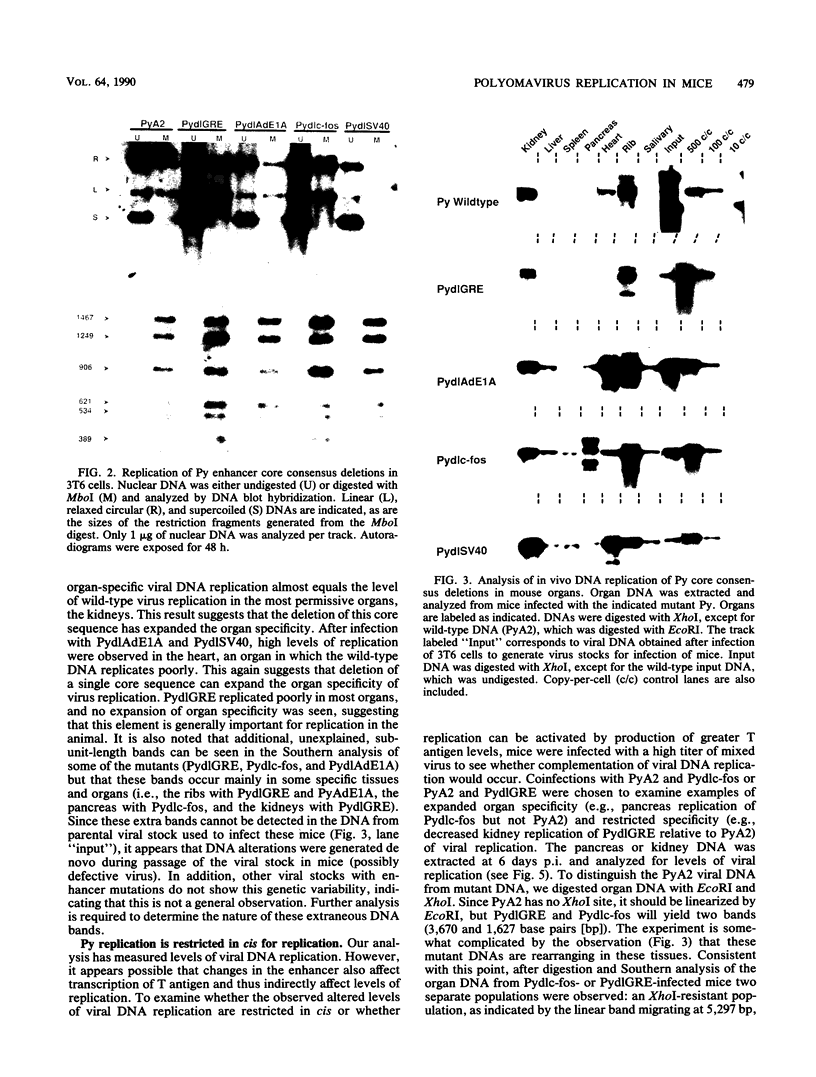
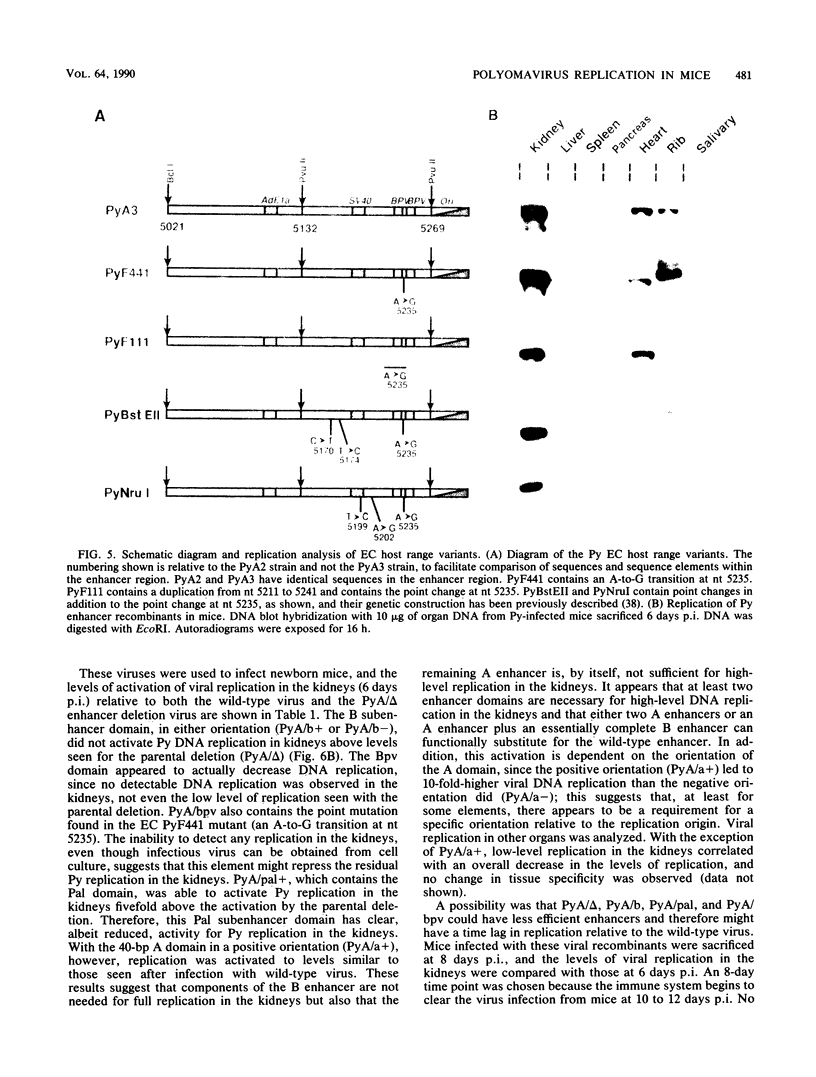
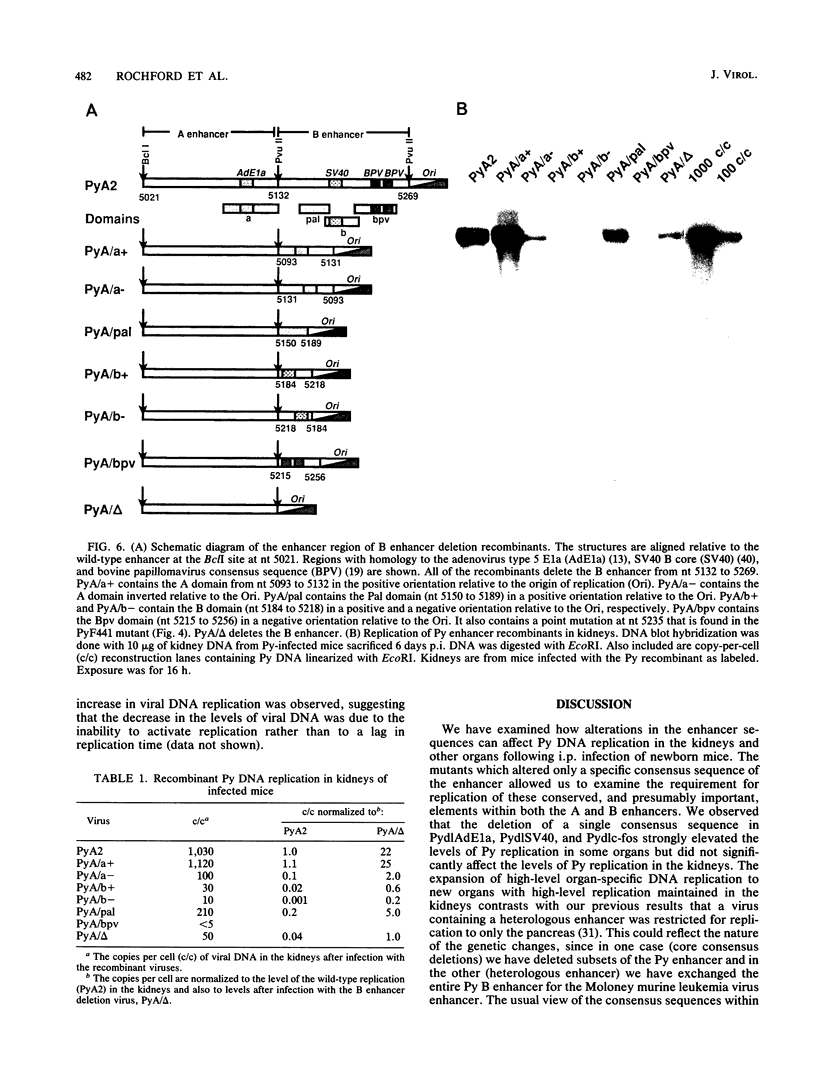
Abstract
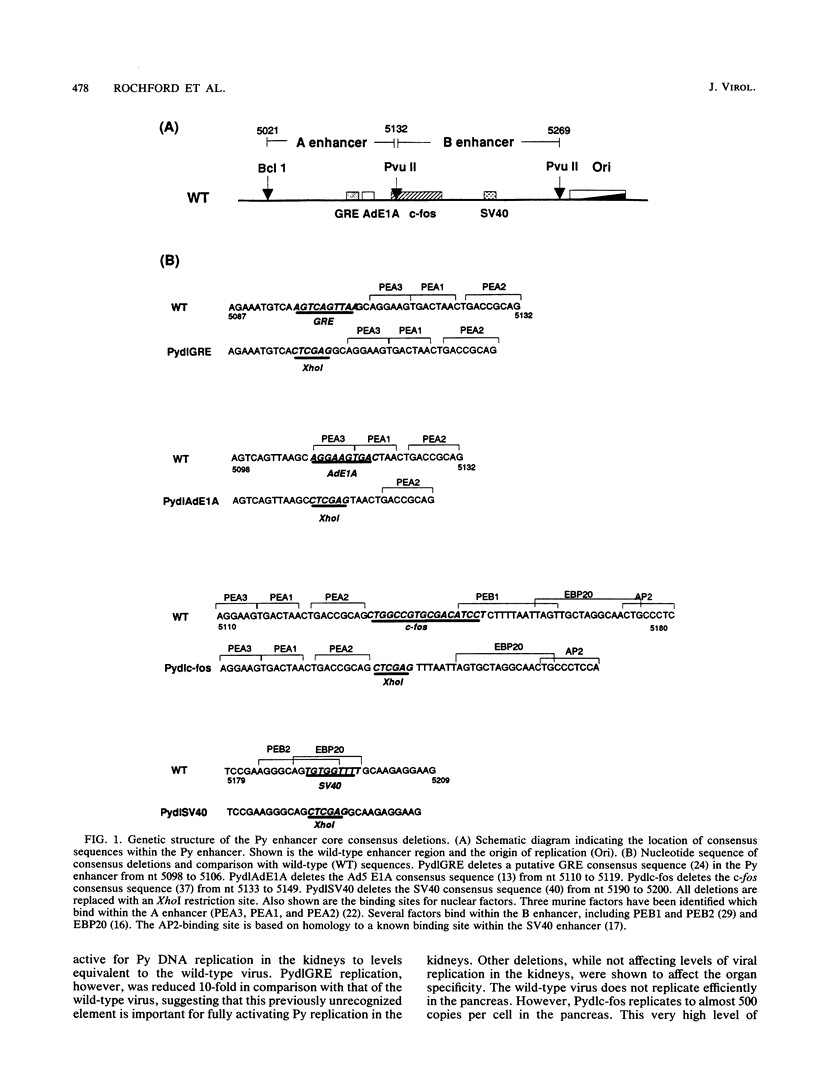
In this report, we describe the first systematic analysis of the genetic requirements for polyomavirus (Py) enhancer-activated viral DNA replication during the acute phase of infection in mice. Four mutants were made which substituted XhoI sites for conserved enhancer consensus sequences (adenovirus type 5 E1A, c-fos, simian virus 40, and a glucocorticoidlike consensus sequence). Viral DNA replication in infected mouse organs was measured by DNA blot analysis. Only the loss of the glucocorticoidlike consensus sequence element significantly reduced Py DNA replication in the kidneys, the primary target organ for viral replication. The loss of the c-fos, adenovirus type 5 E1A, or simian virus 40 consensus sequences, however, expanded organ-specific viral DNA replication, relative to wild-type Py, by allowing high-level replication in the pancreas or heart or both. Analysis of Py variants selected for replication in undifferentiated embryonal carcinoma cell lines (PyF441, PyF111) showed that there was little change in levels of viral DNA replication in kidneys and other organs as compared with those in the wild-type virus. If the entire B enhancer is deleted, only low overall levels of viral replication are observed. Wild-type levels of replication in the kidneys can be reconstituted by addition of a single domain from within the A enhancer (nucleotides 5094 to 5132) to the B enhancer deletion virus, suggesting that a single domain from the A enhancer can functionally substitute for the entire B enhancer. This also indicates that the determinants for kidney-specific replication are not found in the B enhancer.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell B. A., Villarreal L. P. Functional analysis of the individual enhancer core sequences of polyomavirus: cell-specific uncoupling of DNA replication from transcription. Mol Cell Biol. 1988 May;8(5):1993–2004. doi: 10.1128/mcb.8.5.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell B. A., Villarreal L. P. Host species specificity of polyomavirus DNA replication is not altered by simian virus 40 72-base-pair repeats. Mol Cell Biol. 1985 Jun;5(6):1534–1537. doi: 10.1128/mcb.5.6.