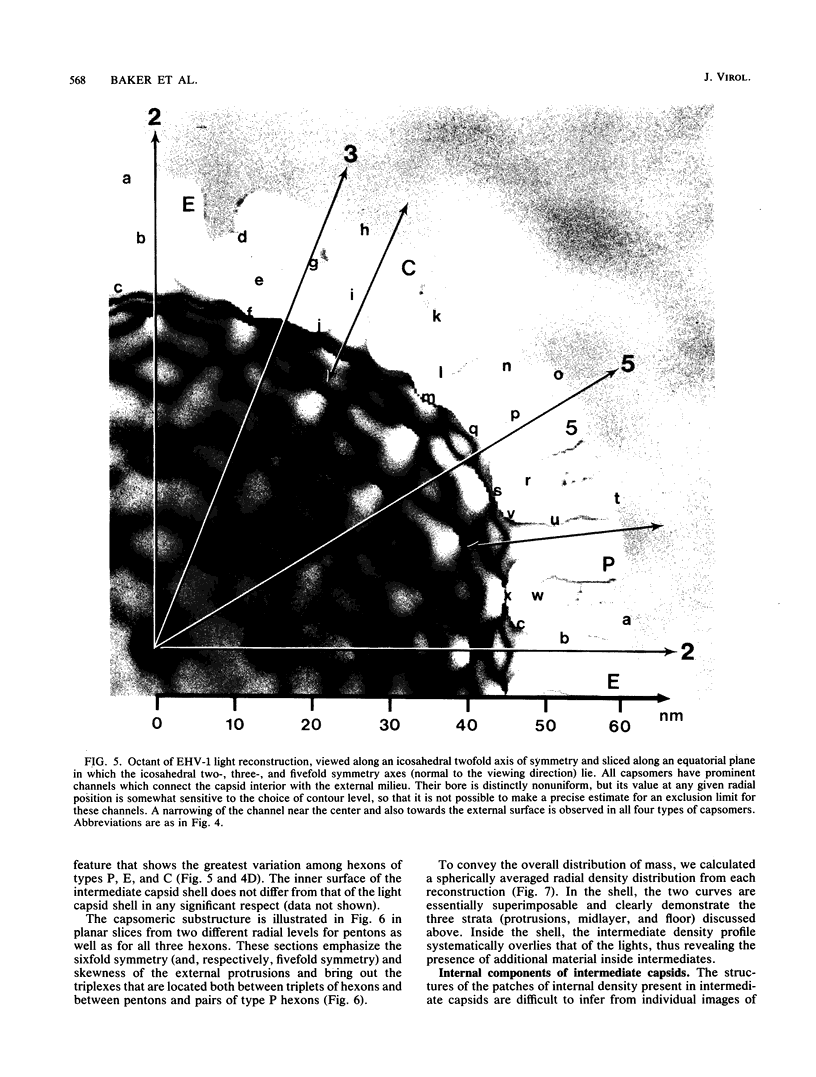
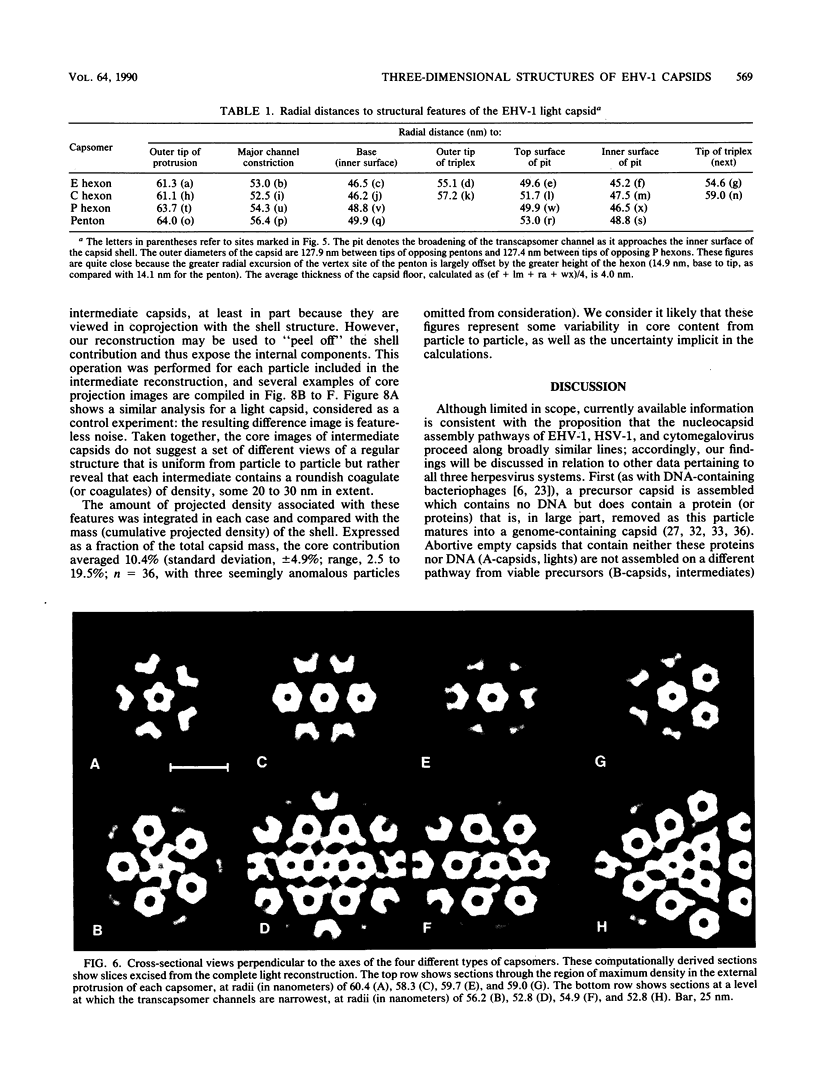
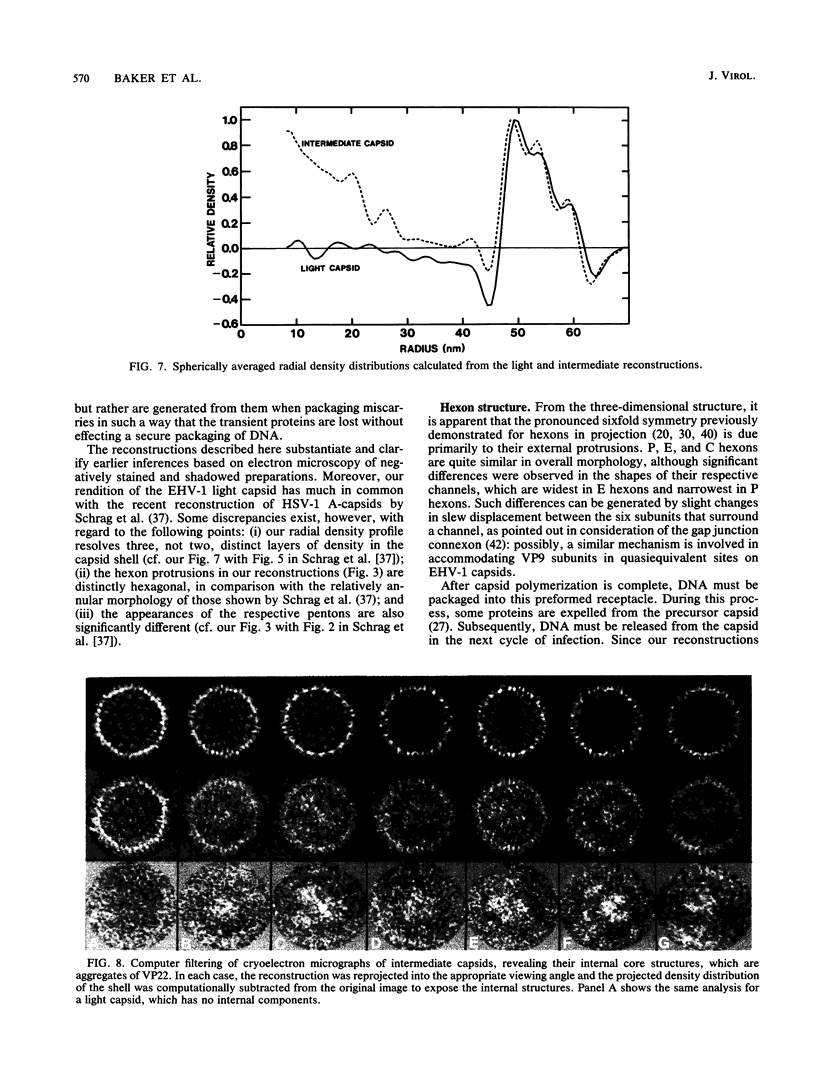
Abstract
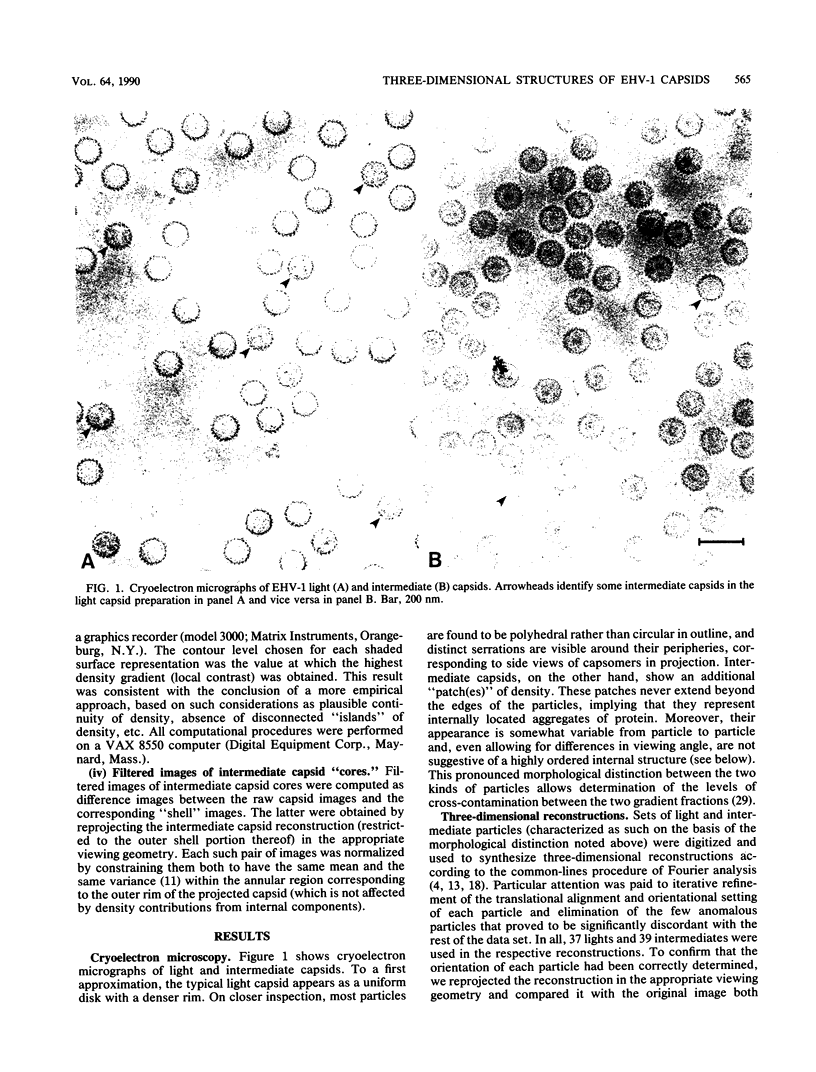
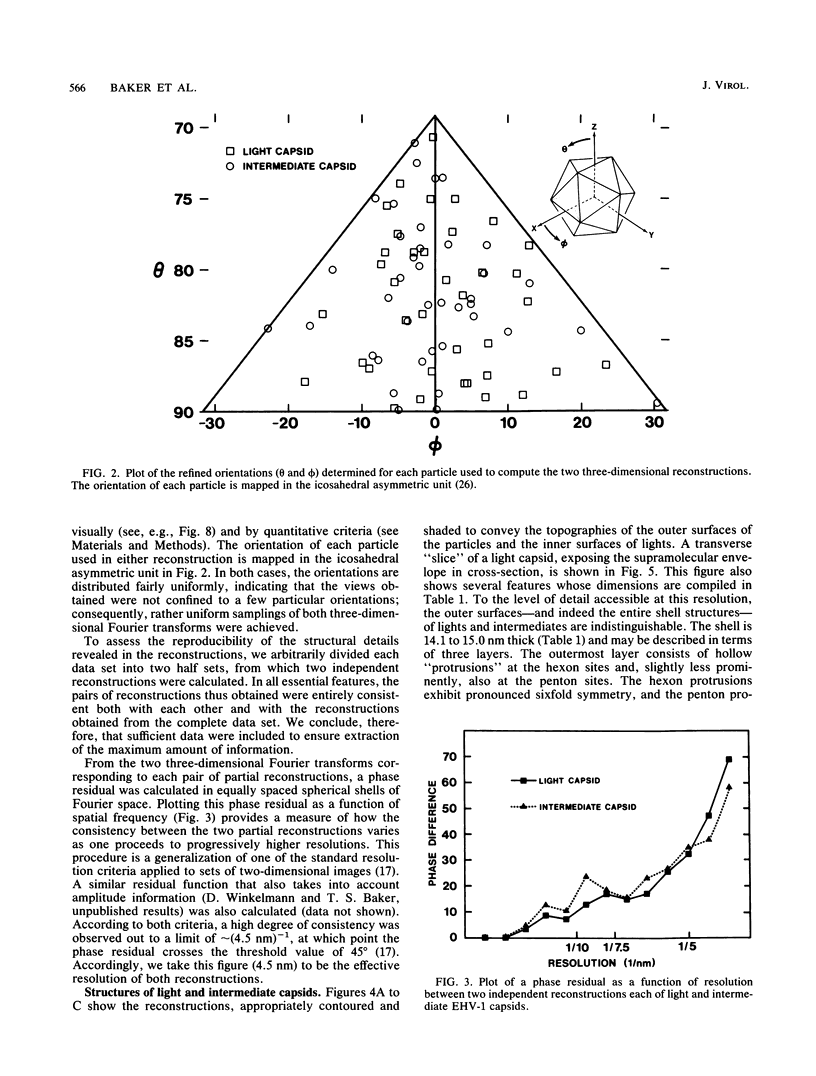
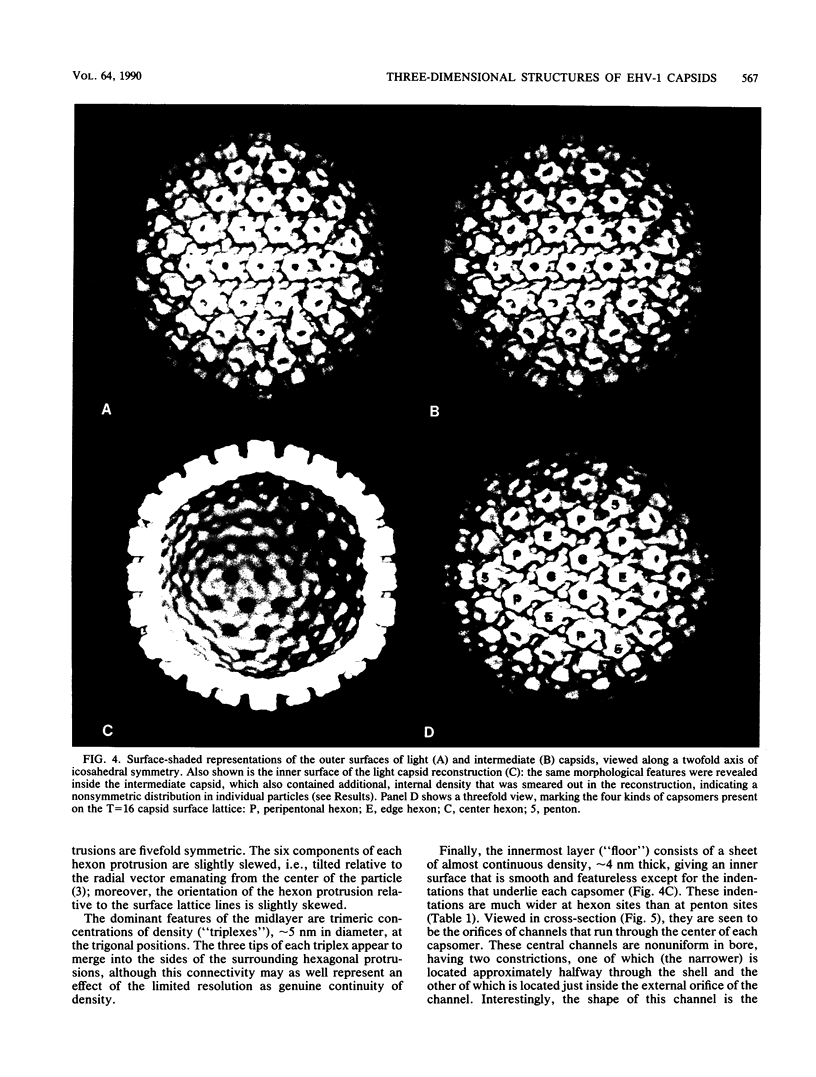
Cryoelectron microscopy and three-dimensional computer reconstruction techniques have been used to compare the structures of two types of DNA-free capsids of equine herpesvirus 1 at a resolution of 4.5 nm. "Light" capsids are abortive, whereas "intermediate" capsids are related to maturable intracellular precursors. Their T = 16 icosahedral outer shells, approximately 125 nm in diameter, are indistinguishable and may be described in terms of three layers of density, totalling 15 nm in thickness. The outermost layer consists of protruding portions of both the hexon and the penton capsomers, rising approximately 5 nm above a midlayer of density. The innermost layer, or "floor," is a 4-nm-thick sheet of virtually continuous density except for the orifices of the channels that traverse each capsomer. Hexon protrusions are distinctly hexagonal in shape, and penton protrusions are pentagonal. The structures of the three kinds of hexons (distinguished according to their positions on the surface lattice) are closely similar but differ somewhat in their respective orientations and in the shapes of their channels. The most prominent features of the midlayer are threefold nodules ("triplexes") at the trigonal lattice points. By analogy with other viral capsids, the triplexes may represent trimers of another capsid protein, possibly VP23 (36 kilodaltons [kDa]) or VP26 (12 kDa). Intermediate capsids differ from light capsids, which are empty, in having one or more internal components. In individual images from which the shell structure has been filtered away, these components are seen to have dimensions of 20 to 30 nm but to lack a visible substructure. This material--which is smeared out in the reconstruction, implying that its distribution is not icosahedrally symmetric or necessarily consistent from particle to particle--consists of aggregates of VP22 (46 kDa). From several lines of evidence, we conclude that this protein is located entirely within the capsid shell. These aggregates may be the remnants of morphogenetic cores retained in capsids interrupted in the process of DNA packaging.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian M., Dubochet J., Lepault J., McDowall A. W. Cryo-electron microscopy of viruses. Nature. 1984 Mar 1;308(5954):32–36. doi: 10.1038/308032a0. [DOI] [PubMed] [Google Scholar]
- Aebi U., van Driel R., Bijlenga R. K., ten Heggeler B., van den Broek R., Steven A. C., Smith P. R. Capsid fine structure of T-even bacteriophages. Binding and localization of two dispensable capsid proteins into the P23* surface lattice. J Mol Biol. 1977 Mar 15;110(4):687–698. doi: 10.1016/s0022-2836(77)80084-3. [DOI] [PubMed] [Google Scholar]
- Baker T. S., Caspar D. L., Hollingshead C. J., Goodenough D. A. Gap junction structures. IV. Asymmetric features revealed by low-irradiation microscopy. J Cell Biol. 1983 Jan;96(1):204–216. doi: 10.1083/jcb.96.