Abstract
It has long been shown that therapeutic ultrasound can be used effectively to ablate solid tumors, and a variety of cancers are presently being treated in the clinic using these types of ultrasound exposures. There is, however, an ever-increasing body of preclinical literature that demonstrates how ultrasound energy can also be used non-destructively for increasing the efficacy of drugs and genes for improving cancer treatment. In this review, a summary of the most important ultrasound mechanisms will be given with a detailed description of how each one can be employed for a variety of applications. This includes the manner by which acoustic energy deposition can be used to create changes in tissue permeability for enhancing the delivery of conventional agents, as well as for deploying and activating drugs and genes via specially tailored vehicles and formulations.
Keywords: high intensity focused ultrasound (HIFU), drug and gene delivery, hyperthermia, acoustic cavitation, acoustic radiation forces
1. Introduction
1.1 Cancer
Human endeavor has made great strides in the treatment of cancer. Whereas a century ago the chances of someone surviving cancer was zero, today two out of every three people diagnosed will still be alive five years later. Although the ‘cure’ for cancer has been far more elusive than once hoped, treatment continues to improve, evident by the ever increasing number of survivors. In the last decade, for example, we have experienced a 10% decline in the number of cancer deaths [1].
Improved cancer treatment is in part linked to the increase in understanding of cellular, genetic and molecular mechanisms, which provide targets for interventions to prevent, detect, eliminate and control the disease [1]. After surgical removal and/or sterilization by radiation of a primary tumor, management of the residual disease is typically carried out using a variety of systemic therapies [2,3]. However, in order for these therapies to be successful, there are two requirements that must be satisfied. First, the particular agent being used must be effective in the in vivo, orthotopic microenvironment of the tumor being treated (not just in cell culture). Secondly, optimal quantities of the agent must reach all cells of the targeted tumor [2].
1.2 Tumor microenvironment – barriers to drug & gene delivery
In the United States, the large majority of deaths due to cancer is the result of solid tumors [4]. For most advanced cancers, chemotherapy remains the treatment of choice. However, chemotherapy is rarely curative, especially for solid tumors [5]. Although these anticancer agents are effective for killing tumor cells in monolayers grown in culture, they are unable to reach all tumor cells that are able to regenerate the tumors in vivo [3]. A number of factors has been identified in the microenvironment of solid tumors that are responsible for non-uniform and insufficient levels of anti-cancer agents being delivered. These occur due to abnormalities in both the vasculature and the extracellular matrix and lead to deficiencies in transvascular and interstitial transport, respectively [6], which ultimately affect the bioavailability and efficacy of chemotherapeutic agents [7].
Compared to normal tissues, blood vessels in tumors are leaky, possessing large gaps between endothelial cells [8]. The vasculature is also chaotic in regards to spatial distribution, microvessel length and diameter [6], and it can be tortuous and saccular, possessing haphazard interconnections, which renders the vessels functionally abnormal [9]. Proliferating tumor cells can also generate solid pressure on blood vessels that will further impair blood flow [9]. Another important characteristic of the tumor microenvironment is the combination of a leaky vasculature and a lack of functional lymphatics, which can create increased interstitial fluid pressures compared to normal tissues. These high pressures are found just past the periphery of solid tumors, being approximately the same as the microvascular pressure. As a result, extravasation of large convection-dependent agents can be severely limited [10]. In tumors, the plasma to interstitial gradient of oncotic pressure is also generally reduced, being yet another factor that can contribute to less than optimal delivery of therapeutic agents [11].
Another often overlooked factor for insufficient delivery of anti-cancer agents to tumor cells is the increase in mean distance between tumor cells and the blood vessels that they supply. Whereas the well-organized, normal tissues of the human body enable most cells to be within a few cell diameters of a blood vessel, this is often not the case in solid tumors. Relatively higher cell proliferation rates in tumors, compared to normal tissues, can result in tumor cells forcing vessels apart, leading to a reduction in vascular density. As a result, populations of cells are created that can be more than 100 µm from blood vessels, a problem that may further be exacerbated by the already poor organization of the tumor vasculature. This phenomenon can lead to limited access of drugs to those distant tumor cells. It can also reduce the delivery of oxygen and create conditions of hypoxia (leading to reduced efficacy of radiation therapy) and the build up of metabolic products (e.g. carbonic and lactic acid), lowering the extracellular pH and potentially affecting the cellular uptake of some drugs [3].
Additional factors in the extracellular matrix (ECM) of tumors can limit interstitial transport and, as a result, further prevent sufficient and uniform distribution of anti-cancer agents; especially large agents such as viral vectors [6]. The ECM is made up of a matrix of proteoglycans, collagens, and additional molecules, which are produced and assembled by stromal and tumor cells [12]. McGuire et al [13] showed that greater collagen content in the ECM required higher infusion pressure to initiate flow in the tumor interstitium. Netti et al [14] demonstrated the inverse relationship between tumor content of fibrillar collagen and interstitial diffusion of large macromolecules. Collagen in the ECM can physically obstruct transport, where the sizes of viral vectors, for example, can be larger than the space between the fibers [6]. The effects of collagen on reducing interstitial transport were established by treatment with collagenase to chemically disrupt the fibers and increase interstitial transport of antibodies [14] and oncolytic viruses [15]. Collagen fibers also bind and stabilize glycosaminoglycan and hyaluronic acid, which can affect interstitial transport by creating resistance to water and solute transport [14]. Dreher et al [16] demonstrated the manner by which the size of agents may effect drug delivery to tumors, where increasing the molecular weight of macromolecules was found to increase their plasma half-life; however it decreased vascular permeability for reduced extravasation, as well as penetration into tumor tissue from the vessel wall.
The tumor microenvironment poses a formidable obstacle to enabling uniform and adequate delivery of anticancer agents. If delivered successfully, anticancer agents could substantially improve the treatment of solid tumors. Considerable effort, therefore, has gone into finding ways to modify the tumor microenvironment for this purpose. McKee et al [15] recently showed using human melanoma xenografts in mice that improved interstitial distribution of oncolytic virus obtained by co-injecting collagenase could also improve growth inhibition of the tumors compared to the virus on its own. Krol et al [17] on the other hand showed that reducing the number of viable cells could also increase the available fraction of drugs. Other strategies that are being developed for enhancing local drug delivery involve external sources of energy in combination with specially designed carriers to respond to that energy for local drug release [18]. Hyperthermia mediated liposomal drug delivery, for example, is one such strategy that has shown much promise for enhancing local drug deployment while minimizing drug distribution outside targeted tissues [19]. In this review, strategies based on combining non-invasive and non-destructive therapeutic ultrasound exposures with anti-cancer agents will be described by specifically using representative publications from the literature that best exemplify these strategies. In some of these, the exposures are used in a unique fashion with specially tailored agents to improve localized deposition. In others, direct effects are created in the tissues to reduce transport barriers as a way to improve the delivery of more conventional agents whose efficacy is limited because of dose limiting toxicity.
2. Therapeutic ultrasound
2.1 Diagnostic US v therapeutic US
Using ultrasound for therapeutic purposes dates back to more than half a century ago, even predating the use of ultrasound for diagnostic imaging [20]. Therapeutic ultrasound is generally described as the use of ultrasound for applications other than imaging or diagnostics [21]. In diagnostic ultrasound, energy deposition in tissues is meant to be minimal in order not to produce biological effects. On the other hand, applications of therapeutic ultrasound are based on depositing ultrasound energy to specifically create effects. These can be mild and non-destructive, such as those generated for healing in physical therapy [22], or more extreme and destructive, such as thermal ablation of tumors [23]. There are presently a large range of therapeutic applications of ultrasound exposures based on the many ways that ultrasound energy can interact with cells and tissues [24]. The mechanisms for producing effects are typically divided into thermal mechanisms and non-thermal mechanisms. In the following section, the three most important ultrasound mechanisms (heat generation, acoustic cavitation, and acoustic radiation forces) for creating bio-effects will briefly be covered as an introduction for describing the manner by which these mechanisms are involved in therapeutic ultrasound applications, especially in regards to enhancing the delivery of therapeutic agents.
2.2 Therapeutic ultrasound devices
Ultrasound exposures may be provided using either plane wave, non-focused transducers, or focused transducers. Non-focused transducers are typically used for physical therapy applications and for enhancing transdermal delivery, also know as sonophoresis [25]. Similar to light, ultrasound waves can be focused onto very small volumes, which greatly increases their intensity – hence the name high intensity focused ultrasound (HIFU). Focused beams are created using spherically-curved transducers, allowing energy to be deposited deep inside the body [20]. The ultrasound wave passes through the skin and other intervening tissues over a wide area producing relative low spatial intensities and consequently creating no damage [26]. At the focus, however, intensities can be 3 to 4 orders of magnitude higher than at the transducer surface [20]. Targeting of HIFU exposures to specific tissues, organs and tumors may be carried out using different imaging modalities: diagnostic (B-scan) ultrasound [27] and magnetic resonance imaging (MRI) [28]. Recent studies have also indicated that computed tomography (CT) [29] and optical 3D tracking [30] can also be used for guiding HIFU exposures; however, these imaging modalities have not yet been incorporated into commercial HIFU devices. The advantages of using extra-corporeal HIFU exposures, for example for tumor ablation, compared to more invasive surgical procedures, are many-fold, and include limited blood loss and infection, elimination of scar formation, and a decreased risk of other complications. Tumor ablation with HIFU can also be provided on an outpatient basis, where cost and recovery times are significantly reduced in comparison to other existing techniques, such as radio frequency ablation, laser, and cryoablation [23]. A comparison of focused and non-focused beams appears in Figure 1, as well the manner by which diagnostic ultrasound can be used for treatment planning.
Figure 1.
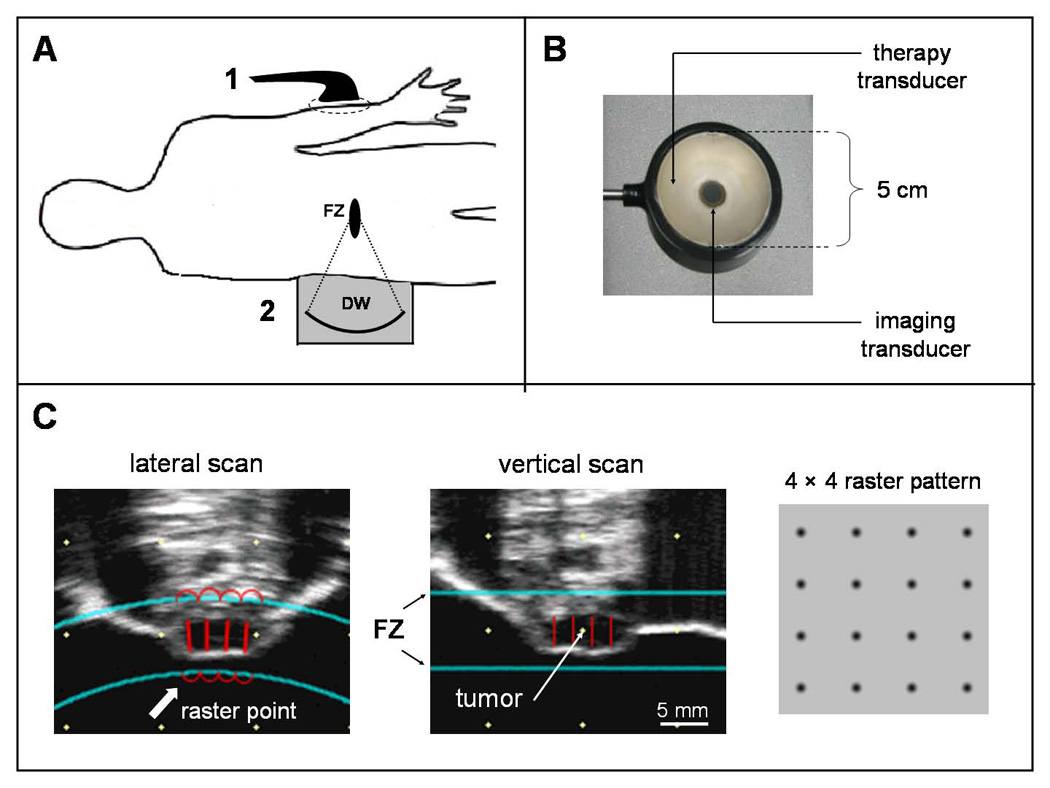
A – a schematic representation showing how non-focused ultrasound exposures using a plane wave transducer (1) are used to treat superficially, whereas a concave tranducer (2), housed in a coupling bath of degassed water (DW), can be used to place the focal zone (FZ) of the beam deeper inside the body; B – tranducer head of a dual probe showing the concave bowl therapeutic transducer for producing a focused beam and the collinear imaging tranducer for treatment planning of the exposure. This device was modified from a Sonoblate 500 (Focus Surgery, Indianapolis, IN) and used for some of the preclinical studies described in this review [57–59,61,142–149,151,153,154,157, 163]; C – representative lateral and vertical B mode ultrasound scans of a subcutaneous tumor in a murine flank. The two long horizontal lines indicate the focal zone (FZ) of the transducer and the shorter vertical lines, the raster (treatment) points for the exposures. Four raster points are positioned in each view, producing a 4 × 4 raster pattern (right) for complete treatment of the tumor. Mice are treated in a bath of degassed water, and the tumor is positioned using a 3 dimensional stage during imaging. Because the depth of the tumor is less than the focal zone, rastering will occur in only 2 dimensions.
