Summary
During nervous system development, spinal commissural axons project towards and across the ventral midline. They are guided in part by netrin-1, made by midline cells, which attracts the axons by activating the netrin receptor DCC, but previous studies suggest that additional receptor components are required. Here we report that the Down Syndrome Cell Adhesion Molecule (DSCAM), a candidate gene implicated in the mental retardation phenotype of Down Syndrome, is expressed on spinal commissural axons, binds netrin-1, and is necessary for commissural axons to grow towards and across the midline. DSCAM and DCC can each mediate a turning response of these neurons to netrin-1. Similarly, Xenopus spinal neurons exogenously expressing DSCAM can be attracted by netrin-1 independently of DCC. These results show that DSCAM is a receptor that can mediate turning responses to netrin-1, and support a key role for netrin/DSCAM signaling in commissural axon guidance in vertebrates.
Keywords: Axon guidance, Midline, Attraction, netrin-1, DSCAM, DCC
Introduction
In the developing nervous system, axons grow long distances over complex terrain by navigating a series of closely spaced intermediate targets, responding to the combined actions of attractive and repulsive guidance cues (reviewed in Tessier-Lavigne and Goodman, 1996; Dickson, 2002). The ventral midline of the central nervous system acts as an important intermediate target for commissural axons in the developing spinal cord. These neurons are born in the dorsal part of the spinal cord, then send axons towards and subsequently across the ventral midline, a region populated by so-called floor plate cells (reviewed in Colamarino and Tessier-Lavigne, 1995). The axons project towards the midline in part because they are attracted by the floor plate-derived chemoattractant netrin-1 (Kennedy et al., 1994; Placzek et al., 1990; Serafini et al., 1994; Tessier-Lavigne et al., 1988). In mice lacking netrin-1, many commissural axon trajectories are foreshortened, fail to invade the ventral spinal cord and are misguided (Serafini et al., 1996). However, some do reach and cross the midline, indicating that other factors cooperate with netrin-1 to guide these axons. Indeed, the evidence indicates that guidance to the midline is also directed in part by the morphogen Sonic Hedgehog (Shh), also made by floor plate cells, which collaborates with netrin-1 in midline attraction (Charron et al., 2003).
Available evidence has indicated that Deleted in Colorectal Carcinomas (DCC) is a key mediator of netrin-1’s functions in commissural axon guidance. DCC is expressed by commissural axons and binds netrin-1, and in DCC knock-out embryos, commissural axons show growth and guidance defects similar to those in netrin-1 deficient mice (Fazeli et al., 1997; Serafini et al., 1996). In vitro evidence also supports a key role. First, netrin-1 was purified on the basis of its ability to stimulate commissural axon outgrowth from explants of rat dorsal spinal cord in collagen gels (Serafini et al., 1994), and the outgrowth-promoting action of netrin-1 on embryonic spinal commissural axons is blocked by antibodies to DCC (Keino-Masu et al., 1996). Thus, DCC is required for netrin-1’s outgrowth-promoting effects. However, netrin-1 can also elicit a turning response in commissural axons when presented from a point source (Kennedy et al., 1994). Interestingly, the same antibody that blocks netrin-1 mediated outgrowth of axons failed to block turning within explants towards a netrin-1 source (Keino-Masu et al., 1996). These results raised the question whether DCC is required for the turning response.
Later experiments examined the role of DCC in mediating netrin responses using Xenopus spinal and retinal axons in dissociated cell culture. In those cultures, netrin-1 causes an increase in the rate of extension of these axons, and, when presented from a point source, can also elicit turning of the axons towards the source. Both activities are blocked by the same antibody to DCC used in the experiments described above (de la Torre, et al., 1997; Ming, et al., 1997; Stein et al 2001), indicating that DCC is necessary for both these effects.
Thus, experiments with Xenopus neurons showed that DCC is both necessary and sufficient for mediating the outgrowth and turning effects of netrin-1 in those neurons in vitro. They did not directly address whether DCC is also required for turning responses of rodent commissural axons towards a netrin source within tissue. Here we have revisited this issue, by asking whether commissural axons express other netrin receptors that could function either alone or together with DCC in mediating these responses.
Down syndrome cell adhesion molecule (DSCAM) is a type I TM orphan receptor comprising ten Immunoglobin (Ig) and six fibronectin type III (FNIII) repeats in its extracellular domain, and an intracellular domain with no identifiable motifs (Yamakawa et al., 1998). The mammalian genome encodes two DSCAM genes, DSCAM and its paralog, DSCAM-like 1 (DSCAM-L1). DSCAM maps to a region on Chromosome 21 (21q22.2- 22.3) that has been shown to contribute to cognitive deficits in individuals with Down Syndrome (DS) (Yamakawa et al., 1998). First insights into the functional contribution of DSCAMs to vertebrate development has come from the characterization of a mouse that carries a spontaneous mutation in the DSCAM gene displaying defects in neurite arborization and mosaic spacing (Fuerst et al., 2008). In addition, Yamagata and Sanes (2008) reported that DSCAMs contribute to laminar targeting in the chick retina.
Drosophila Dscam has been shown to function as an important regulator of development in the fly nervous system, and possesses extraordinary molecular diversity (reviewed in Zipursky et al., 2006). Drosophila Dscam can potentially generate 38,016 different mRNA isoforms through alternative splicing (Schmucker et al., 2000). This remarkable number of unique Dscam isoforms has been shown to contribute to the formation of complex patterns of neuronal connections including axon guidance, targeting, tiling, axon branch formation, and specification and dendrite pattering (Chen et al., 2006; Hughes et al 2007; Millard et al 2007; Schmucker et al., 2000; Zhan et al., 2004; Zhu et al., 2006). Further studies have shown that each isoform binds to itself but does not bind (or only binds poorly) to other isoforms, and that these preferential homophilic binding relations contribute to the formation of complex patterns of neuronal connections (Hattori et al., 2007; Matthews et al., 2007; Meijers et al., 2007; Soba et al., 2007; Wojtowicz et al., 2007). The two mammalian DSCAMs show little if any patterns of alternative splicing (Schmucker et al., 2000), as only two splice forms were identified for DSCAM-L1, both resulting in a deletion in the second Ig domain (Barlow et al., 2002). Although it has been shown that mammalian DSCAMs can mediate homophilic cell adhesion (Agarwala et al., 2001), the complexity of homophilic binding seen in Drosophila clearly cannot be attained in mammals. In addition, the Drosophila genome has four Dscam genes, and only one of the four genes displays this extraordinary molecular diversity. Thus, it is conceivable that DSCAMs may be activated by heterophilic ligands as well.
In our search of additional netrin-binding proteins, we found that DSCAM is expressed by commissural axons and can bind netrin-1. We provide evidence that it can mediate turning responses to netrin-1, both alone and in collaboration with DCC, and propose that it accounts for the missing turning receptor in mammalian commissural axons whose existence was foreshadowed by previous studies. These studies both identify DSCAM as a netrin receptor, and illustrate how members of a family with important homophilic actions can also function as heterophilic receptors as well.
Results
DSCAM is expressed by commissural axons in the developing mammalian spinal cord
To explore whether additional guidance receptors exist that may mediate turning of rodent commissural axons to netrin-1, we initiated a screen and searched for receptors expressed on rat commissural axons during E11 to 13, a time when commissural axons extend and cross the midline.
One approach we took was to search for Ig superfamily members with homology to DCC. To this end, oligonucleotides recognizing the third Ig and second FNIII domains of DCC, a highly conserved region among DCC family members, were used to perform rt-PCR using mRNA derived from rat E13 dorsal spinal cord as a template. PCR products were identified by sequence, and promising candidates were analyzed for their mRNA expression pattern in E13 rat spinal cord. One of the candidate receptors isolated from this screen was DSCAM (see Methods). To explore whether DSCAM contributes to commissural axon growth and guidance, we first characterized its spatial and temporal expression in the developing rat spinal cord. A polyclonal antiserum was generated against the last 100 amino acids of its intracellular domain. Western blot analysis showed that the antiserum specifically recognized DSCAM. A similar size protein was detected by the DSCAM antiserum in E11 and E12 spinal cord extracts (Figure S1). The antiserum was used to stain transverse sections of E12-15 rat spinal cord. DSCAM protein appears to be dynamically regulated during the period of commissural axon guidance to and around the midline. At E12, DSCAM is highly expressed on precrossing commissural axons, as well as on growth cones of pioneer neurons that already have crossed the midline (Figure 1B). During this time of development, many precrossing axons are positive for both DSCAM and TAG-1 (Figure 1D). At E13 DSCAM protein can still be detected on the precrossing portions of commissural axons, but to a lower extent (Figure 1F), while expression increases on axons coursing in the ventral (Figure 1F) and dorsal funiculus (Figure 1F). By E14 (Figure 1J) and E15 (Figure 1M) DSCAM is abundantly expressed in postcrossing axons as well as at low levels in the dorsal root entry zone (drez). In the drez, DSCAM protein appears to be located more ventrally (Figure 1L and 1P). The sites and timing of expression of DSCAM are consistent with a possible role in axon guidance during spinal cord development.