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker T. S., Drak J., Bina M. Reconstruction of the three-dimensional structure of simian virus 40 and visualization of the chromatin core. Proc Natl Acad Sci U S A. 1988 Jan;85(2):422–426. doi: 10.1073/pnas.85.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker T. S., Drak J., Bina M. The capsid of small papova viruses contains 72 pentameric capsomeres: direct evidence from cryo-electron-microscopy of simian virus 40. Biophys J. 1989 Feb;55(2):243–253. doi: 10.1016/S0006-3495(89)82799-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booy F. P., Ruigrok R. W., van Bruggen E. F. Electron microscopy of influenza virus. A comparison of negatively stained and ice-embedded particles. J Mol Biol. 1985 Aug 20;184(4):667–676. doi: 10.1016/0022-2836(85)90312-2. [DOI] [PubMed] [Google Scholar]
- Braun D. K., Roizman B., Pereira L. Characterization of post-translational products of herpes simplex virus gene 35 proteins binding to the surfaces of full capsids but not empty capsids. J Virol. 1984 Jan;49(1):142–153. doi: 10.1128/jvi.49.1.142-153.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASPAR D. L., KLUG A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- Crowther R. A. Procedures for three-dimensional reconstruction of spherical viruses by Fourier synthesis from electron micrographs. Philos Trans R Soc Lond B Biol Sci. 1971 May 27;261(837):221–230. doi: 10.1098/rstb.1971.0054. [DOI] [PubMed] [Google Scholar]
- DeRosier D. J., Moore P. B. Reconstruction of three-dimensional images from electron micrographs of structures with helical symmetry. J Mol Biol. 1970 Sep 14;52(2):355–369. doi: 10.1016/0022-2836(70)90036-7. [DOI] [PubMed] [Google Scholar]