2.3 Applications of therapeutic ultrasound
Applications of therapeutic ultrasound can generally be divided into those that are based on the direct effects of ultrasound energy deposition and those that enhance the delivery of therapeutic agents. In the former group, the various applications can be distinguished in regards to how the ultrasound energy is applied. As a rule, the greater the rate of energy being deposited in the tissue, the more pronounced the effects being generated. On the one hand, low-level energy deposition, using non-focused beams, is typically used for healing purposes in physical therapy. The range of applications for these exposures, which are thought to improve blood flow to the treated region, and hence increase the delivery of nutrients and oxygen as well as the removal of cellular wastes, include the relief of inflammation, muscle spasms and pain, in addition to accelerating healing and increasing range of motion [31]. Other applications include facilitating the repair of fractures [22], and preclinical studies have demonstrated how these exposures can accelerate the recovery of sciatic nerve injury [32]. Higher rates of energy deposition are presently being used to ablate solid tumors, such as prostate cancer [33] and uterine fibroids [34]. These types of exposures, which produce irreversible cell death by the process of coagulative necrosis, are also being evaluated in clinical trials for treating breast and kidney tumors [35], liver tumors [36], testicular cancer [37], and for palliation in patients with bone cancer [38]. The same types of short continuous exposures, generating high levels of heat, are also presently being developed for controlling hemorrhage [39].
Although HIFU exposures for thermal ablation are probably the best-known application of therapeutic ultrasound, one only has to look in the literature to see that the vast majority of novel applications presently being developed are for enhancing the delivery of drugs and genes. The only use of ultrasound presently in the clinic for enhancing drug delivery is sonophoresis (i.e. ultrasound enhanced trans-dermal delivery). Sonophoresis is still, however, restricted to local applications, such as increasing the delivery of anti-inflammatory agents [25], and accelerating the onset of cutaneous anesthesia using local anesthetics [40]. Other applications of ultrasound-mediated delivery presently being developed include improving the delivery of thrombolytic agents [41], opening the blood brain barrier [42], and enhancing the delivery of drugs and genes for the treatment of solid tumors [21]. The last of these applications, using HIFU exposures, is the topic of this review, and will be discussed in regards to the specific ultrasound mechanisms involved with each of the different treatment strategies.
3. Ultrasound treatment strategies for drug and gene delivery to solid tumors
3.1 Heat
Among all the ultrasound mechanisms for producing bio-effects, the generation of heat in tissues due to ultrasound exposure is probably the best known and understood. Heat generation results from the absorption of energy, where the volumetric rate of heat being created is directly proportional to the specific absorption coefficient of the tissue being treated and the intensity and the frequency of the ultrasound wave, and inversely proportional to the specific heat of that particular tissue [43]. The net amount of heat generated in the tissue, and the temperature elevation resulting from it, will depend on the removal of heat through diffusion, which is also tissue dependent, and convection by the vasculature, where perfusion itself can increase as a response to the tissue being heated. Relatively greater heat will be generated at tissue to tissue interfaces due to discontinuities in impedance [44], and also when acoustic cavitation occurs, where the bubbles serve to increase absorption and concentrate acoustic energy [45]. In some cases, where the heating process creates changes in the tissues (e.g. coagulation for ultrasound ablation) and boiling and/or nonlinear acoustic wave propagation occurs, the heating process will become even more complex, and therefore still harder to predict [46].
3.1.1 Heat sensitive liposomes
Using liposomes for encapsulating drugs is an effective way to improve drug delivery to solid tumors, compared to the drug alone, where systemic toxicity is lowered and uptake is increased into cells [47]. Further enhancement of tumor drug accumulation can occur by incorporating lipid-conjugated polyethylene glycol (PEG) into the liposome membrane. This lowers their volume of distribution and prolongs clearance time, due to the protective barrier that PEG provides against interactions with plasma proteins and the reticuloendothelial system [48]. Traditional liposomes are stable in the physiological temperature range; however, they can be designed to undergo a phase change when heated, which renders them more permeable and consequently releases their payload [49]. By combining these thermosensitive liposomes (TSLs) with an external source of heat, such as infrared laser [50] or microwaves [51], a certain degree of targeting is achieved where the TSLs will release their payload where local tissue temperatures are elevated [52]. When combined with localized hyperthermia, TSLs have demonstrated the ability to improve delivery to tumors of various types of anti-cancer agents, including methotrexate [53], cisplatin [54], and doxorubicin [55].
Only a small number of preclinical studies to date have been reported where HIFU exposures, as a source of hyperthermia, were combined with TSLs. In one study, TSLs were loaded with a paramagnetic contrast agent, where the triggering temperature of the liposomes was set to thermal ablation levels (i.e. approximately 57 °C). Using an MR-guided HIFU system in combination with the TSLs and imaging for T1-weighted signal intensity enhancement, the authors demonstrated that they could non-invasively validate that indeed temperature elevations were achieved for ablating the targeted tissue [56].
TSLs that trigger in the non-destructive hyperthermia range (e.g. 39 – 41°C) are more commonly used for the purpose of drug delivery [55]. Dromi et al [57] used these low temperature sensitive liposomes, or LTSLs, with pulsed-HIFU exposures that typically produce temperature elevations of only 4–5 °C [58]. They showed that combining the exposures with the LTSLs could significantly enhance the delivery of doxorubicin in a murine breast cancer tumor xenografts, compared to a commercial non-thermosensitive liposome (i.e. Doxil®). This increase in local drug delivery was subsequently shown to also improve growth inhibition of the tumors. More recently, Patel et al [59] demonstrated that increased spatial rates of drug deployment with the LTSLs could be obtained when using a split focus HIFU transducer, which allows for larger volumes of tissue to be simultaneously treated.
3.1.2 Heat shock protein promoters
One of the requirements of gene therapy is tight control of transgene expression, both spatial and temporal. In the different gene delivery strategies to be discussed, the aim of the procedures is enhancing spatial targeting and efficiency of gene delivery. These strategies, however, do not include mechanisms for temporal control. Tissue specific promoters may also be used to limit transgene expression to targeted tissues, and in that way add a layer of targeting and safety to gene delivery procedures [60]. An example is described below, where a promoter for an esophageal carcinoma was used with pulsed-HIFU exposures with an adenovector-expressing TRAIL [61].
Methods have been developed to temporally control transgene expression, using gene promoters that respond to external chemical factors such as small molecules [62] or different antibiotics [63,64]. One major drawback to the systemic administration of agents such as these is that since they will reach all parts of the body, expression of a gene will occur that was not accurately delivered to the original targeted tissue [65]. Even when intra-tumoral infusions are used for more direct delivery of therapeutic genes, conditions of the administration of the gene carriers, such excess infusion pressure, may lead to systemic leakage from the injection site [66].
In eukaryotic cells, heat shock protein (HSP) transcription is robustly initiated within minutes of exposure to temperature elevations above those for maximum growth. Because of this, heat shock response has been identified as one of the most selective and inducible ways to regulate transcription [65]. There are seven types of HSPs that respond well in terms of inducement to heat; of these HSP70 has demonstrated the best overall response, especially since it is the first to be repressed in the absence of stimulus [67]. Brade et al [68] demonstrated a strong thermal dose response of reporter gene expression when controlled by an HSP70B promoter. In vitro studies showed that increasing heat shock, by increasing the temperature, duration, or both, produced proportional increases in both temporal duration and magnitude of EGFP expression in human prostate cancer cells [69]. Pre-clinical studies have also produced promising results when using HSP promoters to control in vivo transgene expression and consequently improve therapeutic outcomes [65].
Non-destructive HIFU exposures have been shown to cause significant upregulation of a number of genes including glucose regulated proteins (GRP), stress proteins (SP), and HSPs [70]. The potential of HIFU exposures as a source of hyperthermia for turning genes on using HSP70 promoters has been demonstrated in vivo [65,71]. Madio et al [71] were the first to demonstrate how localized hyperthermia (5 to 8 °C, for 45 min) using HIFU exposures could significantly increase levels of HSP70 mRNA in the muscle of rats. Kramer et al [70] also found up-regulation of HSPs in tissues bordering thermal lesions produced by HIFU exposures in the prostate of patients with benign prostate hyperplasia. This phenomenon is thought to be part of one of the proposed mechanisms for inducing cellular immunity against cancer cells as a result of HIFU exposures, where the release of HSPs are known to stimulate the activity of cytotoxic T-cells [23].
Various studies have demonstrated the ability of HSP promoters in response to HIFU exposures to control transgene expression. Liu et al [72] used tumors cells transfected with a bioluminescent reporter gene that was controlled by an HSP70B promoter. Their in vitro experiments used HIFU exposures to create relatively high temperatures (60 °C) for short durations (5 sec), where the thermal dose was just below that for inducing coagulative necrosis. A marked increase in gene expression was observed; however, higher thermal doses above this level decreased the relative number of viable cells. The same exposures were then shown to reproduce these results in vivo [73]. Another study in rodents used tumors stably transfected with a fluorescent reporter gene controlled by an HSP70 promoter [74]. The strategy of using lower temperature elevations for longer periods has also been used to generate similar increases in transgene expression in the liver [75] and in the prostrate [76].
In the above-mentioned studies [74–76], MRI was used to guide the HIFU exposures, which not only enabled more accurate placement of the HIFU beam, but also incorporated automated, real-time feedback control of a predefined temperature-time trajectory to compensate for tissue perfusion and inhomogeneity, two important factors known to effect heat generation. The clear advantage of using an MR-guided HIFU system with heat shock inducible promoters was demonstrated in these studies, where the heat was delivered non-invasively in a tightly controlled manner, both spatially and temporally, and in regards to the thermal dose. A major disadvantage, however, of using MRI is the relatively high operating cost involved, making it not readily available as a research resource for developing gene therapy protocols. See Rome et al [65] for a comprehensive review on using HSP promoters for temporal and spatial control of therapeutic genes, both with and without HIFU exposures.
3.1.3 Thermal ablation and chemotherapy
A number of preclinical studies have indicated the potential of combining HIFU ablation exposures with chemotherapy for the treatment of tumors. These include the use of doxorubicin in liver tumors [77], adriamycin in a neuroblastoma [78], fluorouracil in peritoneal carcinomatosis [79], and paclitaxel and estramustine in a AT2 Dunning adenocarcinoma [80]. It is generally thought that the improved effects of combining HIFU and these agents occurs outside of the immediate treatment zone of the HIFU beam (i.e. where thermal cytotoxicity is not occurring) and is due to either the agents sensitizing the cells to increase the normally sub-therapeutic effects of the HIFU exposures, or conversely, to the sub-lethal HIFU effects that improve the uptake of the agents. Regarding the potential effects of sub-lethal hyperthermia, it has been shown to create changes in cells that can improve the efficacy of anticancer agents, as well as to increase the intercellular concentration of those agents [81]. Acoustic cavitation is also known to occur during thermal ablation with HIFU [45], which can temporarily increase the permeability of cell membranes (i.e. sonoporation, see below) for improved uptake of DNA [82]. Overall, there appears to be sufficient evidence that the increased efficacy of combining chemotherapeutic agents with ablative HIFU exposures (compared to either treatment alone) could be due in part to sub-lethal effects of HIFU, both thermal and non-thermal, for improving the bioavailability of those agents.
3.2 Acoustic cavitation
Acoustic cavitation may be defined as the growth, oscillation, and collapse of small stabilized gas bubbles under the influence of the varying pressure field of a sound wave in a fluid medium. In regards to its potential for producing effects in biological tissues, especially those that enhance drug delivery, it is considered the most important of all the non-thermal ultrasound mechanisms [83]. The growth of cavitating bubbles in an ultrasonic field is by rectified diffusion, where the net amount of gas diffusing into a bubble during its expansion is greater than the diffusion out during contraction. There are two distinct types of acoustic cavitation activity. Non-inertial (or stable) cavitation bubbles persist for a relatively large number of acoustic cycles, where the bubble radius varies about an equilibrium value, determined by the operating frequency. Inertial (or transient) cavitation bubbles grow faster, expanding two to three times their resonant size, where they oscillate unstably, and finally collapse in a single compression half-cycle [84]. Although it has been proposed that damage to biological tissues can theoretically occur from stable cavitation bubbles [85], it is generally accepted that the primary mechanism for structurally altering intact cells is inertial cavitation, where these alterations include both irreversible damage [86] as well as non-destructive increases in membrane permeability [87].
Probably the most important factors affecting acoustic cavitation are the number and availability of small stabilized gas bubbles, also called cavitation nuclei [83,87]. Cavitation activity will increase with a corresponding increase in the number of nuclei available [88], which are ubiquitous in non-degassed water and other liquids, but are considered scarce in animal tissues [83]. Another important factor that will determine whether cavitation will or will not occur is the available physical space for bubbles to form and grow. Because of these factors, it is difficult to induce cavitation within intact cells and in the extra-cellular matrix [89]. On the other hand, the vasculature possesses both the required dimensions and cavitation nuclei for initiation of cavitation when a high enough ultrasound pressure fields exists. More specifically, it is the peak rarefactional pressure of the ultrasound exposure that will control the onset of cavitation activity [90].
