Abstract
Background
Diffusion tensor imaging (DTI) has revealed microstructural aspects of adolescent brain development, the cognitive correlates of which remain relatively uncharacterized.
Method
DTI was used to assess white matter microstructure in 18 typically developing adolescents (ages 16–18). Fractional anisotropy (FA) and mean diffusion (MD) were evaluated within the splenium and body of the corpus callosum in relation to cognitive performance.
Results
Visuospatial construction abilities were associated with white matter integrity in both the splenium and body of the corpus callosum, while only splenium integrity was associated with language and psychomotor function.
Conclusions
Results suggest that, for typically developing adolescents, white matter coherence positively relates to visuospatial, psychomotor, and language skills. These findings may have implications for the cognitive functioning of clinical populations in which typical white matter development is altered.
Keywords: diffusion tensor imaging, adolescence, white matter maturation, microstructure, development, cognition, neuropsychology, splenium, corpus callosum
The corpus callosum (CC), the brain’s largest fiber tract, is the main conduit for cerebral interhemispheric transfer. Organization within the commissure is roughly topographical, with anterior fibers that pass through the rostrum and genu of the CC connecting homologous inferior frontal and parietal regions, and fibers that pass through the posterior splenium linking regions in the temporo-parieto-occipital junction (De Lacoste et al., 1985). Studies of adult patients with developmental or acquired CC damage suggest that CC structural integrity is linked to cognitive performance in various domains including bimanual coordination and visual-motor integration (Berlucchi et al., 1995; Eliassen et al., 2000). Furthermore, structural CC anomalies relate to interhemispheric transfer performance deficits in several developmental disorders including dyslexia (Hynd et al., 1995) and fetal alcohol spectrum disorders (Roebuck et al., 2002). Previous magnetic resonance imaging (MRI) research indicates that the CC has a protracted growth pattern, with development continuing into adolescence, although the slope of this growth curve attenuates by young adulthood (Pujol et al., 1993). Although it is thought that the CC mainly serves an excitatory role in interhemispheric communication, the tract may at times transmit inhibitory impulses (Bloom and Hynd, 2005). In summary, the CC is a relatively late maturing brain structure that subserves a variety of cognitive processes.
Diffusion tensor imaging (DTI) is a magnetic resonance technique in which images are made sensitive to the ongoing random motions of water molecules in tissues. This sensitivity is created by the application of additional magnetic field gradients along a set of specified directions. Diffusion along these gradients causes signal loss that is proportional to the local diffusion and thus, by collecting a set of images with different diffusion encoding gradient directions, the directional dependence of the local diffusion can be mapped. Local variations in diffusion are caused primarily by the interaction of the diffusing water molecules with complex tissue geometry. In white matter, molecular movement is restricted by microstructural barriers, yielding preferred directions of motion, so diffusion is said to be anisotropic (Moseley et al., 2002). By measuring the diffusion along different directions, an estimate of its directional dependence can be derived. However, it should be noted that the movement of water within biological tissue is complicated, and the actual details of diffusion depend on a variety of factors, including extracellular volume fraction (Sen and Basser, 2005), as well as the neuronal volume fraction, axon spacing, and myelin permeability.
Using a simple Gaussian model, the directional dependence of the diffusion can be characterized by a diffusion tensor, a 3 × 3 symmetric matrix characterized by 3 eigenvectors and their associated eigenvalues. The average of the eigenvalues of this tensor is the mean diffusion (MD) in the voxel. The anisotropy of the diffusion is related to the variance in the eigenvalues. This is typically constructed by a normalized variance called the fractional anisotropy (FA), which is proportional to the standard deviation of the eigenvalues divided by the magnitude of the diffusion tensor; FA values range from 0 to 1 (Basser and Pierpaoli, 1996). Higher FA values indicate greater anisotropy, which is generally thought to reflect more coherent tissue structure. MD is an estimate of the local diffusion tensor, or diffusivity, averaged over spatial directions. Accordingly, low FA and high MD values in white matter suggest compromised white matter integrity. Studies of clinical populations have suggested that, at least in some cases, microstructural integrity as revealed by DTI may be a more sensitive indicator of white matter damage than studies of macrostructure based on traditional volumetric analyses of MR images (Ma et al., 2005; Pfefferbaum and Sullivan, 2002; Pfefferbaum et al., 2000).
Dovetailing with morphometric MRI findings, DTI studies indicate that CC structure changes in the context of brain maturation. Specifically, cross-sectional samples of children, adolescents, and young adults show CC increases in FA (Barnea-Goraly et al., 2005; Ben Bashat et al., 2005; Snook et al., 2005) and decreases in MD (Snook et al., 2005) with development, suggesting greater fiber structure coherence and diffusion restriction as the CC matures. In children and adolescents, age-related FA increases in the CC body have been associated with local white matter macrostructure density increases (Barnea-Goraly et al., 2005). Few other studies have directly examined the degree to which white matter macrostructural and microstructural developmental processes share the same trajectory.