Figure 1. DSCAM protein is expressed by commissural axons in the developing rat spinal cord.
At E12 many commissural axons approach the floor plate and a few pioneer axons have begun to cross (A). DSCAM is expressed on precrossing commissural axons (asterisks in B) as well as on growth cones and axons of pioneer neurons (arrow in B). DSCAM expression is maintained in commissural neurons at E13 (F), a time when many axons cross to the contralateral site of the spinal cord and begin to grow longitudinally (E). DSCAM is expressed in precrossing axons (asterisks in F) and on axons coursing in the ventral (arrow in F) and dorsal funiculi (arrowhead in F). By E14 (I) and E15 (M) DSCAM is expressed in postcrossing axons (arrows in J and N) and at low levels in the drez (asterisks in J). In contrast, TAG-1 protein is primarily localized to the precrossing region of commissural axons (C, G) and, although it persists in the ventral funiculus (asterisks in G) it appears to be rapidly downregulated in postcrossing commissural axons (K and O), but maintained in sensory neurons of the dorsal root ganglia and in the drez. At E12 and E13 the majority of precrossing (asterisks in D and H) and crossing commissural axons (arrowhead in D and H) are positive for DSCAM and TAG-1. In the drez, DSCAM expression is found more ventrally (asterisks in J, L, N and P), whereas TAG-1 is more dorsally expressed (arrow in K, L, O and P).
DSCAM is required for commissural axon guidance in vivo
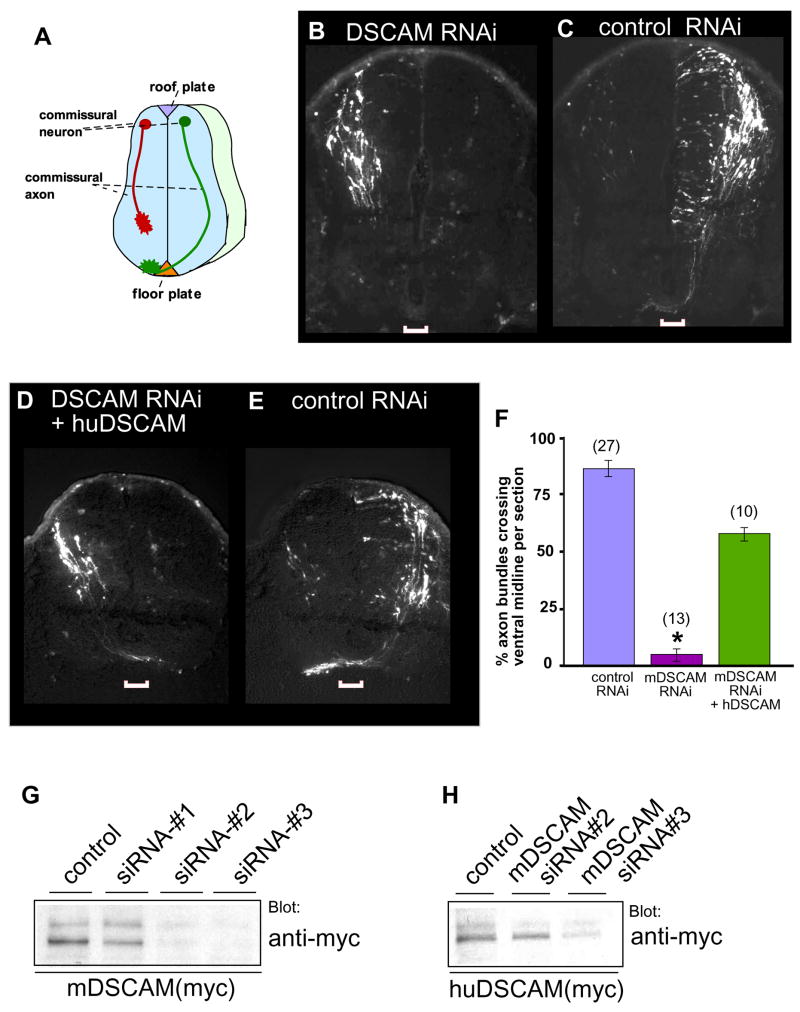
To investigate a potential role for DSCAM in commissural axon pathfinding in vivo we used a whole embryo culture (WEC) system (Osumi et al., 2001), which makes it possible to culture mouse embryos for 2 or 3 days and maintain normal spinal cord development (Chen et al., 2008). As can be seen from Figure 2C, commissural axons transfected with a control siRNA pool projected ventrally near the edge of the spinal cord, successfully reached the ventral midline, and crossed the floor plate. In contrast, commissural axons electroporated with siRNAs specific to mouse DSCAM (Figure 2B, 2F, 2G, S2 and S3) displayed severe defects in axonal trajectories: they appeared foreshortened, reaching the level of the developing motor column but failing to project effectively to the ventral midline or to cross the midline. We next tested if the mDSCAM siRNA knockdown phenotype was specific to DSCAM loss-of-function. Taking advantage of the specificity of the siRNA#2 to murine DSCAM (Figure 2H) we introduced the mDSCAM siRNA together with a cDNA encoding human DSCAM (Yamakawa et al., 1998). As shown in Figure 2D commissural axons expressing human DSCAM reached and crossed the midline, similar to those in wild type or control siRNA treated spinal cords (Figure 2C, 2E). Thus the commissural guidance defects caused by introducing mDSCAM siRNA is specific to DSCAM loss-of-function.
Figure 2.
DSCAM is necessary for the guidance of commissural axons to the ventral midline. (A) Schematic drawing of a transverse section of the spinal cord. A modified whole embryo culture system was used to eliminate DSCAM expression in commissural neurons by RNAi knockdown. mDSCAM siRNA treated axons fail to reach the midline (B), whereas the axons of commissural neurons electroporated with control RNAi reach and cross the midline (C). When a cDNA encoding human DSCAM, insensitive to the mDSCAM siRNA, was co-electroporated, commissural axons reach and cross the midline (D), similar as seen in control siRNA electroporated axons (E). The width of the midline is indicated in each image by the white bracket.
(F) Quantification of axon crossing defects caused due to lack of DSCAM protein. Shown are the percentages of labeled commissural axon bundles that cross the ventral midline per section. Numbers in parentheses indicate the number of embryos quantified for each condition. (*): P<0.0001 (Student t-test) compared to the condition coelectroporated with human DSCAM cDNA. Error bars indicate the standard error of the mean (SEM). (G) mDSCAM siRNA’s #1 and #2 block mDSCAM protein expression as shown by Western blotting, whereas human DSCAM is insensitive to mDSCAM siRNA #2 (H).
The phenotype displayed by axons in DSCAM RNAi knockdown experiments is highly reminiscent of that observed in netrin-1 and DCC mutant embryos, raising the possibility that DSCAM may function with DCC in mediating netrin-1 effects.
