Abstract
Obesity and hypertension have been identified as cardiovascular risk factors that contribute to the progression of end-stage renal disease. To examine the mechanisms by which high fat diet and hypertension contribute to endothelial dysfunction and renal injury, 8 week old male spontaneously hypertensive rats and Wistars were fed high fat (36% fat) or normal fat (7% fat) diet for 10 weeks. High fat diet increased body weight in Wistar and hypertensive rats by 25g and 31g respectively. Systolic blood pressure was higher in the hypertensive rats compared with Wistars, however blood pressure was unaltered by high fat diet. Afferent arteriole response to acetylcholine was impaired in high fat groups after just 3 weeks. Renal macrophage infiltration was increased in the hypertensive high fat group compared with others and monocyte chemoattractant protein-1 excretion was increased in both high fat fed groups. Renal PCR arrays displayed significant increases in 2 inflammatory genes in hypertensives fed a normal diet, 1 gene was increased in high fat fed Wistars, whereas 12 genes were increased in high fat fed hypertensives. Urinary albumin excretion was increased in the hypertensives compared with Wistars, which was further exacerbated by the high fat diet. Glomerular nephrin expression was reduced and desmin was increased by high fat diet in the hypertensives. Our results indicate that endothelial dysfunction precedes renal injury in normotensive and spontaneously hypertensive rats fed a high fat diet, and hypertension with obesity induces a powerful inflammatory response, and disruption of the renal filtration barrier.
Keywords: Obesity, Inflammation, Hypertension, Renal Disease
Introduction
Obesity and hypertension are co-morbid pathological conditions that have been identified as independent risk factors for the development of endothelial dysfunction and renal disease 1. These risk factors are increasing in prevalence at an alarming rate, with more than 30% of the US population classified as obese and one in three adult Americans currently suffering from hypertension. Blood pressure is strongly correlated to body mass index and in the Framingham Offspring Study up to 78% of male hypertensive cases were attributable to obesity 2. Independently obesity increases the risk for chronic kidney disease (CKD) 4 fold 3, hypertensives account for 25% of all CKD patients and obese patients with hypertension are at the greatest risk for developing chronic renal disease 4-6.
Independently hypertension and obesity have been linked with the development of insulin resistance, endothelial dysfunction, inflammation and renal injury 7, 8. However these conditions are commonly found in combination and it is now becoming apparent that the ensuing renal injury and vascular dysfunction is a result of the combination of the two risk factors9. Animal models of obesity and hypertension such as the obese Zucker rat have been shown to develop albuminuria, progressive glomerulosclerosis and endothelial dysfunction, however the mechanisms involved in the development of insulin resistance, vascular dysfunction and renal injury are complex and still not completely understood 10-12.
There is growing evidence that there is a relationship between obesity, hypertension and increased levels of circulating pro-inflammatory cytokines, which have been associated with the development of endothelial dysfunction and renal injury 13, 14. Activation of an inflammatory response has also been observed in animal models of obesity in addition to increased oxidative stress and lipid mediators which can contribute to renal injury 15, 16. Deficiency of the inflammatory cytokine monocyte chemoattractant protein-1 (MCP-1) receptor (Ccr2) gene has been shown to decrease weight gain in high fat fed mice, to reduce adipose tissue macrophage infiltration and improve insulin sensitivity 17. In addition obese MCP-1 (Ccl2) knockout mice are protected from renal inflammation and diabetic renal injury 15.
In light of this evidence we hypothesized that a high fat diet would impair endothelial function and potentially exacerbate renal injury in spontaneously hypertensive rats. We proposed that an inflammatory response to 10 weeks high fat feeding would contribute to the altered endothelial function and renal injury in this model. Therefore, in this study we aimed to investigate how a high fat diet affects renal endothelial and glomerular function and to examine potential mechanisms involved in the development of renal injury in obesity and hypertension.
Methods
Eight week old male Wistar Kyoto (WKY) rats and spontaneously hypertensive rats (SHRs) were divided into groups (n=4−6) and fed ad libitum either normal rat chow containing 7% fat or high fat diet containing 36% fat (#F2685, BioServ, Frenchtown, NJ, USA). Every 7 days rats were weighed, blood glucose measurements were taken from a tail vein using a glucometer and systolic blood pressure was measured using tail-cuff plethysmography.
Hyperinsulinemic-euglycemic clamp experiments, enzyme-linked immunoassays, in vitro perfused juxtamedullary nephron experiments, immunohistochemistry, immunofluorescence, western blots gene expression arrays were carried out. For detailed methods please see supplemental information at http://hyper.ahajournals.org.
Results
Metabolic Parameters
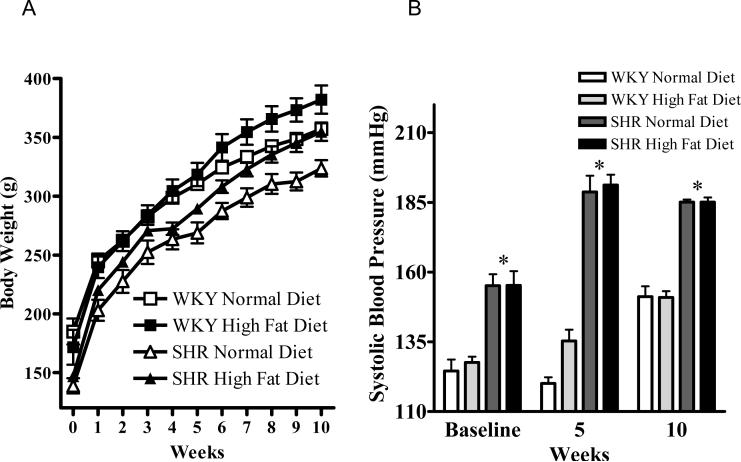
Figure 1 displays the effect of 10 weeks normal or high fat diet on body weight and mean systolic blood pressure in male WKY and SHR. The WKY rats had a higher baseline mean body weight of 185 ±11g compared with the baseline mean body weight of 138 ±6g in the SHR (P<0.01), a pattern which continued throughout the 10 week study that was independent of diet (Figure 1A). Ten weeks high fat diet resulted in a significant increase in body weight in both strains compared with those fed a normal diet (P<0.05). Mean systolic blood pressure increased over the 10 week period in the normal diet fed WKY (from 128 ±5 mmHg to 151 ±4 mmHg) (Figure 1B).