Interestingly, growth within the CC shows a region specific pattern, with volume increases slowing in an anterior to posterior direction (Giedd et al., 1999; Paus et al., 2001). This has led to the conclusion that the splenium may be a later maturing subregion of the CC, compared to anterior portions of the tract (Ben Bashat et al., 2005). The notion of region-specific developmental trajectories within the CC has implications for the maturation of cognitive skills that rely on interhemispheric information transfer. Increasingly, DTI is used to probe associations between white matter maturation, cognitive development, and cognitive decline (see Moseley et al., 2002 for a comprehensive review). Studies of normal aging have associated decreases in white matter FA with poorer performance on a variety of behavioral tasks including those tapping psychomotor, reasoning, and processing speed abilities (Moseley et al., 2002). Other investigations have reported relationships between diffusion and neuropsychological performance in adults with dyslexia (Klingberg et al., 2000) and schizophrenia (Nestor et al., 2004). In children, the development of working memory and reading abilities is related to FA increases in functionally-associated regions of the left frontal and temporal lobes, respectively (Nagy et al., 2004). A study examining the development of cognitive control abilities (Liston et al., 2005) revealed that age-related increases in diffusion restriction of frontal-striatal radiations predicted better performance on a cognitive control task among both children and adults. This suggests that microstructural coherence gains are part of typical maturation in the frontal-striatal circuitry underlying response inhibition.
The purpose of the present study was to relate white matter microstructure of the CC to cognitive performance in a group of typically developing adolescents. Specifically, we examined DTI values in CC regions of interest in conjunction with test scores from a comprehensive neuropsychological test battery assessing 6 cognitive domains: 1) visuospatial, 2) learning and memory, 3) working memory, 4) psychomotor, 5) executive functioning, and 6) language ability. Based on previous research correlating diffusion variables with IQ in a developmental sample (Schmithorst et al., 2005), we generally hypothesized that high FA and low MD white matter values would be associated with better neuropsychological test scores. More specifically, we predicted that subregion-specific dissociable relationships between diffusion properties and behavior would emerge within the CC, based on putative topography of the commissure and its connections with the cortex. That is, structural integrity of CC subregions should relate to those aspects of cognition that are subserved by homologous anatomical regions connected through a particular locus of the CC. Thus, because the splenium interconnects regions of the parietal and temporal cortices (De Lacoste et al., 1985), it was expected that posterior CC functioning would relate to performance on tests of visual-spatial functioning and language. Accordingly, we did not expect the posterior CC to correlate with executive functioning, as these abilities are subserved by the frontal cortices, which are connected by anterior regions of the CC. Additionally, we did not expect tests of memory to relate to CC integrity, as there is some indication from clinical literature that interhemispheric integration of memory function relies on extracallosal transfer mechanisms (Clark & Geffen, 1989).
Methods
Participants
Participants were 18 healthy adolescents ages 16 to 18 recruited by flyer distribution at local high schools as part of a larger study (Medina et al., 2007; Nagel et al., 2006; Schweinsburg et al., 2005). Written consent and assent, approved by the University of California San Diego Human Research Protections Program, were obtained from all participants and, for those under age 18, from their legal guardian. To minimize the potential influence of confounding factors, participants underwent a rigorous screening procedure involving youth and parent interviews to exclude adolescents with histories of prenatal substance exposure, birth complications, medical problems, neurological dysfunction, head injury, learning disability, psychotropic medication use, or substance use. Youths with possible histories of psychiatric problems (including attention, conduct, mood, anxiety, or psychotic disorder) were also excluded, as determined by positive indicators on either youth or parent administration of the NIMH Diagnostic Interview Schedule for Children Predictive Scales (DISC-PS-4.32b; Lucas et al., 2001; Shaffer et al., 2000).
Measures
Structured clinical interview
Adolescent participants and parents were separately administered confidential structured clinical interviews (Brown et al., 1989) by bachelor’s and master’s level psychometrists. Interviews ascertained demographic characteristics, medical and developmental history, and social and academic functioning. Parents (typically the biological mother) were asked about the teen’s family history, fetal and infant development, childhood behavior, and parent education and occupation (Hollingshead, 1965), and were given the Child Behavior Checklist (Achenbach, 2001).
Neuropsychological testing
A 2.5-hour standardized battery of neuropsychological tests was selected to cover a range of cognitive domains, including visuospatial, learning and memory, working memory, psychomotor, executive, and language functioning. The battery consisted of the Digit Symbol and Digit Span subtests of the Wechsler Adult Intelligence Scale-III (WAIS-III; Wechsler, 1997), the Vocabulary and Block Design subtests of the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999), the Tower and Trail Making subtests of the Delis-Kaplan Executive Function System (D-KEFS; Delis et al., 2001), the Paced Auditory Serial Addition Test (PASAT; Gronwall, 1977), the Rey-Osterrieth Complex Figure Test (ROCF; Osterrieth, 1944), the Wide Range Achievement Test, version 3 (WRAT-3; Wilkinson, 1993), and the California Verbal Learning Test-2nd Edition (CVLT-II; Delis et al., 1994). Handedness was assessed with the Edinburgh Handedness Inventory (Oldfield, 1971), and only right-handed individuals were included in the study. Test sequence was standard across participants, with more taxing, timed tasks occurring earlier in the battery and interspersed with less demanding tests. A break was given after 1.5 hours.
