Abstract
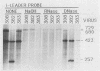
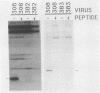
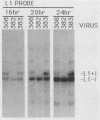
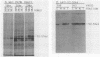
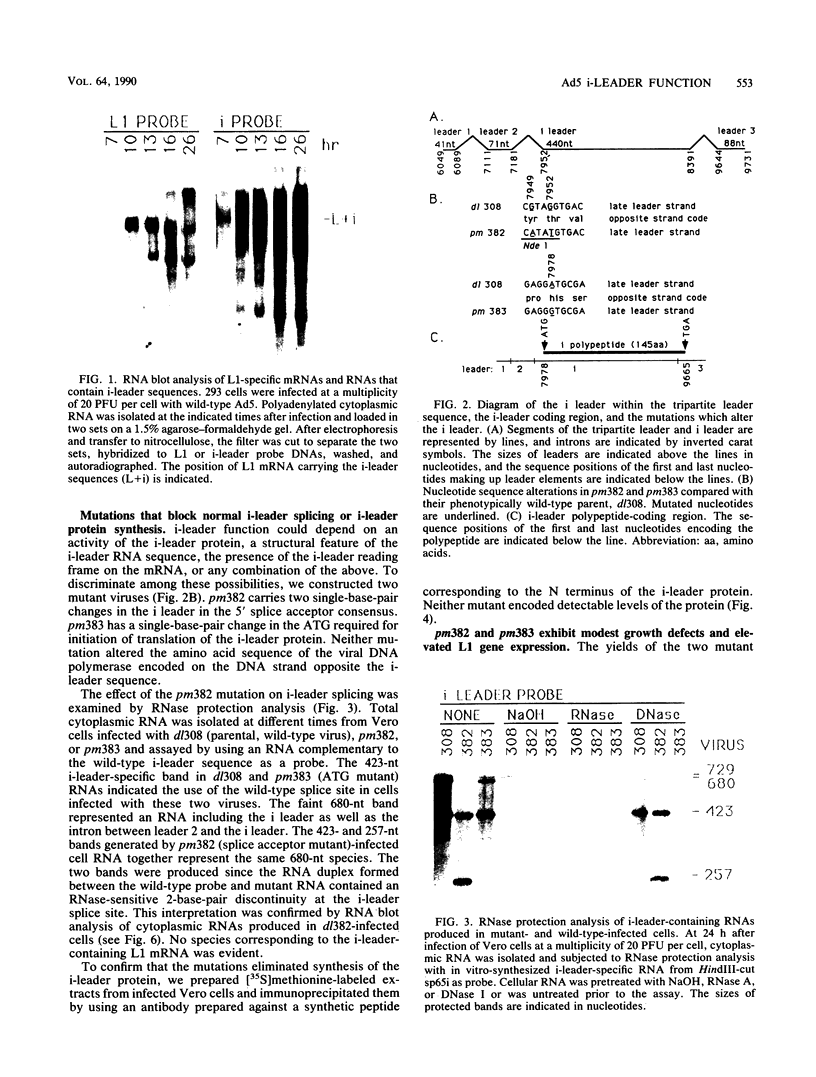
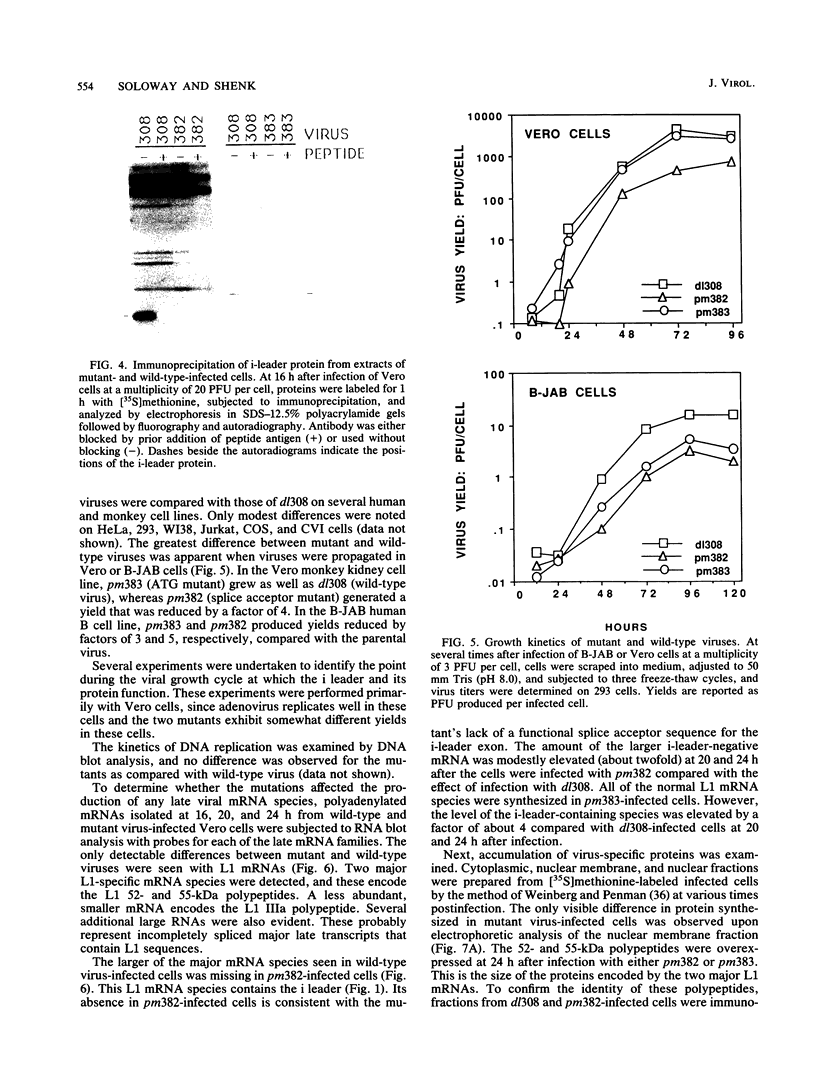
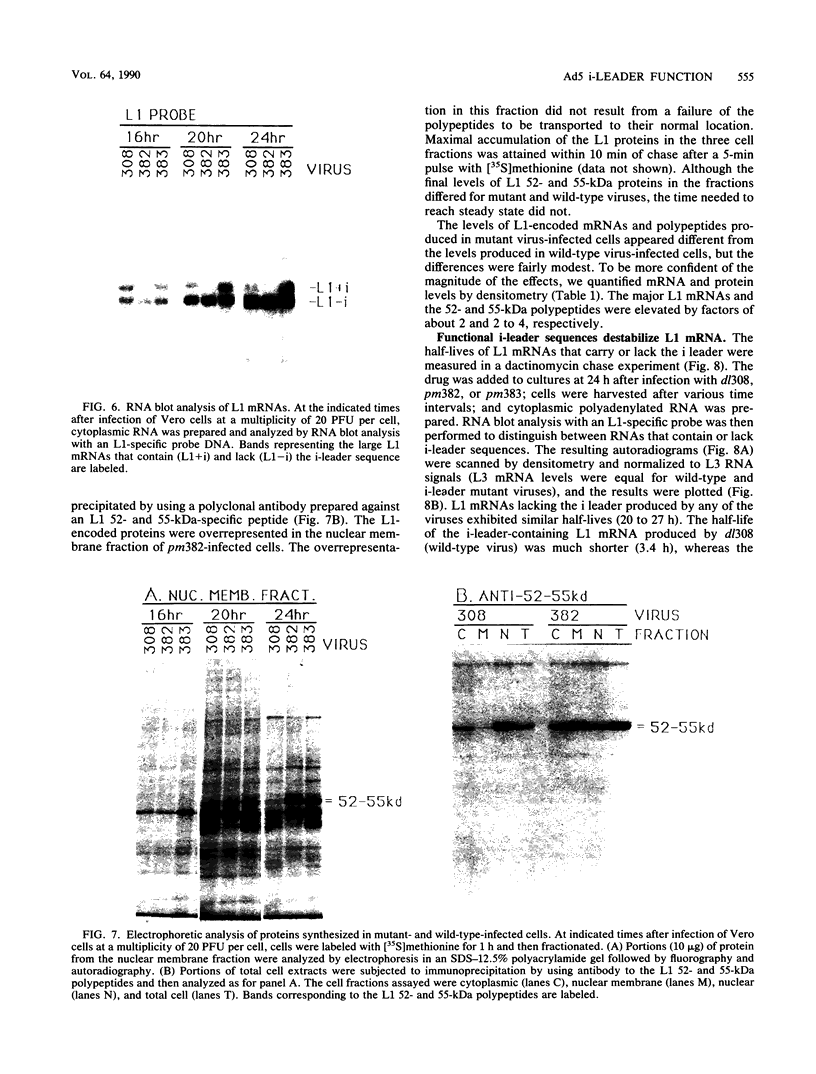
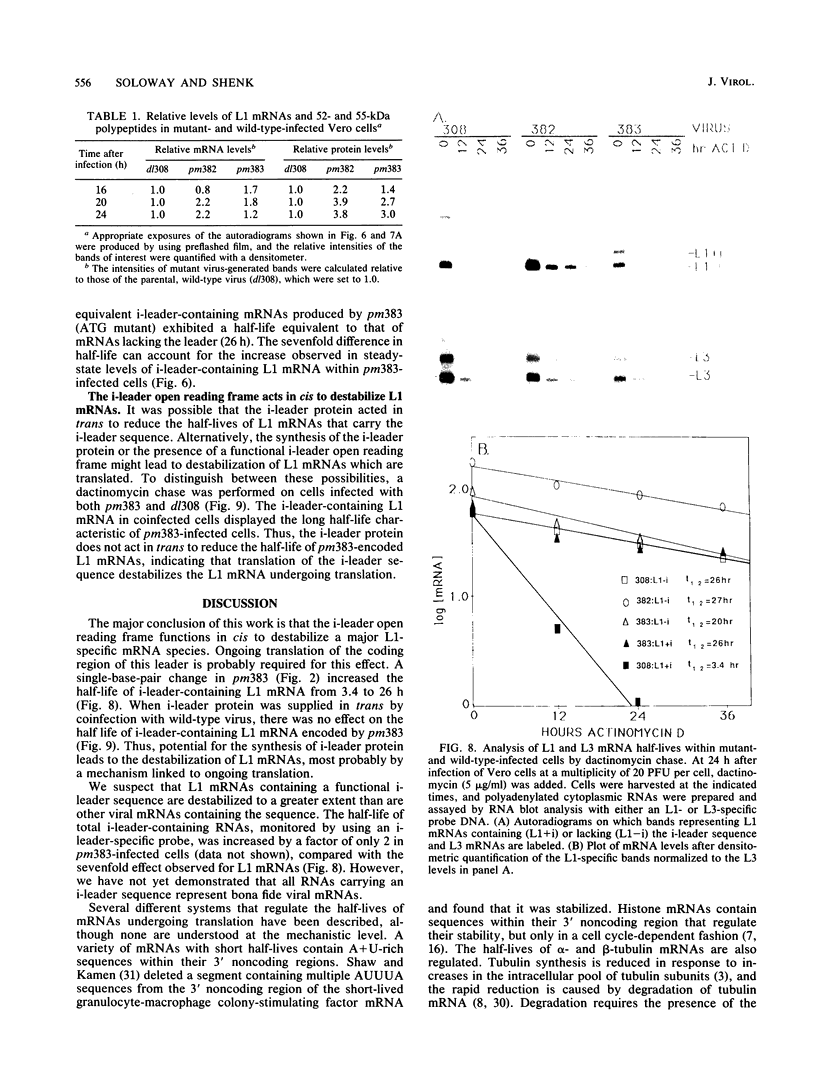
The 440-nucleotide adenovirus type 5 i-leader sequence, encoding a 13.6-kilodalton protein, is located between the second and third components of the tripartite leader sequence. It appears primarily on the L1 family of mRNAs. To study its function, we constructed two point mutations within the i leader. pm382 lacks the wild-type i-leader splice acceptor and failed to splice the leader onto L1 mRNAs. pm383 lacks the ATG used for translation of the i-leader protein; it synthesized i-leader-containing mRNAs, but failed to produce detectable levels of the polypeptide. Both mutants exhibited modestly reduced yields in some but not all cell lines tested and accumulated slightly elevated levels of L1 mRNA and L1 52- and 55-kilodalton proteins in infected cells. Mutant phenotypes were consistently more pronounced in pm382- than in pm383-infected cells. In wild-type virus-infected cells, L1 mRNAs lacking the i leader displayed a half-life of about 26 h, whereas L1 mRNAs containing the leader were much less stable, with a half-life of less than 4 h. In pm383-infected cells (ATG mutant), L1 mRNAs containing the i leader exhibited a half-life of 26 h. The abnormally long half-life of pm383-encoded L1 mRNAs containing a mutant i leader was not reduced by coinfection with wild-type virus, suggesting that synthesis of the i-leader protein leads to destabilization of the i-leader-containing L1 mRNA undergoing translation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akusjärvi G., Persson H. Controls of RNA splicing and termination in the major late adenovirus transcription unit. Nature. 1981 Jul 30;292(5822):420–426. doi: 10.1038/292420a0. [DOI] [PubMed] [Google Scholar]