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell B. A., Villarreal L. P. Tissue specific phenotypes of polyomavirus A and B enhancer transpositions and duplications: positional and nonpositional effects on replication in lymphoid cells. Virus Genes. 1987 Nov;1(1):23–34. doi: 10.1007/BF00125683. [DOI] [PubMed] [Google Scholar]
- Dawe C. J., Freund R., Mandel G., Ballmer-Hofer K., Talmage D. A., Benjamin T. L. Variations in polyoma virus genotype in relation to tumor induction in mice. Characterization of wild type strains with widely differing tumor profiles. Am J Pathol. 1987 May;127(2):243–261. [PMC free article] [PubMed] [Google Scholar]
- De Simone V., Amati P. Replicative cis-advantage of polyomavirus regulatory region mutants in different murine cell lines. J Virol. 1987 May;61(5):1615–1620. doi: 10.1128/jvi.61.5.1615-1620.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Simone V., La Mantia G., Lania L., Amati P. Polyomavirus mutation that confers a cell-specific cis advantage for viral DNA replication. Mol Cell Biol. 1985 Aug;5(8):2142–2146. doi: 10.1128/mcb.5.8.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delli Bovi P., De Simone V., Giordano R., Amati P. Polyomavirus growth and persistence in Friend erythroleukemic cells. J Virol. 1984 Feb;49(2):566–571. doi: 10.1128/jvi.49.2.566-571.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubensky T. W., Villarreal L. P. The primary site of replication alters the eventual site of persistent infection by polyomavirus in mice. J Virol. 1984 May;50(2):541–546. doi: 10.1128/jvi.50.2.541-546.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura F. K., Deininger P. L., Friedmann T., Linney E. Mutation near the polyoma DNA replication origin permits productive infection of F9 embryonal carcinoma cells. Cell. 1981 Mar;23(3):809–814. doi: 10.1016/0092-8674(81)90445-1. [DOI] [PubMed] [Google Scholar]
- Fujimura F. K. Point mutation in the polyomavirus enhancer alters local DNA conformation. Nucleic Acids Res. 1988 Mar 25;16(5):1987–1997. doi: 10.1093/nar/16.5.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing P., Shenk T. The adenovirus type 5 E1A transcriptional control region contains a duplicated enhancer element. Cell. 1983 Jul;33(3):695–703. doi: 10.1016/0092-8674(83)90012-0. [DOI] [PubMed] [Google Scholar]