Procedures
All participants were administered the neuropsychological test battery by a trained, reliable psychometrist in a quiet testing room at the research site. Drawings (e.g., ROCF) were double-scored, and all scores were checked by a separate trained psychometrist and reviewed by a clinical neuropsychologist (BJN or SFT).
Imaging was performed on a 1.5 T GE Signa LX MRI scanner (Milwaukee, MI). Axial images covering the whole brain were acquired with a high angular resolution diffusion encoding sequence using a spiral acquisition (Frank, 2002; Frank, 2001). Diffusion was encoded with a spin echo preparation along 42 diffusion directions with b = 1600 s/mm2 with 4 averages. Image parameters for the DTI sequence were: TR = 3000 ms, TE = 103 ms, and NEX = 4, 128 × 128 matrix, FOV = 25 cm, 32 slices covering all cerebral white matter, voxel size 1.95 × 1.95 × 3.9 mm3, acquisition time=17:36 min. A high-resolution T1-weighted structural image informed structure localization, and was collected in the sagittal plane using an inversion recovery prepared T1-weighted 3D spiral fast spin echo sequence (Wong et al., 2000) (TR = 2000 ms, TE = 16 ms, FOV = 240 mm, 128 continuous slices, resolution = 0.94 × 0.94 mm × 1.33 mm, acquisition time = 8:36 min). The diffusion tensor was estimated for each voxel by fitting the full data from six unknowns in the diffusion tensor via linear regression (Basser et al., 1994a). FA and MD images were created after estimation of the diffusion tensor (Basser et al., 1994b).
Regions of interest (ROIs) were drawn onto the b = 0 T2-weighted image (inherently co-registered with FA and trace images) to cover the splenium, body, and genu of the CC (see Figure 1) using the method described by Pfefferbaum et al. (Pfefferbaum et al., 2005; Pfefferbaum et al., 2000) by two trained raters blind to participant characteristics who achieved excellent reliability (min ICC = .91) using AFNI (afni.nimh.nih.gov)(Cox, 1996; Cox and Hyde, 1997). Alignment was checked by overlaying ROI assignments on the high resolution image; voxels not clearly within white matter according to both the b = 0 image and the high resolution image were removed from the ROI. FA and MD values were extracted and averaged across each ROI. Neuropsychological test scores were scaled to published norms and, if necessary, were inverted (by subtracting the attained score from the sample maximum + 1) so that higher scores indicated better performance for all measures.
Figure 1.
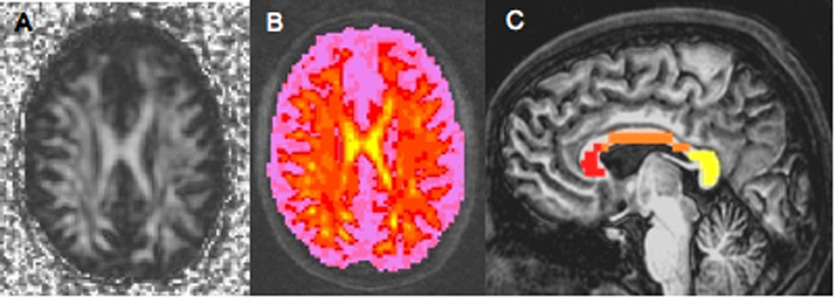
(A) Fractional anisotropy (FA) axial slice from a single subject. (B) Sample FA overlay (yellow=highest; pink=lowest) on high resolution anatomical image. (C) Sample CC regions of interest drawn at diffusion tensor image resolution for the splenium (yellow), body (orange), and genu (red) of the CC, overlaid on high resolution anatomical image.
Statistical Analyses
To test the hypothesis that superior neuropsychological functioning relates to greater callosal microstructural integrity, Pearson correlation coefficients quantified the relationship between diffusion variables (FA and MD in the CC splenium and body) and 6 domains of neuropsychological test performance:(1) visuospatial skills (ROCF copy accuracy and WASI Block Design); (2) learning and memory (CVLT-C list A total and ROCF delay accuracy); (3) working memory (PASAT and WAIS-III Digit Span backward); (4) psychomotor function (WAIS-III Digit Symbol and D-KEFS Trail Making motor speed); (5) executive functioning (D-KEFS Trail Making composite and D-KEFS Towers total achievement); and (6) language (WASI Vocabulary and WRAT-3 Reading). The large number of planned statistical comparisons increased the possibility of detecting spurious relationships. However, it has been argued that Bonferroni-type adjustments excessively control Type I error particularly in the case of correlated outcome measures. Given expected dependencies in the DTI data, we instead examined the binomial distribution of the correlational outcomes. Therefore, the pattern of correlations was tested for statistical significance using Fisher exact test to examine whether more correlations occurred in the predicted direction than would have occurred by chance (Ghahramani, 1996). The predicted direction was based on the hypothesis that better neuropsychological performance would relate positively to FA and negatively to MD. The goal of this analysis was to mitigate the consequences of the inflated error rate associated with multiple comparisons by examining whether the overall pattern of correlations was suggestive of the hypothesized relationship.
Results
Table 1 displays means for sample demography and DTI values. As genu FA was lower and more variable than anticipated, we did not evaluate DTI results from this subregion further. However, splenium and body FA and MD values were stable and within an anticipated range.