DSCAM binds Netrin-1
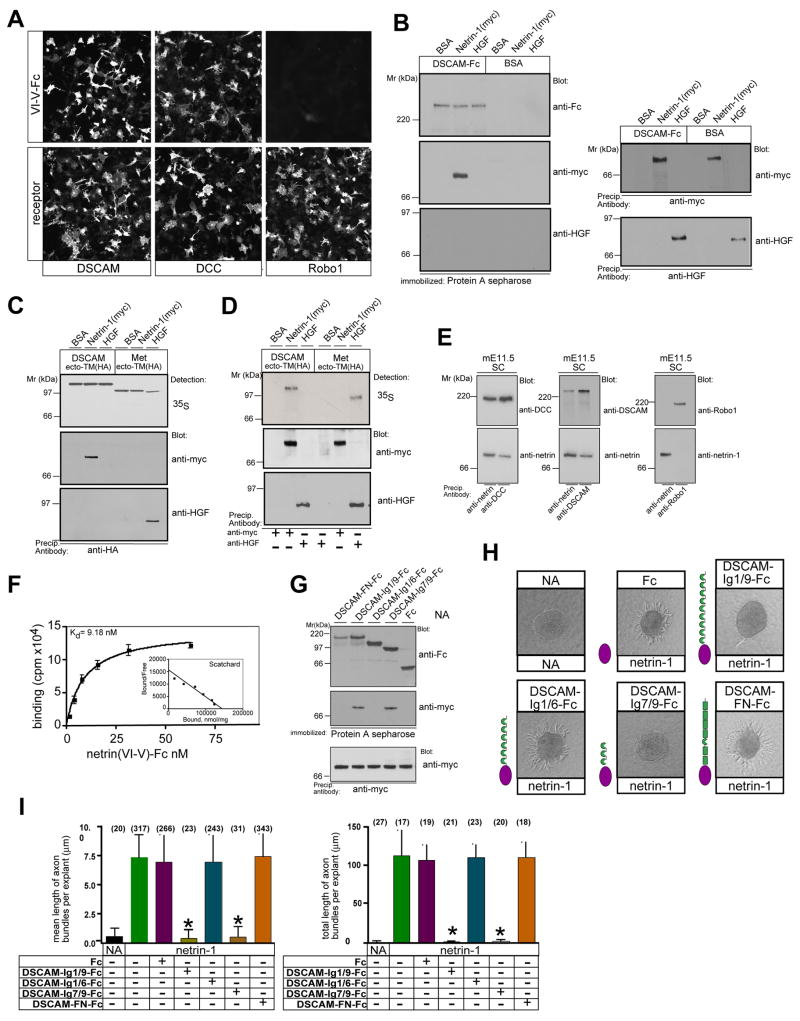
To determine whether DSCAM is a receptor for netrin-1, we examined whether netrin-1 binds cells expressing DSCAM protein in a cell-overlay binding assay (Keino-Masu et al., 1996). As shown in Figure 3A, cells expressing DSCAM showed significant binding to netrin-1-Fc. Netrin-1-Fc bound to these cells to an apparently similar extent as to cells expressing DCC, but did not bind cells expressing Robo1. This result identifies DSCAM as a candidate receptor for netrin-1.
Figure 3.
DSCAM binds netrin-1. (A) COS cells expressing DSCAM or DCC, but not Robo1 bind netrin-1 (top). Receptor expression was verified by immunocytochemistry (bottom). (B–D) Netrin-1 specifically binds to DSCAM. (B) DSCAMecto-Fc or control [(BSA)] protein were incubated with netrin-1, HGF, or BSA and analyzed for protein-protein interaction. Ligand protein was confirmed. (C, D) DN-forms of DSCAM and Met were translated in vitro and evaluated for ligand binding using co-precipitation studies. (E) Netrin-1 associates with DSCAM or DCC in mouse E11.5 spinal cord extracts. (F) Equilibrium binding of netrin-1 to DSCAM expressing cells. 293 cells transfected with mDSCAM or vector alone were incubated with the indicated concentrations of ligand. Binding of netrin-1 (VI-V-Fc) was determined by measuring the radioactivity associated with the cells. Open circles: total binding to DSCAM-expressing cells, open squares total binding to vector expressing cells; closed circles: specific binding. Dissociation constant (Kd) measurement: Scatchard analyses were performed on DSCAM and vector expressing cells. Each point on the graph represents the average of four identical treatment groups. Specific binding curves were fitted using the Hill equation, yielding Kd values of 9.18 nM for DSCAM. (G) DSCAM Ig7/9-Fc is sufficient to bind to netrin-1. Netrin-1 binds only to immobilized DSCAM-Ig1/9-Fc and DSCAM-Ig7/9-Fc. One tenth of the reaction was removed prior incubation and immunoprecipitated using an anti-myc (netrin-1) antibody. (H) DSCAM-Ig1/9-Fc and DSCAM-Ig7/9-Fc block netrin-1 mediated commissural axon outgrowth of E11.5 mouse spinal cord explants. Each micrograph shows a representative image from one out of three independent experiments. (I) Quantification of the mean length of axon bundles per explant and total length of axons per explant (from at least four explants in triplicates). At concentration of 1 μg/ml DSCAM-Ig1/9-Fc and DSCAM-Ig7/9-Fc block completely netrin-1 mediated axon outgrowth. Error bars represent the standard deviation (SD). *Statistically significant change when compared with explants cultured in the presence of netrin-1 without DSCAM-Fc protein (P< 0.0001, Student’s t test). Numbers in parentheses indicate the number of axon bundles (left) or the number of explants (right).
To examine if netrin-1 binds directly to DSCAM we generated a soluble form of the DSCAM ectodomain fused to the Fc portion of human Ig (DSCAMecto-Fc). First, we found that DSCAMecto-Fc precipitated netrin-1 in an in-solution binding assay (Figure 3B). The binding appeared to be specific because DSCAMecto-Fc did not precipitate hepatocyte growth factor (HGF), a ligand for the receptor tyrosine kinase Met, which is of similar size and charge to netrin-1. The possibility that a cofactor from the COS cells used to generate the fusion protein contributed to the interaction was eliminated as an in vitro translated DN-form of DSCAM was capable of precipitating netrin-1 but not HGF (Figure 3C). As a control the DN-form of Met, precipitated HGF, but not netrin-1 (Figure 3C). In reverse experiments, netrin-1 precipitated DSCAM but not the HGF receptor (Figure 3D). We then evaluated if endogenous netrin-1 associates with DSCAM protein in mouse E11.5 spinal cord extracts. Both DSCAM and DCC can be detected in netrin-1 precipitates (Figure 3E). Thus, netrin-1 can directly bind to DSCAM in vitro and in vivo.
The affinity of DSCAM for netrin-1 was estimated in equilibrium binding experiments using netrin-1(VI.V)-Fc (Keino-Masu et al 1996), yielding a dissociation constant of 9.18 nM for DSCAM (Figure 3F). This value is comparable with that observed for the binding of netrin-1(VI.V)-Fc to DCC (Keino-Masu et al 1996).
The immunoglobin domains of DSCAM are sufficient to bind netrin-1 and block netrin-1 mediated outgrowth
We next sought to identify specific subdomains of DSCAM involved in netrin-1 binding. For this, we generated Fc-fusion constructs encoding either Ig domains one to six (DSCAM-Ig1/6-Fc), Ig domains seven to nine (DSCAM-Ig7/9-Fc), Ig domains one to nine (DSCAM-Ig1/9-Fc) or the six Fibronectin (FNIII) repeats that surround the tenth Ig domain (DSCAM-FNIII-Fc), and evaluated binding to netrin-1 using the in-solution binding assay. Netrin-1 bound to DSCAM-Ig1/9-Fc and to DSCAM-Ig7/9-Fc but not to DSCAM-Ig1/6-Fc or DSCAM-FNIII-Fc (Figure 3G). Thus DSCAM-Ig-domains seven to nine seem to be necessary and sufficient for netrin-1 binding.
From this finding, we predicted that DSCAM-Ig1/9-Fc and DSCAM-Ig7/9-Fc should interfere with netrin-1 mediated commissural outgrowth (Serafini et al., 1994). Indeed we found that consistent with the binding data, addition of DSCAM-Ig1/9-Fc or DSCAM-Ig7/9-Fc (as low as 1 μg/ml) blocked netrin-1 mediated outgrowth (Figure 3H). Netrin-1 independent outgrowth was not altered by any of the DSCAM-Fc fusion proteins (Figure S4).
Taken together, these findings suggest that the DSCAM-Ig domains 7–9 are necessary and sufficient for netrin-1 binding.
DSCAM is required for commissural axon growth but acts in parallel to DCC in axon turning
We next examined whether DSCAM is required for the turning responses of rat commissural axons to netrin-1. This netrin-mediated turning activity is demonstrated by culturing pieces of spinal cords adjacent to control cells or cells secreting netrin-1. Under control conditions, the axons grow along a stereotyped trajectory within explants, but when cultured with cells secreting netrin-1, the axons are deflected from this trajectory and turn towards the source (Placzek et al., 1990; Kennedy et al., 1994; Okada et al., 2006). To specifically knockdown DSCAM expression, E11 rat spinal cords were electroporated on the dorsal aspect with a GFP plasmid and with either a control siRNA pool or DSCAM-targeting siRNA#2. COS cell aggregates expressing recombinant netrin-1 were positioned alongside the dorsoventral axis of these electroporated rat E11 whole spinal cord explants and cultured for 48 hours.
