Abstract
Erythropoietin (EPO) is of great interest as a therapy for many of the central nervous system (CNS) diseases and its administration is protective in experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis (MS). Endogenous EPO is induced by hypoxic/ischemic injury, but little is known about its expression in other CNS diseases. We report here that EPO expression in the spinal cord is induced in mouse models of chronic or relapsing-remitting EAE, and is prominently localized to motoneurons. We found a parallel increase of hypoxia-inducible transcription factor (HIF)-1α, but not HIF-2α, at the mRNA level, suggesting a possible role of non-hypoxic factors in EPO induction. EPO mRNA in the spinal cord was co-expressed with interferon (IFN)–γ and tumor necrosis factor (TNF), and these cytokines inhibited EPO production in vitro in both neuronal and glial cells. Given the known inhibitory effect of EPO on neuroinflammation, our study indicates that EPO should be viewed as part of the inflammatory/anti-inflammatory network in MS.
INTRODUCTION
The interest in erythropoietin (EPO) as a neuroprotective mediator has grown since it was found that systemically administered EPO crosses the blood brain barrier and is protective in several animal models of disease, including cerebral and spinal cord traumatic or ischemic injury and peripheral neuropathies (1,2). EPO administration reduced damage-induced neuroinflammation, a common component of all these diseases (3), suggesting that EPO anti-inflammatory action might be important in its pharmacological activity. This hypothesis also is supported by the fact that administration of EPO is effective in experimental autoimmune encephalomyelitis (EAE), an animal model for multiple sclerosis (MS), and a model of inflammatory disease of the central nervous system (CNS) (1,4–6). The importance of this observation was stressed by a clinical trial with EPO in MS patients (7).
These findings raised the question of the expression of endogenous EPO in CNS diseases. EPO typically is induced via the hypoxia-inducible transcription factor (HIF)-1 and HIF-2, and its elevation mediates the increased production of erythrocytes in hypoxic conditions (8). While originally thought to be produced only by the kidney, many tissues now are recognized as expressing EPO (8,9).
In the CNS, EPO is produced following cerebral ischemia (10,11), and plays a crucial role in neuronal survival and in mediating ischemic preconditioning (12, 13).
Microarray studies in MS brain revealed upregulation of genes involved in neural protective mechanisms known to be induced upon ischemic preconditioning, including HIF-1α and vascular endothelial growth factor (VEGF) receptor 1 (14). In addition, expression of HIF-1α has been found in brain lesions of a subtype of MS cases (15), suggesting that a hypoxia-like tissue injury may play a pathogenic role in MS.
Therefore, we investigated the expression of EPO in the spinal cord of mice using two models of EAE: a chronic model induced in C57BL/6 mice by immunization against a peptide of myelin oligodendrocyte glycoprotein (MOG) (6), and a relapsing-remitting model induced in SJL mice by immunizing against myelin proteolipid protein (PLP) peptide (16). Since we found that EPO is co-induced along with interferon (IFN)–γ and tumor necrosis factor (TNF), pro-inflammatory cytokines with a pathogenic role in EAE, we investigated the effect of IFN-γ and TNF on EPO expression in neuronal and glial cells. The results indicate that EPO is induced during EAE and is negatively regulated by IFN-γ and TNF.
MATERIALS AND METHODS
Animal Experiments
Procedures involving animals and their care were conducted in conformity with the institutional guidelines in compliance with national and international laws and policies (17–19). The protocols for the proposed investigation were reviewed and approved by the Animal Care and Use Committees (IACUC) of the Mario Negri Institute for Pharmacological Research.
Chronic EAE was induced in female C57BL/6 mice (6–8 wks; Harlan, Bresso, MI, Italy) by subcutaneous (s.c.) immunization with 200 μg of MOG peptide 35–55 (Multiple Peptide Systems, San Diego, CA, USA) per mouse in incomplete Freund’s adjuvant (Sigma, St Louis, MO, USA) supplemented with 8 mg/mL of M. tuberculosis (H37Ra; Difco Laboratories, Detroit, MI, USA). Mice received 500 ng of pertussis toxin (Sigma) intravenously (i.v.) at the time of immunization and 48 h later. Clinical score was recorded daily as described elsewhere (6).
Relapsing-remitting EAE was induced in female 8- to 12-wk-old SJL mice (Charles River, Calco, LC, Italy) by s.c. immunization with PLP peptide 139–151 (100 μg/mouse, in incomplete Freund’s adjuvant, Sigma, containing 2 mg/mL of heat-killed M. tuberculosis, Difco) as described (16).