A variety of phenomena has been reported to occur when a cavitating bubble collapses in an open medium. Imploding bubbles create large increases in localized temperature, leading to the thermal dissociation of water molecules and the formation of hydroxyl molecules [84]. The ‘sonochemical’ effects that may subsequently occur from the ensuing free radical formation include DNA damage (in aqueous solution), inactivation of proteins and enzymes, and lipid peroxidation. Damage to cell membranes is also thought to occur; however, this effect is considered to be hard to detect, since the mechanical effects (see below) typically dominate in cell lysis [86].
The most pronounced effects of inertial cavitation will occur when the bubbles collapse near a rigid boundary, while surrounded by a relatively large (i.e. compared to the bubble diameter) body of fluid [90]. Compared to a bubble collapsing in an open medium (described above), constraints in fluid flow will be imposed by the boundary, causing asymmetrical collapse of the bubble, where the far side impacts and penetrates the bubble surface closest to the boundary, generating a ‘wall-direct re-entrant jet’. A high-pressure region is created at the penetration interface [91], where jet velocities can be greater than 100 m s−1 and the pressures as high as 109 Pa [92]. High-speed photography has been used to document the manner by which the jets form, compressing and cracking tissue surfaces [93], and rendering them damaged and pitted [93–95]. For a comprehensive review on acoustic cavitation, including factors controlling its activity and relevant bio-effects, see Kimmel [90].
3.2.1 Blood brain barrier disruption
The first reported study employing therapeutic ultrasound in animals involved the use of HIFU exposures to accurately create focal lesions in the brain. Although craniotomies were required for accessing the brain tissue in order to produce these effects, the study demonstrated the potential of therapeutic ultrasound and heralded a new era in biomedical research and development [96]. Not long afterwards, the procedure was being used for human treatment, where specific regions in the basal ganglia were ablated in patients with Parkinson’s disease [97].
Recent advances in multi-modality imaging, together with multi-element array transducers, have enabled the development of prototype ultrasound devices for accurately and safely providing HIFU exposures through the intact skull [20]. In addition to using these exposures for ablating tissue (typically in continuous mode), they have also been used in pulsed mode (which lowers the rate of energy deposition, and hence can be non-destructive) for opening the blood brain barrier (BBB) [98]. More recent developments in this type of procedure have shown that the addition of ultrasound contrast agents (UCAs), as a source of cavitation nuclei, during exposures renders cavitation activity more predictable and also lowers the intensity threshold for its onset [28]. The mechanism by which delivery enhancement occurs is not precisely known. It is hypothesized that the stably oscillating cavitating bubbles, generate mechanical stress in the adjacent blood vessel walls, while causing little or no damage to brain tissue, and in that manner increase vascular permeability both through structural and physiological processes [42]. As a consequence, the BBB is reversibly disrupted, allowing extravasation of therapeutic agents. Past studies have shown that among these agents are some important for the treatment of cancer, including the antibody-based agent Herceptin [99] and liposomal doxorubicin [100]. Both of the agents are clinically relevant for treating malignancies of the brain, but are normally unable to overcome the BBB. The present standard for providing these types of HIFU exposures is by using MRI guidance, where the delivery of gadolinium-based MR contrast agents can be used as a reliable surrogate marker for successful permeability enhancement and optimization of treatment [20]. For a comprehensive review on BBB disruption using HIFU, see [42].
3.2.2 Sonoporation
In contrast to employing stable (i.e. non-inertial) cavitation to alter vascular permeability for increased extravasation and hence improved delivery to whole tissues, inertial cavitation (i.e. collapsing bubbles) can be used to alter the permeability of individual cells for improved delivery of genes and drugs at that level. This process has been termed ‘sonoporation’, where sound energy is employed to enhance the permeability of plasma membranes through the creation of pores. This technique was first demonstrated by Fechheimer et al [101], who exposed cell suspensions of live slime mold amoebae to ultrasound in the presence of fluorescein-labeled dextrans, which because of their size were normally impermeable to the cells. The exposures enabled approximately 40% of the cells to take up the fluorophore, and the process was subsequently reproduced in mammalian cells for delivering DNA [102].
Only recently could the process of sonoporation be observed, where high-speed photography enabled the visualization of asymmetrical bubble collapse and consequent formation of wall-direct re-entrant jets (as described above) for the delivery of molecules to individual cells [103]. van Wamel et al [104] similarly found a direct correlation between cell deformation and consequent increase in cell membrane permeability, where propidium iodide served as a membrane integrity probe. Numerous studies, especially in vitro, have clearly demonstrated how this process can become more efficient with the addition of UCAs, where the stabilized microbubbles serve as cavitation nucleation agents [104]. As will be discussed below, adding UCAs is especially crucial for applications in vivo in order to improve transfection efficiency, where the process is limited by a dearth of cavitation nucleation sites.
Although sonoporation is reversible and therefore non-destructive, the activity of cavitation (especially inertial cavitation) is non-uniform and difficult to control, and ultimately some of the exposed cells will experience irreversible damage that finally leads to cell death [94]. The presence of cavitation due to ultrasound exposure can also cause significant increases in the temperature of the tissue [45, 105]. Cytotoxic temperatures may also occur due to viscous dissipation in the coating of UCAs [106]. As will be discussed below, effective sonoporation has yet to be demonstrated without cell destruction occurring during the process. For a review of sonoporation for gene delivery, see Miller [107].
The goal of gene therapy applications is the transfer of genetic material to the targeted cells, where the transgenes will either alter phenotypes or produce local therapeutic agents, depending on the treatment [6]. Over half of the more than 500 gene therapy trials tracked by the NIH Recombinant Advisory Committee since the first human gene transfer trial in 1998 have been for the treatment of cancer [108]. Safe and efficient gene transfer, however, has been shown to be much more difficult than was previously anticipated [109], and today is considered to be the greatest hurdle for the implementation of gene therapy in human medicine [110,111].
A number of physical methods have been developed for delivering genes. These either enhance membrane permeability, as the case for electroporation [111], infrared laser exposures [112], or increased hydrodynamic pressure (i.e. hydroporation) [113–115], or the transfection relies on more ballistic techniques, where the DNA is physically ‘driven’ into the cells using magnets with superparamagnetic nanoparticles [116] or by coating the DNA onto metal particles (e.g. silver or gold), which are then propelled directly into the skin using compressed gases [117]. Although effective to different degrees, these methods are all associated with one or more factors that are problematic for their widespread implementation, such as being invasive and/or limited to a specific tissue type or anatomical region. Sonoporation, as described above, suffers from neither one of these limitations. The following section will describe the various strategies for sonoporation mediated gene delivery, where the DNA is administered locally, intra-arterially (at a specific organ or tissue), or systemically.
One of the strategies for cancer gene therapy is administering genes locally by intratumoral infusion, which is the most common used method in clinical trials when using viral vectors [66]. The advantages of local administration over systemic delivery include the circumvention of the transvascular barrier (important for large vectors such as viruses), and the generation of transient pressure gradients that can induce convection, as well as tissue deformation, which can increase the connectedness and size of pores in the interstitium. Local administration can also minimize toxicity to normal tissues [6], however, only if conditions of the infusions preclude dissemination of vectors into the leaky tumor microvasculature [66].
Sonoporation was used by Miller and Song [118], who co-injected the reporter gene luciferase with UCAs in murine renal carcinomas and then exposed the tumors to pulsed-HIFU. The authors demonstrated that this type of strategy could significantly increase gene transfection compared to injections without exposures or with exposures but without UCAs. However, this procedure also caused substantial destructive effects, which were evident by the reduced growth rates of the tumors compared to the two other groups. Tumor destruction was apparently due to inertial cavitation, since increasing the HIFU dose, both pulse duration and peak rarefactional pressure amplitude (the most important exposure parameter controlling cavitation activity), caused a decrease in both tumor growth and expression of the reporter gene. Reduced reporter gene expression, in this case, could be attributed to a decrease in the overall number of viable cells and not a lower rate in transfection efficiency. It is interesting that the same exposures without UCAs did not cause significant increases in gene expression compared to sham controls, as occurred with UCAs. These results are in contrast to those of a study in Dunning prostate tumors in rats, into which DNA was injected that encoded for another reporter enzyme, beta-galactosidase. In this study, where comparable exposure amplitudes were used, significant increases in expression were found when using pulsed-HIFU exposures without UCAs, compared to injections alone, apparently due to substantially longer exposures that were employed [109]. The ability to obtain sonoporation by direct injection into the tissues without UCAs (which typically lower the intensity threshold for cavitation to occur) is thought to be a result of the injections themselves providing cavitation nucleation sites through the local destructive effects that the injections created.
Another strategy for using HIFU exposures for enhancing gene transfection is to administer the DNA systemically, followed by exposures in a targeted tissue or organ. Such a procedure has been proposed for treating cardiovascular diseases, where trans-thoracic ultrasound exposures, for example, focused on the heart could assist in the delivery of genes to increase vascularization of the myocardium in order to ameliorate congestive heart failure [119]. Other targets could potentially be skeletal muscle, the blood vessels themselves, or even solid tumors. This type of strategy, however, would require safeguards, such as tissue specific promoters, so that expression of suicide genes would only occur in the tumors designated for treatment. Carriers, such as liposomes or non-viral vectors, would also be necessary to protect the DNA in the serum from clearance and degradation, and in that way increase concentration and circulation time.
Increased gene delivery can also be achieved where DNA is introduced into a large blood vessel of the targeted organ or tissue and then followed by an ultrasound exposure (e.g. focused, non-focused, or intra-luminal). Such a procedure has the advantage of restricting transfection, which, like for systemic administration, is crucial when using suicide genes. It also requires reduced amounts of DNA and UCAs (if being used), where these agents are also concentrated for increasing transfection efficiency. Combining DNA with UCAS to enhance local gene expression in an isolated blood vessel was demonstrated by Hashiya et al [120] using a catheter based ultrasound device in a rat carotid artery model. Similar enhancement with this procedure, compared to administering the DNA alone, was found in a study in the carotid artery of rabbits in which the addition of UCAs further increased expression, indicating that sonoporation was an active mechanism for transfection. The addition of UCAs was also found to decrease the intensity threshold for producing damage in the treated vessels, which occurred in the form of visible hemorrhage in the vessel walls [121]. Although potentially feasible for the treatment of cancer, results of a study using this type of strategy for tumors has yet to be reported in literature.
3.2.3 Drug carriers triggered by cavitation
Over the years, advances in the ability to chemically engineer a wide range of materials and drugs has created a new field of almost unlimited possibilities for using ultrasound to remotely deploy therapeutically relevant agents. Studies, for example, have demonstrated how biodegradable polymers implanted in the skin of rodents could be degraded by superficial ultrasound exposures for controlling the release of drugs incorporated in them [122]. The range of possible factors for controlling release rates include the type of polymeric matrix, the molecular weight of the incorporated drug, as well as the different ultrasound exposure parameters [123]. Recent preclinical studies have shown promising developments for systemically administered drug carriers, where extracorporeal ultrasound exposures are used to create reversible changes in the carriers and consequently release the chemotherapeutic agents that they incorporate. Examples of this type of carrier include polymeric micelles [124] and liposomes [125]. The obvious benefit of using this type of drug/device combination is increasing local drug delivery in a targeted region for improved efficacy, while concomitantly minimizing systemic exposure to the drug and the subsequent side effects associated with its use. The ultrasound mechanism for deploying drugs from the carriers has been shown to be acoustic cavitation; however, the exact manner by which the interactions between the bubbles and the carriers create these effects have yet to thoroughly be elucidated.
3.2.4 Sonodynamic therapy
The energy provided by ultrasound exposures can also be exploited to activate anti-tumor agents; a process known as sonodynamic therapy (SDT). The name SDT is derived from photodynamic therapy (PDT), which is a more established technique using light as an activation source [126]. In fact, photosensitizers (typically employed for PDT) were the first compounds used for evaluating the potential of SDT. In one of the earlier proof of concept studies, synergistic effects were found when combining exposures with the agents in regards to improving growth inhibition of targeted tumors [127]. The mechanisms of SDT for anti-tumor effects are complex and are mediated by acoustic cavitation. Similar to PDT, the agents used for SDT applications typically have low toxicity, and their activation by ultrasound is thought to generate radicals that are capable of initiating chain peroxidation of lipids in cellular membranes. Because light has limited penetration through tissue, PDT is normally restricted for treatment of relatively small tumors on or under the skin, or with endoscopes or fiber optic catheters for reaching the lining of some internal organs [126]. The major advantage of SDT over PDT is that focused ultrasound exposures can non-invasively treat regions deep in the body [128]. For a comprehensive review on SDT see Rosenthal et al. [128].