- Frank J., Verschoor A., Boublik M. Computer averaging of electron micrographs of 40S ribosomal subunits. Science. 1981 Dec 18;214(4527):1353–1355. doi: 10.1126/science.7313694. [DOI] [PubMed] [Google Scholar]
- Fuller S. D. The T=4 envelope of Sindbis virus is organized by interactions with a complementary T=3 capsid. Cell. 1987 Mar 27;48(6):923–934. doi: 10.1016/0092-8674(87)90701-x. [DOI] [PubMed] [Google Scholar]
- Furlong D. Direct evidence for 6-fold symmetry of the herpesvirus hexon capsomere. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2764–2766. doi: 10.1073/pnas.75.6.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine J. W., Honess R. W., Cassai E., Roizman B. Proteins specified by herpes simplex virus. XII. The virion polypeptides of type 1 strains. J Virol. 1974 Sep;14(3):640–651. doi: 10.1128/jvi.14.3.640-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imber R., Tsugita A., Wurtz M., Hohn T. Outer surface protein of bacteriophage lambda. J Mol Biol. 1980 May 25;139(3):277–295. doi: 10.1016/0022-2836(80)90131-x. [DOI] [PubMed] [Google Scholar]
- Ishii T., Yanagida M. Molecular organization of the shell of the Teven bacteriophage head. J Mol Biol. 1975 Oct 5;97(4):655–660. doi: 10.1016/s0022-2836(75)80065-9. [DOI] [PubMed] [Google Scholar]
- Klug A., Finch J. T. Structure of viruses of the papilloma-polyoma type. IV. Analysis of tilting experiments in the electron microscope. J Mol Biol. 1968 Jan 14;31(1):1–12. doi: 10.1016/0022-2836(68)90050-8. [DOI] [PubMed] [Google Scholar]