Real-Time RT-PCR
Total RNA was extracted from spinal cords or cell cultures using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA), retrotranscribed according to standard procedures, and amplified by real-time reverse-transcription (RT)-PCR. All procedures were performed on the ABI PRISM 7300 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). For quantification, we used the comparative threshold cycle ( Ct) method, according to the manufacturer’s guidelines. EPO mRNA was analyzed by the TaqMan method, with gene expression assays for EPO and GAPDH (housekeeping gene) commercially available from Applied Biosystems. Data were expressed as EPO mRNA units, with a cDNA from mouse kidney used as a calibrator (or rat kidney when EPO mRNA was measured in rat glial cells). IFN-γ and TNF mRNA levels were analyzed by the SYBR Green method, with primer sequences for TNF, IFN-γ, and GAPDH as reported (20). Data were expressed as fold induction versus control mice, using one of the controls as a calibrator.
Immunohistochemistry
Animals were sacrificed by intracardial perfusion (21). EPO localization was evaluated along the whole length of the spinal cord. Sections (40 μm-thick) were washed in phosphate-buffered saline (PBS), incubated in 10% blocking serum in PBS at room temperature and exposed to polyclonal rabbit anti-EPO (1:100, H-162, Santa Cruz Biotechnology, Heidelberg, Germany) in 2% goat sera at 4° C. After an overnight incubation, the sections were incubated with biotinylated goat anti-rabbit antibodies (1:100, Vector Laboratories Inc., Burlingame, CA, USA) for 60 min, revealed with avidin-biotin using a peroxidase kit (Vectastain Elite, Vector), and the signal visualized with diaminobenzidine (DAB; Sigma Chemical, Deisenhofen, Germany) under a light microscope.
Free floating immunofluorescence experiments were carried out on 30 μm-thick lumbar spinal cord (L2–L3) sections. Coronal sectioning was preferred to better evaluate the localization of the different markers in specific areas of the grey matter. EPO was detected with polyclonal rabbit anti-EPO and biotinylated goat anti-rabbit antibodies as described above, and the immunofluorescence revealed with Alexa 488 goat anti-rabbit secondary antibody (1:1000, Molecular Probes, Eugene, OR, USA). Neurons were stained with 530-615 NeuroTrace Fluorescent Nissl reagent (1:5000, Molecular Probes). Astrocytes were identified by a mouse anti-GFAP monoclonal antibody (1:1000, Immunological Science, Rome, Italy) followed by an Alexa 546 goat anti-mouse secondary antibody (1:1000, Molecular Probes). Sections were observed with an Olympus Fluoview microscope BX61 with confocal system FV500. To confirm the specificity of the signal, we checked that incubating with either a primary or a secondary antibody alone gave no staining (not shown).
Cell Culture
Human Kelly neuroblastoma cells were cultured and stimulated with the HIF-inducer deferoxamine mesylate (DFX, Sigma) as reported (22), with or without recombinant human IFN-γ (HyCult Biotechnology, Uden, The Netherlands) or recombinant human TNF-α (R&D Systems, Minneapolis, MN, USA). After 24 h of incubation, EPO was measured in the supernatant by Enzyme-Linked ImmunoSorbent Assay (ELISA) (Quantikine EPO, R&D Systems, sensitivity 0.6 mU/mL). All samples were assayed in duplicate. Results were expressed as mU/mL.
Primary cultures of glial cells were prepared from 1- to 2-d-old newborn rats (Sprague-Dawley, Charles River) as described (3). Cells were treated for 24 h with the HIF-inducer dimethyloxalylglycine (DMOG, Frontier Scientific Inc., Logan, UT, USA), with or without recombinant rat IFN-γ (R&D Systems) or recombinant rat TNF-α (R&D Systems), then total RNA was extracted and analyzed for EPO expression by real-time RT-PCR.