Figure 1.
A: Body weight and B: Systolic blood pressure in the WKY and SHR animals fed a normal diet and a high fat diet at baseline and throughout the 10 weeks study. Values are mean ±SEM (n=5). *P<0.05 WKY vs SHR, †P<0.05 high fat diet vs normal diet.
The mean systolic blood pressure of WKY fed a high fat diet increased similarly (from 126 ±2 mmHg to 151 ±2 mmHg) indicating high fat diet had no effect on systolic blood pressure in the WKY rats (Figure 1B). The SHR had a significantly higher mean systolic blood pressure than the WKY rats throughout the study period which was unaffected by diet. These data indicate that up to 10 weeks high fat diet had no effect on systolic blood pressure in either rat strain. The systolic blood pressures measured were at the high end of the normal range for WKY at the corresponding age, which may have been as a result of the tail-cuff technique used which can transiently elevate blood pressure due to increasing stress levels in the rats.
Plasma Leptin and Cholesterol levels
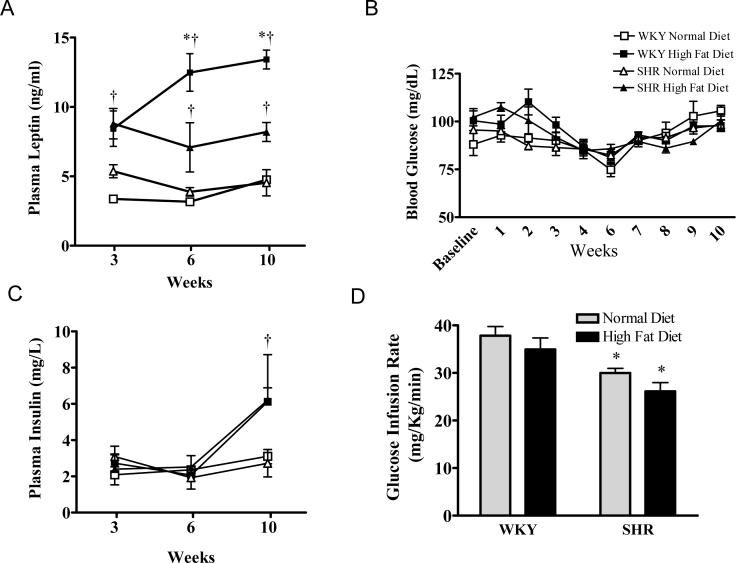
Plasma leptin levels were significantly increased in both the WKY and SHR fed a high fat diet compared with the normal diet fed groups after just 3 weeks (P<0.001) (Figure 2A). Interestingly following 6 weeks high fat diet the WKY displayed an even greater elevation in plasma leptin compared with the similarly treated SHR (P<0.05). This pattern continued until 10 weeks on the respective diets. Plasma cholesterol levels were 44 ± 3 and 64 ± 2 mg/dL in the WKY and SHR respectively fed a normal diet for 3 weeks, and levels were increased to 68 ± 2 and 93 ± 4 mg/dL respectively by 3 weeks high fat diet (P<0.05). Plasma cholesterol levels remained elevated in both WKY and SHR fed a high fat diet throughout the study.
Figure 2.
A: Blood glucose levels in WKY and SHR fed a normal or high fat diet for up to 10 weeks. B: Plasma insulin levels in WKY and SHR fed a normal or high fat diet for 3, 6 or 10 weeks. C: Glucose infusion rates from hyperinsulinemic, euglycemic clamp experiments on WKY and SHR fed a normal or high fat diet for 10 weeks. D: Plasma leptin levels in WKY and SHR fed a normal or high fat diet for 3, 6 or 10 weeks. Values are mean ±SEM (n=5). *P<0.05 WKY vs SHR, †P<0.05 high fat diet vs normal diet.
Blood Glucose levels and Insulin Sensitivity
Insulin sensitivity measurements are presented in Figures 2B-D. There were no differences in blood glucose levels observed throughout the 10 week study period between rat strain or diet (Figure 2B). Plasma insulin levels were similar between the WKY rats and SHR after 3 and 6 weeks on the normal and high fat diet (Figure 2C). But after 10 weeks both WKY and SHR groups fed a high fat diet displayed a significant increase in plasma insulin compared with those on a normal diet (P<0.05).
Hyperinsulinemic euglycemic clamp experiments were carried out to monitor peripheral insulin sensitivity in WKY and SHR fed normal or high fat diet for 10 weeks (Figure 2D). In the SHR a 20% reduction in glucose infusion rate was required compared with the WKY, indicating the development of insulin resistance in SHR (P<0.05). Interestingly 10 weeks high fat feeding did not alter peripheral insulin sensitivity in the WKY or exacerbate the reduction in insulin sensitivity observed in the SHR.
Afferent Arteriole Endothelial Function
Afferent arteriole endothelial function was measured using the in vitro juxtamedullary preparation and the results are depicted in Figure 3. Afferent arteriole endothelial dilatory responses to acetylcholine were significantly impaired in the WKY after just 3 weeks high fat feeding, compared with WKY rats fed a normal diet (P<0.05). Endothelial function was also impaired in the SHR fed a high fat diet for 3 weeks in response to acetylcholine compared with SHR fed a normal diet (P<0.05) (Figure 3A). Endothelial function continued to be impaired in both rat strains as a result of the high fat feeding for up to 10 weeks (Figure 3B) (P<0.05). The SHR group fed a normal diet for 10 weeks displayed an impaired endothelial dilatory response to acetylcholine compared with the WKY fed a normal diet for 10 weeks (P<0.05). Smooth muscle cell dilatory responses to sodium nitroprusside reached 68% ± 10 and 47% ± 9 of baseline in WKY fed a normal and high fat diet respectively for 3 weeks and 73% ± 10 and 69% ± 4 in the normal and high fat fed SHR. These data indicate that it is specifically the endothelial dilatory response that is impaired by high fat in this model.
Figure 3.
Afferent arteriole vascular response to acetylcholine in WKY and SHR fed a normal or high fat diet for A: 3 weeks, B: 10 weeks. Values are mean ±SEM (n=5). *P<0.05 WKY vs SHR, †P<0.05 high fat diet vs normal diet.