- Lee J. Y., Irmiere A., Gibson W. Primate cytomegalovirus assembly: evidence that DNA packaging occurs subsequent to B capsid assembly. Virology. 1988 Nov;167(1):87–96. doi: 10.1016/0042-6822(88)90057-8. [DOI] [PubMed] [Google Scholar]
- Newcomb W. W., Brown J. C., Booy F. P., Steven A. C. Nucleocapsid mass and capsomer protein stoichiometry in equine herpesvirus 1: scanning transmission electron microscopic study. J Virol. 1989 Sep;63(9):3777–3783. doi: 10.1128/jvi.63.9.3777-3783.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb W. W., Brown J. C. Use of Ar+ plasma etching to localize structural proteins in the capsid of herpes simplex virus type 1. J Virol. 1989 Nov;63(11):4697–4702. doi: 10.1128/jvi.63.11.4697-4702.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson N. H., Baker T. S. Magnification calibration and the determination of spherical virus diameters using cryo-microscopy. Ultramicroscopy. 1989 Jul-Aug;30(3):281–297. doi: 10.1016/0304-3991(89)90057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer E. L., Martin M. L., Gary G. W., Jr The ultrastructure of disrupted herpesvirus nucleocapsids. Virology. 1975 May;65(1):260–265. doi: 10.1016/0042-6822(75)90026-4. [DOI] [PubMed] [Google Scholar]
- Perdue M. L., Cohen J. C., Kemp M. C., Randall C. C., O'Callaghan D. J. Characterization of three species of nucleocapsids of equine herpesvirus type-1 (EHV-1). Virology. 1975 Mar;64(1):187–204. doi: 10.1016/0042-6822(75)90091-4. [DOI] [PubMed] [Google Scholar]