RESULTS
EPO Expression Is Increased in the Spinal Cord of Mice with EAE
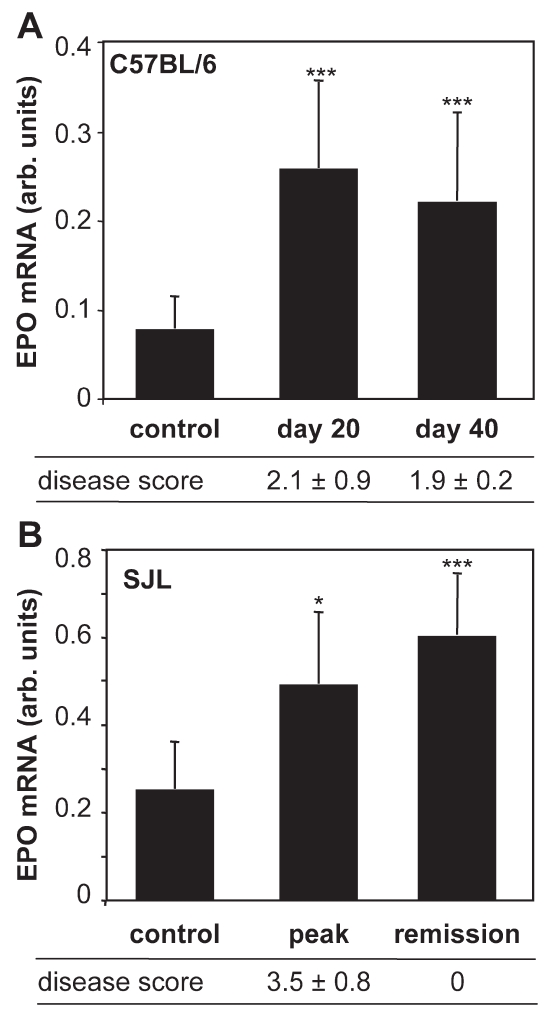
In chronic EAE induced by immunization of C57BL/6 mice with MOG35-55, clinical signs of disease have an onset at d 10–15 after immunization, peak at d 20, and then stabilize (6). By quantitative real-time RT-PCR, we found a marked increase in EPO mRNA expression in the spinal cord of mice with EAE at d 20 as compared with control naive mice (Figure 1A). EPO mRNA levels remained elevated at d 40, when the clinical signs of the disease are stabilized.
Figure 1.
EPO expression in EAE. EPO mRNA was measured in the spinal cord of control and C57BL/6 mice with MOG-induced chronic EAE at the indicated day after the immunization (A) or SJL mice with PLP-induced relapsing-remitting EAE at the peak of the disease, d 14–15 after immunization, and in the remission phase, on d 19–30 after immunization (B). The clinical score of the disease is indicated below each panel. Results are the mean ± standard error of mean (SEM) of values of at least 10 mice per group (A) and at least 5 mice per group (B), with individual samples tested in duplicate. *P < 0.05; ***P < 0.001 versus control, Student t test.
In SJL mice, PLP139-155-induced EAE is characterized by a relapsing-remitting type of disease (16). In this model, EPO expression was increased in the spinal cord at the peak of the disease, and remained elevated in the remission phase (Figure 1B).
Immunohistochemical Localization of EPO in the Spinal Cord
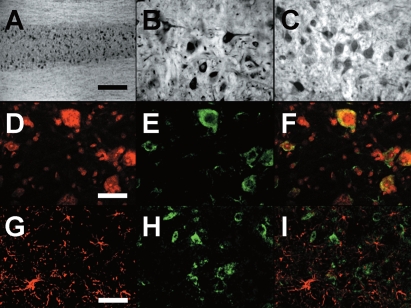
Immunohistochemistry on d 20 after immunization of C57BL/6 mice revealed an almost selective immunoreactivity in the gray matter (central layer), with a spot pattern, and a very low staining in the white matter (external layers) (Figure 2A). Higher magnification of the area corresponding to the ventral horns showed that EPO is localized in cell bodies of neurons with different sizes, likely motoneurons and interneurons (Figure 2B). Although DAB immunohistochemistry does not allow a quantitative evaluation, staining intensity of large-sized neurons appears higher in EAE mice (Figure 2B) compared with healthy controls (Figure 2C). Incubation of sections without the primary antibody allowed us to exclude the presence of a non-specific immunoreactivity due to the biotinylated secondary antibody (not shown).
Figure 2.
Immunohistochemical localization of EPO in the spinal cord. (A) Representative picture showing the pattern of EPO staining along the spinal cord in chronic EAE, 20 d after immunization. The more caudal part of the spinal cord, representing the lumbar tract, is on the left of the picture, and the thoracic regions are on the right. (B–C) Higher magnification pictures showing EPO in the ventral horns of the lumbar spinal cord in EAE (B) and a control mouse (C). (D–F) Nissl staining (D), EPO (E), and their colocalization (F), in the anterior horns of the lumbar spinal cord in EAE. (G–I) GFAP (G), EPO (H), and the merging field obtained by the overlapping of both signals (I), in the anterior horns of the lumbar spinal cord in EAE. Scale bars: A, 500 μm; B–C, 80 μm; D–F, 50 μm; G–I, 60 μm.