- Akusjärvi G., Pettersson J. Sequence analysis of adenovirus DNA. IV. The genomic sequences encoding the common tripartite leader of late adenovirus messenger RNA. J Mol Biol. 1979 Oct 15;134(1):143–158. doi: 10.1016/0022-2836(79)90417-0. [DOI] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Farmer S. R., Penman S. Mechanisms of regulating tubulin synthesis in cultured mammalian cells. Cell. 1979 Jun;17(2):319–325. doi: 10.1016/0092-8674(79)90157-0. [DOI] [PubMed] [Google Scholar]
- Berget S. M., Moore C., Sharp P. A. Spliced segments at the 5' terminus of adenovirus 2 late mRNA. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3171–3175. doi: 10.1073/pnas.74.8.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkner K. L., Schaffhausen B. S., Roberts T. M., Sharp P. A. Abundant expression of polyomavirus middle T antigen and dihydrofolate reductase in an adenovirus recombinant. J Virol. 1987 Apr;61(4):1213–1220. doi: 10.1128/jvi.61.4.1213-1220.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkner K. L., Sharp P. A. Effect of the tripartite leader on synthesis of a non-viral protein in an adenovirus 5 recombinant. Nucleic Acids Res. 1985 Feb 11;13(3):841–857. doi: 10.1093/nar/13.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capasso O., Bleecker G. C., Heintz N. Sequences controlling histone H4 mRNA abundance. EMBO J. 1987 Jun;6(6):1825–1831. doi: 10.1002/j.1460-2075.1987.tb02437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron J. M., Jones A. L., Rall L. B., Kirschner M. W. Autoregulation of tubulin synthesis in enucleated cells. Nature. 1985 Oct 17;317(6038):648–651. doi: 10.1038/317648a0. [DOI] [PubMed] [Google Scholar]
- Castrillo J. L., Carrasco L. Adenovirus late protein synthesis is resistant to the inhibition of translation induced by poliovirus. J Biol Chem. 1987 May 25;262(15):7328–7334. [PubMed] [Google Scholar]