Table 1.
Participant characteristics
| M ± SD or % | |
|---|---|
| Age (range 16–18) | 17.65 ± 1.12 |
| % Female | 39% |
| % Caucasian | 67% |
| Grades completed | 11.22 ± 1.40 |
| Annual household income ($K) | 124.33 ± 75.23 |
| Vocabulary T-score | 55.72 ± 7.93 |
| Corpus callosum splenium FA | 0.82 ± 0.03 |
| Corpus callosum splenium MD | 0.65 ± 0.04 |
| Corpus callosum body FA | 0.69 ± 0.04 |
| Corpus callosum body MD | 0.77 ± 0.04 |
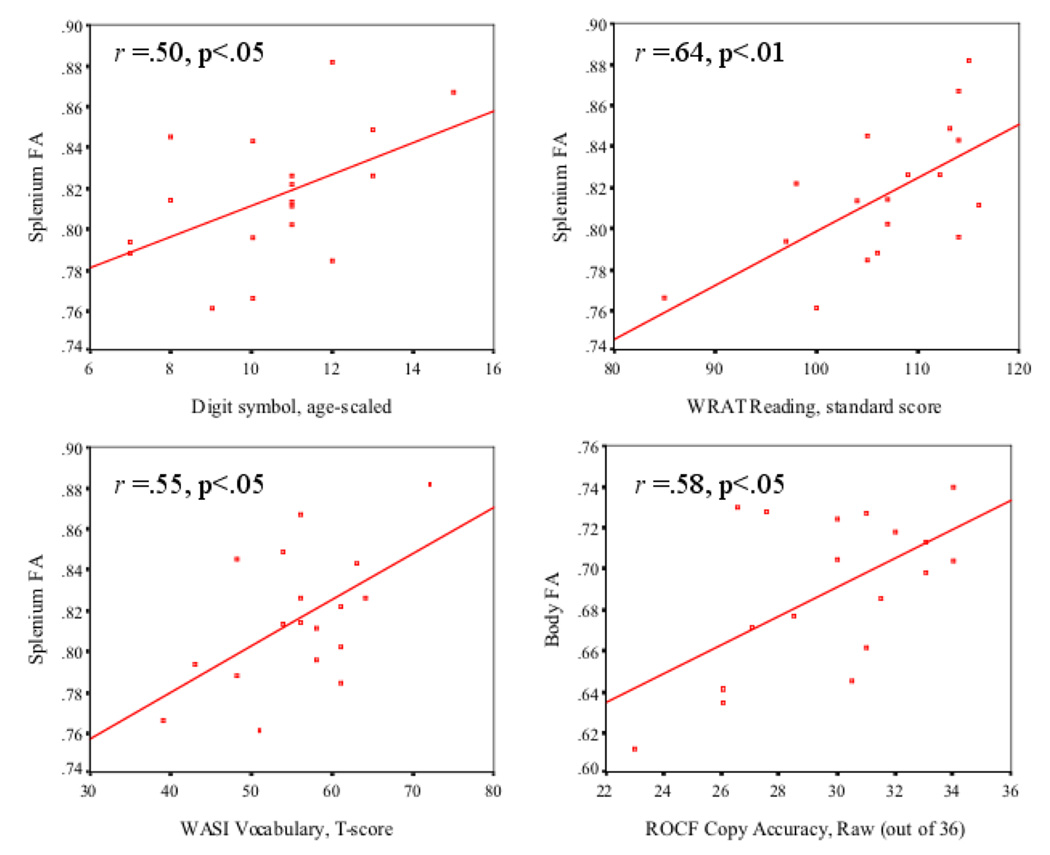
Two-tailed Pearson correlations between diffusion measures and neuropsychological test scores are presented in Table 2. A power analysis revealed that for two-tailed correlations, our sample size yielded adequate power (1-β=.80) to detect a large effect size of .58 at alpha =.05 (Cohen, 1992). Significant correlations were observed between: ROCF copy accuracy and body FA; Digit Symbol and splenium FA; Vocabulary and splenium FA; and Reading and splenium FA. Trends toward statistically significant correlative relationships emerged between Block Design and body FA, ROCF copy accuracy and splenium and body FA and body MD, and Trail Making Test motor speed and splenium MD. See Figure 2 for graphical depiction of select correlations. For the majority of statistically correlative relationships, better neuropsychological scores were associated with increased FA values and decreased MD values. The one exception was the Trail Making motor speed subtest, which showed a trend toward a positive correlation with splenium MD. The pattern of correlations observed for the splenium and body was statistically significant, with 38 of 48 correlations occurring in the predicted direction (Fisher exact test, p<.001).
Table 2.