Within control explants, commissural axons visualized by GFP fluorescence follow their normal stereotyped dorso-ventral trajectory to the ventral midline similar to the one they take in vivo. In the presence of COS cell derived netrin-1, control siRNA-electroporated commissural axons were deflected from a stereotyped dorso-ventral trajectory and turned toward the COS cell aggregate (Figure 4B, 4F). Down-regulation of DCC by RNAi or genetically (Figure 4G, S5) in this assay resulted in inhibition of commissural axon growth. Importantly, however, it did not result in complete block of commissural axon turning to a netrin-1 source, consistent with our finding that antibodies to DCC did not block netrin-induced turning within these explants (Keino-Masu et al., 1996). Interestingly, while the cell bodies of control RNAi-treated commissural neurons migrated ventrally (similar to their in vivo migration paths), commissural neurons electroporated with DCC siRNA failed to migrate ventrally (Figure 4G), an effect already evident at 16 hrs (Figure 4C), consistent with a reported abnormal ventral migration seen in DCC mutant mice (Ding et al., 2005).
Figure 4.
DSCAM promotes axonal growth but is dispensable for cell body migration and for axon turning toward a local source of netrin-1 in whole spinal cord turning assays. (A, J) Schematic representation of commissural axon turning assays and outcome of knockdown experiments. E11 rat spinal cord explants were cocultured with netrin-1 secreting cells post electroporation. Commissural axon trajectories were visualized after 16 hours (B–E) or 48 hours in culture (F–I). (B, F) Netrin-1 elicits directional commissural axon turning in explants electroporated with non-targeting control siRNAs, whereas down-regulation of DCC expression by RNAi inhibits axonal growth and directional axon turning toward a local source of netrin-1, and blocks medial cell body migration, but does not alter commissural neuron number (C, G). Down-regulation of DSCAM inhibits commissural axon outgrowth, but has no effect on directional axon turning toward a local source of netrin-1, and does not block medial migration of neuronal cell bodies (D, H). In DSCAM/DCC double knockdowns axon turning was abolished, whereas commissural axon outgrowth was altered to variant degrees (E, I). Human DSCAM restores the commissural axon growth defect caused by DSCAM RNAi #2 (M) and is sufficient to rescue commissural axon turning towards a point source of netrin-1 in explants in which both DSCAM and DCC are down regulated by siRNA (N). (O) Quantification of the median turning distance of commissural axon. For each explant, turning distance of GFP-labeled commissural axons was measured from the edge of the explant to the most distant GFP-positive axon exhibiting a turning response to netrin-1. Numbers in parentheses indicate the number of explants quantified for each experimental condition. Error bars represent the standard deviation (SD). P values (Student’s t-test) indicate whether the averages between various data sets are statistical significant.
The DSCAM siRNA-electroporated commissural axons also appeared to be significantly shorter compared to control axons (Figure 4H versus 4F). Nevertheless, the degree of axon turning towards the netrin-1 source exhibited by commissural axons electroporated with DSCAM RNAi was similar to turning of axons electroporated with control RNAi (Figure 4H versus 4F). In addition, cell body migration patterns of DSCAM RNAi commissural neurons appeared normal (Figure 4H), suggesting that DSCAM may be dispensable for the ventrally-directed cell body migration process.
Thus, these RNAi data support a model consistent with requirements for both DCC and DSCAM in commissural axon extension and for DCC alone in ventral cell body migration. To test whether DCC and DSCAM were functioning redundantly in turning, or whether there was an additional receptor for turning, we performed double siRNA knockdown of DCC and DSCAM, and found that turning was now abolished (Figure 4I and 4O). The specificity of the DSCAM knockdown was confirmed by the observation that heterologous expression of human DSCAM was sufficient to rescue commissural axon turning caused by DSCAM/DCC siRNA double knockdown (Figure 4N, 4O).
Thus, we propose a model in which DCC and DSCAM function together in mediating rat commissural axon turning within neural epithelium.
A receptor complex of DSCAM and DCC
We next examined whether DSCAM and DCC can associate biochemically. We first examined whether the two proteins are coexpressed in commissural axons. DSCAM and DCC colocalization can be detected in precrossing commissural axons in the rat E12 spinal cord (Figure 5A), however we could not be certain whether all commissural axons co-express DSCAM and DCC. The findings that DSCAM and DCC are co-expressed in vivo raised the possibility that these two proteins may form a receptor complex and may be part of the same signaling complex necessary for the guidance of commissural axons in the spinal cord.
Figure 5.
Netrin-1 triggers the dissociation of a DSCAM/DCC complex. (A) DSCAM (red) and DCC (green) proteins co-localize in precrossing commissural axons in transverse section of the E12 rat spinal cord. Lower panels are close ups (20x) of the ventral spinal cord of section displayed above (10x, Zeiss NLO confocal). (B) Endogenous DSCAM and DCC protein associate in extracts derived from mouse E11.5 dorsal spinal cord. (C) Netrin-1 triggers the dissociation of DSCAM and DCC, but stimulates DCC oligomerization in vitro. (D) Dissociated E11.5 dorsal spinal cords were cultured on laminin, stimulated with netrin-1 (300 ng/ml) for the indicated times and receptors were analyzed for complex formation. Endogenous DSCAM and DCC proteins form a complex in the absence of netrin-1, but not in its presence. (E) DSCAM/DCC complex requires the presence of the TM domain of DSCAM. DCC coprecipitates with DSCAM or a DN-form of DSCAM (DSCAMecto-TM), but not with Met-DSCAM, a chimera composed of the ectodomain of DSCAM fused in-frame with the TM and intracellular domain of Met. (F) DSCAM does not oligomerize in response to netrin. DSCAM(V5) and DSCAM(myc) were evaluated for coprecipitation in vitro.
We then tested whether such a complex exists in mouse E11.5 spinal cord extracts. Indeed, DCC and DSCAM form a receptor complex or are part of a receptor complex in spinal commissural axons (Figure 5B).
To further dissect this interaction, we first sought to analyze if the DSCAM/DCC receptor complex requires netrin-1. As shown in Figure 5C, DSCAM coprecipitated with DCC in the absence of netrin-1, but the receptor complex dissociates in response to netrin-1 stimulation. In contrast, DCC is multimerized in the presence of netrin-1, but not in its absence (Figure 5C, Stein et al. 2001). We next explored if netrin-1 has a similar effect on dissociating an endogenous DSCAM/DCC complex. For this we turned to commissural neuron cultures derived from mouse E11.5 dorsal spinal cord, which have little to no endogenous netrin-1. Similar to the transfected cells, DSCAM forms a complex with DCC in the absence of netrin-1, but not in its presence (Figure 5D).
We next sought to explore the mechanism through which DCC and DSCAM interact. As shown in Figure 5E, DCC failed to associate with a DSCAM-Met chimera (a fusion of the DSCAM ectodomain and the TM and intracellular domain of Met) but associates with a full-length or DN-form of DSCAM suggesting that the interaction requires the presence of the DSCAM TM domain. These findings are consistent with our yeast-two hybrid results, in which we did not observe any interaction of the DCC cytoplasmic domain with that of DSCAM (data not shown). Thus the cytoplasmic domain of DSCAM and DCC are neither sufficient nor necessary for complex formation. Together, our results suggest that DSCAM and DCC associate through a TM interaction in the absence of netrin-1. The functional implication of this interaction is not known, but may allow DCC and DSCAM to contribute to other guidance pathways in a netrin-1 independent fashion (Yu et al., 2002) or may serve as a way to hold DSCAM and DCC in a resting state, until netrin-1 reaches a critical concentration at which both receptors are activated.
These data suggest that DSCAM and DCC form a receptor complex in the absence of netrin-1, whereas the presence of netrin-1 (500 ng/ml) triggers the dissociation of the complex at least in commissural neurons in vitro. These results imply that DSCAM and DCC may signal independently.
We next sought to test whether DSCAM oligomerizes in response to netrin-1 activation. As shown in Figure 5F, DSCAM‘s do not co-precipitate either in the absence or presence of netrin-1. Consistent with this, the intracellular domain of DSCAM in a LexA-based yeast-two hybrid system does not homodimerize (data not shown). Thus, DSCAM may either signal as a monomer or utilizes a novel co-receptor to mediate netrin-1 attraction.
