Abstract
Aims/hypothesis
Physical activity is important in preventing insulin resistance, but it is unclear which dimension of activity confers this benefit. We examined the association of overall level and intensity of physical activity with fasting insulin level, a marker of insulin resistance.
Methods
This was a cross-sectional analysis of the Medical Research Council Ely population-based cohort study (2000–2002). Physical activity energy expenditure (PAEE) in kJ kg−1 min−1 was measured by heart rate monitoring with individual calibration over a period of 4 days. The percentage of time spent above 1.5, 1.75 and 2 times resting heart rate (RHR) represented all light-to-vigorous, moderate-to-vigorous and vigorous activity, respectively.
Results
Data from a total of 643 non-diabetic individuals (319 men, 324 women) aged 50 to 75 years were analysed. In multivariate linear regression analyses, adjusting for age, sex and body fat percentage, PAEE was significantly associated with fasting insulin (pmol/l) (β = −0.875, p = 0.006). Time (% of total) spent above 1.75 × RHR and also time spent above 2 × RHR were both significantly associated with fasting insulin (β = −0.0109, p = 0.007 and β = −0.0365, p = 0.001 respectively), after adjusting for PAEE, age, sex and body fat percentage. Time spent above 1.5 × RHR was not significantly associated with fasting insulin in a similar model (β = −0.0026, p = 0.137).
Conclusions/interpretation
The association between PAEE and fasting insulin level, a marker of insulin resistance, may be attributable to the time spent in moderate-to-vigorous and vigorous activity, but not to time spent in light-intensity physical activity.
Keywords: Activity intensity, Energy expenditure, Insulin resistance, Physical activity
Introduction
Cardiovascular disease (CVD) is now the leading cause of morbidity and mortality both globally as well as in industrialised countries [1, 2]. The increasing CVD burden has been attributed to changing patterns of various lifestyle and resulting aetiological risk factors such as physical inactivity, obesity, glucose intolerance, dyslipidaemia and high blood pressure [3, 4]. These risk factors cluster in individuals in what has recently been termed the metabolic or insulin resistance syndrome [5, 6]. Separately, these factors of the metabolic syndrome are associated with CVD [7, 8], with the association greatly increasing when these factors cluster together [9].
Insulin resistance, considered by some as one of the key components of the metabolic syndrome, is an important factor in the development of type 2 diabetes even in individuals with normal glucose tolerance [10–13]. It is also associated with hypertension, dyslipidaemia and atherosclerotic disease, coronary heart disease and stroke [14–16]. A number of methods have been developed and validated for use in evaluating insulin resistance in humans. The fasting plasma insulin concentration, which is inexpensive and relatively easy to measure, has been shown to be correlated with insulin sensitivity measured by the clamp technique [17, 18]. Fasting insulin has also been shown to identify individuals with the metabolic syndrome [19] and to predict future diabetes in non-diabetic individuals [17].
Several factors have been associated with the development of insulin resistance, including age, obesity [20], body fat distribution [21], dietary factors [22], genetic factors [23], cardiorespiratory fitness and physical inactivity [24]. Previous research has shown that there is an inverse association between physical activity and insulin resistance, although most observational studies have used subjective estimates of physical activity via self-report, some of which only capture a specific dimension of activity such as leisure-time or occupational physical activity [25, 26]. Fewer studies have used objective measures of physical activity [8, 27], which may give a more reliable estimate of physical activity patterns. A recent review of physical activity and insulin resistance [28] highlighted major uncertainties in our understanding of dose–response issues and the subcomponents of activity most closely associated with insulin sensitivity. These subcomponents relate to the type of activity, volume, intensity, duration and frequency. This is an important question, as it may contribute to the design of appropriate preventive health strategies. In particular, it is important to understand whether the association between insulin resistance and activity is explained by total activity or by spending more time doing higher intensity activities, since the consequence of such an observation would be the development of very different approaches to prevention.
We have previously observed associations between physical activity energy expenditure (PAEE) and fasting insulin level [23, 29], a marker of insulin resistance, and between PAEE and clustered metabolic risk [30, 31]. We did not, however, examine the impact of the intensity of physical activity on these associations. Therefore, the aim of the present study was to examine the association of overall level and intensity of physical activity with fasting insulin level in a cross-sectional analysis of the Medical Research Council (MRC) Ely cohort study.
Methods
Participants
The participants for this study were all part of the MRC Ely study, a prospective population-based cohort study of the aetiology and pathogenesis of type 2 diabetes and related metabolic disorders [10, 32]. The present cross-sectional analyses were performed using data from the second follow up (2000–2002).
A total of 678 individuals from the cohort, for whom complete data on free-living heart rate (HR) monitoring were available and who did not have previously diagnosed diabetes, were included in the present report. Based on a 75 g oral glucose tolerance test, the participants were classified according to the 1998 WHO glucose tolerance categories [5] into normal glucose, impaired fasting glucose, impaired glucose tolerance or diabetes. A total of 20 individuals were diagnosed as having type 2 diabetes, 12 did not provide a 2 h glucose measurement and three individuals had no data on fasting insulin; all of these were excluded. Thus, the present analyses include 643 individuals (319 men, 324 women). Ethical approval for the MRC Ely cohort study was obtained from the Cambridge local research ethics committee and all participants provided signed informed consent.