3.3 Acoustic Radiation forces
When applying ultrasound exposures using relatively high amplitudes, conditions of non-linear acoustics will typically prevail. Under such conditions a transfer of momentum may occur from the ultrasound wave to the medium, generating a unidirectional force, also known as a radiation force. Radiation forces are proportional to the absorption coefficient of the medium and the rate of energy being applied and inversely proportional to the speed of sound of the ultrasound wave in the medium. [43]. If radiation forces are large enough, they will be capable of causing local displacements of tissue in the region of the focal zone of the HIFU beam, where the degree of displacement will primarily be determined by the elastic (Young’s) modulus of the tissue [129]. In a fluid medium, radiation forces can produce motion in the form of a steady flow, also known as acoustic streaming [43]. The velocity of the stream is proportional to the attenuation coefficient of the medium, the ultrasound intensity, and the surface area of the transducer and inversely proportional to the speed of sound of the medium and the bulk viscosity [130]. Acoustic streaming has been observed to reduce heating from exposure to ultrasound through the process of increasing convective heat loss [131], as well as of increasing the mass transport of nanoparticles for improved transdermal delivery [95,132].
3.3.1 Interactions with the vasculature and drug carriers
A variety of biological phenomena has been observed to directly occur from the application of acoustic radiation forces. Single pulses, at short duration and low energy, were shown by Mihran et al. [133] to modify the excitability of myelinated sciatic nerves in frogs in vitro. Radiation forces were also found to reduce aortic pressure and induce premature ventricular contraction in the hearts of frogs [134] as well as to interrupt the flow in blood vessels in the eyes of rabbits [135]. In regards to applications for drug delivery, these forces demonstrated the ability to modulate the position and velocity of flow of UCAs in the vasculature, where the agents were redistributed for distances of even centimeters from the luminal space towards the walls of the vessels, even at velocities greater than 0.5 m/s [136]. The ability to do so has enabled a number of unique strategies to be tested such as facilitating receptor-ligand mediated adhesion of drug carrying nanoparticles [137] or deflecting the drug carrying agents towards a vessel’s wall prior to fragmenting them for improving local deposition of the agent [138]. In the latter case, energy release from the fragmented agent could also potentially create effects in the endothelium for enhanced local delivery [139]. The unique manner by which the ultrasound energy interacts with these UCAs is also being employed to design drug carriers that reversibly transform from stealth agents (that evade the immune system for increased circulation) to ones with increased adhesion to further improve binding [140]. For a comprehensive review on how these types of interactions can be used for potential drug and gene delivery applications, see Ferrara et al [139].
3.3.2 Creation of structural effects for enhanced permeability
So far this review has described the manner of operation of the better-known ultrasound mechanisms, heat and cavitation, in terms of how they can be used for enhancing drug and gene delivery. These types of energy deposition enable activation and deployment of drugs and genes, where cavitation (both inertial and non-inertial) can also be used to alter the permeability of cells and blood vessels for improving the delivery of various types of therapeutic agents. To date, however, ultrasound exposures have also been used for enhanced drug delivery where the experimental evidence indicates that the mechanisms are neither thermal nor cavitational. Frenkel et al [141], using non-focused exposures, showed that non-destructive widening of intercellular spaces between epithelial cells could occur at a fluid-tissue interface. Mechanistic investigations into this novel phenomenon indicated that these effects were due to transverse waves generated at the fluid/tissue interface. It is thought that the rapid dampening of these waves (which occurs in soft tissues) creates a steep gradient in shear forces, where the resulting strain works on the relative weak structural elements in the tissue, those of cell to cell junctions and cellular interfaces. Applying these exposures for mass transport purposes demonstrated their ability to increase the penetration of nanoparticles from the fluid medium into the adjacent epithelium, as well as to increase their rate of effective diffusion through the tissues [95].
In regards to pulsed-HIFU exposures, which employ spatial intensities that can be up to three orders of magnitude higher than non-focused exposures (i.e. intensities of 1000 – 2000 W cm2), preclinical investigations using a variety of tumor models have shown how these exposures can enhance the delivery, both locally and systemically, of various agents possessing different formulations (small molecules, DNA, and nanoparticles) for improved anti-tumor effects [21]. Temperature elevations during these low duty cycle (5 & 10%) exposures are found to be no greater than 4 to 5 degrees Celsius in tumors [58,59] and in muscle [142]. Hyperthermia using similar temperature elevations has demonstrated the ability to significantly enhance extravasation; however, this type of treatment typically lasts for 1 hr in order to be effective [49,55]. The pulsed-HIFU exposures (described above) typically occur for 2 min or less; however this same thermal dose provided via a non-ultrasound source was recently found to be ineffective for delivery enhancement typically found with pulsed-HIFU [142].
As described above, acoustic cavitation has been shown to be effective for enhancing extravasation, and indeed is considered the most important mechanism for doing so. In regards to the pulsed-HIFU exposures, the absence of UCAs did not preclude the existence of cavitation. Investigations carried in tumors [143] and muscle [142], monitoring for both harmonic and broadband emissions as an indicator of cavitation activity, showed that cavitation was indeed present; however, it occurred almost exclusively in expected locations such as in vascular-rich regions (i.e. the dermis and outer surface of tumors) and at tissue interfaces (e.g. the skin with the muscle or tumors). These studies showed that in deeper regions, in both tumors and muscle, delivery enhancement (enhanced extravasation and increased interstitial transport) occurred in the absence of acoustic cavitation. Indeed, it was the observed effects on interstitial transport, where there is a dearth of cavitation nuclei as well as a lack of physical space for bubble oscillation and expansion in the interstitium [89], that provided further support for the presence of a non-cavitational mechanism.
If delivery enhancement is occurring in the absence of both a thermal and cavitational mechanism, the question arises as to what is the underlying mechanism responsible for these effects. Frenkel et al [144] first proposed the possibility of displacements generated by acoustic radiation forces. The basis for this mechanism is that within the focal zone displacement in the tissue is non-uniform, and even greater non-uniformity will occur at boundary regions between the focal zone (where displacement is highest) and tissue outside and adjacent to it [129]. Non-uniform displacement between any two adjacent regions of tissue can induce shear forces, where (as described above) the resulting strain can create structural effects for enhancing tissue permeability. What makes this proposed mechanism more feasible is that the magnitude of these displacements typically occurs on the order of tens of microns as determined by simulations [144] and measurements using acoustic radiation force imaging [145].
These pulsed-HIFU exposures have shown a wide range of versatility in terms of the agents and tissue models to which they have been applied in preclinical studies. In murine studies, pre-treatment of the muscle with such exposures enabled delivery enhancement of nanoparticles by increasing extravasation [142,143,145] and interstitial transport [145]. In tumors, an increase in extravasation was also observed for systemically administered fluorescent dextrans [146], plasmid DNA [147], fluorescent nanoparticles [58], and monoclonal antibodies [148]. In therapeutic studies, increased tumor regression was found using direct intra-tumoral injections of TNFα [149] and an adenovirus for delivering TRAIL [61], where in both studies the increase in anti-tumor effects correlated with more widespread induction of necrosis and apoptosis, respectively. Histological analysis of tissues treated with pulsed-HIFU in the absence of contrast agents has shown that the exposures produce gaps between cells of normal tissues [98,150] and muscle fibers [142] in a non-destructive manner. Similar effects in tumors were observed, corresponding to increased penetration and distribution of locally injected nanoparticles [151].
The ability of pulsed-HIFU exposures to improve the efficacy of locally administered agents was demonstrated by Schratzberger et al [152], who found increased levels of gene expression in the muscle of rabbits, albeit these levels were lower than when the injections preceded the exposures where apparently sonoporation occurred. The versatility of these exposures in providing beneficial effects, in addition to enhancing extravasation, was also demonstrated in thrombolytic studies, where improved binding and penetration of tPA in whole blood clots was observed in vitro [153], which would explain increased rates of thrombolysis found in both in vitro [144] and in vivo [154] models. It has been suggested that the increase in intercellular gaps in tumors could not only enhance local interstitial transport, but that the ensuing increase in hydraulic conductivity measured in these tumors [Frenkel, unpublished] would also lead to a reduction in interstitial fluid pressure in the core of tumors, which could improve extravasation of large molecules [155]. Empirical evidence has shown that increases in both systemic and locally administered agents correlate with increased radiation force induced displacements produced by pulsed-HIFU exposures [144,145]. However, more in depth investigations will have to be carried out before this proposed mechanism is shown to be conclusive.
4. Safety considerations
The evidence provided up until now has indicated that there are multiple ways that ultrasound can be used to enhance the delivery and activity of drugs and genes for improving the efficacy of cancer therapy. In order for these strategies to be considered, however, it is necessary that the proposed treatment itself does not worsen the clinical outcome. The large amount of data collected from preclinical studies to date has shown that the thermal dose created by pulsed-HIFU exposures (discussed above) typically generates a temperature elevation of 4 to 5 °C during a 2 min exposure [58,59,142]; this thermal excursion is far below that required to produce any cytotoxic effects [156]. That the exposures were not destructive is backed up by histological analysis of a variety of treated tumor models, where differences, including the incidence of apoptotic cells, between control and treated tumors were not found [58,147,148,151,157]. In these same studies where tumor growth was monitored, those treated with HIFU only were found to grow at rates not significantly slower than untreated controls, providing further evidence of the non-destructive nature of these exposures. The same exposures used for thrombolysis studies similarly showed no destructive effects, both in vitro [144] and in vivo [154].
In addition to accurate guidance of HIFU exposures to targeted tissues for enhancing spatial delivery (as described until now), temporal enhancement is also a requirement in order for these exposures to be deemed safe. A recent study carried out in the flank muscle of mice indicated that longer lag times between exposures and systemic administration of fluorescently labeled nanoparticles (100 and 200 nm in diameter) correlated with fewer extravasated particles in the treated tissue. By 48 hrs post-treatment, the concentration of nanoparticles observed in the tissue was less than 5% of that occurring immediately post-treatment, indicating that indeed the induced effects for enhancing delivery were reversible [143]. In experiments carried out for opening the BBB, enhanced delivery of an MR contrast agent was found to be almost completely reversed (80 to 90%) three hours after treatment [158]. These results of both studies are not at all surprising, seeing that the process by which delivery enhancement is occurring does so without generating destructive biological effects.
Because the majority of deaths associated with cancer are due to metastatic disease and not the primary tumor [159], one of the most important questions that can be asked in regards to the safety of the HIFU exposures is whether or not they enhance the dissemination of neoplastic cells. For continuous exposures used for tumor ablation, the tissue is essentially being ‘fixed’ in situ so this question perhaps seems less pressing. Indeed, one preclinical study using prostate xenograft tumors showed that an increase did not occur in the number of metastases observed in the lungs of animals whose primary tumor was treated with ablative HIFU [160]. Similarly, in two clinical studies in which hepatocellular carcinomas and osteosarcomas were ablated with HIFU, a detectable increase in the number of circulating tumor cells post-treatment was not found for either tumor type [161].
In the case of pulsed-HIFU exposures used for enhancing tissue permeability, the need to determine if indeed the exposures are exacerbating the metastatic process would seem even more critical, since they are non-destructive and are purported to be creating effects such as widening intercellular spaces. Therefore, it is not unreasonable to suspect that by doing so an increase in the number of disseminated cells could occur. Whether or not this would lead to increased numbers of micro-metastases that consequently become clinically relevant metastases is a question that would also have to be addressed.
The metastatic process is a complex one starting with the separation of cells from the primary tumor, their invasion into the surrounding tissues and basement membranes, and eventually their arrival into the vasculature or lymphatics [159,162]. The more that is learned of the importance of host-tumor microenvironment interactions, the greater the preference for orthotopic tumors over ectopic models for studying metastasis [162]. Shih et al. [163] recently used a human melanoma cell line grown orthotopically (i.e. by intradermal inoculation) in immunocompromised mice to determine if pulsed-HIFU exposures, used for enhancing drug and gene delivery, were enhancing metastasis. Preliminary results have indicated that no adverse effects (i.e. an increase in metastases observed in the lungs) were occurring. A second study was also recently carried out using a similar cell line and exposures, where an increase in metastasis was found to occur [164]. The importance, however, of the latter study’s results is difficult to assess, since the tumors were not grown orthotopically, but instead ectopic tumors were grown subcutaneously in the mice.
5. Conclusions
Since the first experiments were done using ultrasound exposures in biological tissue, an increasing body of knowledge has been acquired improving the understanding of the many ways that ultrasound energy can interact with tissues, therapeutic agents and carriers for enhancing the treatment of cancer and other diseases. (Figure 2). Representative examples of these different types of treatments appear in Table 1. Together with advances in the technology of applying this energy and guiding and monitoring it, a variety of ultrasound-based therapeutic applications have been developed and continue to be proposed. In order to continue to develop such applications and improve those that presently exist, an in-depth and basic understanding of the ultrasound mechanisms discussed herein will be required. This will ultimately be achieved by continuing to collect both preclinical and clinical data, along with continuing to develop mathematical models and computer simulations, which together will enable more efficient optimization of the treatments in regards to the multiple exposure parameters that may be selected. Moreover, by having a comprehensive understanding of the way by which the acoustic and physical characteristics of the tissues are involved in these mechanisms, more intelligent translation of effective exposures from one tissue type to another will also be possible.
Figure 2.