Involvement of DSCAM in Xenopus neuron responses to netrin-1
We next turned to the Xenopus axon turning assay to determine whether DSCAM can also participate in netrin responses in those cells. In that assay, the growth cones of these neurons in culture are exposed to gradients of soluble factors established by repetitive pulsatile release from a glass micropipette (Zhang et al., 1994). This assay has been used to characterize growth cone responses to several guidance cues, including brain-derived neurotrophic factor (BDNF), netrin-1 and Slit2 (Ming et al., 1997; Stein and Tessier-Lavigne, 2001). When growth cones from stage 22 embryos are exposed to gradients of netrin-1, they turn and extend towards the point source of netrin-1, and their rate of growth is increased, dependent of DCC function (Ming et al., 1997, Stein et al., 2001). Netrin receptors other than DCC have not been detected in stage 22 Xenopus spinal neurons, including Xenopus Unc5a-d, neogenin or the Adenosine-2b receptor (Suresh et al in preparation). In fact, we failed to detect DSCAM expression in stage 22 neurons by single cell rt-PCR (data not shown), indicating that DCC is the only known netrin receptor expressed in stage 22 Xenopus spinal neurons.
Despite the lack of DSCAM expression in these neurons, we tested whether a DN-form of DSCAM could block netrin responses in these neurons. Whereas uninjected neurons turned towards a netrin source (Figure 6A), neurons expressing the DN-DSCAM did not (Figure 6B). This inhibitory action was selective for responses to netrin-1, since turning responses to BDNF were not affected (Figure 6B). As a further control, neurons from embryos injected with full-length DSCAM showed normal responses to both netrin-1 and BDNF (Figure 6A). Thus, a DN-form of DSCAM can selectively block netrin responses in these cells.
Figure 6. DSCAM guides growth cones independent of DCC.
DSCAM protein does not alter netrin-mediated attraction of monopolar and bipolar stage 22 Xenopus growth cones. (A) Growth cones derived from embryos heterologously expressing mouse DSCAM display a similar pattern of chemoattraction and extension to netrin-1 and BDNF as observed in wild type growth cones. (B) In contrast, a DN-form of DSCAM blocks netrin-1, but not BDNF mediated guidance. (C) Wild type, but not DSCAM expressing growth cones depend on DCC-function to mediate chemoattraction to netrin-1. For all experiments described in this figure, growth cones were exposed for a 1-hour period to gradients of control medium (NA), netrin-1 (5 μg/ml) or BDNF (50 μg/ml). (Top) Distribution of turning angles, and (middle) net neurite extension are presented in scatter plots, each symbol represents the response of an individual neuron. (Bottom) Cumulative distribution plots of turning angles for all conditions are shown. Numbers in parentheses represent the total number of growth cones tested in each condition. * indicates statistical significant changes compared with wild-type neurons (P< 0.0001, Student’s t test).
The apparent lack of DSCAM expression in these neurons allowed us to test whether DSCAM is sufficient to mediate turning. First we studied cells in which we had overexpressed DSCAM. In normal cells from uninjected embryos, we found that the antibody to DCC blocks the axonal responses to netrin-1 (Figure 6C, Ming et al., 1997). However, in cells from embryos injected with full-length DSCAM mRNA, we found that the response to netrin-1 was no longer blocked in the presence of this antibody (Figure 6D). Similar, neurites failed to respond to netrin-1 when endogenous DCC was knocked down by a specific Xenopus DCC morpholino, but the loss of netrin-1 attraction was rescued by heterologous expression of mouse DSCAM in DCC-deficient growth cones (Figure S6).
This result provides evidence that DSCAM can mediate a turning response independent of DCC in these neurons.
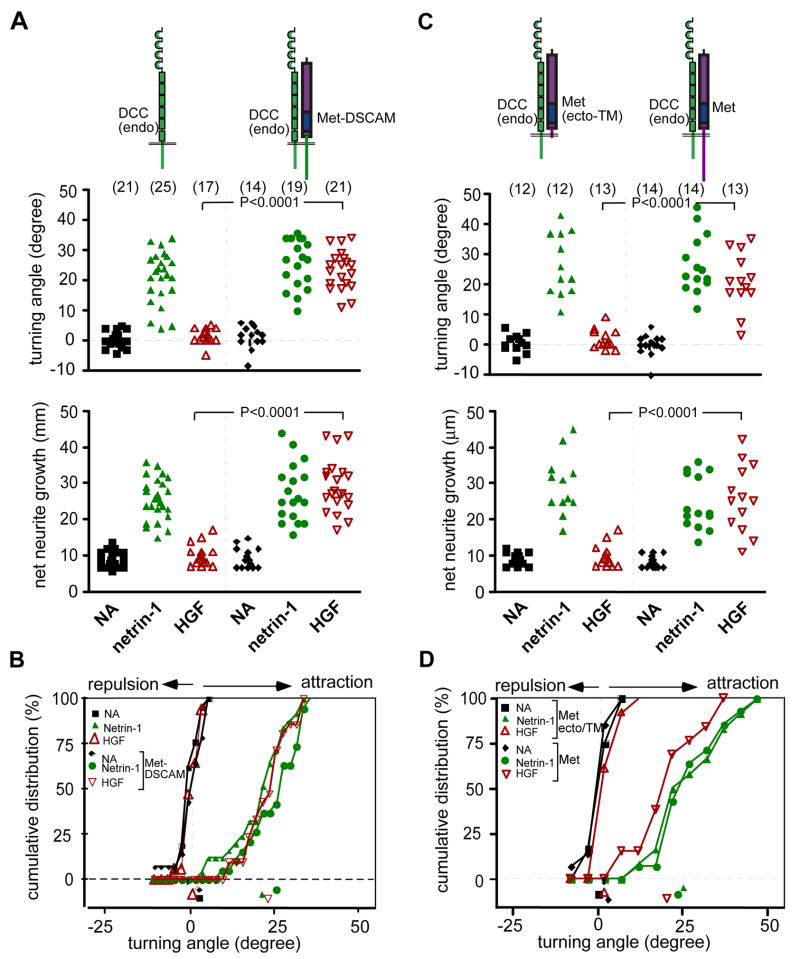
Further evidence that DSCAM can mediate a turning response came from using a chimeric receptor in which the ectodomain of the HGF receptor Met replaced that of DSCAM. We tested the function of this Met-DSCAM chimera by expressing it in stage 22 Xenopus spinal neurons and evaluating their response in the Xenopus assay. This assay relies on the observations that wild type stage 22 spinal neurons with monopolar or bipolar morphology do not respond to HGF (Figure 7A, Stein et al., 2001), and that netrin-1 and HGF can attract axons heterologously expressing Met and increase their rate of extension but a DN-form of Met cannot transduce an attractive response to HGF (Figure 7B, Stein et al., 2001). Thus, this effect requires the presence of the intracellular domain of Met. When the Met-DSCAM chimera was introduced into these spinal neurons, growth cones turned and extended towards the HGF source (Figure 7A). In addition, the presence of the Met-DSCAM chimera in these growth cones did not alter endogenous DCC signaling, since growth cones responded to netrin-1 gradients with similar turning angles and neurite extension to those seen for wild type control neurons (Figure 7A) or Met expressing neurons (Figure 7B). These results suggest that the intracellular domain of DSCAM in the context of a Met-chimeric receptor can transduce signals leading to growth cone attraction and axon elongation.
Figure 7.
The intracellular domain of DSCAM signals chemoattraction. (A) Wild type spinal neurons derived from stage 22 Xenopus embryos do not respond to HGF, whereas HGF triggers attraction in growth cones heterologously expressing a Met-DSCAM chimera. Distribution of turning angles (top) and net neurite extension (bottom) of all assayed neurons presented as scatter plots in response to culture medium (NA), netrin-1 (5 μg/ml) or HGF (10 μg/ml). Numbers in parentheses represent the total number of growth cones tested in each condition. (B) Cumulative distribution plot of turning angles for all conditions are shown. Percentage value refers to the percentage of growth cones with angular positions less than a given angle. (C) Turning angle (top) and net neurite extension (bottom) and cumulative distribution (D) of growth cones expressing either a DN-form or full-length mouse Met (right of dashed line). Growth cones were exposed to gradients with the indicated ligand as in (A). * statistical significant changes compared with wild-type neurons (P< 0.0001, Student’s t test.
Taken together, these results indicated that full-length DSCAM is capable of mediating a turning response in Xenopus neurons, and that its cytoplasmic domain is sufficient for this effect in the context of a chimeric receptor, even though DSCAM may not normally be expressed by these neurons.
Discussion
Our results indicate that DSCAM binds netrin-1 and can mediate turning responses to this cue independent of DCC in rodent commissural neurons, where it is normally expressed, and in Xenopus spinal neurons, where it does not appear to be normally expressed. In addition, in the developing rodent spinal cord it appears, like DCC, to be required for the normal growth and guidance of commissural axons towards the midline. These results implicate DSCAM as a netrin receptor regulating axon guidance independently of, and in collaboration with, DCC.