When Nissl was used to identify neurons (Figure 2D, red), EPO staining (Figure 2E, green) co-localized with it (Figure 2F, merge), showing a cytoplasmic localization. On the other hand, co-localization experiments using GFAP to stain astrocytes (Figure 2G, red) and EPO (Figure 2H, green) confirmed the lack of expression of EPO by astrocytes (Figure 2I, merge).
HIF-1α Expression Is Induced in EAE
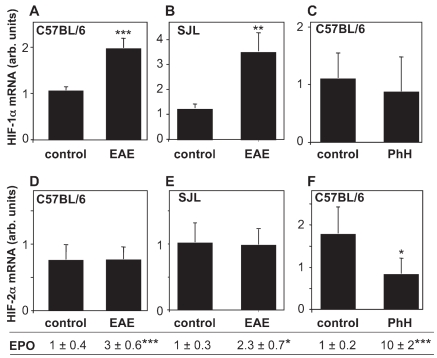
We investigated the mRNA expression of HIF-1α and HIF-2α in the spinal cord of mice with chronic EAE (C57BL/6) or relapsing-remitting EAE (SJL), and correlated it with the increased expression of EPO mRNA. For purpose of comparison, we also measured HIFs and EPO mRNA in the spinal cord of mice treated with phenylhydrazine (PhH), which also is known to induce EPO expression in the CNS (23). In both models of EAE, a significant increase of HIF-1α, but not HIF-2α, expression was observed (Figure 3), accompanied by a two- to threefold induction in EPO mRNA (see above and Figure 3). On the contrary, PhH did not increase spinal cord HIF-1α and HIF-2α mRNA, even though it strongly induced EPO expression (controls, 0.11 ± 0.02; PhH, 1.01 ± 0.15; P < 0.001, n = 4), acting through stabilization of the HIF protein, as reported (24).
Figure 3.
HIF-1α expression is induced in EAE. HIF-1α mRNA was measured in the spinal cord of control and C57BL/6 mice with MOG-induced EAE at d 20 after immunization (A), SJL mice with PLP-induced EAE at the peak of the disease, d 14–15 after immunization (B) or C57BL/6 healthy mice 24 h after treatment with phenylhydrazine (PhH; 150 mg/Kg, i.p., panel C). In the same mice, HIF-2α mRNA was also measured (D–F). EPO mRNA levels, expressed as fold induction versus control, are indicated below. Results are the mean ± SEM (n = 5: panels A,B,D,E; n = 4: panels C,F), with individual samples tested in duplicate. *P < 0.05; **P < 0.01; ***P < 0.001 versus control, Student t test.
IFN-γ and TNF Are Co-Induced with EPO and Inhibit its Expression in Neuronal and Glial Cells in vitro
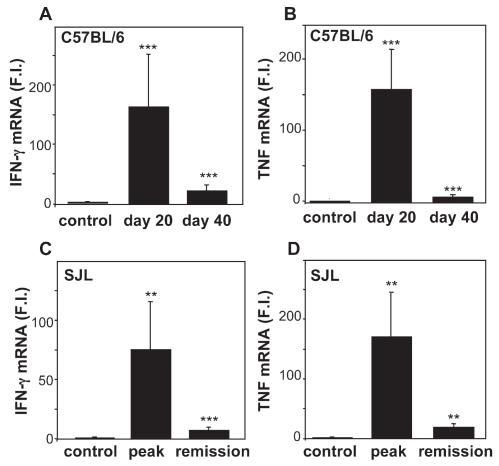
We also measured the expression of the two inflammatory cytokines IFN-γ and TNF in the spinal cord. This was high at the peak of the disease and then, unlike EPO, declined both in chronic EAE in C57BL/6 mice (Figure 4A,4B) and in relapsing-remitting EAE in SJL mice (Figure 4C,4D).
Figure 4.
Expression of IFN-γ and TNF in EAE. IFN-γ and TNF expression in spinal cords of C57BL/6 mice with MOG-induced EAE (A,B), or in SJL mice with PLP-induced EAE (C,D) at the time points described in the legend to Figure. 1. Results (fold induction [FI] versus control) are the mean ± SEM of values of at least 10 mice per group (A,B) and at least 5 mice per group (C,D), with individual samples tested in duplicate. *P < 0.05; **P < 0.01; ***P < 0.001 versus control, Student t test.