Inflammatory Markers
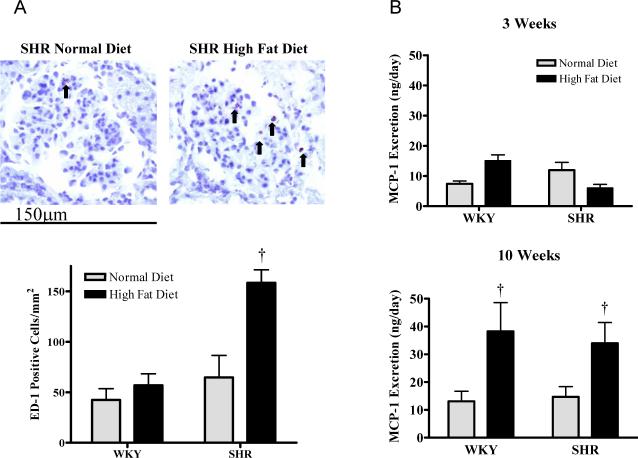
Figure 4A displays representative pictures of renal glomeruli, which show macrophages were present in the kidneys of SHR fed a normal diet for 10 weeks. In contrast SHR fed a high fat diet for 10 weeks displayed an increase in macrophage infiltration compared with the other groups (P<0.05). Plasma levels of the inflammatory marker monocyte chemoattractant protein (MCP)-1 were 23 ± 1 and 22 ± 2 ng/ml in the normal diet fed WKY and SHR respectively and levels were not different at 28 ± 2 and 18 ± 1 ng/ml in the high fat fed WKY and SHR. Urinary MCP-1 levels were not altered by 3 weeks high fat diet in either rat strain; in contrast, MCP-1 protein excretion was significantly increased by 10 weeks high fat diet in both strains compared with the those fed a normal diet (P<0.05)(Figure 4B).
Figure 4.
A: Renal cortical immunohistochemistry in WKY and SHR showing ED-1 positive cells and mean positive cell counts, 400x magnification. B: Urinary monocyte chemoattractant protein-1 concentration in rats fed for 3 or 10 weeks. Values are mean ±SEM (n=5). †P<0.05 high fat diet vs normal diet.
Gene Expression Profiling of Inflammatory Cytokines and Receptors
Real-time PCR arrays were used to profile mRNA expression of 84 inflammatory cytokines and receptors in the kidney cortex. In calculating fold changes in gene expression, the normal diet WKY were used as a control (baseline) for both normal diet SHR group and high fat diet WKY group. The high fat diet fed WKY group was then used as a control for the high fat diet SHR group. Gene expression was considered to be significantly upregulated when fold increases were greater than 2.5 above the respective control group and p<0.05. In the high fat diet WKY group Ccr1 (2.6) mRNA was upregulated in comparison to the normal diet WKY group (Figure 5A). In the normal diet SHR group mRNA of Ccl22 (3.2) and Ccl19 (4.1) were upregulated when compared to the normal diet WKY group (Figure 5B). The high fat diet SHR group had 12 genes upregulated compared with the high fat diet WKY group (Figure 5C): Bcl6 (4.6), Ccl11(3.4), Ccl25 (12.9), Cxcl (7.6), Cxcl2 (6.2), Il18 (2.6), Il1a (4.0), Il1f6 (18.2), Il3 (8.9), Il4 (6.2), Il5 (4.9), and Il5ra (4.7). There were several other genes increased between 1.5-fold and 2.5-fold (please see Table S1 http://hyper.ahajournals.org).
Figure 5.
Real-time PCR array data showing mean renal cortical mRNA expression of 84 inflammatory cytokines genes (n=3). A: WKY high fat (HF) vs WKY normal diet (ND) showing changes in gene expression as a result of 10 weeks high fat diet in normotensive rats. B: SHR ND vs WKY ND showing changes in gene expression as a result of hypertension. C: SHR HF vs WKY HF showing changes in gene expression as a result of 10 weeks high fat diet in hypertensive rats.
Renal Injury Markers
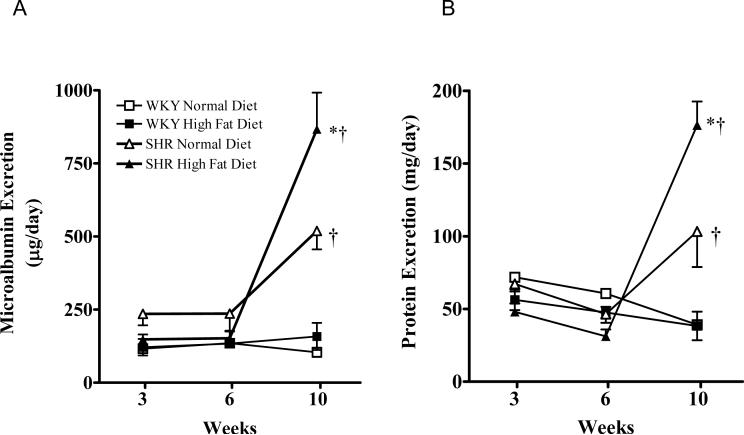
Urinary albumin excretion was unchanged after 10 weeks high fat feeding in the WKY rats (Figure 6A). After 3 and 6 weeks of either diet the SHR displayed albumin excretory levels similar to those observed in the WKY rats. After 10 weeks however, the SHR fed a normal diet developed significantly higher albumin excretion levels than the WKY (P<0.05). In addition the SHR fed a high fat diet for 10 weeks displayed an even greater increase in urinary albumin compared with the normal diet fed SHR (P<0.05). Urinary protein excretion followed a similar pattern to that observed for albumin excretion (Figure 6B). The SHR fed a normal diet for 10 weeks exhibited increased protein excretion compared with the WKY groups and 10 weeks high fat diet further exacerbated this proteinuria (P<0.05).
Figure 6.
A: Urinary microalbumin excretion in WKY and SHR fed a normal or high fat diet for 3, 6 or 10 weeks. B: Urinary protein excretion in WKY and SHR fed a normal or high fat diet for 3, 6 or 10 weeks.
The expression of the slit diaphragm protein nephrin was evaluated qualitatively by immunofluorescence on kidney sections from WKY and SHR fed normal or high fat diet for 10 weeks (Figure 7A) and quantitatively by western blot on isolated glomeruli (Figure 7B). Ten weeks high fat diet significantly reduced glomerular nephrin protein expression in both the WKY and the SHR compared with those fed a normal diet (P<0.05). Glomerular desmin was also measured by immunofluorescence on frozen kidney sections and by western blot on isolated glomeruli (Figures 7C and D). Glomerular desmin expression was significantly increased in glomeruli from SHR fed a high fat diet for 10 weeks compared with the other groups (P<0.001).