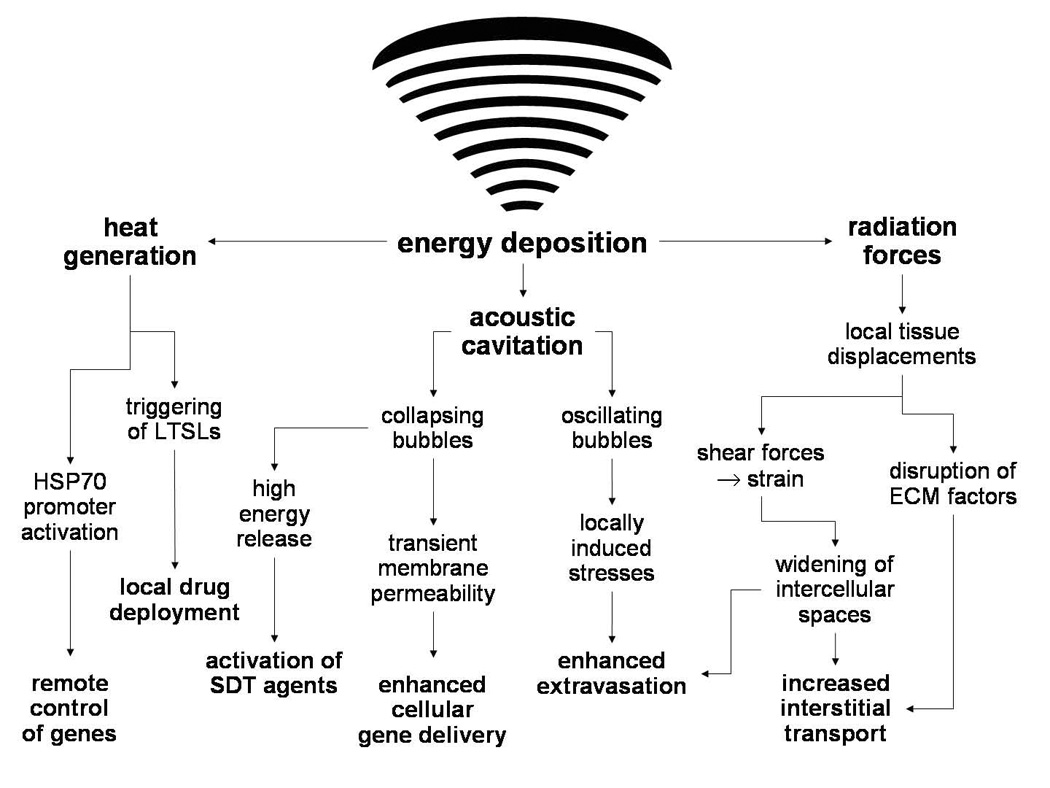
A schematic representation of the manner by which energy deposition from focused ultrasound exposures can act through various mechanisms to enhance the delivery or activity of drugs and genes. HSP – heat shock protein; LTSLs – low temperature sensitive liposomes; SDT – sonodynamic therapy; ECM – extracellular matrix.
Table 1.
Ultrasound mediated delivery/activation strategies. Cited references are those that appear in this review and which were chosen as representative of those strategies.
| Mechanism | Refs. | |
|---|---|---|
| Enhanced local deployment of drugs: | ||
| US exposures follow systemic administration of drugs loaded in LTSLs | HT | 57,59 |
| Increased transgene expression: | ||
| US exposures follow delivery of DNA possessing HSP70 promoters | HT | 73–76 |
| Enhanced drug & MAb delivery through the BBB: | ||
| Transcranial US exposures follow systemic administration of drugs and UCAs | ACNI | 99,100 |
| Enhanced delivery of DNA to intact cells: | ||
| US exposures follow local administration of DNA | ACI | |
| - with UCAs | 118 | |
| - without UCAs | 109 | |
| Enhanced delivery of DNA to intact cells: | ||
| US exposures follow systemic administration of DNA with UCAs | ACI | 119 |
| Enhanced delivery of DNA to intact cells: | ||
| US exposures follow intra-arterial administration of DNA | ACI | |
| - with UCAs | 120, 121 | |
| - without UCAs | 121 | |
| Enhanced local deployment of drugs: | ||
| US exposures follow polymeric micelles administration | ACI/NI? | 124 |
| US exposures follow liposome administration | 125 | |
| Enhanced activation of agents: | ||
| US exposures follow systemic administration of SDT agent | ACI | 127 |
| Enhanced binding of targeted agents: | ||
| US exposures following systemic administration of agents | ARF | 140 |
| Enhanced delivery of drugs, MAbs, DNA, nanoparticles: | 58,142, 143, 145–148 | |
| US exposures precede systemic administration of agents | ARF | |
| Enhanced delivery of viral vectors, DNA, nanoparticles: | 61, 145, 149, 151, 152 | |
| US exposures precede local administration of agents | ARF | |
HT – hyperthermia; AC – acoustic cavitation; ARF – acoustic radiation force; NI – non-inertial; I – inertial; LTSLs – low temperature sensitive liposomes; HSP – heat shock protein; BBB – blood brain barrier; UCAs – ultrasound contrast agents; MAbs – monoclonal antibodies.
Acknowledgements
The author would like to thank Ms Hilary Hancock for her editing contributions to this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.von Eschenbach AC. A vision for the National Cancer Institute Program in the United States. Nat Rev Cancer. 2004;4(10):820–828. doi: 10.1038/nrc1458. [DOI] [PubMed] [Google Scholar]
- 2.Jain RK. Delivery of molecular and cellular medicine to solid tumors. J Control Release. 1998;53:49–67. doi: 10.1016/s0168-3659(97)00237-x. [DOI] [PubMed] [Google Scholar]
- 3.Minchinton AI, Tannock IF. Drug penetration in solid tumors. Nat Rev Cancer. 2006;6(8):583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 4.North American Association of Central Cancer Registries Annotated Bibliography of Research and Publications. Multi-registry Cancer Incidence & Mortality Data in the United States & Canada. 2007 February; http://www.naaccr.org/filesystem/pdf/BiblioFeb 2007B.pdf.
- 5.Lake RA, Robinson BW. Immunotherapy and chemotherapy - a practical partnership. Nat Rev Cancer. 2005;5(5):397–405. doi: 10.1038/nrc1613. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Yuan F. Delivery of viral vectors to tumor cells: extracellular transport, systemic distribution, and strategies for improvement. Ann Biomed Eng. 2006;34(1):114–127. doi: 10.1007/s10439-005-9007-2. [DOI] [PubMed] [Google Scholar]
- 7.Galmarini CM, Galmarini FC. Multidrug resistance in cancer therapy: role of the tumor microenvironment. Curr Opin Investig Drugs. 2003;4(12):1416–1421. [PubMed] [Google Scholar]
- 8.McDonald DM, Choyke PL. Imaging of angiogenesis: from microscopic to clinic. Nat Med. 2003;9(6):713–725. doi: 10.1038/nm0603-713. [DOI] [PubMed] [Google Scholar]
- 9.Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 10.Boucher Y, Baxter LT, Jain RK. Interstitial pressure gradients in tissue-isolated and subcutaneous tumors: implications for therapy. Cancer Res. 1990;50(15):4478–4484. [PubMed] [Google Scholar]
- 11.Di Paolo A, Bocci G. Drug distribution in tumors: mechanisms, role in drug resistnace, and methods for modification. Curr Oncol Rep. 2007;9(2):109–114. doi: 10.1007/s11912-007-0006-3. [DOI] [PubMed] [Google Scholar]
- 12.Mow VC, Mak AF, Lai WM, Rosenberg LC, Tang LH. Viscoelastic properties of proteoglycan subunits and aggregates in varying solution concentrations. J Biomech. 1984;17(5):325–338. doi: 10.1016/0021-9290(84)90027-7. [DOI] [PubMed] [Google Scholar]
- 13.McGuire S, Zaharoff D, Yuan F. Nonlinear dependence of hydraulic conductivity on tissue deformation during intratumoral infusion. Ann Biomed Eng. 2006;34(7):1173–1181. doi: 10.1007/s10439-006-9136-2. [DOI] [PubMed] [Google Scholar]
- 14.Netti PA, Berk DA, Swartz MA, Grodzinsky AJ, Jain RK. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000;60:2497–2503. [PubMed] [Google Scholar]
- 15.McKee TD, Grandi P, Mok W, Alexandrakis G, Insin N, Zimmer JP, Bawendi MG, Boucher Y, Breakefield XO, Jain RK. Degradation of fibrillar collagen in a human melanoma xenograft improves the efficacy of an oncolytic herpes simplex virus vector. Cancer Res. 2006;66(5):2509–2513. doi: 10.1158/0008-5472.CAN-05-2242. [DOI] [PubMed] [Google Scholar]
- 16.Dreher MR, Liu W, Michelich CR, Dewhirst MW, Yuan F, Chilkoti A. Tumor vascular permeability, accumulation, and penetration of macromolecular drug carriers. J Natl Cancer Inst. 2006;98(5):335–344. doi: 10.1093/jnci/djj070. [DOI] [PubMed] [Google Scholar]
- 17.Krol A, Dewhirst MW, Yual F. Effects of cell damage and glycosaminoglycan degradation on available extravascular space of different dextrans in a rat fibrosarcoma. Intl J Hyperthermia. 2003;19(2):154–164. doi: 10.1080/02656730210166519. [DOI] [PubMed] [Google Scholar]
- 18.Shukla GS, Krag DN. Selective delivery of therapeutic agents for the diagnosis and treatment of cancer. 2006;6(1):39–54. doi: 10.1517/14712598.6.1.39. [DOI] [PubMed] [Google Scholar]
- 19.Ponce AM, Vujaskovic Z, Yuan F, Needham D, Dewhirst MW. Hyperthermia mediated liposomal drug delivery. Int J Hyperthermia. 2006;22(3):205–213. doi: 10.1080/02656730600582956. [DOI] [PubMed] [Google Scholar]
- 20.Clement GT. Perspectives in clinical uses of high-intensity focused ultrasound. Ultrasonics. 2004;42(10):1087–1093. doi: 10.1016/j.ultras.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Frenkel V, Li KC. Potential role of pulsed-high intensity focused ultrasound in gene therapy. Future Oncol. 2006;2(1):111–119. doi: 10.2217/14796694.2.1.111. [DOI] [PubMed] [Google Scholar]
- 22.Warden SJ, Fuchs RK, Kessler CK, Avin KG, Cardinal RE, Stewart RL. Ultrasound produced by a conventional therapeutic ultrasound unit accelerates fracture repair. Phys Ther. 2006;86(8):1118–1127. [PubMed] [Google Scholar]
- 23.Kennedy JE. High-intensity focused ultrasound in the treatment of solid tumors. Nat Rev Cancer. 2005;5:321–327. doi: 10.1038/nrc1591. [DOI] [PubMed] [Google Scholar]
- 24.Mitragotri S. Healing sound: the use of ultrasound in drug delivery and other therapeutic applications. Nat Rev Drug Discov. 2005;4(3):255–260. doi: 10.1038/nrd1662. [DOI] [PubMed] [Google Scholar]
- 25.Mitragotri S, Kost J. Low-frequency sonophoresis: a review. Adv Drug Deliv Rev. 2004;56(5):589–601. doi: 10.1016/j.addr.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 26.Hill CR, ter Haar GR. High intensity focused ultrasound - potential for cancer treatment. Br J Radiol. 1995;68(816):1296–1303. doi: 10.1259/0007-1285-68-816-1296. [DOI] [PubMed] [Google Scholar]
- 27.Koch MO, Gardner T, Cheng L, Fedewa RJ, Seip R, Sangvhi NT. Phase I/II trial of high intensity focused ultrasound for the treatment of previously untreated localized prostate cancer. J Urol. 2007;178(6):2366–2370. doi: 10.1016/j.juro.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 28.McDannold N, Hynynen K. Quality assurance and system stability of a clinical MRI-guided focused ultrasound system: four-year experience. Med Phys. 2006;33(11):4307–4313. doi: 10.1118/1.2352853. [DOI] [PubMed] [Google Scholar]
- 29.Wood BJ, Yanof J, Frenkel V, Viswanathan A, Dromi S, Kruecker J, Bauer C, Li KCP. CT and Ultrasound Guided Stereotactic High Intensity Focused Ultrasound (HIFU); Proc Int’l Sym Ther US; Boston. 2005. [Google Scholar]
- 30.Vo H, Patriciu A, Luk A, Frenkel V, Wood BJ. CT-guided high intensity focused ultrasound ablation with optical 3D tracking; Proc IEEE Ultrasonics; New York. 2007. [Google Scholar]
- 31.Byl NN. The use of ultrasound as an enhancer for transcutaneous drug delivery: phonophoresis. Phys Ther. 1995;75(6):539–553. doi: 10.1093/ptj/75.6.539. [DOI] [PubMed] [Google Scholar]
- 32.Mourad PD, Lazar DA, Curra FP, Mohr BC, Andrus KC, Avellino AM, McNutt LD, Crum LA, Kliot M. Ultrasound accelerates functional recovery after peripheral nerve damage. Neurosurgery. 2001;48(5):1136–1140. doi: 10.1097/00006123-200105000-00035. [DOI] [PubMed] [Google Scholar]
- 33.Thuroff S, Chaussy C, Vallancien G, Wieland W, Kiel HJ, Le Duc A, Desgrandchamps F, De La Rosette JJ, Gelet A. High-intensity focused ultrasound and localized prostate cancer: efficacy results from the European multicentric study. J Endourol. 2003;17(8):673–677. doi: 10.1089/089277903322518699. [DOI] [PubMed] [Google Scholar]
- 34.Stewart EA, Gedroyc WM, Tempany CM, Quade BJ, Inbar Y, Ehrenstein T, Shushan A, Hindley JT, Goldin RD, David M, Sklair M, Rabinovici J. Focused ultrasound treatment of uterine fibroid tumors: safety and feasibility of a noninvasive thermoablative technique. Am J Obstet Gynecol. 2003;189(1):48–54. doi: 10.1067/mob.2003.345. [DOI] [PubMed] [Google Scholar]
- 35.Wu F, Wang ZB, Chen WZ, Bai J, Zhu H, Qiao TY. Preliminary experience using high intensity focused ultrasound for the treatment of patients with advanced stage renal malignancy. J Urol. 2003;170(6 Pt 1):2237–2240. doi: 10.1097/01.ju.0000097123.34790.70. [DOI] [PubMed] [Google Scholar]
- 36.Kennedy JE, Wu F, ter Haar GR, Gleeson FV, Phillips RR, Middleton MR, Cranston D. High-intensity focused ultrasound for the treatment of liver tumors. Ultrasonics. 2004;42(1–9):931–935. doi: 10.1016/j.ultras.2004.01.089. [DOI] [PubMed] [Google Scholar]