- Chow L. T., Broker T. R., Lewis J. B. Complex splicing patterns of RNAs from the early regions of adenovirus-2. J Mol Biol. 1979 Oct 25;134(2):265–303. doi: 10.1016/0022-2836(79)90036-6. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Gelinas R. E., Broker T. R., Roberts R. J. An amazing sequence arrangement at the 5' ends of adenovirus 2 messenger RNA. Cell. 1977 Sep;12(1):1–8. doi: 10.1016/0092-8674(77)90180-5. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolph P. J., Racaniello V., Villamarin A., Palladino F., Schneider R. J. The adenovirus tripartite leader may eliminate the requirement for cap-binding protein complex during translation initiation. J Virol. 1988 Jun;62(6):2059–2066. doi: 10.1128/jvi.62.6.2059-2066.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falvey E., Ziff E. Sequence arrangement and protein coding capacity of the adenovirus type 2 "i" leader. J Virol. 1983 Jan;45(1):185–191. doi: 10.1128/jvi.45.1.185-191.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Green M., Brackmann K. H., Lucher L. A., Symington J. S., Kramer T. A. Human adenovirus 2 E1B-19K and E1B-53K tumor antigens: antipeptide antibodies targeted to the NH2 and COOH termini. J Virol. 1983 Dec;48(3):604–615. doi: 10.1128/jvi.48.3.604-615.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson T. B., Soloway P. D., Ornelles D. A., Doerfler W., Shenk T. Adenovirus L1 52- and 55-kilodalton proteins are required for assembly of virions. J Virol. 1989 Sep;63(9):3612–3621. doi: 10.1128/jvi.63.9.3612-3621.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N., Shenk T. An adenovirus type 5 early gene function regulates expression of other early viral genes. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3665–3669. doi: 10.1073/pnas.76.8.