Physical activity
Physical activity was objectively measured by HR monitoring. PAEE was assessed using the flex HR method with individual calibration of the HR–energy expenditure (EE) relationship using indirect calorimetry [33] during rest and submaximal exercise as previously described [34, 35]. Briefly, resting oxygen uptake 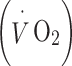 and carbon dioxide production
and carbon dioxide production 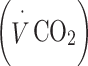 were measured in a supine position for at least 6 min. Heart rate (in beats per minute, bpm),
were measured in a supine position for at least 6 min. Heart rate (in beats per minute, bpm), 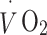 and
and 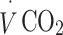 were then assessed during an incremental treadmill exercise protocol. The protocol comprised 5-min stages of walking, starting at 4.0 km/h and 0% gradient, then increasing to 5% gradient, 10% gradient (4.0 km/h) and finally 5.6 km/h at 10% gradient.
were then assessed during an incremental treadmill exercise protocol. The protocol comprised 5-min stages of walking, starting at 4.0 km/h and 0% gradient, then increasing to 5% gradient, 10% gradient (4.0 km/h) and finally 5.6 km/h at 10% gradient. 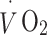 and
and 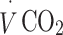 were continuously measured throughout the test (Vista XT metabolic system; Vacumed, Ventura, CA, USA). Participants breathed through a facemask (Hans Rudolph, Kansas City, MO, USA) and expired air was measured with a turbine flowmeter, carbon dioxide concentration with an infrared sensor and oxygen concentration with a fast differential paramagnetic sensor. Flow-rate, carbon dioxide concentration and oxygen concentration data were analysed with Vista Turbofit software (Vacumed) to calculate
were continuously measured throughout the test (Vista XT metabolic system; Vacumed, Ventura, CA, USA). Participants breathed through a facemask (Hans Rudolph, Kansas City, MO, USA) and expired air was measured with a turbine flowmeter, carbon dioxide concentration with an infrared sensor and oxygen concentration with a fast differential paramagnetic sensor. Flow-rate, carbon dioxide concentration and oxygen concentration data were analysed with Vista Turbofit software (Vacumed) to calculate 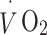 and
and 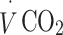 . Gas analysers were calibrated with gases of known composition and the turbine flowmeter was calibrated with a 3 l syringe before each measurement.
. Gas analysers were calibrated with gases of known composition and the turbine flowmeter was calibrated with a 3 l syringe before each measurement.
At any point of the test, the procedure was stopped if (1) the individual’s HR exceeded 90% of age-predicted maximum HR (220—age); (2) the individual’s HR exceeded 80% of maximum HR for over 2 min; or (3) the individual developed breathlessness, chest pain or symptoms of presyncope, or (4) he or she asked to stop.
The flex HR point was calculated as the mean of the highest HR during rest and the lowest HR during exercise. Resting HR (RHR) was calculated as the average of the individual HR data points during supine rest.
Free-living HR (Polar Electro, Kempele, Finland) was measured on a minute-by-minute basis during the waking hours over 4 days. Data from the individual calibration were used to predict minute-by-minute EE for each participant [36]. PAEE was calculated by subtracting mean resting EE from average EE (expressed in kJ kg−1 min−1).
The individual minute-by-minute HR data were processed after categorising into bands of 1.5 to 1.75, 1.75 to 2.0 and above 2.0 × RHR, and then collapsed into bands of time spent above 1.5 × RHR, above 1.75 × RHR and above 2.0 × RHR. These time-estimates were expressed as a percentage of the total number of free-living data points. Internally, we compared these intensity categories against intensity categories from accelerometry (Actigraph; MTI, Fort Walton Beach, FL, USA) in an independent sample of adults (n = 265). Accelerometer counts of 101–1951, 1952–5723 and above 5723 counts/minute were considered as light, moderate and rigorous intensity activities respectively. The mean difference in percentage of time spent in activity between HR monitoring (above 1.5 RHR) and accelerometry was 3.19% (95% CI 0.14–6.25). The mean difference in time spent in moderate-to-vigorous and vigorous activity between the two methods was 2.37% (95% CI 1.41–3.34) and 0.88% (95% CI 0.66–1.11) respectively. Time spent at 1.5 to 1.75 RHR was weakly significantly correlated with time spent at light intensity activity from accelerometry (r = 0.14, p = 0.043). Time spent at 1.75 to 2.0 RHR and time spent above 2.0 RHR were significantly correlated with time spent at moderate and vigorous activity from accelerometry, respectively (r = 0.31, p < 0.0001; r = 0.45, p < 0.0001).
In the present aetiological analyses, three time estimates were considered: time spent above 1.5 × RHR, above 1.75 × RHR and above 2.0 × RHR. The reasoning behind this was that these categorisations would have clinical utility without the need for expensive equipment.
Cardiorespiratory fitness
An estimate of aerobic fitness was obtained by linear extrapolation of the individually observed HR–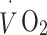 relationship up to age-predicted maximum HR.
relationship up to age-predicted maximum HR.
Fasting insulin
All participants were asked to refrain from eating and drinking from 22:00 hours on the previous evening and fasting blood samples were collected between 08:30 and 09:30 hours on the next day. The plasma samples were immediately separated in a cooled centrifuge at 4°C. After centrifugation (∼1,400×g), the plasma samples were divided into aliquots, placed in the freezer at −20°C, then packed in dry ice and transported to the biochemistry laboratory where they were stored at −70°C within 4 h of collection. Plasma insulin was measured by two-site immunometric assays with either 125I or alkaline phosphatase labels. Cross-reactivity was <0.2% with intact proinsulin at 400 pmol/l and <1% with 32–33 split proinsulin at 400 pmol/l. Inter-assay CVs were 6.6% at 28.6 pmol/l (n = 99), 4.8% at 153.1 pmol/l (n = 102) and 6.0% at 436.7 pmol/l (n = 99) respectively.