Pearson 2-tailed correlations between corpus callosum diffusion and neuropsychological measures
| Correlation with DTI values | ||||||||
|---|---|---|---|---|---|---|---|---|
| Splenium | Body | |||||||
| Neuropsychological variable | FA | p | MD | p | FA | p | MD | p |
| Rey-Osterrieth Complex Figure copy accuracy | .46§ | .06 | −.11 | ns | .58* | .01 | −.47§ | .05 |
| WASI Block Design T-score | .37 | ns | .05 | ns | .44§ | .07 | −.29 | ns |
| CVLT-C list A total T-score | .24 | ns | −.19 | ns | .01 | ns | −.20 | ns |
| Rey-Osterrieth Complex Figure delay accuracy | −.02 | ns | .18 | ns | .33 | ns | −.14 | ns |
| Paced Auditory Serial Addition Task total | .21 | ns | −.04 | ns | .28 | ns | −.37 | ns |
| WAIS-III Digits backward | .13 | ns | .11 | ns | .11 | ns | −.01 | ns |
| WAIS-III Digit Symbol scaled score | .50* | .04 | −.08 | ns | .15 | ns | −.21 | ns |
| D-KEFS Trail Making Motor Speed scaled score | −.17 | ns | .47§ | .05 | −.10 | ns | −.01 | ns |
| D-KEFS Trail Making total scaled score | .30 | ns | .10 | ns | .22 | ns | −.19 | ns |
| D-KEFS Towers total scaled score | .22 | ns | −.27 | ns | −.04 | ns | .16 | ns |
| WASI Vocabulary T-score | .55* | .02 | −.15 | ns | .30 | ns | −.33 | ns |
| WRAT-3 Reading standard score | .64* | <.01 | −.18 | ns | .27 | ns | −.37 | ns |
Note: For all neuropsychological scores, higher scores reflect better performance. DTI: diffusion tensor imaging; FA: fractional anisotropy; MD: mean diffusion; ns: non-significant (p≥.08).
p < .05
trend (p = .05 – .07)
Figure 2.
Scatterplots for corpus callosum subregion fractional anisotropy (FA) and neuropsychological scores.
Confirmatory analyses used multiple regression to examine whether FA values in the CC splenium or body accounted for significant variance in cognitive domains in which a significant correlative relationship was observed (visuospatial skills, language, and psychomotor functioning). Performance on the visuospatial domain significantly accounted for variance in body FA (overall model p<.05; adjusted R2 = 28%), and performance on the language domain accounted for significant variance in splenium FA (overall model p<.05; adjusted R2 = 36%). Psychomotor speed did not account for significant variance in splenium FA.
Lastly, because ongoing brain development is expected within the age range of our sample, the associations between age and the splenium and body CC diffusion variables were explored. A significant relationship between splenium MD and age was observed (r=-.52; p=.03), while other age-diffusion relationships were non-significant. Based on these results, a post-hoc hierarchical regression analysis was conducted to examine how age might influence the result relating splenium MD to cognitive performance. Age was entered into the regression model first, followed by Trail Making motor speed. Overall model fit was significant (p<.05; adjusted R2 = 42%), and follow-up analyses indicated that age was a significant predictor of variance in the MD of the splenium (p<.05), while Trail Making motor speed performance was not.
Discussion
We observed relationships between microstructure of the CC and performance on tests of visuospatial cognition, language, and psychomotor function, while other abilities, such as memory, were unrelated to diffusion within the CC. These results suggest that white matter coherence relates to the maturation of certain cognitive abilities in adolescence. Moreover, these results provide some indication of CC subregion specific brain-behavior relationships, as microstructure of the CC splenium and body related to visuospatial skills, while language and psychomotor performance were associated only with the splenium.
Previous research on alcoholic adults has related better visuospatial ability with increased anisotropy and decreased diffusion in the posterior corpus callosum (Pfefferbaum et al., 2005). Our results concur with this finding, though in our typically developing adolescent sample both the CC body and splenium were related to visuospatial abilities, while in Pfefferbaum and colleagues’ adult sample, only the CC splenium was related to performance on a visuospatial task. Given the age and health discrepancies between the samples, white matter maturational status could be a possible reason for this difference, though another explanation could rest on the type of visuospatial task used. The present study used two tasks that are visuo-constructive in nature, while the Pfefferbaum et al. study used a test of nonverbal perceptual reasoning; this raises the possibility that selective aspects of visuospatial cognition may have distinct anatomical correlates within the CC. Regardless, findings from both studies support the conclusion that posterior CC microstructural integrity supports visuospatial cognition.
Given that splenial fibers interconnect temporo-parieto-occipital regions (De Lacoste et al., 1985), our finding associating splenial microstructure with reading ability corroborates extant literature suggesting that reading is subserved by temporo-parietal regions. More specifically, prior DTI studies have indicated that white matter coherence in these regions impacts reading ability. FA was decreased bilaterally in white matter of the temporo-parietal junction in adults with a history of dyslexia (Klingberg et al., 2000). Moreover, reading performance correlated with FA in the left temporal lobe of adults with and with out dyslexia (Klingberg et al., 2000), and in typically developing children and adolescents (Nagy et al., 2004). In addition to implications with regard to reading ability, the association observed in our sample between verbal ability and callosal integrity is also of interest because tests of language function are thought to be reliable indicators of general cognitive ability (Lezak et al., 2004). This suggests that CC microstructural organization within posterior regions may be especially important in honing intellectual skills.