The temporal co-induction of EPO, IFN-γ, and TNF in the spinal cord prompted us to investigate the effect of these cytokines on EPO expression in neuronal cells. To this end, we used Kelly human neuroblastoma cells, known to produce significant amounts of EPO protein, detectable by ELISA (22). IFN-γ or TNF, alone or in combination, did not induce EPO production in Kelly cells (not shown). On the contrary, when EPO was induced with the HIF-stabilizer DFX, IFN-γ dose-dependently inhibited DFX-induced EPO secretion (Figure 5A). TNF alone did not affect DFX-induced EPO production, but synergized with IFN-γ (Figure 5B). The observed synergy is consistent with the hypothesis that lack of inhibition by TNF alone could be due, at least partially, to low expression of TNF receptor 1 (TNFR1), which is known to be induced by IFN-γ. In fact, we found that, in our conditions, IFN-γ increased TNFR1 mRNA levels at 4 h (three-fold induction measured by real-time RT-PCR, data not shown).
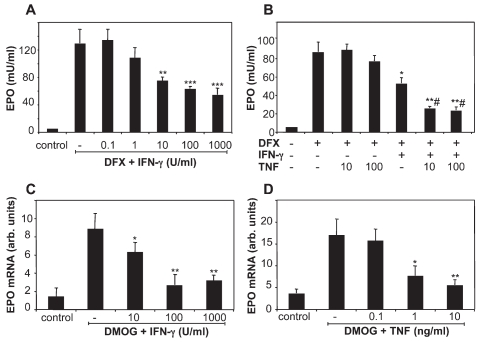
Figure 5.
EPO expression is downregulated by inflammatory cytokines in vitro. Kelly neuroblastoma cells were incubated for 24 h with 200 μM deferoxamine mesylate (DFX) in the presence or absence of IFN-γ (A), or TNF at the indicated concentrations (10 or 100 ng/mL), with or without preincubation with 10 U/mL IFN-γ (B), and EPO secretion was measured by ELISA. Rat glial cells were incubated for 24 h with 500 μM dimethyloxalylglycine (DMOG) and the indicated concentrations of IFN-γ (C) or TNF (D), and EPO expression was analyzed by real-time RT-PCR. Results are the mean ± SEM of triplicate samples tested in duplicate. *P < 0.05; **P < 0.01; ***P < 0.001 versus DFX or DMOG alone; #P < 0.01 versus DFX + IFN-γ; Student t test.
Although immunolocalization of EPO in EAE suggests it is produced mainly by neurons, others reported that glial cells also express EPO (10,11,25,26). We thus studied the effect of IFN-γ and TNF on EPO expression in rat primary glial cells, where EPO was induced with the HIF-stabilizer DMOG. In these experiments, EPO was measured by RT-PCR because its levels in the culture supernatants were undetectable by ELISA. As shown in Figure 5, panels C and D, both IFN-γ and TNF markedly and dose-dependently inhibited EPO expression in glial cells.
DISCUSSION
While administration of EPO is protective in models of EAE (1,4–6) and possibly in MS patients (7), as well as in other CNS diseases (1,2), induction of endogenous EPO in the CNS has only been reported for hypoxic/ischemic pathologies, where EPO is induced as a result of the activation of HIF by hypoxia (10–12).
Our study shows that EPO is expressed in the CNS of mice with EAE, and immunohistochemistry indicated that it was associated mainly with neurons. The finding that neuronal cells are the main source of EPO expression in the spinal cord is in agreement with a previous study in the development of CNS (27).
In consideration of the anti-inflammatory action of exogenous EPO administration in EAE (6), the induction of endogenous EPO reported here suggests that EPO may be part of a protective response, along with anti-inflammatory cytokines such as interleukin-4 (IL-4), interleukin-10 (IL-10), and transforming growth factor (TGF)-β—whose expression correlates with recovery from EAE (28–30). Of note, considering the neuronal localization of EPO expression, others reported that neurons can express neurotrophic or protective cytokines: motoneurons can express brain-derived neurotrophic factor (BDNF) (31), and TGF-β is expressed by neurons during the remission phase of EAE (30).