Figure 7.
A: Immunofluorescence showing glomerular nephrin expression in WKY and SHR fed a normal or high fat diet for 10 weeks, 400x magnification. B: Western blot of glomerular nephrin protein expression in WKY and SHR fed a normal or high fat diet for 10 weeks. Values are mean ±SEM (n=5). †P<0.05 high fat diet vs normal diet. C: Immunofluorescence presenting glomerular desmin expression in WKY and SHR fed a normal or high fat diet for 10 weeks, 400x magnification. D: Western blot of glomerular desmin protein expression in WKY and SHR fed a normal or high fat diet for 10 weeks. Values are mean ±SEM (n=5). †P<0.001 high fat diet vs normal diet.
Discussion
Currently obesity and hypertension independently affect over 30 percent of the US population 3.
There is a direct link between obesity, hypertension and the development of CKD the mechanisms of which are still little understood 4. Spontaneously hypertensive rats experience many of the symptoms that are present in obese humans with hypertension when fed a high fat diet. They gain weight, develop endothelial dysfunction and with continued high fat diet, renal injury and inflammation ensue.
Interestingly we observed significant endothelial dysfunction after just 3 weeks high fat diet in both normotensive and hypertensive rats, 7 weeks before changes in albumin excretion or inflammatory cytokine expression. We observed no differences in dilatory response to sodium nitroprusside, which indicated that the impaired response to acetylcholine was a result of endothelial dysfunction and not smooth muscle dysfunction. Plasma leptin and cholesterol levels were increased as a result of 3 weeks high fat diet in both rat strains, which may have contributed to the development of endothelial dysfunction observed as a result of the high fat diet. High leptin levels have been shown to reduce dilatory response to acetylcholine 18. A potential mechanism for leptin and cholesterol induced vascular dysfunction is through an upregulation of reactive oxygen species generation, which can disrupt the endogenous vasoactive response to acetylcholine 19, 20. There is evidence that endothelial dysfunction is linked with the development of renal injury and the presence of endothelial dysfunction is a predictor of cardiovascular risk and severity of renal disease 21. In this study we observed endothelial dysfunction 7 weeks prior to the development of renal injury and renal inflammation in the SHRs fed a high fat diet but not in the WKY rats. These data would indicate that the endothelial dysfunction is not a clear predictor of renal injury in this model but in combination with hypertension and a high fat diet may have contributed to the renal injury observed.
Visceral obesity has been linked with the development of insulin resistance, type-2 diabetes and renal injury by way of increased circulating free fatty acids and adipokines 22, 23.
High insulin levels can induce renal hemodynamic changes, glomerular hypertrophy, mesangial cell proliferation and autoregulation can be disrupted, lowering the pressure threshold for renal damage 24-26. An increased incidence of insulin resistance has been reported in hypertensive patients as well as animal models of hypertension such as the SHR 7, 8, 27, 28. In this study we observed a significant increase in plasma insulin levels in both WKY and SHR fed a high fat diet compared with those fed a normal diet. However, euglycemic hyperinsulinemic clamp experiments did not indicate a reduction in peripheral insulin sensitivity as a result of 10 weeks high fat diet. Therefore we conclude that the WKY and SHR receiving a high fat diet have a level of insulin resistance which may be classified as pre-diabetic.
There is growing support for the hypothesis that obesity, hypertension and CKD are all inflammatory diseases and that the renal injury observed in obesity and hypertension are contributed to by increased expression of pro-inflammatory cytokines 29, 30. Cytokines produced by the adipose tissue in obesity such as IL-1β, TNFα, IL-6 and MCP-1 have been associated with the progression of endothelial dysfunction and renal injury 31-33. We observed no effect of 10 weeks high fat diet on systolic blood pressure in either the WKY or SHR. However we did observe significant albuminuria and proteinuria in the SHR groups fed the normal diet, which was further exacerbated by high fat. The exacerbation of the renal injury in the absence of changes in blood pressure indicates the high fat diet impaired renal function as a result of pressure independent mechanisms. Renal blood flow and pressure was not directly measured however, so a change in blood pressure transmission to the kidney as a result of the high fat diet can not be ruled out. One possible mechanism responsible for the development of the renal injury observed is enhanced expression of inflammatory cytokines which we found to be upregulated in the SHR group fed the high fat diet.
Inflammatory cytokines are characteristically produced by macrophages 14. An increase in the number of macrophages present in the adipose tissue as well as in the kidney is apparent in animal models of obesity 34. In obesity the adipose tissue recruits mature macrophages leading to the secretion of these inflammatory cytokines in higher levels than in lean animals 35. In animal models of hypertension there is also often an increase in the expression of inflammatory cytokines accompanied by a reduction in renal function 36, 37. We observed an increase in macrophage infiltration into the kidney cortex of the SHR fed a high fat diet compared to other groups. The elevation in macrophage infiltration therefore was as a result of the combination of obesity and hypertension present in the SHR high fat fed group. MCP-1 production is stimulated by the presence of adipokines and is a potent chemotactic factor involved in the recruitment of monocytes to the site of inflammation. We observed increases in MCP-1 excretion in both rat strains with 10 weeks high fat diet, however plasma MCP-1 levels were unchanged suggesting that the inflammation was subacute and isolated to the kidney.