Anthropometry
Height without shoes was measured using a standard rigid stadiometer. Body weight was measured using standard calibrated clinical weight scales in light clothes to the nearest 0.1 kg.
Waist and hip circumferences were measured using a D-loop non-stretch fibreglass tape measure with participants in light indoor clothing. The waist circumference was measured at the mid-point between the lower costal margin and the level of the anterior superior iliac crests. The hip circumference was measured at the level of the greater trochanters. The circumferences were measured in cm to the nearest 0.1 cm.
Body composition was assessed by impedance using a standard bioimpedance technique (Bodystat 1500; Bodystat, Isle of Man, UK) according to the manufacturer’s instructions. This method has previously been shown to be valid [37].
Statistical analyses
Analyses were done using STATA version 9.2 SE software (StataCorp, College Station, Texas, USA). Baseline descriptive characteristics of the study participants are presented as means with standard deviations or as numbers with proportions and are stratified by sex and/or age groups. The distribution of fasting insulin concentration was right-skewed and therefore normalised by logarithmic transformation. The central tendency was reported as geometric mean with 95% CIs.
The association between the distribution of physical activity intensity and PAEE was explored first by examining the correlation between PAEE and time spent in the different HR zones, and second by stratifying PAEE into quartiles and using one-way ANOVA to test for differences between these quartiles of the time spent in the HR zones.
In the multivariate linear regression models, the effect of PAEE (entered as a continuous variable) on log fasting insulin was explored with adjustment for age and sex, both with and without adjustment for body fat percentage. To these two models, we then added the time spent in each of the RHR zones in turn.
Results
Characteristics of the participants
A total of 643 individuals (319 men, 324 women) for whom data on percentage of time spent in different HR zones were available were included in these analyses. The characteristics of the study population are presented in Table 1, stratified by sex. There was no significant difference in the mean age by sex. Men reported a higher prevalence of alcohol consumption and smoking than women (80.5 vs 73% and 14.4 vs 12.2% respectively). On average, men were heavier than women and had a larger waist circumference and WHR, whereas body fat percentage was higher in women. Some 50% of men and 38% of women were overweight (BMI ≥ 25 and <30 kg/m2) while 19 and 18% respectively were obese (BMI ≥ 30 kg/m2).
Table 1.
Baseline characteristics by sex; the MRC Ely Study, 2000–2002
| Men (n = 319) | Women (n = 324) | |||||
|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | |
| Age (years) | 57.9 | 4.6 | 50.2–75.0 | 57.5 | 4.0 | 50.1–73.8 |
| Weight (kg) | 84.0 | 12.8 | 55–131 | 69.9 | 13.1 | 45–126.5 |
| BMI (kg/m2) | 27.1 | 3.8 | 18.5–40.8 | 26.4 | 4.6 | 17.8–46.3 |
| WHR | 0.94 | 0.06 | 0.80–1.11 | 0.81 | 0.06 | 0.59–1.02 |
| Body fat (%) | 25.2 | 4.8 | 7.3–44.5 | 37.8 | 5.9 | 20.4–56.4 |
| FPG (mmol/l) | 4.7 | 0.6 | 3.7–6.9 | 4.4 | 0.5 | 3.4–6.6 |
| Fasting insulin (pmol/l) | 57.9 | 32.3 | 8.0–249.0 | 47.7 | 30.6 | 4.9–261.0 |
| Fitness (ml kg−1 min−1) | 43.5 | 9.1 | 23.3–84.5 | 34.8 | 9.1 | 3.5–78.8 |
| PAEE (kJ kg−1 min−1 ) | 0.14 | 0.07 | 0–0.33 | 0.09 | 0.06 | 0–0.30 |
Participants: n = 643
FPG, fasting plasma glucose
A total of 12% of women compared with 16% of men had impaired glucose tolerance. Women on average had a significantly lower fasting insulin level than men (47.7 vs 57.9 pmol/l; p < 0.001). Total EE and PAEE were significantly higher in men than in women (p < 0.001), a difference that persisted after normalising PAEE by body weight.
The mean (±SD) flex HR was 81 ± 11 bpm or on average 1.2 × RHR. Flex HR was almost always lower than 1.5 × RHR (in 98% of the individuals). RHR was significantly negatively correlated with cardiorespiratory fitness (r = −0.4, p < 0.001). The mean resting energy expenditure was 0.09 ± 0.02 kJ kg−1 min−1 (1.3 metabolic equivalents [MET]) and the energy expenditure corresponding to the three HR thresholds of 1.5, 1.75 and 2 × RHR was 0.35 ± 0.08, 0.46 ± 0.1, and 0.56 ± 0.13 kJ kg−1 min−1 (or 4.9, 6.5, and 7.9 MET) respectively. Table 2 presents data, stratified by sex, on the distribution of physical activity intensity as percentage of time spent at HR zones 1.5 to 1.75, 1.75 to 2.0 and above 2.0 × RHR. Men generally spent more time than women in each of the three HR zones considered (p < 0.01). Both men and women spent on average (±SD) 14.7% (±13.9%) of their waking hours above 1.5 × RHR. Median (interquartile range) time spent above 1.75 RHR was 1.3% (0.2–4.4%) and that spent above 2 × RHR was 0.1% (0–0.6%).
