Abstract
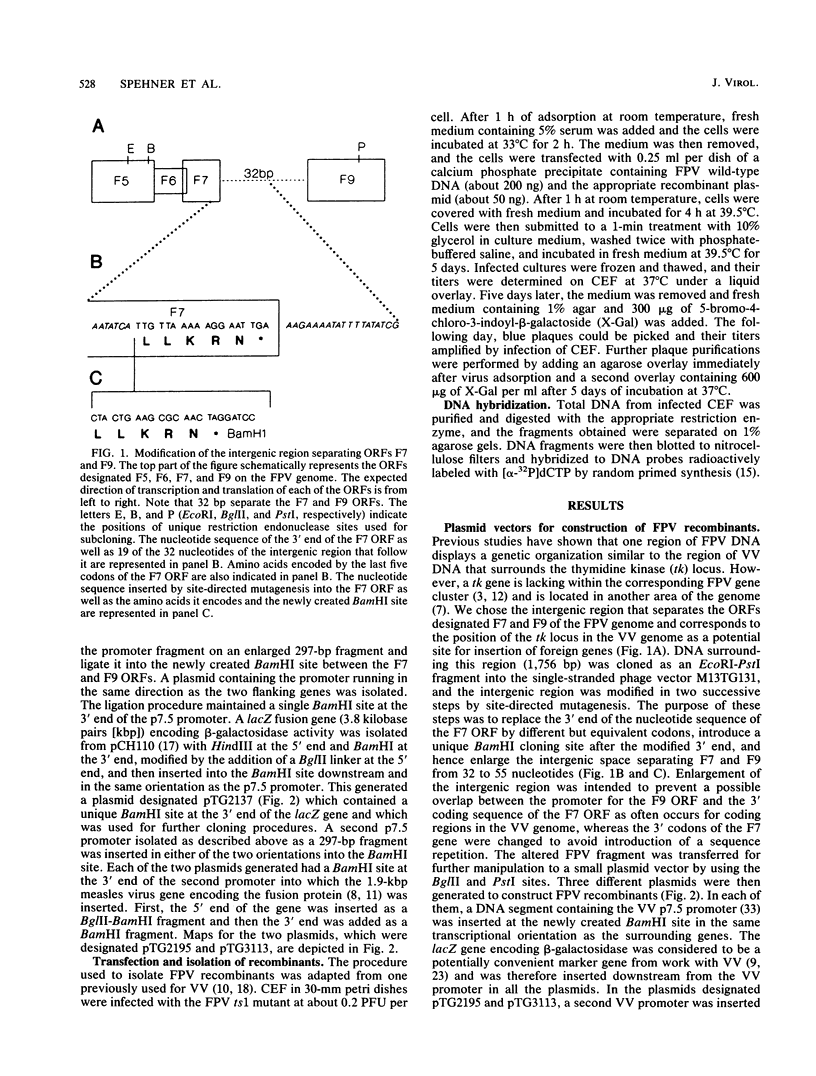
A DNA fragment from fowlpox virus cloned on a plasmid vector was modified to contain foreign DNA inserts within an intergenic region. In a first step, a 32-base-pair intergenic region from the fowlpox virus genome corresponding to the position of the thymidine kinase locus in the vaccinia virus genome was enlarged to 55 base pairs by site-directed mutagenesis. A unique restriction endonuclease site introduced upstream of the intergenic region was then used to insert various foreign DNA fragments. The lacZ gene encoding beta-galactosidase and the measles virus gene encoding the fusion protein were positioned downstream of two vaccinia virus p7.5 promoter elements in either a direct repeat or inverted repeat orientation. Foreign DNA inserts contained within the fowlpox virus sequence were transferred to the viral genome by homologous recombination occurring in cells infected with a fowlpox virus temperature-sensitive mutant and transfected with both wild-type viral DNA and plasmid DNA. Recombinant viruses were selected for the expression of beta-galactosidase activity by screening for blue plaques in the presence of a chromogenic substrate. Stable recombinants expressing both the lacZ gene and the unselected measles gene were obtained when the p7.5 promoter was present as an inverted repeat. However, when the p7.5 promoter was in the direct repeat orientation, viral recombinants which initially expressed both gene inserts readily deleted the lacZ gene flanked by the promoter repeat. The methods described enable precise insertion and deletion of foreign genes in the fowlpox virus genome and could be applied to other intergenic regions of the same virus as well as other poxviruses.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball L. A. High-frequency homologous recombination in vaccinia virus DNA. J Virol. 1987 Jun;61(6):1788–1795. doi: 10.1128/jvi.61.6.1788-1795.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binns M. M., Stenzler L., Tomley F. M., Campbell J., Boursnell M. E. Identification by a random sequencing strategy of the fowlpoxvirus DNA polymerase gene, its nucleotide sequence and comparison with other viral DNA polymerases. Nucleic Acids Res. 1987 Aug 25;15(16):6563–6573. doi: 10.1093/nar/15.16.6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binns M. M., Tomley F. M., Campbell J., Boursnell M. E. Comparison of a conserved region in fowlpox virus and vaccinia virus genomes and the translocation of the fowlpox virus thymidine kinase gene. J Gen Virol. 1988 Jun;69(Pt 6):1275–1283. doi: 10.1099/0022-1317-69-6-1275. [DOI] [PubMed] [Google Scholar]
- Boyle D. B., Coupar B. E. A dominant selectable marker for the construction of recombinant poxviruses. Gene. 1988 May 15;65(1):123–128. doi: 10.1016/0378-1119(88)90424-6. [DOI] [PubMed] [Google Scholar]
- Boyle D. B., Coupar B. E. Construction of recombinant fowlpox viruses as vectors for poultry vaccines. Virus Res. 1988 Jun;10(4):343–356. doi: 10.1016/0168-1702(88)90075-5. [DOI] [PubMed] [Google Scholar]