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman R. J. Identification of the components necessary for adenovirus translational control and their utilization in cDNA expression vectors. Proc Natl Acad Sci U S A. 1985 Feb;82(3):689–693. doi: 10.1073/pnas.82.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klessig D. F. Two adenovirus mRNAs have a common 5' terminal leader sequence encoded at least 10 kb upstream from their main coding regions. Cell. 1977 Sep;12(1):9–21. doi: 10.1016/0092-8674(77)90181-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis J. B., Anderson C. W. Proteins encoded near the adenovirus late messenger RNA leader segments. Virology. 1983 May;127(1):112–123. doi: 10.1016/0042-6822(83)90376-8. [DOI] [PubMed] [Google Scholar]
- Logan J., Shenk T. Adenovirus tripartite leader sequence enhances translation of mRNAs late after infection. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3655–3659. doi: 10.1073/pnas.81.12.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucher L. A., Symington J. S., Green M. Biosynthesis and properties of the adenovirus 2 L1-encoded 52,000- and 55,000-Mr proteins. J Virol. 1986 Mar;57(3):839–847. doi: 10.1128/jvi.57.3.839-847.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachter J. S., Yen T. J., Cleveland D. W. Autoregulation of tubulin expression is achieved through specific degradation of polysomal tubulin mRNAs. Cell. 1987 Oct 23;51(2):283–292. doi: 10.1016/0092-8674(87)90155-3. [DOI] [PubMed] [Google Scholar]
- Parker B. A., Stark G. R. Regulation of simian virus 40 transcription: sensitive analysis of the RNA species present early in infections by virus or viral DNA. J Virol. 1979 Aug;31(2):360–369. doi: 10.1128/jvi.31.2.360-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger M. F., Cleveland D. W. Retention of autoregulatory control of tubulin synthesis in cytoplasts: demonstration of a cytoplasmic mechanism that regulates the level of tubulin expression. J Cell Biol. 1985 Nov;101(5 Pt 1):1941–1952. doi: 10.1083/jcb.101.5.