Table 2.
Proportion of time spent in different RHR zones (%) during free-living; the MRC Ely Study, 2000–2002
| Percentage of time spent | |||||
|---|---|---|---|---|---|
| n a | Median | Range | Mean | SD | |
| Men (n = 319) | |||||
| 1.5–1.75 RHR | 314 | 9.6 | 0–50.1 | 12.0 | 9.9 |
| 1.75–2 RHR | 282 | 1.6 | 0–25.6 | 3.4 | 4.6 |
| >2 RHR | 202 | 0.1 | 0–16.5 | 1.1 | 2.3 |
| Women (n = 324) | |||||
| 1.5–1.75 RHR | 319 | 8.2 | 0–50.5 | 10.4 | 9.2 |
| 1.75–2 RHR | 276 | 0.8 | 0–26.4 | 2.1 | 3.1 |
| >2 RHR | 184 | 0.1 | 0–11.4 | 0.5 | 1.3 |
Participants: n = 643
a n=number of individuals with non-zero values
Associations between fasting insulin, PAEE and activity intensity
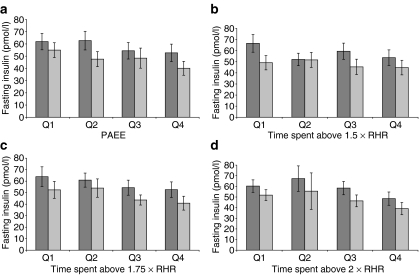
PAEE was significantly correlated with the percentage of time spent above 1.5 × RHR (r = 0.61, p < 0.001), above 1.75 × RHR (r = 0.49, p < 0.001), and above 2 × RHR (r = 0.35, p < 0.001). Figure 1 shows that there was a trend towards a decreasing fasting insulin level across sex-specific quartiles of PAEE, from the first quartile to the fourth. A similar tendency was seen for sex-specific quartiles of time spent above 1.75 × RHR and above 2.0 × RHR, but not for time spent above 1.5 × RHR.
Fig. 1.
Mean (95% CI) fasting insulin by sex-specific quartiles of PAEE (a) and percentage time spent at different RHR zones as indicated (b–d). Dark grey bars, men; light grey bars, women. The MRC Ely Study, 2000–2002. n = 643
The results of the linear regression analyses of log fasting insulin and PAEE are presented in various models in Table 3. Since the direction of association of the initial analyses was the same for men and women, all remaining analyses are presented in the entire population after adjustment for sex. PAEE was significantly negatively associated with log fasting insulin in an unadjusted model (β = −1.15, p < 0.001). In multivariate regression adjusting for age and sex, and then including body fat percentage (models 1 and 2), the association of log fasting insulin with PAEE remained highly statistically significant (β = −1.87, p < 0.001 and β = −0.875, p = 0.006 respectively). In the latter model, log fasting insulin was significantly positively associated with body fat percentage and negatively associated with being a woman (p < 0.001).
Table 3.
Independent associations between physical activity intensity and insulin resistance; the MRC Ely Study, 2000–2002
| β | t | p value | R2 | |
|---|---|---|---|---|
| Model 1 | 0.0819 | |||
| PAEE (kJ kg−1 min−1) | −1.8701 | −5.53 | <0.001 | |
| Age (years) | 0.0003 | 0.06 | 0.95 | |
| Sex (female) | −0.2981 | −6.59 | <0.001 | |
| Model 1a | 0.083 | |||
| PAEE (kJ kg−1 min−1) | −1.6504 | −3.91 | <0.001 | |
| Time above 1.5 RHR (%) | −0.0017 | −0.87 | 0.385 | |
| Model 1b | 0.0914 | |||
| PAEE (kJ kg−1 min−1) | −1.42 | −3.74 | <0.001 | |
| Time above 1.75 RHR (%) | −0.0116 | −2.57 | 0.01 | |
| Model 1c | 0.0991 | |||
| PAEE (kJ kg−1 min−1) | −1.4882 | −4.22 | <0.001 | |
| Time above 2 RHR (%) | −0.0427 | −3.49 | 0.001 | |
| Model 2 | 0.2626 | |||
| PAEE (kJ kg−1 min−1) | −0.875 | −2.78 | 0.006 | |
| Age (years) | −0.0065 | −1.44 | 0.151 | |
| Sex (female) | −0.8471 | −14.15 | <0.001 | |
| Body fat (%) | 0.0466 | 12.45 | <0.001 | |
| Model 2a | 0.2651 | |||
| PAEE (kJ kg−1 min−1) | −0.5319 | −1.36 | 0.173 | |
| Time above 1.5 RHR (%) | −0.0026 | −1.49 | 0.137 | |
| Model 2b | 0.2709 | |||
| PAEE (kJ kg−1 min−1) | −0.4535 | −1.3 | 0.195 | |
| Time above 1.75 RHR (%) | −0.0109 | −2.7 | 0.007 | |
| Model 2c | 0.2751 | |||
| PAEE (kJ kg−1 min−1) | −0.5598 | −1.72 | 0.087 | |
| Time above 2 RHR (%) | −0.0365 | −3.32 | 0.001 |
Participants: n = 643
β is the regression coefficient (log pmol/l per unit increase in exposure)
t is the t statistic which corresponds to the regression coefficient divided by its standard error
Models 1, 1a, 1b and 1c are adjusted for age and sex
Models 2, 2a, 2b and 2c are adjusted for age, sex and body fat percentage
The effect of time spent at different HR zones on the association between PAEE and log fasting insulin was investigated by including these HR zones individually in models 1 and 2. The introduction of time spent above 1.5 × RHR into model 2 (model 2a) attenuated the association between PAEE and log fasting insulin, and in this model neither PAEE (β = −0.532, p = 0.173) nor time spent above 1.5 × RHR (β = −0.003, p = 0.137) was significant after adjusting for age, sex and body fat percentage. Similarly, in model 2b, where time spent above 1.75 × RHR was introduced into model 2, the observed negative association between PAEE and log fasting insulin was attenuated (β = −0.454, p =0.195). After adjustment for PAEE, age, sex and body fat percentage, we saw a statistically significant negative association between time spent above 1.75 × RHR and log fasting insulin (β = −0.011, p = 0.007). The inclusion of time spent above 2 × RHR also attenuated the association between log fasting insulin and PAEE (β = −0.56, p = 0.087). Furthermore, time spent above 2 × RHR (model 2c) was significantly negatively associated with log fasting insulin (β = −0.037, p = 0.001) after adjusting for PAEE and the same confounders as above. The explained variance in log fasting insulin varied between 8 and 10% for the models excluding body fat percentage (models 1, 1a, 1b and 1c) and between 26 and 28% for the models that included body fat percentage (models 2, 2a, 2b, 2c).