- 37.Kratzik C, Schatzl G, Lackner J, Marberger M. Transcutaneous high-intensity focused ultrasonography can cure testicular cancer in solitary testis. Urology. 2006;67(6):1269–1273. doi: 10.1016/j.urology.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 38.Catane R, Beck A, Inbar Y, Rabin T, Shabsin N, Hengst S, Prerrer RM, Hanannel A, Dogadkin O, Liberman B, Kopelman D. MR-guided focused ultrasound surgery (MRgFUS) for the palliation of pain in patients with bone metastases-preliminary clinical experience. Ann Oncol. 2007;18(1):162–167. doi: 10.1093/annonc/mdl335. [DOI] [PubMed] [Google Scholar]
- 39.Zderic V, Vaezy S. Hemorrhage control using high intensity focused ultrasound. Int J Hyperthermia. 2007;23(2):1–9. doi: 10.1080/02656730601169779. [DOI] [PubMed] [Google Scholar]
- 40.Katz NP, Shapiro DE, Herrmann TE, Kost J, Custer LM. Rapid onset of cutaneous anesthesia with EMLA cream after pretreatment with a new ultrasound-emitting device. Anesth Analg. 2004;98(2):371–376. doi: 10.1213/01.ANE.0000099716.02783.C4. [DOI] [PubMed] [Google Scholar]
- 41.Pfaffenberger S, Devcic-Kuhar B, Kastl SP, Huber K, Maurer G, Wojta J, Gottsauner-Wolf M. Ultrasound thrombolysis. Tromb Haemost. 2005;94(1):26–36. doi: 10.1160/TH04-12-0818. [DOI] [PubMed] [Google Scholar]
- 42.Hynynen K. Focused ultrasound for blood-brain disruption and delivery of therapeutic molecules into the brain. Expert Opin Drug Deliv. 2007;4(1):27–35. doi: 10.1517/17425247.4.1.27. [DOI] [PubMed] [Google Scholar]
- 43.Nyborg WL. Mechanisms for bioeffects of ultrasound relevant to therapeutic applications. In: Wu J, Nyborg WL, editors. Emerging Therapeutic Ultrasound. New Jersey: World Scientific Publishing Co.; 2006. [Google Scholar]
- 44.Haken BA, Frizzell LA, Carstensen EL. Effect of mode conversion on ultrasonic heating at tissue interfaces. J Ultrasound Med. 1992;11(8):393–405. doi: 10.7863/jum.1992.11.8.393. [DOI] [PubMed] [Google Scholar]
- 45.Sokka SD, King R, Hynynen K. MRI-guided gas bubble enhanced ultrasound heating in in vivo rabbit thigh. Phys Med Biol. 2003;48(2):223–241. doi: 10.1088/0031-9155/48/2/306. [DOI] [PubMed] [Google Scholar]
- 46.Khokhlova VA, Bailey MR, Reed JA, Cunitz BW, Kaczkowski PJ, Crum LA. Effects of nonlinear propagation, cavitation, and boiling in lesion formation by high intensity focused ultrasound in a gel phantom. J Acoust Soc Amer. 2006;119(3):1834–1848. doi: 10.1121/1.2161440. [DOI] [PubMed] [Google Scholar]
- 47.Allen TM. Liposomes: opportunities in drug delivery. Drugs. 1997;(54):8–14. doi: 10.2165/00003495-199700544-00004. [DOI] [PubMed] [Google Scholar]
- 48.Allen C, Dos Santos N, Gallagher R, Chiu GN, Shu Y, Li WM, Johnstone SA, Janoff AS, Mayer LD, Webb MS, Bally MB. Controlling the physical behavior and biological performance of liposome formulations through use of surface grafted poly(ethylene glycol) Biosci Rep. 2002;22(2):225–550. doi: 10.1023/a:1020186505848. [DOI] [PubMed] [Google Scholar]
- 49.Kong G, Dewhirst MW. Hyperthermia and Liposomes. Intl J Hyperthermia. 1999;15:345–370. doi: 10.1080/026567399285558. [DOI] [PubMed] [Google Scholar]
- 50.Salomir R, Palussiere J, Fossheim SL, Rogstad A, Wiggen UN, Grenier N, Moonen CT. Local delivery of magnetic resonance (MR) contrast agent in kidney using thermosensitive liposomes and MR imaging-guided local hyperthermia: a feasibility study in vivo. J Magn Reson Imaging. 2005;22:534–540. doi: 10.1002/jmri.20416. [DOI] [PubMed] [Google Scholar]
- 51.Khoobehi GA, Peyman MR, Niesman M. Oncel, Hyperthermia and temperature-sensitive liposomes: selective delivery of drugs into the eye. Jpn J Ophthalmol. 1989;33:405–412. [PubMed] [Google Scholar]
- 52.Gaber MH, Wu NZ, Hong K, Huang SK, Dewhirst MW, Papahadjopoulos D. Thermosensitive liposomes: extravasation and release of contents in tumor microvascular networks. Int J Radiat Oncol Biol Phys. 1996;36(5):1177–1187. doi: 10.1016/s0360-3016(96)00389-6. [DOI] [PubMed] [Google Scholar]
- 53.Weinstein JN, Magin RL, Cysk RL, Zaharko DS. Treatment of solid L1210 murine tumors with local hyperthermia and temperature-sensitive liposomes containing methotrexate. Cancer Res. 1980;40:1388–1395. [PubMed] [Google Scholar]
- 54.Nishita T. Heat-sensitive liposomes containing cisplatin and localized hyperthermia in treatment of murine tumor. Osaka City Med J. 1998;44:73–83. [PubMed] [Google Scholar]
- 55.Kong G, Anyarambhatla G, Petros WP, Braun RD, Colvin OM, Needham D, Dewhirst MW. Efficacy of liposomes and hyperthermia in a human tumor xenograft model: importance of triggered drug release. Cancer Res. 2000;60(24):6950–6957. [PubMed] [Google Scholar]
- 56.McDannold N, Fossheim SL, Rasmussen H, Martin H, Vykhodtseva N, Hynynen K. Heat-activated liposomal MR contrast agent: initial in vivo results in rabbit liver and kidney. Radiology. 2004;230(3):743–752. doi: 10.1148/radiol.2303021713. [DOI] [PubMed] [Google Scholar]
- 57.Dromi S, Frenkel V, Luk A, Traughber B, Angstadt M, Bur M, Poff J, Xie J, Libutti SK, Li KC, Wood BJ. Pulsed-high intensity focused ultrasound and low temperature sensitive liposomes for enhanced targeted drug delivery and anti-tumor effect. Clin Cancer Res. 2007;13(9):2722–2727. doi: 10.1158/1078-0432.CCR-06-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frenkel V, Etherington A, Greene M, Quijano J, Xie J, Hunter F, Dromi S, Li KC. Delivery of liposomal Doxorubicin (Doxil®) in a breast cancer tumor model: investigation of potential enhancement by pulsed-high intensity focused ultrasound exposure. Acad Radiol. 2006;13(4):469–479. doi: 10.1016/j.acra.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 59.Patel P, Luk A, Durrani AK, Dromi S, Angstad M, Wood BJ, Frenkel V. In vitro and in vivo evaluations of increased spatial heat deposition using a split focus High Intensity Ultrasound (HIFU) Transducer. Intl J Hyperthermia. doi: 10.1080/02656730802064621. (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Greenberger S, Shaish A, Varda-Bloom N, Levanon K, Breitbart E, Goldberg I, Barshack I, Hodish I, Yaacov N, Bangio L, Goncharov T, Wallach D, Harats D. Transcription-controlled gene therapy against tumor angiogenesis. J Clin Inv. 2004;113(7):1017–1024. doi: 10.1172/JCI20007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patel P, Wood BJ, Frenkel V, Nguyen D. Enhancement of AdVgTRAIL Gene Therapy using Pulsed-High Intensity Focused Ultrasound (HIFU) in a Human Esophageal Carcinoma Model in Mice; Proc RSNA; Chicago. 2007. [Google Scholar]
- 62.Clackson T. Controlling mammalian gene expression with small molecules. Curr Opin Chem Biol. 1997;1(2):210–218. doi: 10.1016/s1367-5931(97)80012-9. [DOI] [PubMed] [Google Scholar]
- 63.Iida, Chen ST, Friedmann T, Yee JK. Inducible gene expression by retrovirus-mediated transfer of a modified tetracycline-regulated system. J Virol. 1996;70(9):6054–6059. doi: 10.1128/jvi.70.9.6054-6059.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Szulc J, Wiznerowics M, Sauvain MO, Trono D, Aebischer P. A versatile tool for conditional gene expression and knockdown. Nat Methods. 2006;3(2):109–116. doi: 10.1038/nmeth846. [DOI] [PubMed] [Google Scholar]
- 65.Rome C, Couillaud F, Moonen CT. Spatial and temporal control of expression of therapeutic genes using heat shock protein promoters. Methods. 2005;35(2):188–198. doi: 10.1016/j.ymeth.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 66.Wang Y, Yang Z, Liu S, Kon T, Krol A, Li CY, Yuan F. Characterisation of systemic dissemination of nonreplicating adenoviral vectors from tumours in local gene delivery. Br J Cancer. 2005;92(8):1414–1420. doi: 10.1038/sj.bjc.6602494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.DiDomenico BJ, Bugaisky GE, Lindquist S. Heat shock and recovery are mediated by different translational mechanisms. Proc Natl Acad Sci U S A. 1982;79(20):6181–6185. doi: 10.1073/pnas.79.20.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brade AM, Ngo D, Szmitko P, Li PX, Liu FF, Klamut HJ. Heat-directed gene targeting of adenoviral vectors to tumor cells. Cancer Gene Ther. 2000;7(12):1566–1574. doi: 10.1038/sj.cgt.7700267. [DOI] [PubMed] [Google Scholar]
- 69.Borrelli MJ, Schoenherr DM, Wong A, Bernock LJ, Corry PM. Heat-activated transgene expression from adenovirus vectors infected into human prostate cancer cells. Cancer Res. 2001;61(3):1113–1121. [PubMed] [Google Scholar]
- 70.Kramer G, Steiner GE, Grobl M, Hrachowitz K, Reithmayr F, Paucz L, Newman M, Madersbacher S, Gruber D, Susani M, Marberger M. Response to sublethal heat treatment of prostatic tumor cells and of prostatic tumor infiltrating T-cells. Prostate. 2004;58(2):109–120. doi: 10.1002/pros.10314. [DOI] [PubMed] [Google Scholar]
- 71.Madio DP, van Gelderen P, DesPres D, Olson AW, de Zwart JA, Fawcett TW, Holbrook NJ, Mandel M, Moonen CT. On the feasibility of MRI-guided focused ultrasound for local induction of gene expression. J Magn Reson Img. 1998;8(1):101–104. doi: 10.1002/jmri.1880080120. [DOI] [PubMed] [Google Scholar]
- 72.Liu Y, Kon T, Li C, Zhong P. High intensity focused ultrasound-induced gene activation in sublethally injured tumor cells in vitro. J Acoust Soc Am. 2005;118(5):3328–3336. doi: 10.1121/1.2041247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu Y, Kon T, Li C, Zhong P. High intensity focused ultrasound-induced gene activation in solid tumors. J Acoust Soc Am. 2006;120(1):492–501. doi: 10.1121/1.2205129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guilhon E, Voisin P, de Zwart JA, Quesson B, Salomir R, Maurange C, Bouchaud V, Smirnov P, de Verneuil H, Vekris A, Canioni P, Moonen CT. Spatial and temporal control of transgene expression in vivo using a heat-sensitive promoter and MRI-guided focused ultrasound. J Gene Med. 2003;5(4):333–342. doi: 10.1002/jgm.345. [DOI] [PubMed] [Google Scholar]
- 75.Plathow C, Lohr F, Divkovic G, Rademaker G, Farhan N, Peshke P, Zuna I, Debus J, Claussen CD, Kauczor HU, Li CY, Jenne J, Huber P. Focal gene induction in the liver of rats by a heat-inducible promoter using focused ultrasound hyperthermia: preliminary results. Invest Radiol. 2005;40(11):729–735. doi: 10.1097/01.rli.0000184763.62578.06. [DOI] [PubMed] [Google Scholar]
- 76.Silcox CE, Smith RC, King R, McDannold N, Bromley P, Walsh K, Hynynen K. MRI-guided ultrasonic heating allows spatial control of exogenous luciferase in canine prostate. Ultrasound Med Biol. 2005;31(7):965–970. doi: 10.1016/j.ultrasmedbio.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 77.Yang R, Reilly CR, Rescorla FJ, Faught PR, Sanghvi NT, Fry FJ, Franklin TD, Jr, Lumeng L, Grosfeld JL. High-intensity focused ultrasound in the treatment of experimental liver cancer. Arch Surg. 1991;126(8):1002–1009. doi: 10.1001/archsurg.1991.01410320088012. [DOI] [PubMed] [Google Scholar]