- Perdue M. L., Kemp M. C., Randall C. C., O'Callaghan D. J. Studies of the molecular anatomy of the L-M cell strain of equine herpes virus type 1: proteins of the nucleocapsid and intact virion. Virology. 1974 May;59(1):201–216. doi: 10.1016/0042-6822(74)90216-5. [DOI] [PubMed] [Google Scholar]
- Preston V. G., Coates J. A., Rixon F. J. Identification and characterization of a herpes simplex virus gene product required for encapsidation of virus DNA. J Virol. 1983 Mar;45(3):1056–1064. doi: 10.1128/jvi.45.3.1056-1064.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayment I., Baker T. S., Caspar D. L., Murakami W. T. Polyoma virus capsid structure at 22.5 A resolution. Nature. 1982 Jan 14;295(5845):110–115. doi: 10.1038/295110a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rixon F. J., Cross A. M., Addison C., Preston V. G. The products of herpes simplex virus type 1 gene UL26 which are involved in DNA packaging are strongly associated with empty but not with full capsids. J Gen Virol. 1988 Nov;69(Pt 11):2879–2891. doi: 10.1099/0022-1317-69-11-2879. [DOI] [PubMed] [Google Scholar]
- Schrag J. D., Prasad B. V., Rixon F. J., Chiu W. Three-dimensional structure of the HSV1 nucleocapsid. Cell. 1989 Feb 24;56(4):651–660. doi: 10.1016/0092-8674(89)90587-4. [DOI] [PubMed] [Google Scholar]
- Sherman G., Bachenheimer S. L. Characterization of intranuclear capsids made by ts morphogenic mutants of HSV-1. Virology. 1988 Apr;163(2):471–480. doi: 10.1016/0042-6822(88)90288-7. [DOI] [PubMed] [Google Scholar]
- Steven A. C., Couture E., Aebi U., Showe M. K. Structure of T4 polyheads. II. A pathway of polyhead transformation as a model for T4 capsid maturation. J Mol Biol. 1976 Sep 5;106(1):187–221. doi: 10.1016/0022-2836(76)90307-7. [DOI] [PubMed] [Google Scholar]