A correlation was also observed between better performance on a psychomotor processing speed task and higher splenial FA. This suggests that optimal information processing speed is supported by white matter maturation of posterior callosal fibers, as indexed by FA. Future whole brain analyses will provide more precise localization of white matter tract relationships to cognitive function, as the CC correlations here may be attributable to FA in regions not assessed. It should be noted that the positive relationship between splenium MD and motor speed performance on a trail-making test was unexpected. However, results from a post-hoc analysis indicate that motor speed was not a significant predictor of splenium MD once participant age (range: 16–18 years) was considered. This suggests that age may explain this unexpected relationship, and underscores the need to evaluate brain-behavior relationships among adolescents in the context of ongoing maturational processes.
In summary, our findings generally support the hypothesis that neuropsychological performance relates positively to callosal FA and negatively to MD. Thus, these data provide evidence that typical cognitive development is supported by white matter maturation, as measured by DTI. The brain structure-function relationships observed in the present study suggest that optimal cognitive functioning in adolescents is correlated with a high degree of white matter coherence and thus high values of FA in the CC. Moreover, certain abilities (visuospatial, language, and psychomotor functioning) were related to CC structural coherence, while many abilities examined were not.
This study is limited by several factors. An important limitation rests on our modest sample size, which diminishes statistical power to detect relationships with smaller effect sizes. Thus, negative findings relating callosal microstructure to some neuropsychological domains should be interpreted cautiously until they are replicated in a larger sample. This study uses a cross-sectional design, which cannot directly evaluate developmental trends. Additionally, DTI measurements, in general, are compromised by susceptibility distortions within the images from which the diffusion tensor is derived. Field mapping was not available at the time of data collection. Our current DTI studies, using a higher field strength, acquire field maps that are used to unwarp the diffusion-weighted images. In addition to visual inspection, which showed artifact in the anterior portion of some diffusion images, the standard deviations associated with DTI genu measures in our sample are about twice that of the other two subregions. A study examining subregional differences in CC fiber density noted histological differences in the genu compared to other areas of the CC, including more unmyelinated axons, smaller median axonal diameter, and increased fiber density (Aboitiz et al., 1992). Thus, differences in fiber density among CC subregions may account for local signal differences within the diffusion tensor images. Also, because we did not gate diffusion image acquisition to the cardiac cycle, the noise we observed might be due to involuntary movement artifact caused by pulsatile flow of cerebrospinal fluid (Pierpaoli et al., 2003). The nature and magnitude of brain movements due to pulsatile flow vary by anatomical region (Greitz et al., 1992), so it is possible that the anterior CC was differentially affected by physiological noise. Accordingly, diffusion values in the genu were not pursued further with statistical analysis. A final limitation concerns current DTI analytic methodology. The standard method of DTI employed uses a simple Gaussian model (Basser and Pierpaoli, 1996), which is insufficient to model diffusion in voxels that contain multiple fiber directions (Frank, 2001; Frank, 2002). This can result in decreased anisotropy measures in regions that, although highly organized, contain fiber crossings with multiple directions. In addition, more complete models may distinguish between diffusion of water molecules that is "restricted" (i.e., within neurons) versus "hindered" (i.e., between neurons), which cannot be ascertained using the basic DTI model of Gaussian diffusion. Rectifying these limitations, however, requires high b-values not attainable on the system and a subsequent increase in averages, and hence imaging time, which is not permissible for adolescent subjects.
Despite these limitations, the current study addresses an important niche in the brain-cognition and developmental literature. Data examining white matter anisotropy in children and adolescents have found CC FA reductions in several clinical populations such as autism (Barnea-Goraly et al., 2004), developmental delay (Filippi et al., 2003), and fetal alcohol syndrome (Ma et al., 2005). However, few studies in developmentally compromised populations have examined the relationship between CC microstructure and behavior by correlating DTI-derived values with performance on a comprehensive neuropsychological test battery. The data presented in this report provide an initial benchmark indicating an appreciable range of white matter integrity within a typically developing adolescent sample, and linking white matter coherence with superior neurocognitive performance. Future studies should expand upon these findings by examining a wider age range and by acquiring whole-brain DTI to provide a more comprehensive picture of how white matter integrity relates to cognition in adolescence.