- 78.Yang R, Reilly CR, Rescorla FJ, Sanghvi NT, Fry FJ, Franklin TD, Jr, Grosfeld JL. Effects of high intensity focused ultrasound in the treatment of experimental neuroblastoma. J Pediatr Surg. 1992;27(2):246–250. doi: 10.1016/0022-3468(92)90321-w. [DOI] [PubMed] [Google Scholar]
- 79.Prat F, Chapelon JY, el Fadil FA, Theillere Y, Ponchon T, Cathignol D. In vivo effects of cavitation alone or in combination with chemotherapy in a peritoneal carcinomatosis in the rat. Br J Cancer. 1993;68(1):13–17. doi: 10.1038/bjc.1993.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Paparel P, Curiel L, Chesnais S, Ecochard R, Chapelon JY, Gelet A. Synergistic inhibitory effect of high-intensity focused ultrasound combined with chemotherapy on Dunning adenocarcinoma. Brit J Ultrasound. 2005;95(6):881–885. doi: 10.1111/j.1464-410X.2005.05420.x. [DOI] [PubMed] [Google Scholar]
- 81.de Bree E, Theodoropoulos PA, Rosing H, Michalakis J, Romanos J, Beijnen JH, Tsiftsis DD. Treatment of ovarian cancer using intraperitoneal chemotherapy with taxanes: from laboratory bench to bedside. Cancer Treat Rev. 2006;32(6):471–482. doi: 10.1016/j.ctrv.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 82.Deng CX, Sieling F, Pan H, Cui J. Ultrasound-induced cell membrane porosity. Ultrasound Med Biol. 2004;30(4):519–526. doi: 10.1016/j.ultrasmedbio.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 83.Barnett SB, ter Haar GR, Ziskin MC, Nyborg WL, Maeda K, Bang J. Current status of research on biophysical effects of ultrasound. Ultrasound Med Biol. 1994;20(3):205–208. doi: 10.1016/0301-5629(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 84.Suslick KS. Homogenous sonochemistry. In: Suslick KS, editor. Ultrasound: It’s Chemical, Physical and Biological Effects. New York: VCH Publishers Inc; 1988. [Google Scholar]
- 85.Lewin PA, Bjorno L. Acoustically induced shear stresses in the vicinity of microbubbles in tissue. J Acoust Soc Am. 1982;1(3):728–734. [Google Scholar]
- 86.Riesz P, Kondo T. Free radical formation induced by ultrasound and its biological implications. Free Radic Biol Med. 1992;13:247–270. doi: 10.1016/0891-5849(92)90021-8. [DOI] [PubMed] [Google Scholar]
- 87.Bommannan, Menon GK, Okuyama H, Elias PM, Guy RH. Sonophoresis: II. Examination of the mechanism(s) of ultrasound enhanced transdermal drug delivery. Pharm Res. 1992;9(8):1043–1047. doi: 10.1023/a:1015806528336. [DOI] [PubMed] [Google Scholar]
- 88.Crum LA. Tensile strength of water. Nature. 1979;(278):148–149. [Google Scholar]
- 89.Simonin JP. On the mechanisms of in vitro and in vivo phonophoresis. J Control Release. 1995;33:125–141. [Google Scholar]
- 90.Kimmel E. Cavitation bioeffects. Crit Rev Biomed Eng. 2006;34(2):105–161. doi: 10.1615/critrevbiomedeng.v34.i2.10. [DOI] [PubMed] [Google Scholar]
- 91.Zhang S, Duncan JH, Chamine GL. The final stage of collapse of a cavitation bubble near a rigid wall. J Fluid Mech. 1993;257:147–181. [Google Scholar]
- 92.Dear JP, Field JE, Walton AJ. Gas compression and jet formation in cavities collapsed by a shock wave. Nature. 1988;(377):505–508. [Google Scholar]
- 93.Kodama T, Takayama K. Dynamic behavior of bubbles during extracorporeal shock-wave lithotripsy. Ultrasound Med Biol. 1998;24(5):723–728. doi: 10.1016/s0301-5629(98)00022-2. [DOI] [PubMed] [Google Scholar]
- 94.Frenkel V, Kimmel E, Iger Y. Ultrasound-induced cavitation damage to external epithelia of fish skin. Ultrasound Med Biol. 1999;25(8):1295–1303. doi: 10.1016/s0301-5629(99)00069-1. [DOI] [PubMed] [Google Scholar]
- 95.Frenkel V, Kimmel E, Iger Y. Ultrasound-facilitated transport of silver chloride (AgCl) particles in fish skin. J Control Release. 2000;68:251–261. doi: 10.1016/s0168-3659(00)00264-9. [DOI] [PubMed] [Google Scholar]
- 96.Fry WJ. Intense ultrasound in investigations of the central nervous system. Adv Biol Med Phys. 1958;6:281–348. doi: 10.1016/b978-1-4832-3112-9.50012-8. [DOI] [PubMed] [Google Scholar]
- 97.Fry FJ, Ades HW, Fry WJ. Production of reversible changes in the central nervous system by ultrasound. Science. 1958;127(3289):83–84. doi: 10.1126/science.127.3289.83. [DOI] [PubMed] [Google Scholar]
- 98.Mesiwala AH, Farrell L, Wenzel HJ, Silbergeld DL, Crum LA, Winn HR, Mourad PD. High-intensity focused ultrasound selectively disrupts the blood brain barrier in vivo. Ultrasound Med Biol. 2002;28(3):389–400. doi: 10.1016/s0301-5629(01)00521-x. [DOI] [PubMed] [Google Scholar]
- 99.Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc Natl Acad Sci USA. 2006;103(31):11719–11723. doi: 10.1073/pnas.0604318103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Treat LH, McDannold N, Vykhodtseva N, Hynynen K. Transcranial MRI-guided focused ultrasound-induced blood-brain barrier opening in rats; Proc IEEE Ultrasonics; Montreal. 2004. [Google Scholar]
- 101.Fechheimer M, Denny C, Murphy RF, Taylor DL. Measurement of cytoplasmic pH in Dictyostelium discoideum by using a new method for introducing macromolecules into living cells. Eur J Cell Biol. 1986;40(2):242–247. [PubMed] [Google Scholar]
- 102.Fechheimer M, Boylan JF, Parker S, Sisken JE, Patel GL, Zimmer SG. Transfection of mammalian cells with plasmid DNA by scrape loading and sonication loading. Proc Natl Acad Sci U S A. 1987;84(23):8463–8467. doi: 10.1073/pnas.84.23.8463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ohl CD, Arora M, Ikink R. Sonoporation from jetting cavitation bubbles. Biophys J. 2006;91(11):4285–4295. doi: 10.1529/biophysj.105.075366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.van Wamel, Kooiman K, Harteveld M. Vibrating microbubbles poking individual cells: drug transfer into cells via sonoporation. J Control Release. 2006;112(2):149–155. doi: 10.1016/j.jconrel.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 105.Hynynen K. The threshold for thermally significant cavitation in dog's thigh muscle in vivo. Ultrasound Med Biol. 1991;17(2):157–169. doi: 10.1016/0301-5629(91)90123-e. [DOI] [PubMed] [Google Scholar]
- 106.Stride E, Saffari N. The potential for thermal damage posed by microbubble ultrasound contrast agents. Ultrasonics. 2004;42:907–913. doi: 10.1016/j.ultras.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 107.Miller DL. Ultrasound-mediated gene therapy. In: Wu J, Nyborg WL, editors. Emerging Therapeutic Ultrasound. New Jersey: World Scientific Publishing Co.; 2006. [Google Scholar]
- 108.Gottesman MM. Cancer gene therapy: an awkward adolescence. Cancer Gene Ther. 2003;10:501–508. doi: 10.1038/sj.cgt.7700602. [DOI] [PubMed] [Google Scholar]
- 109.Huber PE, Pfisterer P. In vitro and in vivo transfection of plasmid DNA in the Dunning prostate tumor R3327-AT1 is enhanced by focused ultrasound. Gene Ther. 2000;7(17):1516–1525. doi: 10.1038/sj.gt.3301242. [DOI] [PubMed] [Google Scholar]
- 110.Ng, Liu Y. Therapeutic ultrasound: its application in drug delivery. Med Res Rev. 2002;22(2):204–223. doi: 10.1002/med.10004. [DOI] [PubMed] [Google Scholar]
- 111.Pringle IA, McLachlan G, Collie DD, Sumner-Jones SG, Lawton AE, Tennant P, Baker A, Gordon C, Blundell R, Varathalingam A, Davies LA, Schmid RA, Cheng SH, Porteous DJ, Gill DR, Hyde SC. Electroporation enhances reporter gene expression following delivery of naked plasmid DNA to the lung. J Gene Med. 2007;9(5):369–380. doi: 10.1002/jgm.1026. [DOI] [PubMed] [Google Scholar]
- 112.Zeira E, Manevitch A, Khatchatouriants A, Pappo O, Hyam E, Darash-Yahana M, Tavor E, Honigman A, Lewis A, Galun E. Femtosecond infrared laser-an efficient and safe in vivo gene delivery system for prolonged expression. Mol Ther. 2003;8(2):342–350. doi: 10.1016/s1525-0016(03)00184-9. [DOI] [PubMed] [Google Scholar]
- 113.Zhang G, Gao X, Song YK, Vollmer R, Stolz DB, Gasiorowski JZ, Dean DA, Liu D. Hydroporation as the mechanism of hydrodynamic delivery. Gene Ther. 2004;11(8):675–682. doi: 10.1038/sj.gt.3302210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Raake P, von Degenfeld G, Hinkel R, Vachenauer R, Sandner T, Beller S, Andrees M, Kupatt C, Schuler G, Boekstegers P. Myocardial gene transfer by selective pressure-regulated retroinfusion of coronary veins: comparison with surgical and percutaneous intramyocardial gene delivery. J Am Coll Cardiol. 2004;44(5):1124–1129. doi: 10.1016/j.jacc.2004.05.074. [DOI] [PubMed] [Google Scholar]
- 115.Vigen KK, Hegge JO, Zhang G, Mukherjee R, Braun S, Grist TM, Wolff JA. Magnetic resonance imaging-monitored plasmid DNA delivery in primate limb muscle. Hum Gene Ther. 2007;18(3):257–268. doi: 10.1089/hum.2006.115. [DOI] [PubMed] [Google Scholar]
- 116.Chorny, Polyak B, Alferiev IS, Walsh K, Friedman G, Levy RJ. Magnetically driven plasmid DNA delivery with biodegradable polymeric nanoparticles. FASEB J. 2007;21(10):2510–2519. doi: 10.1096/fj.06-8070com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Haynes JR, McCabe DE, Swain WF. Particle-mediated nucleic acid immunization. J Biotechnol. 1996;44(1–3):37–42. doi: 10.1016/0168-1656(96)80298-7. [DOI] [PubMed] [Google Scholar]
- 118.Miller DL, Song J. Tumor growth reduction and DNA transfer by cavitation-enhanced high-intensity focused ultrasound in vivo. Ultrasound Med Biol. 2003;29(6):887–893. doi: 10.1016/s0301-5629(03)00031-0. [DOI] [PubMed] [Google Scholar]
- 119.Unger EC, Hersh E, Vannan M, McCreery T. Gene delivery using ultrasound contrast agents. Echocardiography. 2001;18(4):355–361. doi: 10.1046/j.1540-8175.2001.00355.x. [DOI] [PubMed] [Google Scholar]
- 120.Hashiya N, Aoki M, Tachibana K, Taniyama Y, Yamasaki K, Hiraoka K, Makino H, Yasufumi K, Ogihara T, Morishita R. Local delivery of E2F decoy oligodeoxynucleotides using ultrasound with microbubble agent (Optison) inhibits intimal hyperplasia after balloon injury in rat carotid artery model. Biochem Biophys Res Commun. 2004;317(2):508–514. doi: 10.1016/j.bbrc.2004.03.070. [DOI] [PubMed] [Google Scholar]
- 121.Huber PE, Mann MJ, Melo LG, Ehsan A, Kong D, Zhang L, Resvani M, Peschke P, Jolesz F, Dzau VJ, Hynynen K. Focused ultrasound (HIFU) induces localized enhancement of reporter gene expression in rabbit carotid. Gene Ther. 2003;10:1600–1607. doi: 10.1038/sj.gt.3302045. [DOI] [PubMed] [Google Scholar]