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Symington J. S., Lucher L. A., Brackmann K. H., Virtanen A., Pettersson U., Green M. Biosynthesis of adenovirus type 2 i-leader protein. J Virol. 1986 Mar;57(3):848–856. doi: 10.1128/jvi.57.3.848-856.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmappaya B., Weinberger C., Schneider R. J., Shenk T. Adenovirus VAI RNA is required for efficient translation of viral mRNAs at late times after infection. Cell. 1982 Dec;31(3 Pt 2):543–551. doi: 10.1016/0092-8674(82)90310-5. [DOI] [PubMed] [Google Scholar]
- Thummel C., Tjian R., Hu S. L., Grodzicker T. Translational control of SV40 T antigen expressed from the adenovirus late promoter. Cell. 1983 Jun;33(2):455–464. doi: 10.1016/0092-8674(83)90427-0. [DOI] [PubMed] [Google Scholar]
- Virtanen A., Aleström P., Persson H., Katze M. G., Pettersson U. An adenovirus agnogene. Nucleic Acids Res. 1982 Apr 24;10(8):2539–2548. doi: 10.1093/nar/10.8.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R. A., Penman S. Small molecular weight monodisperse nuclear RNA. J Mol Biol. 1968 Dec;38(3):289–304. doi: 10.1016/0022-2836(68)90387-2. [DOI] [PubMed] [Google Scholar]
- Yen T. J., Gay D. A., Pachter J. S., Cleveland D. W. Autoregulated changes in stability of polyribosome-bound beta-tubulin mRNAs are specified by the first 13 translated nucleotides. Mol Cell Biol. 1988 Mar;8(3):1224–1235. doi: 10.1128/mcb.8.3.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zain S., Sambrook J., Roberts R. J., Keller W., Fried M., Dunn A. R. Nucleotide sequence analysis of the leader segments in a cloned copy of adenovirus 2 fiber mRNA. Cell. 1979 Apr;16(4):851–861. doi: 10.1016/0092-8674(79)90100-4. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis: a simple method using two oligonucleotide primers and a single-stranded DNA template. DNA. 1984 Dec;3(6):479–488. doi: 10.1089/dna.1.1984.3.479. [DOI] [PubMed] [Google Scholar]