Acknowledgments
This work was supported by the VA Research Service, research grants from NIH (AA13419, DA15228, and DA021182 to SFT, and MH064729 to LRF), and a NIAAA research training grant (T32 AA013525 for SLF and ADS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aboitz F, Scheibel AB, Fisher RS, Zaidel E. Fiber composition of the human corpus callosum. Brain Research. 1992;598:143–153. doi: 10.1016/0006-8993(92)90178-c. [DOI] [PubMed] [Google Scholar]
- Achenbach T, Rescorla L. Manual for the ASEBA School Age Forms and Profiles. Burlington, VT: University of Vermont, Research Center for Children Youth & Families; 2001. [Google Scholar]
- Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biological Psychiatry. 2004;55:323–326. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, Dant C, Reiss AL. White matter development during childhood and adolescence: A cross-sectional diffusion tensor imaging study. Cerebral Cortex. 2005;15:1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. Journal of magnetic resonance. Series B. 1994a;103:247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophysics Journal. 1994b;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. Journal of magnetic resonance. Series B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Ben Bashat D, Ben Sira L, Graif M, Pianka P, Hendler T, Cohen Y, Assaf Y. Normal white matter development from infancy to adulthood: Comparing diffusion tensor and high b value diffusion weighted MR images. Journal of magnetic resonance imaging. 2005;21:503–511. doi: 10.1002/jmri.20281. [DOI] [PubMed] [Google Scholar]
- Berlucchi G, Aglioti S, Marzi C, Tassinari G. Corpus callosum and simple visuomotor integration. Neuropsychologia. 1995;33:923–936. doi: 10.1016/0028-3932(95)00031-w. [DOI] [PubMed] [Google Scholar]
- Bloom J, Hynd G. The role of the corpus callosum in interhemispheric transfer of information: Excitation or inhibition? Neuropsychology Review. 2005;15:59–71. doi: 10.1007/s11065-005-6252-y. [DOI] [PubMed] [Google Scholar]
- Brown SA, Vik PW, Creamer VA. Characteristics of relapse following adolescent substance abuse treatment. Addictive Behaviors. 1989;14:291–300. doi: 10.1016/0306-4603(89)90060-9. [DOI] [PubMed] [Google Scholar]
- Cascio CJ, Gerig G, Piven J. Diffusion tensor imaging: Application to the study of the developing brain. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:213–223. doi: 10.1097/01.chi.0000246064.93200.e8. [DOI] [PubMed] [Google Scholar]
- Clark CR, Geffen GM. Corpus callosum surgery and recent memory: A review. Brain. 1989;112:165–175. doi: 10.1093/brain/112.1.165. [DOI] [PubMed] [Google Scholar]
- Cohen J. Quantitative methods in psychology: A power primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox RW, Hyde JS. Software tools for analysis and visualization of fMRI data. NMR in Biomedicine. 1997;10:171–178. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<171::aid-nbm453>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- De Lacoste M, Kirkpatrick J, Ross E. Topography of the human corpus callosum. Journal of Neuropathology and Experimental Neurology. 1985;44:578–591. doi: 10.1097/00005072-198511000-00004. [DOI] [PubMed] [Google Scholar]
- Delis D, Kaplan E, Kramer J. Delis-Kaplan Executive Function Scale. San Antonio, TX: Psychological Corporation; 2001. [Google Scholar]
- Delis D, Kramer J, Kaplan E, Ober B. Manual for the California Verbal Learning Test-Children's Version. San Antonio, TX: Psychological Corporation; 1994. [Google Scholar]
- Eliassen J, Baynes K, Gazzaniga M. Anterior and posterior callosal contributions to simultaneous bimanual movements of the hands and fingers. Brain. 2000;123:2501–2511. doi: 10.1093/brain/123.12.2501. [DOI] [PubMed] [Google Scholar]
- Filippi CG, Lin DD, Tsiouris AJ, Watts R, Packard AM, Heier LA, Ulug AM. Diffusion-tensor MR imaging in children with developmental delay: Preliminary findings. Radiology. 2003;229:44–50. doi: 10.1148/radiol.2291020049. [DOI] [PubMed] [Google Scholar]
- Frank LR. Anisotropy in high angular resolution diffusion-weighted MRI. Magnetic Resonance in Medicine. 2001;45:935–939. doi: 10.1002/mrm.1125. [DOI] [PubMed] [Google Scholar]
- Frank LR. Characterization of anisotropy in high angular resolution diffusion-weighted MRI. Magnetic Resonance in Medicine. 2002;47:1083–1099. doi: 10.1002/mrm.10156. [DOI] [PubMed] [Google Scholar]
- Ghahramani S. Fundamentals of Probability. Upper Saddle River, New Jersey: Prentice Hall; 1996. [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Rajapakse JC, Vaituzis AC, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Development of the human corpus callosum during childhood and adolescence: A longitudinal MRI study. Progress in Neuropsychopharmacology & Biological Psychiatry. 1999;23:571–588. doi: 10.1016/s0278-5846(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Greitz D, Wirestam R, Franck A, Nordell B, Thomsen C, Stahlberg F. Pulsatile brain movement and associated hydrodynamics studied by magnetic resonance phase imaging. The Monro-Kellie doctrine revisited. Neuroradiology. 1992;34:370–380. doi: 10.1007/BF00596493. [DOI] [PubMed] [Google Scholar]
- Gronwall D. Paced Auditory Serial-Addition Task: A measure of recovery from concussion. Perceptual and Motor Skills. 1977;44:367–373. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Two-Factor Index of Social Position. New Haven, CT: Yale University Press; 1965. [Google Scholar]