DSCAM mediates turning responses to netrin-1
In a previous study, we found that an antibody to DCC failed to block the turning of commissural axons within dorsal spinal cord explants towards a netrin source, even though it could block netrin-stimulated outgrowth of axons from explants into a collagen matrix (Keino-Masu et al., 1996). We show here that knockdown of DCC by RNAi has the same effect as the antibody: it blocks outgrowth of commissural axons from explants but not turning of these axons within explants in response to netrin-1. These results could be explained if commissural axons express an additional netrin receptor that can mediate turning in response to netrin-1 independently of DCC, but which is not sufficient to mediate outgrowth in response to netrin-1 independently of DCC.
Our results suggest that DSCAM is this receptor. It is expressed by commissural axons and binds netrin-1. Furthermore, knockdown of DSCAM by siRNA by itself does not block turning within explants in response to netrin-1, but when both DSCAM and DCC are knocked down, turning is completely abolished. Taken together, these results suggest that DSCAM and DCC are each sufficient to mediate turning, that they can function independently to mediate this effect, and that together they account for the receptors that mediate this turning response.
Interestingly, in a whole embryo culture system, knockdown of DSCAM produces a phenotype very similar to that seen when DCC function is lost by gene knockout, namely a profound impairment of the growth of these axons towards the floor plate. Thus, in contrast to the turning response, that is intact when either one is knocked down individually, the growth response requires both DSCAM and DCC.
One possibility is that the growth response in these neurons requires a receptor complex of DCC and DSCAM; alternatively DCC and DSCAM may function independently and not need to form a receptor complex to mediate a growth response, but the amounts expressed in these neurons may be limiting, such that loss of either impairs axon growth.
Our findings show that DCC and DSCAM are capable of forming a receptor complex in dorsal spinal cord tissue (Figure 5B), but only approximately 10–20% of endogenous DSCAM and DCC protein are found in a receptor complex. In addition, in dissociated dorsal spinal cord cultures as well as in transfected cells, DSCAM and DCC form a complex in the absence of netrin-1, but the presence of netrin-1 triggers the complex to dissociate (Figure 5C, D). Thus our data strongly support a mechanism in which DSCAM and DCC signal independent. Support for this model also comes from the Xenopus turning assay, in which either DCC or DSCAM are sufficient to signal netrin-1 mediated outgrowth and turning responses (Figure 6, Figure 7, S6).
The ability of DSCAM to mediate a turning response to netrin-1 is further supported by heterologous expression studies in stage 22 Xenopus spinal neurons, which do not appear to express DSCAM, at least as assessed by rt-PCR. The turning response of these neurons to netrin-1 is normally blocked by the antibody to DCC (supporting further the absence of DSCAM in these cells), but when DSCAM is expressed heterologously in these cells, the turning response is no longer blocked by the antibody; consistent with the ability of DSCAM to mediate turning independent of DCC. This finding is further reinforced by the observation that heterologous expression of a Met-DSCAM chimera in these neurons makes them responsive to HGF in the turning assay (Figure 7).
Together, these results identify DSCAM as a netrin receptor capable of mediating turning responses to netrins, which is also required for the normal growth of these axons within the developing rodent spinal cord.
DSCAM engages a heterophilic ligand for guidance
Our results show that mammalian DSCAM can function as a receptor for the heterophilic ligand netrin-1. It will be of interest to determine whether other heterophilic ligands can bind and activate DSCAM as well. In addition, some functions of vertebrate DSCAMs are may mediated by homophilic binding, indeed in the vertebrate retina DSCAM may contribute by self-avoidance to arborization and to preserve mosaic spacing (Fuerst et al., 2008) similar to what has been documented in Drosophila (reviewed in Zipursky et al., 2006). The ectodomain of Drosophila and mammalian DSCAM have significant homology, and both receptors are capable of binding themselves homophilically (Schmucker et al., 2000). DSCAM in both species displays a conserved ectodomain structure, however only Drosophila Dscam was shown to potentially generate large numbers of splice versions. Recent studies suggest a model in which each DSCAM isoform preferentially binds to the same isoform on opposing cell surfaces and mediates repulsive responses (Hughes et al., 2007; Matthews et al., 2007; Soba et al., 2007). However not all functional contributions of Drosophila Dscam appear to be explicable by homophilic interaction, suggesting that Dscam may utilize heterophilic binding partners as well (Chen et al., 2006; Zhan et al., 2004). Indeed, in Dscam mutants the BN axons display defects in recognizing P2, an intermediate target, and it has been suggested that Dscam recognizes a local attractive signal at this target – perhaps a netrin (Schmucker et al., 2000).
DSCAM and Down syndrome
DSCAM is one of the ~275 genes on chromosome 21, extra copies of which result in a number of significant clinical phenotypes including cognitive defects (Antonarakis and Epstein, 2006). DSCAM maps to chromosome 21q22, the locus associated with the DS mental retardation phenotype (Yamakawa et al., 1998). Changes in neuronal wiring are thought be one of the causes leading to impairment of cognitive function in DS, and postmortem studies have revealed altered axon, dendrite and dendritic spine morphology and complexity (Antonarakis and Epstein, 2006). Given the spatial and temporal expression patterns of DSCAM during development (Agarwala et al., 2001; Barlow et al., 2001), and its function as an axon guidance receptor, elevated levels of DSCAM may contribute to changes in neuronal wiring which ultimately cause altered cognitive function.
Conclusion
Drosophila Dscam has been implicated widely in regulating the formation of neuronal connections, but whether DSCAM plays a similar role in mammalian development has not been well established. Our data provide biochemical and functional evidence that mammalian DSCAM signals axon guidance in response to heterophilic activation by netrin-1. Future studies will establish whether DSCAM functions only in parallel to or also in a complex with DCC, whether it has other co-receptors and heterophilic binding partners, and whether it participates in other aspects of nervous system wiring including dendrite development and synapse formation and if overexpression may contributes to DS.
Experimental Procedures
Construction of Recombinant Fusion Proteins and Plasmids
A detailed description of all constructs used in this study is deposit online or available on request. Briefly, constructs were made in pGEX-5X3 (Pharmacia) to generate GST-fusion proteins, and the COS cell expression vectors pCDNA3, pSEC-C (Invitrogen) and pCMV-Tag4b (Clontech). Various ectodomain or intracellular domain fragments were derived by PCR from rat DCC, mouse Met, mouse DSCAM cDNAs (accession numbers U68725, P16056, NM031174, respectively).
RT-PCR screen to identify Ig-receptors expressed in commissural axons
Total RNA was isolated from rat E13 dorsal spinal cords (DSC). mRNA was reverse transcribed using random hexamer primers (Invitrogen) and AMV-RT (Promega). Single strand product was used as a template for PCR amplification using Forward-primer (5′-CAN CAR WSN GAY GAR CYN WSN GCN CYN CYN-3′) and Reverse primer (5′-RTT RTT RAA NGC NCK NAG RCT NAT NAC RTA-3′). The PCR reaction consisted of 5 min 95°C, followed by 35 cycles of 45 sec at 95°C, 1 min 56°C, and 2 min at 72°C, and completed by a 10 min extension at 72°C. A 500 bp fragment was purified, cloned into pCRII-TOPO (Invitrogen) and were sequence analyzed. Candidate clones encoding receptors were evaluated for their expression by in situ hybridization.
Production and purification of recombinant DSCAM-Fc
293T cells were transfected with various mDSCAM-Fc constructs using Fugene 6 and conditioned for 7 days and concentrated by size exclusion.
Recombinant Netrin-1
Recombinant netrin-1 was purified as described before (Serafini et al., 1994). Functional evaluated for its ability to trigger neurite outgrowth of DSC explants (Serafini et al., 1994) and to attract Xenopus spinal neurons as described (Stein et al., 2001).
Transfection and Immunoprecipitation
Cos cells were transfected with the respective expression constructs using Fugene-6 (Roche). About 60 hr post tansfection medium cells were stimulated with either 500 ng/ml netrin-1 [(+) netrin-1] or BSA [(−)] for 10 or 30 min at 37°C, and lysed in 1 ml WG-buffer (Stein et al., 2001). Lysates were incubated with the indicated antibodies for 8–10 hr at 4°C, complexes were recovered and washed extensively on Protein A-sepharose beads. IP’s were separated on 8% SDS-PAGE and Western blots incubated with the indicated antibodies and analyzed with ECL reagent (Amersham).