The protective action of EPO in MS may be exerted also on other pathways than the inflammatory cytokine network. In fact, others have shown upregulation of VEGF, another target of HIF-1α, in the spinal cords of rats with EAE (32) and in MS active plaques from autopsy samples (14,32), and it was suggested that upregulated VEGF in MS might contribute to the increased permeability of the blood-brain barrier (32). In this context, EPO was reported to counteract the increase in permeability induced by VEGF in an in vitro model of blood-brain barrier (33).
The mechanisms that upregulate EPO expression in EAE remain to be elucidated. The hypoxia-induced upregulation of EPO is modulated at the transcriptional level by HIF. The predominant mode of HIF induction by hypoxia is at the level of protein stability. In particular, the α subunits of HIF (HIF-1α and HIF-2α) are stabilized and accumulate upon hypoxia (34). In addition, several in vivo studies showed increased levels of HIF-1α mRNA (35,36). We found that EPO induction in EAE is paralleled by an elevation of HIF-1α, but not HIF-2α, mRNA. Of note, PhH, that induces EPO expression in the CNS secondary to anemia (23), had no effect on HIF-1α mRNA even if EPO induction was more marked than in EAE, confirming previous studies showing that it acts by increasing HIF at the protein level (24). Indeed PhH even decreased HIF-2α expression, possibly through a negative feedback mechanism.
Recently, possible mechanisms for HIF-1α mRNA induction by hypoxia have been characterized (37), and it also has been reported that non-hypoxic inflammatory mediators, including reactive oxygen species, may activate HIF-1α at the transcriptional level, possibly through NF-κB induction (38). In particular, inflammatory stimuli can induce HIF-1α mRNA in macrophages (39) and microglial cells (40), and these mechanisms might play a role in the increase of EPO expression in EAE. Of note, the effect of inflammatory stimuli on HIF transcriptional activity in macrophages is mediated by specific increase of HIF-1α, and not HIF-2α, mRNA (39), paralleling our finding in the inflamed spinal cord in EAE.
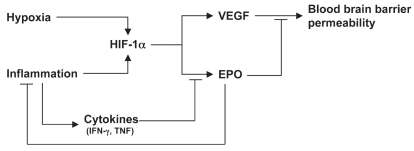
Interestingly, we also show that TNF and IFN-γ inhibit EPO expression in neuronal and glial cell, in agreement with previous reports showing an inhibitory effect of inflammatory cytokines on EPO expression outside the CNS (41). These data, together with the known inhibitory effect of EPO on neuroinflammation (3,6), reveal a cross-talk between EPO and inflammatory cytokines in the CNS, outlined in Figure 6. This circuit may have important implications in the pathogenesis of CNS inflammatory diseases, and could be exploited therapeutically with strategies aimed at upregulating the EPO-mediated protective response.
Figure 6.
Endogenous EPO in the context of the cytokine network in MS.
ACKNOWLEDGMENTS
This study was supported in part by the Kenneth S Warren Institute, Ossining, NY, the Fondazione CARIPLO, Milan, Italy (to PG), the French Centre National de la Recherche Scientifique (CNRS) and the French Ministère de L’Enseignement Supérieur et de la Recherche (to EP and M Bernaudin).
Footnotes
Contributed by: Anthony Cerami.
Online address: http://www.molmed.org
REFERENCES
- 1.Brines ML, et al. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci U S A. 2000;97:10526–31. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghezzi P, Brines M. Erythropoietin as an antiapoptotic, tissue-protective cytokine. Cell Death Differ. 2004;11:S37–S44. doi: 10.1038/sj.cdd.4401450. [DOI] [PubMed] [Google Scholar]
- 3.Villa P, et al. Erythropoietin selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. J Exp Med. 2003;198:971–5. doi: 10.1084/jem.20021067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sattler MB, et al. Neuroprotective effects and intracellular signaling pathways of erythropoietin in a rat model of multiple sclerosis. Cell Death Differ. 2004;11(Suppl 2):S181–92. doi: 10.1038/sj.cdd.4401504. [DOI] [PubMed] [Google Scholar]