- Herbomel P., Bourachot B., Yaniv M. Two distinct enhancers with different cell specificities coexist in the regulatory region of polyoma. Cell. 1984 Dec;39(3 Pt 2):653–662. doi: 10.1016/0092-8674(84)90472-0. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Johnson P. F., Landschulz W. H., Graves B. J., McKnight S. L. Identification of a rat liver nuclear protein that binds to the enhancer core element of three animal viruses. Genes Dev. 1987 Apr;1(2):133–146. doi: 10.1101/gad.1.2.133. [DOI] [PubMed] [Google Scholar]
- Jones N. C., Rigby P. W., Ziff E. B. Trans-acting protein factors and the regulation of eukaryotic transcription: lessons from studies on DNA tumor viruses. Genes Dev. 1988 Mar;2(3):267–281. doi: 10.1101/gad.2.3.267. [DOI] [PubMed] [Google Scholar]
- Katinka M., Yaniv M., Vasseur M., Blangy D. Expression of polyoma early functions in mouse embryonal carcinoma cells depends on sequence rearrangements in the beginning of the late region. Cell. 1980 Jun;20(2):393–399. doi: 10.1016/0092-8674(80)90625-x. [DOI] [PubMed] [Google Scholar]
- Lusky M., Berg L., Weiher H., Botchan M. Bovine papilloma virus contains an activator of gene expression at the distal end of the early transcription unit. Mol Cell Biol. 1983 Jun;3(6):1108–1122. doi: 10.1128/mcb.3.6.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthman H., Magnusson G. High efficiency polyoma DNA transfection of chloroquine treated cells. Nucleic Acids Res. 1983 Mar 11;11(5):1295–1308. doi: 10.1093/nar/11.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Martin M. E., Piette J., Yaniv M., Tang W. J., Folk W. R. Activation of the polyomavirus enhancer by a murine activator protein 1 (AP1) homolog and two contiguous proteins. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5839–5843. doi: 10.1073/pnas.85.16.5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melin F., Pinon H., Reiss C., Kress C., Montreau N., Blangy D. Common features of polyomavirus mutants selected on PCC4 embryonal carcinoma cells. EMBO J. 1985 Jul;4(7):1799–1803. doi: 10.1002/j.1460-2075.1985.tb03853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miksicek R., Heber A., Schmid W., Danesch U., Posseckert G., Beato M., Schütz G. Glucocorticoid responsiveness of the transcriptional enhancer of Moloney murine sarcoma virus. Cell. 1986 Jul 18;46(2):283–290. doi: 10.1016/0092-8674(86)90745-2. [DOI] [PubMed] [Google Scholar]
- Mueller C. R., Mes-Masson A. M., Bouvier M., Hassell J. A. Location of sequences in polyomavirus DNA that are required for early gene expression in vivo and in vitro. Mol Cell Biol. 1984 Dec;4(12):2594–2609. doi: 10.1128/mcb.4.12.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller C. R., Muller W. J., Hassell J. A. The polyomavirus enhancer comprises multiple functional elements. J Virol. 1988 May;62(5):1667–1678. doi: 10.1128/jvi.62.5.1667-1678.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller W. J., Dufort D., Hassell J. A. Multiple subelements within the polyomavirus enhancer function synergistically to activate DNA replication. Mol Cell Biol. 1988 Nov;8(11):5000–5015. doi: 10.1128/mcb.8.11.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller W. J., Mueller C. R., Mes A. M., Hassell J. A. Polyomavirus origin for DNA replication comprises multiple genetic elements. J Virol. 1983 Sep;47(3):586–599. doi: 10.1128/jvi.47.3.586-599.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette J., Yaniv M. Two different factors bind to the alpha-domain of the polyoma virus enhancer, one of which also interacts with the SV40 and c-fos enhancers. EMBO J. 1987 May;6(5):1331–1337. doi: 10.1002/j.1460-2075.1987.tb02372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rochford R., Campbell B. A., Villarreal L. P. A pancreas specificity results from the combination of polyomavirus and Moloney murine leukemia virus enhancer. Proc Natl Acad Sci U S A. 1987 Jan;84(2):449–453. doi: 10.1073/pnas.84.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruley H. E., Fried M. Sequence repeats in a polyoma virus DNA region important for gene expression. J Virol. 1983 Jul;47(1):233–237. doi: 10.1128/jvi.47.1.233-237.