- Boyle D. B., Coupar B. E., Gibbs A. J., Seigman L. J., Both G. W. Fowlpox virus thymidine kinase: nucleotide sequence and relationships to other thymidine kinases. Virology. 1987 Feb;156(2):355–365. doi: 10.1016/0042-6822(87)90415-6. [DOI] [PubMed] [Google Scholar]
- Boyle D. B., Coupar B. E. Identification and cloning of the fowlpox virus thymidine kinase gene using vaccinia virus. J Gen Virol. 1986 Aug;67(Pt 8):1591–1600. doi: 10.1099/0022-1317-67-8-1591. [DOI] [PubMed] [Google Scholar]
- Buckland R., Gerald C., Barker R., Wild T. F. Fusion glycoprotein of measles virus: nucleotide sequence of the gene and comparison with other paramyxoviruses. J Gen Virol. 1987 Jun;68(Pt 6):1695–1703. doi: 10.1099/0022-1317-68-6-1695. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drillien R., Spehner D., Kirn A., Giraudon P., Buckland R., Wild F., Lecocq J. P. Protection of mice from fatal measles encephalitis by vaccination with vaccinia virus recombinants encoding either the hemagglutinin or the fusion protein. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1252–1256. doi: 10.1073/pnas.85.4.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drillien R., Spehner D. Physical mapping of vaccinia virus temperature-sensitive mutations. Virology. 1983 Dec;131(2):385–393. doi: 10.1016/0042-6822(83)90506-8. [DOI] [PubMed] [Google Scholar]
- Drillien R., Spehner D., Villeval D., Lecocq J. P. Similar genetic organization between a region of fowlpox virus DNA and the vaccinia virus HindIII J fragment despite divergent location of the thymidine kinase gene. Virology. 1987 Sep;160(1):203–209. doi: 10.1016/0042-6822(87)90061-4. [DOI] [PubMed] [Google Scholar]
- Esposito J., Condit R., Obijeski J. The preparation of orthopoxvirus DNA. J Virol Methods. 1981 Feb;2(3):175–179. doi: 10.1016/0166-0934(81)90036-7. [DOI] [PubMed] [Google Scholar]
- Falkner F. G., Moss B. Escherichia coli gpt gene provides dominant selection for vaccinia virus open reading frame expression vectors. J Virol. 1988 Jun;62(6):1849–1854. doi: 10.1128/jvi.62.6.1849-1854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Franke C. A., Rice C. M., Strauss J. H., Hruby D. E. Neomycin resistance as a dominant selectable marker for selection and isolation of vaccinia virus recombinants. Mol Cell Biol. 1985 Aug;5(8):1918–1924. doi: 10.1128/mcb.5.8.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C. V., Jacob P. E., Ringold G. M., Lee F. Expression and regulation of Escherichia coli lacZ gene fusions in mammalian cells. J Mol Appl Genet. 1983;2(1):101–109. [PubMed] [Google Scholar]
- Kieny M. P., Lathe R., Drillien R., Spehner D., Skory S., Schmitt D., Wiktor T., Koprowski H., Lecocq J. P. Expression of rabies virus glycoprotein from a recombinant vaccinia virus. Nature. 1984 Nov 8;312(5990):163–166. doi: 10.1038/312163a0. [DOI] [PubMed] [Google Scholar]