The real-time PCR array data clearly shows that hypertension and obesity independently increase renal inflammatory gene expression however in combination the two risk factors have a compound effect resulting in a far greater number of inflammatory cytokines to be markedly upregulated. In particular in the SHR HF group we observed a large increase in mRNA levels of interleukin 1α (Il1α), a gene synthesized by activated macrophages that is associated with diabetic nephropathy and end stage renal disease 38. There is evidence that Il1α can activate NF[.kappa]B and plays a role in the genesis of inflammation by augmenting the transcription of proinflammatory genes 39. Interleukin IL18 (interferon-gamma-inducing factor) was also upregulated in the SHR HF group and has been identified as a diabetes candidate gene which plays an important role in energy homeostasis and insulin sensitivity 40. Ccl25 (TECK) a gene that acts through the chemokine receptor CCR9, was also upregulated in the SHR HF group compared with the WKY HF rats which is another macrophage chemotactic factor that also recruits monocytes and T-cells to the site of inflammation 41. There were a number of genes such as Bcl6, Ccl11, Cxcl1, and the interleukins; Il1f6, Il3, Il4, Il5 and Il5ra that are involved in inflammatory processes that were also significantly upregulated. In addition genes such as chemokine ligands Ccl12, Ccl21b, and chemokine receptors Ccr3, Ccr8, interleukins Il10, Il11, Il13, Il17b Il1f5 and receptors; Il1r2, Il8ra and Il8rb with smaller fold increases were also upregulated. While these genes are known to be inflammatory cytokines some of their detailed functions are less well understood, in this study we show they are associated with obesity and hypertension.
Disruption of the renal filtration barrier is closely associated with albuminuria. The podocyte associated protein nephrin has been linked with filtration barrier integrity and a downregulation in nephrin expression has been observed in animal models of hypertension, obesity and renal injury 42-44. Nephrin ran as a doublet on the western blot which has been previously reported 45. We observed a significant reduction in glomerular nephrin protein expression as a result of 10 weeks high fat diet in the WKY and SHR. Our data indicate that the downregulation in nephrin contributed to the breakdown of the filtration barrier and in combination with the inflammation observed in the high fat fed SHR reduced the ability of the slit diaphragm to prevent the leakage of albumin into the urine. A disassociation between the nephrin expression and albuminuria was observed, indicating that other mechanisms, for example inflammation, may be important in the development or exacerbation of renal injury. Ten weeks high fat diet with hypertension induced an increase in glomerular desmin expression, which has previously been reported in models of renal injury 46. The disassociation of the histological data from the albuminuria indicates that a combination of mechanisms may be responsible for the development of renal injury in this model.
Perspectives
In this study we provide evidence that high fat diet is a powerful stimulus for renal endothelial dysfunction in both a normotensive and a hypertensive rat model and exacerbates renal injury. We show that 10 weeks high fat diet in combination with hypertension results in a marked inflammatory response characterized by renal macrophage infiltration and increased MCP-1 excretion. The combination of high fat diet and hypertension was a strong stimulus for the upregulation of inflammatory cytokine mRNA compared with the high fat diet or hypertension alone. The identification of the role of inflammatory genes in response to hypertension with obesity may identify potential therapeutic targets for the protection of renal function in metabolic syndrome.
Supplementary Methods
All animal studies were approved by the Medical College of Georgia institutional review committee according to the National Institutes of Health guidelines for care and use of laboratory animals. Eight week old Wistar Kyoto and Spontaneously Hypertensive Rats were purchased from Charles River (Wilmington, MA, USA). Animals were housed in the Animal Services Unit at MCG with a 12 hour light-dark cycle, with free access to tap water. Rats were acclimatized for 7 days before use. In all experiments a minimum of four rats per group were used.
After 3, 6 and 10 weeks groups of WKY and SHR fed a normal or high fat diet were placed in metabolic cages for 24 hours for the collection of urine then euthanized for the collection of plasma and kidney tissue, stored at −80°C for subsequent analysis. At the same time points separate groups of rats underwent hyperinsulinemic euglycemic clamp experiments to monitor systemic insulin sensitivity.
In Vitro Assays and Enzyme Linked Immunoassays
Urinary protein excretion was measured using the BCA assay (Pierce, Rockford, IL, USA). Plasma free cholesterol levels were measured using the commercially available kit from Wako Diagnostics (Richmond VA, USA). Urinary microalbumin was measured by enzyme-linked immuno assay (ELISA) (SPI-bio, France). Urinary and plasma MCP-1 was measured by ELISA (BD-Biosciences, USA). Plasma insulin was measured by ELISA (Mercodia, USA) and plasma leptin levels were also measured using a commercially available ELISA (Linco/Millipore Billerica, MA, USA).
Hyperinsulinemic-Euglycemic Clamp Experiments
Male WKY and SHR were fasted overnight prior to the acute clamp experiments (n=4). Blood glucose measurements were taken from the tail vein and then rats were anesthetized with Inactin (1.2mg/100g body weight i.p). The jugular vein was cannulated for the infusion of 10% glucose in saline. The left femoral vein was canulated for the infusion of 0.01U/min insulin and the left femoral artery was cannulated for blood glucose measurements every 5 minutes using a glucometer. The glucose infusion rate was adjusted to maintain blood glucose levels at 125mg/dL for 90 minutes. The mean glucose infusion rate for the final 60 minutes was used to determine insulin sensitivity.
In Vitro Perfused Juxtamedullary Nephron Experiments
Experiments were conducted, in vitro, using the perfused juxtamedullary nephron technique, as previously described 18. Male WKY rats and SHR (n=4) were anesthetized with pentobarbital sodium (40 mg/kg body weight i.p). The kidney was perfused with Tyrode buffer solution containing 5.2% BSA and a complement of l-amino acids. The perfusate was consistently perfused with 95% O2-5% CO2. Perfusion pressure was set at 110 mmHg and monitored continuously. The inner cortical surface of the kidney was superfused with warmed (37°C) Tyrode buffer containing 1% bovine serum albumin. Vessel inner diameters were viewed by a video-microscopy and measured using an image shearing monitor. An afferent arteriole was selected and after 20 minutes equilibration the vessel was constricted using 1 mmol/L phenylephrine added to the superfusate. The kidney was removed and sectioned along the longitudinal axis, with care taken to leave the papilla intact on the dorsal two-thirds of the kidney. The vasculature was isolated as previously described18. The vessel diameter was measured and recorded as baseline. Acetylcholine was added to the perfusate to make a final concentration of 1×10−8 mmol/L then the concentration of acetylcholine was increased to 1×10−7 mmol, 1×10−6 and finally 1×10−5 mmol/L. Mean vessel diameter was recorded for 15 minutes at each concentration of acetylcholine. The vessel was perfused with 1% BSA for 15 minutes in the absence of acetylcholine, followed by 15 minutes incubation with sodium nitroprusside in order to exclude the contribution of the smooth muscle to any differences in dilatory response to acetylcholine.