Tissue immunoprecipitation
For each tissue IP DSC tissue was isolated from 10–12 E11.5 mouse embryos, and lysed in WG buffer. Precleared lysates were incubated with the indicated antibodies for 6 hr at 4°C. IPs were recovered and analyzed as described above.
Dissociated commissural neuron cultures
For primary embryonic cultures DSC tissue from about 30 E11.5 mouse embryos was isolated and dissociated with Cell Dissociation solution (Invitrogen) and trituration. Neurons were seeded in commissural medium (F12, 2% FBS, N3 supplement, 20 mM Glucose) at a density of 5× 105cells per cm2 on PDL/laminin and cultured for 12 hr at 37°C.
Equilibrium-binding Assay
Equilibrium binding assays were done as described before (Keinu-Masu et al 1996). A detailed description of this assay is deposit online. Dissociation constants were determined using Graph Pad Prism 4 Software.
Binding Experiments
Cos-7 cells were transfected with cDNAs encoding mDSCAM, rDCC, rRobo1 or pSEC-B (Invitrogen) using Fugene 6. Seventy hr after transfection, the cells were incubated with 2 μg/ml recombinant myc epitope tagged netrin-1 in binding buffer at 37°C for 1 hr, washed three times with PBS/1% HINGS, and fixed with methanol (50% Methanol/50%PBS for 2 min followed by 100% Methanol for 2 min). Rinsed cells were incubated with anti-myc (1μg/ml) for 1 hr followed by three washes with PBS/1% HINGS. Binding was detected by fluorescence staining.
In-Solution Binding Assay
Purified Fc, DCC-Fc or DSCAM-Fc fusion proteins were immobilized on protein A sepharose beads. Beads were then incubated with myc-epitope tagged netrin-1, recombinant hepatocyte growth factor (HGF, Calbiochem) or BSA for 2 hr at 4°C, followed by three washes with WG buffer. The bound proteins were recovered and analyzed by Western blotting with the indicated antibodies. For ligand IPs, one fifth of each of the above reaction was precipitated for 4 hr at 4°C and analyzed by Western blotting as above.
DSCAM and Met constructs were transcribed and translated in vitro in the presence of microsomal membranes and 35S-Cysteine (GE Healthcare) using the TNT-kit (Promega). Translated proteins were incubated with either netrin-1, recombinant HGF, or BSA for 2 hr at 4°C in WG buffer, followed by IPs with the indicated antibodies for 4 hr at 4°C. Complexes were collected on Protein A sepaharose beads, washed and analyzed by autoradiography or Western blotting.
Generation and characterization of Polyclonal Antibodies
For the GST fusion antibodies mDSCAM AAs 1913–2013 were cloned into pGEX-5X3 and recombinant protein used to generate polyclonal rabbit antibodies (Protein Tech, Chicago, IL).
Immunohistochemistry
Timed pregnant embryos were collected, fixed in 4% PFA overnight. Sections were permeabilized in 0.5% Desoxycholic Acid in 1x PBS (20 min at RT), rinsed with 1x PBS, blocked in PHD (1x PBS/2% goat serum/0.1% Desoxycholic Acid) and incubated overnight with antibodies to DSCAM (1:800), anti-DCC (1 μg/ml) (Calbiochem, AF5), TAG-1 conditioned medium (1:400) (monoclonal 4D7, DHSB, Iowa). After three 15 min washes at RT with PBS, sections were incubated with the respective secondary antibodies diluted in PBS/2% HINGS and washed prior mounting in Fluromount (Southern Biotech).
Commissural Axon Outgrowth Assays
Explants of E11.5 mouse DSCs were isolated and cultured as described previously (Serafini et al., 1994). When indicated, outgrowth of commissural axons was elicited by adding 300 ng/ml of recombinant netrin-1 to the culture medium. Explants were fixed and analyzed 16–18 hr post medium addition.
Xenopus Embryo Microinjection, Cell Culture and Xenopus Turning Assay
Cultures of Xenopus spinal neurons prepared from neural tube tissue of stage 22 embryos and growth cone turning assays were performed as described (Stein et al., 2001) using netrin-1 (5 μg/ml), HGF (Calbiochem; 10 μg/ml) or BDNF (Calbiochem; 50 μg/ml). Anti-DCC (AF5, Calbiochem; 2 μg/ml), DSCAM-Fc or Fc was added to the medium 30 min before initiating the gradient. A detailed description could be found in the supplementary section.
RNAi in whole spinal cord explant system
E11 rat embryos were placed in L15 medium and siRNAs together with GFP encoding plasmids were injected into the central canal of neural tubes. SiRNAs and plasmids were delivered to dorsal progenitor cells by electroporation to the dorsal side of the embryo. Whole spinal cord explants were dissected out, embedded into a 3D collagen gel matrix and cultured in Opti-MEM/F12 medium with netrin-1 and 5% horse serum at 37°C in a 5% CO2 environment. COS cell aggregates expressing chick netrin-1 were positioned along the dorso-ventral axis of the whole spinal cord explants. Sixteen and 48 hr after electroporation GFP-labeled axons and cell bodies of commissural neurons were visualized by fluorescence microscopy.
The following siRNA sequences targeting rat DSCAM were used in this experiment:
rDSCAM siRNA#1 GGA CCU GAG CUU AGG ACA A
rDSCAM siRNA#2 GUG GGA GAG GAA GUG AUU U
rDSCAM siRNA#3 ACA CAA UGG AGA CCA UAG A
In control experiments siCONTROL non-targeting siRNA#1 (D-001210-01-20, Dharmacon) was used. For rescue experiments, 1 μg of human DSCAM cDNA was co-electoroporated together with rat DCC siRNA pool and rat DSCAM siRNA#2 to the dorsal aspect of the embryo.
Quantification
For each explant, turning distance of GFP-labeled commissural axons was measured from the edge of the explant to the most distant GFP-positive axon exhibiting a turning response to netrin-1. Statistical analysis was performed using GraphPad prizm.
RNAi in the whole embryo culture system
E9.5 mouse embryos were dissected out of the yolk sac and amnion membrane in DMEM medium. Control siRNAs together with GFP (or RFP) were injected into neural tube and electroporated laterally to one side of the embryo. Targeting siRNAs and RFP (or GFP) encoding plasmid were then injected into the neural tube and electroporated to the opposite side of the embryo. Whole mouse embryos were cultured in rat serum for two days in 65% and 95% oxygen environment. Fluorescent microscopy was used to detect GFP- and RFP-labeled commissural axons on transverse sections of the spinal cord. The following siRNA sequences targeting mouse DSCAM were used in this experiment:
mDSCAM siRNA#1 GGA CCU GAG UUU AGG ACA A
mDSCAM siRNA#2 GUG GGA GAG GAA GUG AUA U
mDSCAM siRNA#3 ACA CAA UGG AGA CCA UAG A
siCONTROL Non-Targeting siRNA #1 (D-001210-01-20, Dharmacon).
Rescue experiments were conducted as described above, accept that mDSCAM siRNA#2 was co-electroporated with human DSCAM and RFP cDNAs into one side of the E9.5 mouse embryo. Control siRNA and GFP (or RFP) were electroporated to the opposite side of the embryo.
Quantification has been done in the following way. Transverse sections of the spinal cord were subdivided into the dorsal, medial and ventral quadrants. Counts of the labeled commissural axon bundles entering the floorplate (FP) were taken within the ventral quadrants of the spinal cord sections. The total number of the labeled commissural axon bundles per section was determined by counting axon bundles that extend within the medial quadrants of the spinal cord sections. Percentage of commissural axon bundles crossing the FP was determined by taking the ratio of the number of axon bundles entering the FP in the ventral quadrant to the total number of axon bundles extending within the medial quadrant.