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekikawa K., Levine A. J. Isolation and characterization of polyoma host range mutants that replicate in nullipotential embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1100–1104. doi: 10.1073/pnas.78.2.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeda E., Arrand J. R., Smolar N., Walsh J. E., Griffin B. E. Coding potential and regulatory signals of the polyoma virus genome. Nature. 1980 Jan 31;283(5746):445–453. doi: 10.1038/283445a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tang W. J., Berger S. L., Triezenberg S. J., Folk W. R. Nucleotides in the polyomavirus enhancer that control viral transcription and DNA replication. Mol Cell Biol. 1987 May;7(5):1681–1690. doi: 10.1128/mcb.7.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R. Identification of a protein-binding site that mediates transcriptional response of the c-fos gene to serum factors. Cell. 1986 Aug 15;46(4):567–574. doi: 10.1016/0092-8674(86)90882-2. [DOI] [PubMed] [Google Scholar]
- Tseng R. W., Fujimura F. K. Multiple domains in the polyomavirus B enhancer are required for productive infection of F9 embryonal carcinoma cells. J Virol. 1988 Aug;62(8):2890–2895. doi: 10.1128/jvi.62.8.2890-2895.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldman G. M., Lupton S., Kamen R. Polyomavirus enhancer contains multiple redundant sequence elements that activate both DNA replication and gene expression. Mol Cell Biol. 1985 Apr;5(4):649–658. doi: 10.1128/mcb.5.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiher H., König M., Gruss P. Multiple point mutations affecting the simian virus 40 enhancer. Science. 1983 Feb 11;219(4585):626–631. doi: 10.1126/science.6297005. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Satake M., Ito Y. Two overlapping sequence motifs within the polyomavirus enhancer are independently the targets of stimulation by both the tumor promoter 12-O-tetradecanoylphorbol-13-acetate and the Ha-ras oncogene. J Virol. 1989 Mar;63(3):1040–1048. doi: 10.1128/jvi.63.3.1040-1048.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villiers J., Schaffner W., Tyndall C., Lupton S., Kamen R. Polyoma virus DNA replication requires an enhancer. Nature. 1984 Nov 15;312(5991):242–246. doi: 10.1038/312242a0. [DOI] [PubMed] [Google Scholar]