- Steven A. C., Roberts C. R., Hay J., Bisher M. E., Pun T., Trus B. L. Hexavalent capsomers of herpes simplex virus type 2: symmetry, shape, dimensions, and oligomeric status. J Virol. 1986 Feb;57(2):578–584. doi: 10.1128/jvi.57.2.578-584.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart M., Vigers G. Electron microscopy of frozen-hydrated biological material. Nature. 1986 Feb 20;319(6055):631–636. doi: 10.1038/319631a0. [DOI] [PubMed] [Google Scholar]
- Unwin P. N., Zampighi G. Structure of the junction between communicating cells. Nature. 1980 Feb 7;283(5747):545–549. doi: 10.1038/283545a0. [DOI] [PubMed] [Google Scholar]
- Vernon S. K., Lawrence W. C., Cohen G. H. Morphological components of herpesvirus. I. Intercapsomeric fibrils and the geometry of the capsid. Intervirology. 1974;4(4):237–248. doi: 10.1159/000149968. [DOI] [PubMed] [Google Scholar]
- Vogel R. H., Provencher S. W., von Bonsdorff C. H., Adrian M., Dubochet J. Envelope structure of Semliki Forest virus reconstructed from cryo-electron micrographs. Nature. 1986 Apr 10;320(6062):533–535. doi: 10.1038/320533a0. [DOI] [PubMed] [Google Scholar]
- WILDY P., RUSSELL W. C., HORNE R. W. The morphology of herpes virus. Virology. 1960 Oct;12:204–222. doi: 10.1016/0042-6822(60)90195-1. [DOI] [PubMed] [Google Scholar]
- Wurtz M., Kistler J., Hohn T. Surface structure of in vitro assembled bacteriophage lambda polyheads. J Mol Biol. 1976 Feb 15;101(1):39–56. doi: 10.1016/0022-2836(76)90065-6. [DOI] [PubMed] [Google Scholar]