Finally, we re-examined all associations with further adjustment for aerobic fitness, and also substituted body fat percentage by waist circumference as a confounding factor; the results remained unchanged (data not shown).
Discussion
This study investigated the association between objectively measured PAEE and fasting insulin level as a measure of insulin resistance, specifically exploring the effect of physical activity intensity using a simple RHR-derived definition on the observed association. PAEE was significantly and inversely associated with fasting insulin independently of sex, age and body fat. However, this association was mainly explained by an association of moderate and vigorous physical activity with fasting insulin.
Our results are consistent with previous studies, suggesting that physical activity is negatively associated with fasting insulin level. Many of these studies have been based on subjective reports of physical activity using diaries or questionnaires with very different methodologies [38–40]. Some of these studies only assessed a specific category of activity such as occupational or leisure-time activity, but not the total amount or intensity of activity. A few other studies have used objective methods of measuring physical activity, including doubly-labelled water [8, 27], indirect calorimetry [27], accelerometers [8, 30, 31], and HR monitoring [23, 29]. Dvorak et al [27], using the doubly-labelled water method, found no significant association between PAEE and fasting insulin. This could be due to the fact that they did not correct PAEE for differences in body size or adjust for body weight in their analyses.
This is a cross-sectional study, limiting inferences of causality and its direction. However, it has been shown that physically active men were less likely to develop the metabolic syndrome than sedentary men [41, 42] and that PAEE predicts progression towards the metabolic syndrome [43]. Moreover, data from trials have demonstrated that physical activity leads to improved insulin sensitivity or reduced insulin resistance [44, 45]. A previous randomised controlled trial reported on the effect of interventions based on activity intensity and volume (three groups + control) on insulin resistance/insulin sensitivity in participants with normal glucose tolerance [44]. That study suggested that fasting insulin increased significantly in the control group and decreased in the low-volume/moderate-intensity and high-volume/high-intensity groups, whereas the decrease in the low-volume/high-intensity group was not statistically significant. The authors concluded that weekly exercise duration was an important variable influencing changes in insulin sensitivity. These findings are similar to ours, showing that increase in overall physical activity results in a decrease in fasting insulin, even though in that study, the intensity of the exercise training did not significantly influence insulin sensitivity.
Different biological mechanisms have been proposed through which physical activity exerts its action on insulin resistance [28]. These mechanisms include the upregulation of the rate-limiting enzymes of glycolysis and proteins in the insulin-signalling cascade, the lowering of triacylglycerol and NEFA, stimulated endothelial release of nitric oxide and the modulation of appetite via the leptin pathway. Acute muscle contraction allocates glucose transporter-4 to the cell membrane through an insulin-independent mechanism. This acute effect makes it difficult to investigate the effect of physical activity on insulin sensitivity or glucose transport, since observations could be attributable to the most recent bout of activity rather than to a chronic effect.
Collinearity between PAEE and time spent above 1.5, 1.75 and 2.0 × RHR could be a possible reason for the attenuation of the association between fasting insulin and PAEE when HR zone variables were included in the model. This is unlikely since in the multivariate regression models, no substantial increase in the standard error of the coefficient of PAEE was seen when time spent at 1.5 to 1.75, 1.75 to 2.0 or above 2.0 × RHR was individually added to the model, which would have indicated collinearity [46].
After adjusting for age, sex, body composition and PAEE, percentage time spent above 1.75 × RHR and above 2 × RHR was inversely associated with fasting insulin. This means that moderate-to-vigorous (above 1.75 × RHR) and vigorous (above 2.0 × RHR) activity were inversely associated with fasting insulin independently of PAEE. In contrast, light physical activity was not independently associated with fasting insulin. This may mean that moderate-to-vigorous physical activity is more important than light-intensity activity in relation to insulin resistance, although we acknowledge that light activity may be measured with a greater degree of error than moderate and vigorous activity. We note that intensity of activity in this context should be interpreted as relative intensity, due to the normalising effect of RHR and the strong correlation between cardiorespiratory fitness and RHR. This also explains the robustness of our results to additional adjustment for fitness and stresses the clinical utility of this simple measure of physical activity intensity.