- 5.Li W, et al. Beneficial effect of erythropoietin on experimental allergic encephalomyelitis. Ann Neurol. 2004;56:767–77. doi: 10.1002/ana.20274. [DOI] [PubMed] [Google Scholar]
- 6.Savino C, et al. Delayed administration of erythropoietin and its non-erythropoietic derivatives ameliorates chronic murine autoimmune encephalomyelitis. J Neuroimmunol. 2006;172:27–37. doi: 10.1016/j.jneuroim.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Ehrenreich H, et al. Exploring recombinant human erythropoietin in chronic progressive multiple sclerosis. Brain. 2007;130:2577–88. doi: 10.1093/brain/awm203. [DOI] [PubMed] [Google Scholar]
- 8.Jelkmann W. Erythropoietin after a century of research: younger than ever. Eur J Haematol. 2007;78:183–205. doi: 10.1111/j.1600-0609.2007.00818.x. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki R, Masuda S, Nagao M. Pleiotropic functions and tissue-specific expression of erythropoietin. News Physiol Sci. 2001;16:110–3. doi: 10.1152/physiologyonline.2001.16.3.110. [DOI] [PubMed] [Google Scholar]
- 10.Bernaudin M, et al. A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J Cereb Blood Flow Metab. 1999;19:643–51. doi: 10.1097/00004647-199906000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Siren AL, et al. Erythropoietin and erythropoietin receptor in human ischemic/hypoxic brain. Acta Neuropathol (Berl) 2001;101:271–6. doi: 10.1007/s004010000297. [DOI] [PubMed] [Google Scholar]
- 12.Sakanaka M, et al. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci U S A. 1998;95:4635–40. doi: 10.1073/pnas.95.8.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prass K, et al. Hypoxia-induced stroke tolerance in the mouse is mediated by erythropoietin. Stroke. 2003;34:1981–6. doi: 10.1161/01.STR.0000080381.76409.B2. [DOI] [PubMed] [Google Scholar]
- 14.Graumann U, Reynolds R, Steck AJ, Schaeren-Wiemers N. Molecular changes in normal appearing white matter in multiple sclerosis are characteristic of neuroprotective mechanisms against hypoxic insult. Brain Pathol. 2003;13:554–73. doi: 10.1111/j.1750-3639.2003.tb00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aboul-Enein F, et al. Preferential loss of myelin-associated glycoprotein reflects hypoxia-like white matter damage in stroke and inflammatory brain diseases. J Neuropathol Exp Neurol. 2003;62:25–33. doi: 10.1093/jnen/62.1.25. [DOI] [PubMed] [Google Scholar]
- 16.Pedotti R, et al. An unexpected version of horror autotoxicus: anaphylactic shock to a self-peptide. Nat Immunol. 2001;2:216–22. doi: 10.1038/85266. [DOI] [PubMed] [Google Scholar]
- 17.Official Gazette of the Italian Republic, supplement 40. Legislative Decree 116. (February 18, 1992).
- 18.European Economic Community Council Directive 86/609. Official Journal L 358:1. (December 12, 1987).
- 19.Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press; 1996. p. 128. [Google Scholar]
- 20.Overbergh L, Valckx D, Waer M, Mathieu C. Quantification of murine cytokine mRNAs using real time quantitative reverse transcriptase PCR. Cytokine. 1999;11:305–12. doi: 10.1006/cyto.1998.0426. [DOI] [PubMed] [Google Scholar]
- 21.Bigini P, Mennini T. Immunohistochemical localization of TNFalpha and its receptors in the rodent central nervous system. Methods Mol Med. 2004;98:73–80. doi: 10.1385/1-59259-771-8:073. [DOI] [PubMed] [Google Scholar]
- 22.Stolze I, et al. Hypoxia-inducible erythropoietin gene expression in human neuroblastoma cells. Blood. 2002;100:2623–8. doi: 10.1182/blood-2001-12-0169. [DOI] [PubMed] [Google Scholar]
- 23.Liu C, Shen K, Liu Z, Noguchi CT. Regulated human erythropoietin receptor expression in mouse brain. J Biol Chem. 1997;272:32395–400. doi: 10.1074/jbc.272.51.32395. [DOI] [PubMed] [Google Scholar]
- 24.Rankin EB, et al. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest. 2007;117:1068–77. doi: 10.1172/JCI30117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marti HH, et al. Erythropoietin gene expression in human, monkey and murine brain. Eur J Neurosci. 1996;8:666–76. doi: 10.1111/j.1460-9568.1996.tb01252.x. [DOI] [PubMed] [Google Scholar]