Supplementary Material
Acknowledgments
We thank Pietro De Camilli and Paul Forscher for helpful discussion and comments to the manuscript, and J. Korenberg for the human DSCAM cDNA, J. Round for the Robo antiserum. The work was supported by the predoctoral program in genetics (T32-GM07499) to AL, an instrumentation grant (NIH-S10-RR22636) and grants from the Klingenstein Foundation, March of Dimes Foundation (#5-FY04-28), McKnight Scholar Award and a NSF-Career Award (IOB-0545891) to ES, and by the Howard Hughes Medical Institute (to MTL). AN and MTL were supported by Genentech.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agarwala KL, Subramaniam G, Tsutsumi Y, Suzuki T, Amano K, Yamakawa K. Cloning and functional characterization of DSCAML1 a novel DSCAM-like cell adhesion molecule that mediates intracellular adhesion. Biochem Biophys Res Commun. 2001;285:760–772. doi: 10.1006/bbrc.2001.5214. [DOI] [PubMed] [Google Scholar]
- Antonarakis SE, Epstein CJ. The challenge of Down syndrome. Trends in Molecular Medicine. 2006;10:473–9. doi: 10.1016/j.molmed.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Barlow GM, Micales B, Chen XN, Lyons GE, Korenberg JR. Mammalian DSCAMs: roles in the development of the spinal cord, cortex, and cerebellum? Biochem Biophys Res Commun. 2002;293:881–891. doi: 10.1016/S0006-291X(02)00307-8. [DOI] [PubMed] [Google Scholar]
- Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell. 2003;113:11–23. doi: 10.1016/s0092-8674(03)00199-5. [DOI] [PubMed] [Google Scholar]
- Chen BE, Kondo M, Garnier A, Watson FL, Puettmann-Holgado R, Lamar DR, Schmucker D. The molecular diversity of Dscam is functionally required for neuronal wiring specificity in Drosophila. Cell. 2006;125:607–20. doi: 10.1016/j.cell.2006.03.034. [DOI] [PubMed] [Google Scholar]
- Chen Z, Gore BB, Long H, Ma L, Tessier-Lavigne M. Alternative splicing of the Robo3 axon guidance receptor governs the midline switch from attraction to repulsion. Neuron. doi: 10.1016/j.neuron.2008.02.016. in press. [DOI] [PubMed] [Google Scholar]
- Colamarino SA, Tessier-Lavigne M. The role of the floor plate in axon guidance. Annu Rev Neurosci. 1995;18:497–529. doi: 10.1146/annurev.ne.18.030195.002433. [DOI] [PubMed] [Google Scholar]
- Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298:1959–64. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- Ding YQ, Kim JY, Xu YS, Rao Y, Chen ZF. Ventral migration of early born neurons requires DCC and is essential for the projections of primary afferents in the spinal cord. Development. 2005;132:2047–56. doi: 10.1242/dev.01798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazeli A, Dickinson SL, Hermiston ML, Tighe RV, Steen RG, et al. Phenotype of mice lacking functional deleted in colorectal cancer (Dcc) gene. Nature. 1997;386:796–804. doi: 10.1038/386796a0. [DOI] [PubMed] [Google Scholar]
- Fuerst PG, Koizumi A, Masland RH, Burgess RW. Neurite arborization and mosaic spacing in the mouse retina requires DSCAM. Nature. 2008;451:470–474. doi: 10.1038/nature06514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes ME, Bortnick R, Tsubouchi A, Baumer P, Kondo M, Uemura T, Schmucker D. Homophilic Dscam interactions control complex dendrite morphogenesis. Neuron. 2007;54:417–27. doi: 10.1016/j.neuron.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinu-Masu K, Masu M, Hinck L, Leonardo ED, Chan S-Y, Culotti JG, Tessier-Lavigne M. Deleted in colorectal cancer (DCC) encodes a netrin receptor. Cell. 1997;87:175–185. doi: 10.1016/s0092-8674(00)81336-7. [DOI] [PubMed] [Google Scholar]
- Kennedy TE, Serafini T, de la Torre JR, Tessier-Lavigne M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell. 1994;78:425–435. doi: 10.1016/0092-8674(94)90421-9. [DOI] [PubMed] [Google Scholar]
- Matthews BJ, Kim ME, Flanagan JJ, Hattori D, Clemens JC, Zipursky SL, Grueber WB. Dendrite self-avoidance is controlled by Dscam. Cell. 2007;129:593–604. doi: 10.1016/j.cell.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Meijers R, Puettmann-Holgado R, Skiniotis G, Liu JH, Walz T, Wang JH, Schmucker D. Structural basis of Dscam isoform specificity. Nature. 2007;449:487–91. doi: 10.1038/nature06147. [DOI] [PubMed] [Google Scholar]
- Millard SS, Flanagan JJ, Pappu KS, Wu W, Zipursky SL. Dscam2 mediates axonal tiling in the Drosophila visual system. Nature. 2007;447:720–4. doi: 10.1038/nature05855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming GL, Song HJ, Berninger B, Holt CE, Tessier-Lavigne M, Poo MM. cAMP-dependent growth cone guidance by netrin-1. Neuron. 1997;19:1225–1235. doi: 10.1016/s0896-6273(00)80414-6. [DOI] [PubMed] [Google Scholar]
- Okada A, Charron F, Morin S, Shin DS, Wong K, Fabre PJ, Tessier-Lavigne M, McConnell SK. Boc is a receptor for sonic hedgehog in the guidance of commissural axons. Nature. 2006;444:369–373. doi: 10.1038/nature05246. [DOI] [PubMed] [Google Scholar]
- Osumi N, Inoue T. Gene transfer into cultured mammalian embryos by electroporation. Methods. 2001;24:35–42. doi: 10.1006/meth.2001.1154. [DOI] [PubMed] [Google Scholar]
- Placzek M, Tessier-Lavigne M, Jessell T, Dodd J. Orientation of commissural axons in vitro in response to a floor plate-derived chemoattractant. Development. 1990;110:19–30. doi: 10.1242/dev.110.1.19. [DOI] [PubMed] [Google Scholar]
- Schmucker D, Clemens JC, Shu H, Worby CA, Xiao J, Muda M, Dixon JE, Zipursky SL. Drosophila Dscam is an axon guidance receptor exhibiting extraordinary molecular diversity. Cell. 2000;101:671–684. doi: 10.1016/s0092-8674(00)80878-8. [DOI] [PubMed] [Google Scholar]
- Serafini T, Kennedy TE, Galko MJ, Mirzayan C, Jessell TM, Tessier-Lavigne M. The Netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell. 1994;78:409–424. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, Tessier-Lavigne M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- Soba P, Zhu S, Emoto K, Younger S, Yang SJ, Yu HH, Lee T, Jan LY, Jan YN. Drosophila sensory neurons require Dscam for dendritic self-avoidance and proper dendritic field organization. Neuron. 2007;54:403–16. doi: 10.1016/j.neuron.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein E, Zou Y, Poo MM, Tessier-Lavigne M. Binding of DCC by netrin-1 to mediate axon guidance independent of Adenosine A2B receptor activation. Science. 2001;291:1976–1982. doi: 10.1126/science.1059391. [DOI] [PubMed] [Google Scholar]
- Stein E, Tessier-Lavigne M. Hierarchical organization of guidance receptors: silencing of Netrin attraction by slit through a Robo/DCC receptor complex. Science. 2001;291:1928–1938. doi: 10.1126/science.1058445. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Placzek M, Lumsden AG, Dodd J, Jessell TM. Chemotropic guidance of developing axons in the mammalian central nervous system. Nature. 1988;336:775–778. doi: 10.1038/336775a0. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Sanes J. DSCAM and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature. 2008;451:465–469. doi: 10.1038/nature06469. [DOI] [PubMed] [Google Scholar]
- Yamakawa K, Huot YK, Haendelt MA, Hubert R, Chen XN, Lyons GE, Korenberg JR. DSCAM: a novel member of the immunoglobulin superfamily maps in a Down syndrome region and is involved in the development of the nervous system. Hum Mol Genet. 1998;2:227–237. doi: 10.1093/hmg/7.2.227. [DOI] [PubMed] [Google Scholar]
- Yu TW, Hao JC, Lim W, Tessier-Lavigne M, Bargmann CL. Shared receptors in axon guidance: SAX-3/Robo signals via UNC-34/enabled and a netrin-independent Unc-40/DCC function. Nature Neurscience. 2002;5:1147–54. doi: 10.1038/nn956. [DOI] [PubMed] [Google Scholar]
- Zhan XL, Clemens JC, Neves G, Hattori D, Flanagan JJ, Hummel T, Vasconcelos ML, Chess A, Zipursky SL. Analysis of Dscam diversity in regulating axon guidance in Drosophila mushroom bodies. Neuron. 2004;43:673–86. doi: 10.1016/j.neuron.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Zhu H, Hummel T, Clemens JC, Berdnik D, Zipursky SL, Luo L. Dendritic patterning by Dscam and synaptic partner matching in the Drosophila antennal lobe. Nat Neurosci. 2006;9:349–55. doi: 10.1038/nn1652. [DOI] [PubMed] [Google Scholar]
- Zipursky SL, Wojtowicz WM, Hattori D. Got diversity? Wiring the fly brain with Dscam. Trends Biochem Sci. 2006;10:581–8. doi: 10.1016/j.tibs.2006.08.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.