Due to the moderate sample size of this study and the consistency of the associations reported, chance alone is unlikely to explain the observed associations. The individuals in this study are part of the MRC Ely cohort study [10, 32], which is an ongoing population-based cohort study. These participants are a subgroup of the initial volunteers who have been followed up, and includes particularly those who participated in the calibration test procedure. This means that there is a possible healthy sample selection bias. This bias could limit the generalisability of these findings to other populations and maybe to the target population, but it should not affect the internal validity of the associations observed. This population sample was, however, similar to the general population of England in terms of the prevalence of overweight and obesity, which are major risk factors for insulin resistance. The prevalence of overweight and obesity in this sample was 69% in men and 56% in women, and is similar to the national rates of 68% in men and 56% in women [47].
The effects of potential confounders were considered in the analyses. In the descriptive analyses, the results are presented stratified by sex [48, 49]. In the multivariate regression models, adjustments were made for age, sex and body composition when exploring the association between PAEE and fasting insulin. In addition, PAEE was included in the model as a covariate (confounder or mediator), when the effect of intensity of activity on fasting insulin was analysed, implying that the observed associations between different activity intensities and fasting insulin was independent of overall activity. However, insulin resistance is a multifactorial phenotype involving many other factors. Our multivariate models explained between 26 and 28% of the total variance of fasting insulin, with the physical activity variables accounting for 2 to 3% of the total variance.
In these analyses, the proportion of total awake hours spent in HR zones 1.5 to 1.75, 1.75 to 2.0 and above 2.0 × RHR was used as proxy for light, moderate and vigorous activity, respectively. The use of individual RHR in the calculation of time spent at the specified HR zones takes into account between-individual differences in aerobic fitness, and as HR monitors are widely available, our results may be more meaningful at the consumer level and in clinical practice than results based on intensity in units of body movement and energy per time as assessed by more cumbersome procedures (e.g. accelerometry or indirect calorimetry).
In conclusion, this study has provided further evidence of the benefits of physical activity on fasting insulin levels, a measure of insulin resistance. We have previously reported that physical activity is inversely associated with fasting insulin, even after adjustments for age, sex and body composition [23, 29]. However, the results of the present study suggest that the beneficial effect of PAEE on fasting insulin level may be attributable to the time spent in moderate and vigorous activity and not so much to the time spent on light intensity activity. Public health strategies to reduce insulin resistance in middle-aged individuals could therefore encourage people to increase their overall PAEE by devoting more time to moderate and perhaps even vigorous activities.
Acknowledgements
We are grateful to the volunteers for giving their time. We are also grateful to J. Luan for statistical advice; to S. Hennings, S. Emms and E. L. De Rolfe, who coordinated the study and collected data; and to K. Westgate, who assisted with the analysis of physical activity data. The MRC, UK, provided funding for the MRC Ely Study.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- bpm
beats per min
- CVD
cardiovascular diseases
- EE
energy expenditure
- HR
heart rate
- MET
metabolic equivalent task
- MRC
Medical Research Council
- PAEE
physical activity energy expenditure
- RHR
resting heart rate
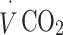
carbon dioxide production
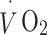
oxygen uptake
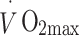
maximum oxygen uptake
References
- 1.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 2.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 4.Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997;349:1436–1442. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- 5.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 6.Balkau B, Charles MA, Drivsholm T, et al. Frequency of the WHO metabolic syndrome in European cohorts, and an alternative definition of an insulin resistance syndrome. Diabetes Metab. 2002;28:364–376. [PubMed] [Google Scholar]
- 7.Hedblad B, Nilsson P, Engstrom G, Berglund G, Janzon L. Insulin resistance in non-diabetic subjects is associated with increased incidence of myocardial infarction and death. Diabet Med. 2002;19:470–475. doi: 10.1046/j.1464-5491.2002.00719.x. [DOI] [PubMed] [Google Scholar]