- 26.Grasso G, et al. Erythropoietin and erythropoietin receptor expression after experimental spinal cord injury encourages therapy by exogenous erythropoietin. Neurosurgery. 2005;56:821–7. doi: 10.1227/01.neu.0000156493.00904.7e. [DOI] [PubMed] [Google Scholar]
- 27.Knabe W, Siren AL, Ehrenreich H, Kuhn HJ. Expression patterns of erythropoietin and its receptor in the developing spinal cord and dorsal root ganglia. Anat Embryol (Berl) 2005;210:209–19. doi: 10.1007/s00429-005-0019-3. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy MK, Torrance DS, Picha KS, Mohler KM. Analysis of cytokine mRNA expression in the central nervous system of mice with experimental autoimmune encephalomyelitis reveals that IL-10 mRNA expression correlates with recovery. J Immunol. 1992;149:2496–505. [PubMed] [Google Scholar]
- 29.Racke MK, et al. Evidence of endogenous regulatory function of transforming growth factor-beta 1 in experimental allergic encephalomyelitis. Int Immunol. 1992;4:615–20. doi: 10.1093/intimm/4.5.615. [DOI] [PubMed] [Google Scholar]
- 30.Issazadeh S, Navikas V, Schaub M, Sayegh M, Khoury S. Kinetics of expression of costimulatory molecules and their ligands in murine relapsing experimental autoimmune encephalomyelitis in vivo. J Immunol. 1998;161:1104–12. [PubMed] [Google Scholar]
- 31.Fumagalli E, Bigini P, Barbera S, De Paola M, Mennini T. Riluzole, unlike the AMPA antagonist RPR119990, reduces motor impairment and partially prevents motoneuron death in the wobbler mouse, a model of neurodegenerative disease. Exp Neurol. 2006;198:114–28. doi: 10.1016/j.expneurol.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Proescholdt MA, Jacobson S, Tresser N, Oldfield EH, Merrill MJ. Vascular endothelial growth factor is expressed in multiple sclerosis plaques and can induce inflammatory lesions in experimental allergic encephalomyelitis rats. J Neuropathol Exp Neurol. 2002;61:914–25. doi: 10.1093/jnen/61.10.914. [DOI] [PubMed] [Google Scholar]
- 33.Martinez-Estrada OM, et al. Erythropoietin protects the in vitro blood-brain barrier against VEGF-induced permeability. Eur J Neurosci. 2003;18:2538–44. doi: 10.1046/j.1460-9568.2003.02987.x. [DOI] [PubMed] [Google Scholar]
- 34.Semenza GL. HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol. 2001;13:167–71. doi: 10.1016/s0955-0674(00)00194-0. [DOI] [PubMed] [Google Scholar]
- 35.Wiener CM, Booth G, Semenza GL. In vivo expression of mRNAs encoding hypoxia-inducible factor 1. Biochem Biophys Res Commun. 1996;225:485–8. doi: 10.1006/bbrc.1996.1199. [DOI] [PubMed] [Google Scholar]
- 36.Palmer LA, Semenza GL, Stoler MH, Johns RA. Hypoxia induces type II NOS gene expression in pulmonary artery endothelial cells via HIF-1. Am J Physiol. 1998;274:L212–9. doi: 10.1152/ajplung.1998.274.2.L212. [DOI] [PubMed] [Google Scholar]
- 37.Belaiba RS, et al. Hypoxia upregulates hypoxia-inducible factor-1alpha transcription by involving phosphatidylinositol 3-kinase and nuclear factor kappaB in pulmonary artery smooth muscle cells. Mol Biol Cell. 2007;18:4691–7. doi: 10.1091/mbc.E07-04-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonello S, et al. Reactive oxygen species activate the HIF-1alpha promoter via a functional NFkappaB site. Arterioscler Thromb Vasc Biol. 2007;27:755–61. doi: 10.1161/01.ATV.0000258979.92828.bc. [DOI] [PubMed] [Google Scholar]
- 39.Mi Z, et al. Synergystic induction of HIF-1alpha transcriptional activity by hypoxia and lipopolysaccharide in macrophages. Cell Cycle. 2008;7:232–41. doi: 10.4161/cc.7.2.5193. [DOI] [PubMed] [Google Scholar]
- 40.Oh YT, et al. Lipopolysaccharide induces hypoxia-inducible factor-1 alpha mRNA expression and activation via NADPH oxidase and Sp1-dependent pathway in BV2 murine microglial cells. Neurosci Lett. 2008;431:155–60. doi: 10.1016/j.neulet.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 41.Jelkmann W, Pagel H, Wolff M, Fandrey J. Monokines inhibiting erythropoietin production in human hepatoma cultures and in isolated perfused rat kidneys. Life Sci. 1992;50:301–8. doi: 10.1016/0024-3205(92)90338-p. [DOI] [PubMed] [Google Scholar]