ED-1 Immunohistochemistry
Five μm frozen kidney sections were cut and incubated overnight at room temperature with mouse anti-rat CD-68 primary antibody 1:100 (Serotec, Raleigh, NC, USA) followed by the secondary antibody goat anti-mouse IgG HRP 1:50 (Serotec, Raleigh, NC, USA) for 1 hour at room temperature. Slides were incubated with AEC substrate chromogen (DAKO, Carpinteria, CA, USA) for 20 minutes, rinsed and counterstained with Mayers hematoxylin for 30 seconds. Photographs were taken at 400X magnification and CD-68 positive cells were counted in a blinded fashion. The number of positive cells was calculated per square millimeter.
Nephrin and Desmin Immunofluorescence
Five μm frozen kidney sections were incubated over night at room temperature with goat anti-human nephrin primary antibody 1:50 (sc-19000, Santa Cruz Biotechnology, CA, USA) followed by rabbit anti-goat Cy-3 fluorescent tagged secondary antibody 1:400 for 1 hour (Zymed, CA, USA). Slides were counterstained with 300nmol DAPI (Invitrogen, CA, USA) for 1 minute and mounted using Prolong Gold anti-fade (Invitrogen, CA, USA). Desmin immunofluorescence was carried out using a 1: 50 dilution of mouse anti-human desmin primary antibody (Dako, CA, USA) followed by a 1:800 dilution of FITC tagged goat anti-mouse secondary antibody (Zymed, CA, USA). Photographs were taken at 400X maginification.
Glomeruli Isolation and Western Blotting
Kidneys were perfused with cold phosphate buffered saline (PBS) pH 7.4, removed and pressed through a sieve with 180μm diameter holes, filtered through a 200mm filter using a vacuum and suspended in PBS. The suspension was then filtered using a vacuum through a 70μm filter and the flow-through discarded. The tissue remaining on the filter was resuspended in PBS, centrifuged and the pellet containing glomeruli was then solubilized with RIPA buffer (Upstate, USA) containing 1% protease inhibitor. For nephrin western blots 50μg glomerular protein was run on an 8% Tris-glycine gel for 2 hours and primary goat anti-nephrin antibody (N-20, Santa Cruz, CA, USA) was incubated 1:200 and mouse anti-rat HSP90 1:1000 for one hour. This was followed by secondary donkey anti-goat and goat anti-mouse IgG HRP antibody 1:5000 for one hour. HSP90 was used as a loading control for nephrin because of its molecular weight and the use of an 8% gel. For desmin western blots, 20ug glomerular protein was run on a 10% Tris-glycine gel for 1.5 hours and primary mouse anti-human desmin antibody (clone D33 DAKO, CA, USA) was incubated 1:100 and mouse anti rat β-actin 1:10,000 for one hour followed by secondary goat anti-mouse IgG HRP antibody 1:5000 for one hour.
Real-time Polymerase Chain Reaction (PCR) Array Gene Expression Profiling
Total RNA was extracted from 20mg kidney cortex using the RNeasy® Plus Mini kit (Qiagen, CA, USA) according to the manufacturer's protocol. RNA concentrations were determined using absorbance at 260nm. Reverse-transcription was performed on 2 g of RNA from each sample using the RT2 PCR Array First Strand Kit (SuperArray Bioscience, MD, USA). Each cDNA synthesis reaction was diluted before being added to an RT2 Real-Time SYBR Green PCR Mastermix (SuperArray Bioscience, MD, USA) which was aliquoted onto a 96-well PCR Array plate, one sample per plate; each well contained a primer pair for a different gene or control. Thermal cycling and real-time detection were done with a Bio-Rad iCycler (Bio-Rad Laboratories, Hercules, CA): step 1) 95°C for 10 minutes, step 2) 95°C for 15 seconds followed by 60°C for 60 seconds (repeated 40 times). Melt-curve analysis was completed after each PCR reaction. Threshold cycle (Ct) values were normalized to a set of housekeeping genes to get a Ct value and fold-changes were calculated using the equation: (2-Ct test) · (2-Ct control)−1. Student's T-test was used for statistical analysis and changes greater than ±2 and p<0.05 were considered significant. Superarray results were confirmed by real-time PCR on three genes present on the arrays, picked at random.
Statistics
Data presented are mean ± SEM. Data was analyzed by 2-way analysis of variance for repeated measures followed by the Bonferoni and Dunn post-hoc test. The significance of difference in experiments with just one variable is calculated using an unpaired t-test. A value of P<0.05 was considered significant.
Sources of Funding
This work was supported by National Institute of Health grant HL59699 & HL074167.
Footnotes
Disclosures None.
Supplementary Material
Supplemental Table S1. Results from inflammatory gene Superarrays (n=2−3). Outlined data indicates a minimum of a two fold change in gene expression with significance (p<0.05).
References
- 1.Muller DN, Shagdarsuren E, Park JK, Dechend R, Mervaala E, Hampich F, Fiebeler A, Ju X, Finckenberg P, Theuer J, Viedt C, Kreuzer J, Heidecke H, Haller H, Zenke M, Luft FC. Immunosuppressive treatment protects against angiotensin II-induced renal damage. The American journal of pathology. 2002 Nov;161(5):1679–1693. doi: 10.1016/S0002-9440(10)64445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garrison RJ, Kannel WB, Stokes J,, 3rd, Castelli WP. Incidence and precursors of hypertension in young adults: the Framingham Offspring Study. Preventive medicine. 1987 Mar;16(2):235–251. doi: 10.1016/0091-7435(87)90087-9. [DOI] [PubMed] [Google Scholar]
- 3.NHANES http://www.cdc.gov/nchs/nhanes.htm.
- 4.NIDDK http://win.niddk.nih.gov/statistics/index.htm#preval.
- 5.AHA http://www.americanheart.org/presenter.jhtml?identifier=4621.