- 8.Yao M, Lichtenstein AH, Roberts SB, et al. Relative influence of diet and physical activity on cardiovascular risk factors in urban Chinese adults. Int J Obes Relat Metab Disord. 2003;27:920–932. doi: 10.1038/sj.ijo.0802308. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan NM. The deadly quartet. Upper-body obesity, glucose intolerance, hypertriglyceridemia, and hypertension. Arch Intern Med. 1989;149:1514–1520. doi: 10.1001/archinte.149.7.1514. [DOI] [PubMed] [Google Scholar]
- 10.Wareham NJ, Byrne CD, Williams R, Day NE, Hales CN. Fasting proinsulin concentrations predict the development of type 2 diabetes. Diabetes Care. 1999;22:262–270. doi: 10.2337/diacare.22.2.262. [DOI] [PubMed] [Google Scholar]
- 11.Feskens EJ, Tuomilehto J, Stengard JH, Pekkanen J, Nissinen A, Kromhout D. Hypertension and overweight associated with hyperinsulinaemia and glucose tolerance: a longitudinal study of the Finnish and Dutch cohorts of the Seven Countries Study. Diabetologia. 1995;38:839–847. doi: 10.1007/s001250050361. [DOI] [PubMed] [Google Scholar]
- 12.Ohmura T, Ueda K, Kiyohara Y, et al. The association of the insulin resistance syndrome with impaired glucose tolerance and NIDDM in the Japanese general population: the Hisayama study. Diabetologia. 1994;37:897–904. doi: 10.1007/BF00400945. [DOI] [PubMed] [Google Scholar]
- 13.Eriksson KF, Lindgarde F. Poor physical fitness, and impaired early insulin response but late hyperinsulinaemia, as predictors of NIDDM in middle-aged Swedish men. Diabetologia. 1996;39:573–579. doi: 10.1007/BF00403304. [DOI] [PubMed] [Google Scholar]
- 14.Howard G, O’Leary DH, Zaccaro D, et al. Insulin sensitivity and atherosclerosis. The Insulin Resistance Atherosclerosis Study (IRAS) Investigators. Circulation. 1996;93:1809–1817. doi: 10.1161/01.cir.93.10.1809. [DOI] [PubMed] [Google Scholar]
- 15.Saad MF, Rewers M, Selby J, et al. Insulin resistance and hypertension: the Insulin Resistance Atherosclerosis study. Hypertension. 2004;43:1324–1331. doi: 10.1161/01.HYP.0000128019.19363.f9. [DOI] [PubMed] [Google Scholar]
- 16.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 17.Hanson RL, Pratley RE, Bogardus C, et al. Evaluation of simple indices of insulin sensitivity and insulin secretion for use in epidemiologic studies. Am J Epidemiol. 2000;151:190–198. doi: 10.1093/oxfordjournals.aje.a010187. [DOI] [PubMed] [Google Scholar]
- 18.Cheng C, Campbell KL, Kushner H, Falkner BE. Correlation of oral glucose tolerance test-derived estimates of insulin sensitivity with insulin clamp measurements in an African-American cohort. Metabolism. 2004;53:1107–1112. doi: 10.1016/j.metabol.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Vaccaro O, Masulli M, Cuomo V, et al. Comparative evaluation of simple indices of insulin resistance. Metabolism. 2004;53:1522–1526. doi: 10.1016/j.metabol.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 20.Ferrannini E. Physiological and metabolic consequences of obesity. Metabolism. 1995;44:15–17. doi: 10.1016/0026-0495(95)90313-5. [DOI] [PubMed] [Google Scholar]
- 21.Mooy JM, Grootenhuis PA, de Vries H, Bouter LM, Kostense PJ, Heine RJ. Determinants of specific serum insulin concentrations in a general Caucasian population aged 50 to 74 years (the Hoorn Study) Diabet Med. 1998;15:45–52. doi: 10.1002/(SICI)1096-9136(199801)15:1<45::AID-DIA503>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 22.Feskens EJ, Loeber JG, Kromhout D. Diet and physical activity as determinants of hyperinsulinemia: the Zutphen Elderly Study. Am J Epidemiol. 1994;140:350–360. doi: 10.1093/oxfordjournals.aje.a117257. [DOI] [PubMed] [Google Scholar]
- 23.Franks PW, Luan J, Browne PO, et al. Does peroxisome proliferator-activated receptor gamma genotype (Pro12ala) modify the association of physical activity and dietary fat with fasting insulin level? Metabolism. 2004;53:11–16. doi: 10.1016/j.metabol.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Wareham NJ, Wong MY, Day NE. Glucose intolerance and physical inactivity: the relative importance of low habitual energy expenditure and cardiorespiratory fitness. Am J Epidemiol. 2000;152:132–139. doi: 10.1093/aje/152.2.132. [DOI] [PubMed] [Google Scholar]
- 25.Byberg L, Zethelius B, McKeigue PM, Lithell HO. Changes in physical activity are associated with changes in metabolic cardiovascular risk factors. Diabetologia. 2001;44:2134–2139. doi: 10.1007/s001250100022. [DOI] [PubMed] [Google Scholar]
- 26.Aadahl M, Kjaer M, Jorgensen T. Associations between overall physical activity level and cardiovascular risk factors in an adult population. Eur J Epidemiol. 2007;22:369–378. doi: 10.1007/s10654-006-9100-3. [DOI] [PubMed] [Google Scholar]
- 27.Dvorak RV, Tchernof A, Starling RD, Ades PA, DiPietro L, Poehlman ET. Respiratory fitness, free living physical activity, and cardiovascular disease risk in older individuals: a doubly labeled water study. J Clin Endocrinol Metab. 2000;85:957–963. doi: 10.1210/jc.85.3.957. [DOI] [PubMed] [Google Scholar]
- 28.Wareham JN, Brage S, Franks PW, Abbott RA. Physical activity and insulin resistance. In: Kumar S, O’Rahilly S, editors. Insulin resistance: insulin action and its disturbances in disease. Chichester: Wiley; 2005. pp. 317–400. [Google Scholar]