- Lathe R., Vilotte J. L., Clark A. J. Plasmid and bacteriophage vectors for excision of intact inserts. Gene. 1987;57(2-3):193–201. doi: 10.1016/0378-1119(87)90122-3. [DOI] [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. Vaccinia virus: a selectable eukaryotic cloning and expression vector. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7415–7419. doi: 10.1073/pnas.79.23.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panicali D., Grzelecki A., Huang C. Vaccinia virus vectors utilizing the beta-galactosidase assay for rapid selection of recombinant viruses and measurement of gene expression. Gene. 1986;47(2-3):193–199. doi: 10.1016/0378-1119(86)90063-6. [DOI] [PubMed] [Google Scholar]
- Panicali D., Paoletti E. Construction of poxviruses as cloning vectors: insertion of the thymidine kinase gene from herpes simplex virus into the DNA of infectious vaccinia virus. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4927–4931. doi: 10.1073/pnas.79.16.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J. F., Esteban M. Plaque size phenotype as a selectable marker to generate vaccinia virus recombinants. J Virol. 1989 Feb;63(2):997–1001. doi: 10.1128/jvi.63.2.997-1001.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J. F., Rodriguez D., Rodriguez J. R., McGowan E. B., Esteban M. Expression of the firefly luciferase gene in vaccinia virus: a highly sensitive gene marker to follow virus dissemination in tissues of infected animals. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1667–1671. doi: 10.1073/pnas.85.5.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shida H., Tochikura T., Sato T., Konno T., Hirayoshi K., Seki M., Ito Y., Hatanaka M., Hinuma Y., Sugimoto M. Effect of the recombinant vaccinia viruses that express HTLV-I envelope gene on HTLV-I infection. EMBO J. 1987 Nov;6(11):3379–3384. doi: 10.1002/j.1460-2075.1987.tb02660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. L., Mackett M., Moss B. Infectious vaccinia virus recombinants that express hepatitis B virus surface antigen. Nature. 1983 Apr 7;302(5908):490–495. doi: 10.1038/302490a0. [DOI] [PubMed] [Google Scholar]
- Spyropoulos D. D., Roberts B. E., Panicali D. L., Cohen L. K. Delineation of the viral products of recombination in vaccinia virus-infected cells. J Virol. 1988 Mar;62(3):1046–1054. doi: 10.1128/jvi.62.3.1046-1054.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J., Weinberg R., Kawaoka Y., Webster R. G., Paoletti E. Protective immunity against avian influenza induced by a fowlpox virus recombinant. Vaccine. 1988 Dec;6(6):504–508. doi: 10.1016/0264-410x(88)90101-6. [DOI] [PubMed] [Google Scholar]
- Taylor J., Weinberg R., Languet B., Desmettre P., Paoletti E. Recombinant fowlpox virus inducing protective immunity in non-avian species. Vaccine. 1988 Dec;6(6):497–503. doi: 10.1016/0264-410x(88)90100-4. [DOI] [PubMed] [Google Scholar]
- Tomley F., Binns M., Campbell J., Boursnell M. Sequence analysis of an 11.2 kilobase, near-terminal, BamHI fragment of fowlpox virus. J Gen Virol. 1988 May;69(Pt 5):1025–1040. doi: 10.1099/0022-1317-69-5-1025. [DOI] [PubMed] [Google Scholar]
- Venkatesan S., Baroudy B. M., Moss B. Distinctive nucleotide sequences adjacent to multiple initiation and termination sites of an early vaccinia virus gene. Cell. 1981 Sep;25(3):805–813. doi: 10.1016/0092-8674(81)90188-4. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]