- 6.Kramer H, Luke A, Bidani A, Cao G, Cooper R, McGee D. Obesity and prevalent and incident CKD: the Hypertension Detection and Follow-Up Program. Am J Kidney Dis. 2005 Oct;46(4):587–594. doi: 10.1053/j.ajkd.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Lind L, Berne C, Lithell H. Prevalence of insulin resistance in essential hypertension. Journal of hypertension. 1995 Dec;13(12 Pt 1):1457–1462. [PubMed] [Google Scholar]
- 8.Shimamoto K, Ura N. Mechanisms of insulin resistance in hypertensive rats. Clin Exp Hypertens. 2006 Aug;28(6):543–552. doi: 10.1080/10641960600851900. [DOI] [PubMed] [Google Scholar]
- 9.Hall JE, Kuo JJ, da Silva AA, de Paula RB, Liu J, Tallam L. Obesity-associated hypertension and kidney disease. Current opinion in nephrology and hypertension. 2003 Mar;12(2):195–200. doi: 10.1097/00041552-200303000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Xu ZG, Lanting L, Vaziri ND, Li Z, Sepassi L, Rodriguez-Iturbe B, Natarajan R. Upregulation of angiotensin II type 1 receptor, inflammatory mediators, and enzymes of arachidonate metabolism in obese Zucker rat kidney: reversal by angiotensin II type 1 receptor blockade. Circulation. 2005 Apr 19;111(15):1962–1969. doi: 10.1161/01.CIR.0000161831.07637.63. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi K, Kanda T, Homma K, Tokuyama H, Okubo K, Takamatsu I, Tatematsu S, Kumagai H, Saruta T. Altered renal microvascular response in Zucker obese rats. Metabolism: clinical and experimental. 2002;51(12):1553–1561. doi: 10.1053/meta.2002.36311. [DOI] [PubMed] [Google Scholar]
- 12.Coimbra TM, Janssen U, Grone HJ, Ostendorf T, Kunter U, Schmidt H, Brabant G, Floege J. Early events leading to renal injury in obese Zucker (fatty) rats with type II diabetes. Kidney international. 2000 Jan;57(1):167–182. doi: 10.1046/j.1523-1755.2000.00836.x. [DOI] [PubMed] [Google Scholar]
- 13.Cindik N, Baskin E, Agras PI, Kinik ST, Turan M, Saatci U. Effect of obesity on inflammatory markers and renal functions. Acta Paediatr. 2005 Dec;94(12):1732–1737. doi: 10.1111/j.1651-2227.2005.tb01845.x. [DOI] [PubMed] [Google Scholar]
- 14.Eardley KS, Zehnder D, Quinkler M, Lepenies J, Bates RL, Savage CO, Howie AJ, Adu D, Cockwell P. The relationship between albuminuria, MCP-1/CCL2, and interstitial macrophages in chronic kidney disease. Kidney international. 2006 Apr;69(7):1189–1197. doi: 10.1038/sj.ki.5000212. [DOI] [PubMed] [Google Scholar]
- 15.Chow FY, Nikolic-Paterson DJ, Ma FY, Ozols E, Rollins BJ, Tesch GH. Monocyte chemoattractant protein-1-induced tissue inflammation is critical for the development of renal injury but not type 2 diabetes in obese db/db mice. Diabetologia. 2007 Feb;50(2):471–480. doi: 10.1007/s00125-006-0497-8. [DOI] [PubMed] [Google Scholar]
- 16.Sarafidis PA, Ruilope LM. Insulin resistance, hyperinsulinemia, and renal injury: mechanisms and implications. American journal of nephrology. 2006;26(3):232–244. doi: 10.1159/000093632. [DOI] [PubMed] [Google Scholar]
- 17.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW,, Jr CCR2 modulates inflammatory and metabolic effects of high-fat feeding. The Journal of clinical investigation. 2006 Jan;116(1):115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knudson JD, Dincer UD, Dick GM, Shibata H, Akahane R, Saito M, Tune JD. Leptin resistance extends to the coronary vasculature in prediabetic dogs and provides a protective adaptation against endothelial dysfunction. American journal of physiology. 2005 Sep;289(3):H1038–1046. doi: 10.1152/ajpheart.00244.2005. [DOI] [PubMed] [Google Scholar]
- 19.Wassmann S, Laufs U, Baumer AT, Muller K, Ahlbory K, Linz W, Itter G, Rosen R, Bohm M, Nickenig G. HMG-CoA reductase inhibitors improve endothelial dysfunction in normocholesterolemic hypertension via reduced production of reactive oxygen species. Hypertension. 2001 Jun;37(6):1450–1457. doi: 10.1161/01.hyp.37.6.1450. [DOI] [PubMed] [Google Scholar]
- 20.Yamagishi SI, Edelstein D, Du XL, Kaneda Y, Guzman M, Brownlee M. Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A. The Journal of biological chemistry. 2001 Jul 6;276(27):25096–25100. doi: 10.1074/jbc.M007383200. [DOI] [PubMed] [Google Scholar]
- 21.Ochodnicky P, Vettoretti S, Henning RH, Buikema H, Van Dokkum RP, de Zeeuw D. Endothelial dysfunction in chronic kidney disease: determinant of susceptibility to end-organ damage and therapeutic response. Journal of nephrology. 2006 May-Jun;19(3):246–258. [PubMed] [Google Scholar]
- 22.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006 Dec 14;444(7121):881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 23.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006 Dec 14;444(7121):840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 24.Guo M, Ricardo SD, Deane JA, Shi M, Cullen-McEwen L, Bertram JF. A stereological study of the renal glomerular vasculature in the db/db mouse model of diabetic nephropathy. Journal of anatomy. 2005 Dec;207(6):813–821. doi: 10.1111/j.1469-7580.2005.00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall JE, Brands MW, Dixon WN, Smith MJ,, Jr. Obesity-induced hypertension. Renal function and systemic hemodynamics. Hypertension. 1993 Sep;22(3):292–299. doi: 10.1161/01.hyp.22.3.292. [DOI] [PubMed] [Google Scholar]
- 26.Griffin KA, Bidani AK. Hypertensive renal damage: insights from animal models and clinical relevance. Current hypertension reports. 2004 Apr;6(2):145–153. doi: 10.1007/s11906-004-0091-8. [DOI] [PubMed] [Google Scholar]