- 29.Harding AH, Williams DE, Hennings SH, Mitchell J, Wareham NJ. Is the association between dietary fat intake and insulin resistance modified by physical activity? Metabolism. 2001;50:1186–1192. doi: 10.1053/meta.2001.26702. [DOI] [PubMed] [Google Scholar]
- 30.Ekelund U, Franks PW, Sharp S, Brage S, Wareham NJ. Increase in physical activity energy expenditure is associated with reduced metabolic risk independent of change in fatness and fitness. Diabetes Care. 2007;30:2101–2106. doi: 10.2337/dc07-0719. [DOI] [PubMed] [Google Scholar]
- 31.Ekelund U, Griffin SJ, Wareham NJ. Physical activity and metabolic risk in individuals with a family history of type 2 diabetes. Diabetes Care. 2007;30:337–342. doi: 10.2337/dc06-1883. [DOI] [PubMed] [Google Scholar]
- 32.Williams DR, Wareham NJ, Brown DC, et al. Undiagnosed glucose intolerance in the community: the Isle of Ely Diabetes Project. Diabet Med. 1995;12:30–35. doi: 10.1111/j.1464-5491.1995.tb02058.x. [DOI] [PubMed] [Google Scholar]
- 33.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ceesay SM, Prentice AM, Day KC, et al. The use of heart rate monitoring in the estimation of energy expenditure: a validation study using indirect whole-body calorimetry. Br J Nutr. 1989;61:175–186. doi: 10.1079/BJN19890107. [DOI] [PubMed] [Google Scholar]
- 35.Livingstone MB, Prentice AM, Coward WA, et al. Simultaneous measurement of free-living energy expenditure by the doubly labeled water method and heart-rate monitoring. Am J Clin Nutr. 1990;52:59–65. doi: 10.1093/ajcn/52.1.59. [DOI] [PubMed] [Google Scholar]
- 36.Wareham NJ, Hennings SJ, Prentice AM, Day NE. Feasibility of heart-rate monitoring to estimate total level and pattern of energy expenditure in a population-based epidemiological study: the Ely Young Cohort Feasibility Study 1994–5. Br J Nutr. 1997;78:889–900. doi: 10.1079/BJN19970207. [DOI] [PubMed] [Google Scholar]
- 37.Simpson JA, Lobo DN, Anderson JA, et al. Body water compartment measurements: a comparison of bioelectrical impedance analysis with tritium and sodium bromide dilution techniques. Clin Nutr. 2001;20:339–343. doi: 10.1054/clnu.2001.0398. [DOI] [PubMed] [Google Scholar]
- 38.Marshall JA, Bessesen DH, Hamman RF. High saturated fat and low starch and fibre are associated with hyperinsulinaemia in a non-diabetic population: the San Luis Valley Diabetes Study. Diabetologia. 1997;40:430–438. doi: 10.1007/s001250050697. [DOI] [PubMed] [Google Scholar]
- 39.Irwin ML, Mayer-Davis EJ, Addy CL, et al. Moderate-intensity physical activity and fasting insulin levels in women: the Cross-Cultural Activity Participation Study. Diabetes Care. 2000;23:449–454. doi: 10.2337/diacare.23.4.449. [DOI] [PubMed] [Google Scholar]
- 40.Mayer-Davis EJ, D’Agostino R, Jr, Karter AJ, et al. Intensity and amount of physical activity in relation to insulin sensitivity: the Insulin Resistance Atherosclerosis Study. JAMA. 1998;279:669–674. doi: 10.1001/jama.279.9.669. [DOI] [PubMed] [Google Scholar]
- 41.Laaksonen DE, Lakka HM, Salonen JT, Niskanen LK, Rauramaa R, Lakka TA. Low levels of leisure-time physical activity and cardiorespiratory fitness predict development of the metabolic syndrome. Diabetes Care. 2002;25:1612–1618. doi: 10.2337/diacare.25.9.1612. [DOI] [PubMed] [Google Scholar]
- 42.Lakka TA, Laaksonen DE, Lakka HM, et al. Sedentary lifestyle, poor cardiorespiratory fitness, and the metabolic syndrome. Med Sci Sports Exerc. 2003;35:1279–1286. doi: 10.1249/01.MSS.0000079076.74931.9A. [DOI] [PubMed] [Google Scholar]
- 43.Ekelund U, Brage S, Franks PW, Hennings S, Emms S, Wareham NJ. Physical activity energy expenditure predicts progression toward the metabolic syndrome independently of aerobic fitness in middle-aged healthy Caucasians: the Medical Research Council Ely Study. Diabetes Care. 2005;28:1195–1200. doi: 10.2337/diacare.28.5.1195. [DOI] [PubMed] [Google Scholar]
- 44.Houmard JA, Tanner CJ, Slentz CA, Duscha BD, McCartney JS, Kraus WE. Effect of the volume and intensity of exercise training on insulin sensitivity. J Appl Physiol. 2004;96:101–106. doi: 10.1152/japplphysiol.00707.2003. [DOI] [PubMed] [Google Scholar]
- 45.Kang J, Robertson RJ, Hagberg JM, et al. Effect of exercise intensity on glucose and insulin metabolism in obese individuals and obese NIDDM patients. Diabetes Care. 1996;19:341–349. doi: 10.2337/diacare.19.4.341. [DOI] [PubMed] [Google Scholar]
- 46.Kirkwood BR, Sterne JAC. Essential medical statistics. 2. Cambridge: Blackwell Science; 2004. [Google Scholar]
- 47.Department of Health (2003) Body mass: by sex, 2001: Social Trends 33. Office of National Statistics, Health Survey for England. Available from http://www.statistics.gov.uk/STATBASE/Expodata/Spreadsheets/D6420.xls, accessed 20 December 2007
- 48.Chou P, Lin KC, Lin HY, Tsai ST. Gender differences in the relationships of serum uric acid with fasting serum insulin and plasma glucose in patients without diabetes. J Rheumatol. 2001;28:571–576. [PubMed] [Google Scholar]
- 49.Oh JY, Barrett-Connor E, Wedick NM. Sex differences in the association between proinsulin and intact insulin with coronary heart disease in nondiabetic older adults: the Rancho Bernardo Study. Circulation. 2002;105:1311–1316. doi: 10.1161/hc1102.105565. [DOI] [PubMed] [Google Scholar]