- 122.Kost J, Leong K, Langer R. Ultrasound-enhanced polymer degradation and release of incorporated substances. Proc Natl Acad Sci. 1989;86(20):7663–7666. doi: 10.1073/pnas.86.20.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lavon, Kost J. Mass transport enhancement by ultrasound in non-degradable polymeric controlled release systems. J Control Release. 1998;54(1):1–7. doi: 10.1016/s0168-3659(97)00112-0. [DOI] [PubMed] [Google Scholar]
- 124.Husseini GA, Diaz de la Rosa MA, Gabuji T, Zeng Y, Christensen DA, Pitt WG. Release of doxorubicin from unstabilized and stabilized micelles under the action of ultrasound. J Nanosci Nanotechnol. 2007;7(3):1028–1033. doi: 10.1166/jnn.2007.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schroeder, Avnir Y, Weisman S, Najajreh Y, Gabizon A, Talmon Y, Kost J, Barenholz Y. Controlling liposomal drug release with low frequency ultrasound: mechanism and feasibility. Langmuir. 2007;23(7):4019–4025. doi: 10.1021/la0631668. [DOI] [PubMed] [Google Scholar]
- 126.Palumbo G. Photodynamic therapy and cancer: a brief sightseeing tour. Expert Opin Drug Deliv. 2007;4(2):131–418. doi: 10.1517/17425247.4.2.131. [DOI] [PubMed] [Google Scholar]
- 127.Yumita N, Sasaki K, Umemura S, Nishigaki R. Sonodynamically induced antitumor effect of gallium-porphyrin complex, ATX-70. Jpn J Cancer Res. 1996;87(3):310–306. doi: 10.1111/j.1349-7006.1996.tb00222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rosenthal, Sostaric JZ, Riesz P. Sonodynamic therapy - a review of the synergistic effects of drugs and ultrasound. Ultrason Sonochem. 2004;11(6):349–363. doi: 10.1016/j.ultsonch.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 129.Lizzi FL, Muratore R, Deng CX, Ketterling JA, Alam SK, Mikaelian S, Kalisz A. Radiation-force technique to monitor lesions during ultrasonic therapy. Ultrasound Med Biol. 2003;29(11):1593–1605. doi: 10.1016/s0301-5629(03)01052-4. [DOI] [PubMed] [Google Scholar]
- 130.Dymling SO, Persson HW, Hertz TG, Lindstrom K. A new ultrasonic method for fluid property measurements. Ultrasound Med Biol. 1991;17(5):497–500. doi: 10.1016/0301-5629(91)90186-z. [DOI] [PubMed] [Google Scholar]
- 131.Wu J, Winkler AJ, O'Neill TP. Effect of acoustic streaming on ultrasonic heating. Ultrasound Med Biol. 1994;20(2):195–201. doi: 10.1016/0301-5629(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 132.Frenkel V, Gurka R, Liberzon A, Shavit U, Kimmel E. Preliminary investigations of ultrasound induced acoustic streaming using particle image velocimetry. Ultrasonics. 2001;39(3):153–156. doi: 10.1016/s0041-624x(00)00064-0. [DOI] [PubMed] [Google Scholar]
- 133.Mihran RT, Barnes FS, Wachtel H. Temporally-specific modification of myelinated axon excitability in vitro following a single ultrasound pulse. Ultrasound Med Biol. 1990;16(3):297–309. doi: 10.1016/0301-5629(90)90008-z. [DOI] [PubMed] [Google Scholar]
- 134.Dalecki D, Raeman CH, Child SZ, Carstensen EL. Effects of pulsed ultrasound on the frog heart: III. The radiation force mechanism. Ultrasound Med Biol. 1997;23(2):275–285. doi: 10.1016/s0301-5629(96)00209-8. [DOI] [PubMed] [Google Scholar]
- 135.Lizzi FL, Coleman DJ, Driller J, Franzen LA, Leopold M. Effects of pulsed ultrasound on ocular tissue. Ultrasound Med Biol. 1981;7(3):245–252. doi: 10.1016/0301-5629(81)90035-1. [DOI] [PubMed] [Google Scholar]
- 136.Dayton PA, Allen JS, Ferrara KW. The magnitude of radiation force on ultrasound contrast agents. J Acoust Soc Am. 2002;112(5 Pt 1):2183–2192. doi: 10.1121/1.1509428. [DOI] [PubMed] [Google Scholar]
- 137.Tartis MS, McCallan J, Lum AF, Labell R, Stieger SM, Matsunaga TO, Ferrara KW. Therapeutic effects of paclitaxel-containing ultrasound contrast agents. Ultrasound Med Biol. 2006;32(11):1771–1880. doi: 10.1016/j.ultrasmedbio.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 138.Shortencarier MJ, Dayton PA, Bloch SH, Schumann PA, Matsunaga TO, Ferrara KW. A method for radiation-force localized drug delivery using gas filled lipospheres. IEEE Trans Ultrason Ferrelectr Freq Control. 2004;51(7):822–831. doi: 10.1109/tuffc.2004.1320741. [DOI] [PubMed] [Google Scholar]
- 139.Ferrara K, Pollard R, Borden M. Ultrasound microbubble contrast agents: fundamentals and application to gene and drug delivery. Annu Rev Biomed Eng. 2007;9:415–447. doi: 10.1146/annurev.bioeng.8.061505.095852. [DOI] [PubMed] [Google Scholar]
- 140.Borden MA, Sarantos MR, Stieger SM, Simon SI, Ferrara KW, Dayton PA. Ultrasound radiation force modulates ligand availability on targeted contrast agents. Mol Imaging. 2006;5(3):139–147. [PMC free article] [PubMed] [Google Scholar]
- 141.Frenkel V, Kimmel E, Iger Y. Ultrasound-induced intercellular space widening in fish epidermis. Ultrasound Med Biol. 2000;26(3):473–480. doi: 10.1016/s0301-5629(99)00164-7. [DOI] [PubMed] [Google Scholar]
- 142.O’Neill BE, Vo H, Angstadt M, Quinn TP, Wood BJ, Frenkel V. Investigations into the potential contribution of a thermal mechanism for pulsed-high intensity focused ultrasound mediated delivery; Proc IEEE Ultrasonics; New York. 2007. [Google Scholar]
- 143.O’Neill BE, Frenkel V, Li KCP, Quinn TP. Pulsed HIFU for enhanced drug delivery: a study of possible mechanisms; Proc Int’l Sym Ther US; Oxford. 2006. [Google Scholar]
- 144.Frenkel V, Oberoi J, Park M, Deng C, Stone MJ, Neeman Z, Wood BJ, Horne MK, 3rd, Li KCP. Pulsed-high intensity ultrasound (HIFU) enhances thrombolysis in an in vitro model. Radiology. 2006;239(1):86–93. doi: 10.1148/radiol.2391042181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Frenkel V, Deng C, O'Neill BE, Quijano J, Stone MJ, Dromi S, Hunter F, Xie J, Quinn TP, Wood BJ, Li KCP. Pulsed-high intensity focused ultrasound (HIFU) exposures for enhanced delivery of therapeutics: mechanisms and applications; Proc Int’l Sym Ther US; Boston. 2005. [Google Scholar]
- 146.Yuh EL, Shulman SG, Mehta SA, Xie J, Chen L, Frenkel V, Bednarski MD, Li KC. Delivery of systemic chemotherapeutic agent to tumors by using focused ultrasound: study in a murine model. Radiology. 2005;234(2):431–437. doi: 10.1148/radiol.2342030889. [DOI] [PubMed] [Google Scholar]
- 147.Dittmar KM, Xie J, Hunter F, Trimble C, Bur M, Frenkel V, Li KC. Pulsed high-intensity focused ultrasound enhances systemic administration of naked DNA in squamous cell carcinoma model: initial experience. Radiology. 2005;235(2):541–546. doi: 10.1148/radiol.2352040254. [DOI] [PubMed] [Google Scholar]
- 148.Khaibullina, Jang B, Sun H, Le N, Yu S, Frenkel V, Carrasquillo JA, Pastan I, Li KC, Paik CH. Pulsed High Intensity Focused Ultrasound Enhances Uptake of Radiolabeled Monoclonal Antibody to Human Epidermoid Tumor in Nude Mice. J Nuc Med. doi: 10.2967/jnumed.107.046888. (In press) [DOI] [PubMed] [Google Scholar]
- 149.Quijano J, Colunga A, Xie J, Frenkel V, Li KCP. Enhanced Regression in a Squamous Cell Carcinoma Murine Tumor Model Using Pulsed-High Intensity Focused Ultrasound (HIFU) and Naked TNF-a Plasmid; Proc Soc Mol Img; Cologne. 2005. [Google Scholar]
- 150.Seidl, Steinbach P, Wörle K, Hofstädter F. Induction of stress fibres and intercellular gaps in human vascular endothelium by shock-waves. Ultrasonics. 1994;32(5):397–400. doi: 10.1016/0041-624x(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 151.Traughber B, Frenkel V, Xie J, Poff JA, Quijano J, Khaibullina A, Colunga A, Li KCP. Pulsed-high intensity focused ultrasound (HIFU) exposures combined with intratumoral injection of TNF-a plasmid enhance growth inhibition in a murine squamous cell carcinoma model; Proc Int’l Sym Ther US; Oxford. 2006. [Google Scholar]
- 152.Schratzberger P, Krainin JG, Schratzberger G, Silver M, Ma H, Kearney M, Zuk RF, Brisken AF, Losordo DW, Isner JM. Transcutaneous ultrasound augments naked DNA transfection of skeletal muscle. Mol Ther. 2002;6(5):576–583. [PubMed] [Google Scholar]
- 153.Stone MJ, Frenkel V, Oberoi J, Wood BJ, Horne MK, III, Li KCP. Pulsed-High Intensity Focused Ultrasound (HIFU)-enhanced Thrombolysis in Vitro: Proof of Concept and Investigation of Mechanism; Proc RSNA; Chicago. 2005. [Google Scholar]
- 154.Stone MJ, Frenkel V, Dromi S, Lewis RP, Li KCP, Horne MK, III, Wood BJ. Pulsed-high intensity focused ultrasound enhanced t-PA mediated thrombolysis in a novel in vivo clot model, a pilot study. Thrombosis Res. doi: 10.1016/j.thromres.2007.03.023. (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Boucher Y, Brekken C, Netti PA, Baxter LT, Jain RK. Intratumoral infusion of fluid: estimation of hydraulic conductivity and implications for the delivery of therapeutic agents. Br J Cancer. 1998;78(11):1442–1448. doi: 10.1038/bjc.1998.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dewey WC. Arrhenius relationships from the molecule and cell to the clinic. Intl J Hyperthermia. 1994;10(4):457–483. doi: 10.3109/02656739409009351. [DOI] [PubMed] [Google Scholar]
- 157.Poff, Traughber BJ, Allen C, Colunga A, Xie J, Chen Z, Frenkel V, Wood BJ, van Waes C, Li KCP. Pulsed-high intensity focused ultrasound enhances tumor growth inhibition by bortezomib in a murine squamous cell carcinoma model; Proc Int’l Sym Ther US; Oxford. 2006. [Google Scholar]
- 158.Hynynen K, McDannold N, Sheikov NA, Jolesz FA, Vykhodtseva N. Local and reversible blood-brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. Neuroimage. 2005;24(1):12–20. doi: 10.1016/j.neuroimage.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 159.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411(6835):375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 160.Oosterhof GO, Cornel EB, Smits GA, Debruyne FM, Schalken JA. Influence of high-intensity focused ultrasound on the development of metastases. Eur Urol. 1997;32(1):91–95. [PubMed] [Google Scholar]
- 161.Wu F, Wang ZB, Jin CB, Zhang JP, Chen WZ, Bai J, Zou JZ, Zhu H. Circulating tumor cells in patients with solid malignancy treated by high-intensity focused ultrasound. Ultrasound Med Biol. 2004;30(4):511–517. doi: 10.1016/j.ultrasmedbio.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 162.Kanna C, Hunter K. Modeling metastasis in vivo. Carcinogenesis. 2005;26(3):513–523. doi: 10.1093/carcin/bgh261. [DOI] [PubMed] [Google Scholar]
- 163.Shih J, Pollock C, Angstadt M, Wood BJ, Frenkel V. The Effects of Pulsed High-Intensity Focused Ultrasound Exposures on Metastases; Proc World Conf Interv Oncol; Washington, DC. 2007. [Google Scholar]
- 164.Miller DL, Dou C. The potential for enhancement of mouse melanoma metastasis by diagnostic and high-amplitude ultrasound. Ultrasound Med Biol. 2006;32(7):1097–1101. doi: 10.1016/j.ultrasmedbio.2006.03.013. [DOI] [PubMed] [Google Scholar]