- 27.Frontoni S, Bracaglia D, Gigli F. Relationship between autonomic dysfunction, insulin resistance and hypertension, in diabetes. Nutr Metab Cardiovasc Dis. 2005 Dec;15(6):441–449. doi: 10.1016/j.numecd.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Shankar RR, Wu Y, Shen HQ, Zhu JS, Baron AD. Mice with gene disruption of both endothelial and neuronal nitric oxide synthase exhibit insulin resistance. Diabetes. 2000 May;49(5):684–687. doi: 10.2337/diabetes.49.5.684. [DOI] [PubMed] [Google Scholar]
- 29.Neels JG, Olefsky JM. Inflamed fat: what starts the fire? The Journal of clinical investigation. 2006 Jan;116(1):33–35. doi: 10.1172/JCI27280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koh KK, Han SH, Quon MJ. Inflammatory markers and the metabolic syndrome: insights from therapeutic interventions. Journal of the American College of Cardiology. 2005 Dec 6;46(11):1978–1985. doi: 10.1016/j.jacc.2005.06.082. [DOI] [PubMed] [Google Scholar]
- 31.Chudek J, Adamczak M, Nieszporek T, Wiecek A. The adipose tissue as an endocrine organ--a nephrologists' perspective. Contributions to nephrology. 2006;151:70–90. doi: 10.1159/000095320. [DOI] [PubMed] [Google Scholar]
- 32.Shoelson SE, Lee J, Yuan M. Inflammation and the IKK beta/I kappa B/NF-kappa B axis in obesity- and diet-induced insulin resistance. Int J Obes Relat Metab Disord. 2003 Dec;27(Suppl 3):S49–52. doi: 10.1038/sj.ijo.0802501. [DOI] [PubMed] [Google Scholar]
- 33.Steinberg HO, Tarshoby M, Monestel R, Hook G, Cronin J, Johnson A, Bayazeed B, Baron AD. Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. The Journal of clinical investigation. 1997 Sep 1;100(5):1230–1239. doi: 10.1172/JCI119636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW,, Jr. Obesity is associated with macrophage accumulation in adipose tissue. The Journal of clinical investigation. 2003 Dec;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol. 2004 Nov;15(11):2792–2800. doi: 10.1097/01.ASN.0000141966.69934.21. [DOI] [PubMed] [Google Scholar]
- 36.Franco M, Martinez F, Rodriguez-Iturbe B, Johnson RJ, Santamaria J, Montoya A, Nepomuceno T, Bautista R, Tapia E, Herrera-Acosta J. Angiotensin II, interstitial inflammation, and the pathogenesis of salt-sensitive hypertension. Am J Physiol Renal Physiol. 2006 Dec;291(6):F1281–1287. doi: 10.1152/ajprenal.00221.2006. [DOI] [PubMed] [Google Scholar]
- 37.Vieira JM,, Jr., Rodrigues LT, Mantovani E, Delle H, Mattar AL, Malheiros DM, Noronha IL, Fujihara CK, Zatz R. Statin monotherapy attenuates renal injury in a salt-sensitive hypertension model of renal disease. Nephron. 2005;101(4):p82–91. doi: 10.1159/000087576. [DOI] [PubMed] [Google Scholar]
- 38.Bensen JT, Langefeld CD, Hawkins GA, Green LE, Mychaleckyj JC, Brewer CS, Kiger DS, Binford SM, Colicigno CJ, Allred DC, Freedman BI, Bowden DW. Nucleotide variation, haplotype structure, and association with end-stage renal disease of the human interleukin-1 gene cluster. Genomics. 2003 Aug;82(2):194–217. doi: 10.1016/s0888-7543(03)00123-x. [DOI] [PubMed] [Google Scholar]
- 39.Werman A, Werman-Venkert R, White R, Lee JK, Werman B, Krelin Y, Voronov E, Dinarello CA, Apte RN. The precursor form of IL-1alpha is an intracrine proinflammatory activator of transcription. Proceedings of the National Academy of Sciences of the United States of America. 2004 Feb 24;101(8):2434–2439. doi: 10.1073/pnas.0308705101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Netea MG, Joosten LA, Lewis E, Jensen DR, Voshol PJ, Kullberg BJ, Tack CJ, van Krieken H, Kim SH, Stalenhoef AF, van de Loo FA, Verschueren I, Pulawa L, Akira S, Eckel RH, Dinarello CA, van den Berg W, van der Meer JW. Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nature medicine. 2006 Jun;12(6):650–656. doi: 10.1038/nm1415. [DOI] [PubMed] [Google Scholar]
- 41.Norment AM, Bogatzki LY, Gantner BN, Bevan MJ. Murine CCR9, a chemokine receptor for thymus-expressed chemokine that is up-regulated following pre-TCR signaling. J Immunol. 2000 Jan 15;164(2):639–648. doi: 10.4049/jimmunol.164.2.639. [DOI] [PubMed] [Google Scholar]
- 42.Macconi D, Bonomelli M, Benigni A, Plati T, Sangalli F, Longaretti L, Conti S, Kawachi H, Hill P, Remuzzi G, Remuzzi A. Pathophysiologic implications of reduced podocyte number in a rat model of progressive glomerular injury. The American journal of pathology. 2006 Jan;168(1):42–54. doi: 10.2353/ajpath.2006.050398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Forbes JM, Bonnet F, Russo LM, Burns WC, Cao Z, Candido R, Kawachi H, Allen TJ, Cooper ME, Jerums G, Osicka TM. Modulation of nephrin in the diabetic kidney: association with systemic hypertension and increasing albuminuria. Journal of hypertension. 2002 May;20(5):985–992. doi: 10.1097/00004872-200205000-00034. [DOI] [PubMed] [Google Scholar]
- 44.Blanco S, Bonet J, Lopez D, Casas I, Romero R. ACE inhibitors improve nephrin expression in Zucker rats with glomerulosclerosis. Kidney Int Suppl. 2005 Jan;(93):S10–14. doi: 10.1111/j.1523-1755.2005.09303.x. [DOI] [PubMed] [Google Scholar]
- 45.Yan K, Khoshnoodi J, Ruotsalainen V, Tryggvason K. N-linked glycosylation is critical for the plasma membrane localization of nephrin. J Am Soc Nephrol. 2002 May;13(5):1385–1389. doi: 10.1097/01.asn.0000013297.11876.5b. [DOI] [PubMed] [Google Scholar]
- 46.Nagase M, Shibata S, Yoshida S, Nagase T, Gotoda T, Fujita T. Podocyte injury underlies the glomerulopathy of Dahl salt-hypertensive rats and is reversed by aldosterone blocker. Hypertension. 2006 Jun;47(6):1084–1093. doi: 10.1161/01.HYP.0000222003.28517.99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1. Results from inflammatory gene Superarrays (n=2−3). Outlined data indicates a minimum of a two fold change in gene expression with significance (p<0.05).