- Hynd G, Hall J, Novey E, Eliopulos D, Black K, Gonzalez J, et al. Dyslexia and corpus callosum morphology. Archives of Neurology. 1995;52:32–38. doi: 10.1001/archneur.1995.00540250036010. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Hedehus M, Temple E, Salz T, Gabrieli JD, Moseley M, et al. Microstructure of temporo-parietal white matter as a basis for reading ability: Evidence from diffusion tensor magnetic resonance imaging. Neuron. 2000;25:493–500. doi: 10.1016/s0896-6273(00)80911-3. [DOI] [PubMed] [Google Scholar]
- Lezak M, Howieson D, Loring D. Neuropsychological Assessment. 4th ed. New York: Oxford University Press; 2004. [Google Scholar]
- Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, et al. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cerebral Cortex. 2005;16:553–560. doi: 10.1093/cercor/bhj003. [DOI] [PubMed] [Google Scholar]
- Lucas CP, Zhang H, Fisher PW, Shaffer D, Reigier DA, Narrow WE, et al. The DISC Predictive Scales (DPS): Efficiently screening for diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:443–449. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- Ma X, Coles CD, Lynch ME, Laconte SM, Zurkiya O, Wang D, et al. Evaluation of corpus callosum anisotropy in young adults with fetal alcohol syndrome according to diffusion tensor imaging. Alcoholism: Clinical and Experimental Research. 2005;29:1214–1222. doi: 10.1097/01.alc.0000171934.22755.6d. [DOI] [PubMed] [Google Scholar]
- Medina KL, Nagel BJ, Park A, McQueeny T, Tapert SF. Depressive symptoms in adolescents: Associations with white matter volume and marijuana use. Journal of Child Psychology and Psychiatry. 2007;48:592–600. doi: 10.1111/j.1469-7610.2007.01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley M, Bammer R, Illes J. Diffusion-tensor imaging of cognitive performance. Brain and Cognition. 2002;50:396–413. doi: 10.1016/s0278-2626(02)00524-9. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Medina KL, Yoshii J, Schweinsburg AD, Moadab I, Tapert SF. Age-related changes in prefrontal white matter volume across adolescence. NeuroReport. 2006;17:1427–1431. doi: 10.1097/01.wnr.0000233099.97784.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. Journal of Cognitive Neuroscience. 2004;16:1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Kubicki M, Gurrera RJ, Niznikiewicz M, Frumin M, McCarley RW, et al. Neuropsychological correlates of diffusion tensor imaging in schizophrenia. Neuropsychology. 2004;18:629–637. doi: 10.1037/0894-4105.18.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Osterrieth P. Le test de copie d'une figure complexe. Archiv fur Psychologie. 1944;30:206–356. [Google Scholar]
- Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A. Maturation of white matter in the human brain: A review of magnetic resonance studies. Brain Research Bulletin. 2001;54:255–266. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Dysmorphology and microstructural degradation of the corpus callosum: Interaction of age and alcoholism. Neurobiology of Aging. 2005;27:994–1009. doi: 10.1016/j.neurobiolaging.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Microstructural but not macrostructural disruption of white matter in women with chronic alcoholism. Neuroimage. 2002;15:708–718. doi: 10.1006/nimg.2001.1018. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Adalsteinsson E, Lim KO, Moseley M. In vivo detection and functional correlates of white matter microstructural disruption in chronic alcoholism. Alcoholism: Clinical and Experimental Research. 2000;24:1214–1221. [PubMed] [Google Scholar]
- Pierpaoli C, Marenco S, Rohde G, Jones DK, Barnett AS. Analyzing the contribution of cardiac pulsation to the variability of quantities derived from the diffusion tensor. Paper presented at the Proceedings of the 11th Meeting of the International Society for Magnetic Resonance Imaging; Toronto, Canada. 2003. [Google Scholar]
- Pujol J, Vendrell P, Junque C, Marti-Vilalta JL, Capdevila A. When does human brain development end? Evidence of corpus callosum growth up to adulthood. Annals of Neurology. 1993;34:71–75. doi: 10.1002/ana.410340113. [DOI] [PubMed] [Google Scholar]
- Roebuck T, Mattson S, Riley E. Interhemispheric transfer in children with heavy prenatal alcohol exposure. Alcoholism: Clinical and Experimental Research. 2002;26:1863–1871. doi: 10.1097/01.ALC.0000042219.73648.46. [DOI] [PubMed] [Google Scholar]
- Sen PN, Basser PJ. A model for diffusion in white matter in the brain. Biophysical Journal. 2005;89:2927–2938. doi: 10.1529/biophysj.105.063016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: A cross-sectional diffusion-tensor MR imaging study. Radiology. 2002;222:212–218. doi: 10.1148/radiol.2221010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Cognitive functions correlate with white matter architecture in a normal pediatric population: A diffusion tensor MRI study. Human Brain Mapping. 2005;26:139–147. doi: 10.1002/hbm.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Cheung EH, Brown GG, Brown SA, Tapert SF. fMRI response to spatial working memory in adolescents with comorbid marijuana and alcohol use disorders. Drug and Alcohol Dependence. 2005;79:201–210. doi: 10.1016/j.drugalcdep.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Snook L, Paulson LA, Roy D, Phillips L, Beaulieu C. Diffusion tensor imaging of neurodevelopment in children and young adults. Neuroimage. 2005;26:1164–1173. doi: 10.1016/j.neuroimage.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale-III. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. Manual for the Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Wilkinson GS. Manual for The Wide Range Achievement Test. Third edition. Wilmington, DE: Wide Range, Inc; 1993. [Google Scholar]
- Wong EC, Luh W-M, Buxton RB, Frank LR. Single slab high resolution 3D whole brain imaging using spiral FSE. Proceedings of the International Society for Magnetic Resonance Imaging. 2000;8:683. [Google Scholar]