulomatous disease in common variable immunodeficiency. Ann Intern Med. 1997;127:613–617. doi: 10.7326/0003-4819-127-8_part_1-199710150-00005. [DOI] [PubMed] [Google Scholar]
- 56.Warnatz K, Denz A, Drager R, et al. Severe deficiency of switched memory B cells (CD27(+)IgM(-)IgD(-)) in subgroups of patients with common variable immunodeficiency: a new approach to classify a heterogeneous disease. Blood. 2002;99:1544–1551. doi: 10.1182/blood.v99.5.1544. [DOI] [PubMed] [Google Scholar]
- 57.Stewart DM, McAvoy MJ, Hilbert DM, Nelson DL. B lymphocytes from individuals with common variable immunodeficiency respond to B lymphocyte stimulator (BLyS protein) in vitro. Clin Immunol. 2003;109:137–143. doi: 10.1016/s1521-6616(03)00215-8. [DOI] [PubMed] [Google Scholar]
- 58.Mackay F, Browning JL. BAFF: a fundamental survival factor for B cells. Nat Rev Immunol. 2002;2:465–475. doi: 10.1038/nri844. [DOI] [PubMed] [Google Scholar]
- 59.Mackay F, Schneider P, Rennert P, Browning J. BAFF AND APRIL: a tutorial on B cell survival. Annu Rev Immunol. 2003;21:231–264. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 60.Scapini P, Nardelli B, Nadali G, et al. G-CSF-stimulated neutrophils are a prominent source of functional BLyS. J Exp Med. 2003;197:297–302. doi: 10.1084/jem.20021343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schiemann B, Gommerman JL, Vora K, et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 62.Vallerskog T, Heimburger M, Gunnarsson I, et al. Differential effects on BAFF and APRIL levels in rituximab-treated patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Res Ther. 2006;8:R167. doi: 10.1186/ar2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lavie F, Miceli-Richard C, Ittah M, Sellam J, Gottenberg JE, Mariette X. Increase of B-cell activating factor of the TNF family (BAFF) after rituximab: insights into a new regulating system of BAFF production. Ann Rheum Dis. 2006 doi: 10.1136/ard.2006.060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nishio M, Endo T, Tsukada N, et al. Nurselike cells express BAFF and APRIL, which can promote survival of chronic lymphocytic leukemia cells via a paracrine pathway distinct from that of SDF-1alpha. Blood. 2005;106:1012–1020. doi: 10.1182/blood-2004-03-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haiat S, Billard C, Quiney C, Ajchenbaum-Cymbalista F, Kolb JP. Role of BAFF and APRIL in human B-cell chronic lymphocytic leukaemia. Immunology. 2006;118:281–292. doi: 10.1111/j.1365-2567.2006.02377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Novak AJ, Darce JR, Arendt BK, et al. Expression of BCMA, TACI, and BAFF-R in multiple myeloma: a mechanism for growth and survival. Blood. 2004;103:689–694. doi: 10.1182/blood-2003-06-2043. [DOI] [PubMed] [Google Scholar]
- 67.Moreaux J, Legouffe E, Jourdan E, et al. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood. 2004;103:3148–3157. doi: 10.1182/blood-2003-06-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chiu A, Xu W, He B, et al. Hodgkin lymphoma cells express TACI and BCMA receptors and generate survival and proliferation signals in response to BAFF and APRIL. Blood. 2006 doi: 10.1182/blood-2006-04-015958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kinlen LJ, Webster AD, Bird AG, et al. Prospective study of cancer in patients with hypogammaglobulinaemia. Lancet. 1985;1:263–266. doi: 10.1016/s0140-6736(85)91037-2. [DOI] [PubMed] [Google Scholar]
- 70.Sander CA, Medeiros LJ, Weiss LM, Yano T, Sneller MC, Jaffe ES. Lymphoproliferative lesions in patients with common variable immunodeficiency syndrome. Am J Surg Pathol. 1992;16:1170–1182. doi: 10.1097/00000478-199212000-00004. [DOI] [PubMed] [Google Scholar]
- 71.Ariatti C, Vivenza D, Capello D, et al. Common-variable immunodeficiency-related lymphomas associate with mutations and rearrangements of BCL-6: pathogenetic and histogenetic implications. Hum Pathol. 2000;31:871–873. doi: 10.1053/hupa.2000.7626. [DOI] [PubMed] [Google Scholar]
- 72.Gompels MM, Hodges E, Lock RJ, et al. Lymphoproliferative disease in antibody deficiency: a multi-centre study. Clin Exp Immunol. 2003;134:314–320. doi: 10.1046/j.1365-2249.2003.02253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


