Abstract
Smoking appears to increase overall levels of stress, despite self-reports that men and women smoke to control symptoms of anxiety. The overall incidence of anxiety disorders is also significantly higher in women. This study examined whether behavioral sensitivity to chronic nicotine varies across sexes in mice. Male and female C57BL/6J mice were exposed chronically to nicotine in the drinking water (50, 100, or 200 µg/ml) and tested for locomotor activation and anxiety-like behavior in the elevated plus maze (EPM). Female mice were less sensitive to locomotor activation. Whereas both males and females showed increases in locomotor activity at the highest (200 µg/ml) concentration of nicotine, only males showed locomotor activation at the middle (100 µg/ml) concentration. The decreased sensitivity in females could not be explained by reduced nicotine intake compared to males. In the EPM, nicotine produced an anxiogenic-like response in females, but had no effect in males. Treatment with the high (200 µg/ml) dose of nicotine reduced the amount of time spent in the open arms of the EPM in female, but not male mice. No differences in the anxiogenic-like response to chronic nicotine was observed between β2-subunit knockout and wildtype mice, suggesting that β2-subunit containing nicotinic receptors do not mediate the anxiogenic-like response to chronic nicotine in females. This shows that female mice have an anxiogenic-like response to chronic nicotine, but are less sensitive to nicotine’s psychostimulant properties, which may be related to the increased relapse to smoking following abstinence in women.
Keywords: C57BL/6J mice, nicotine, elevated plus maze, locomotor activity, nicotinic acetylcholine receptor, knockout mice
INTRODUCTION
Tobacco smoking is the leading preventable cause of death in the US [44]. Although men smoke more than women, the decline in smoking prevalence has been slower for women [38]. Women have less success quitting smoking, and are more likely to relapse, than men [2]. These factors put women at increased risk for smoking related illness and warrant the investigation of biologically-based sex differences in nicotine responses.
Women may show reduced abstinence because nicotine replacement is less effective in women than men [23,45]. Studies by Perkins and colleagues suggest smoking behavior in women is reinforced less by nicotine intake and more by non-nicotine factors [33,34]. Negative mood also may play a greater role in smoking relapse for women [5].
In animal models, positive reinforcing effects of nicotine may be greater in female than male rodents. Females show faster acquisition of intravenous nicotine self-administration, higher break points on a progressive ratio schedule [11] and greater preference for nicotine in a two-bottle choice task [25,28]. Female rodents may self administer nicotine at higher rates because nicotine is more reinforcing, or because of differences in sensitivity. In mice, females were less sensitive to nicotine’s locomotor depressant effects [18], but female rats showed increased sensitivity to nicotine’s locomotor stimulant effects [4,14,17,20,39–41].
Nicotine has complex effects on anxiety, with both anxiolytic and anxiogenic effects of nicotine that vary depending on the behavioral model, nicotine dose, route or time course of administration (reviewed in [35]). With respect to sex, female rats were more sensitive to nicotine’s anxiolytic effects in the social interaction test [8], but less sensitive in the elevated plus maze (EPM) [12].
Here, we explore sex differences in behavioral responses to chronic nicotine in mice on measures of sensitivity (locomotor activation) and anxiety (elevated plus maze). Previous studies have shown that β2-subunit containing nicotinic acetylcholine receptors (β2* nAChRs, * represents other subunits) are essential for positive reinforcing [36] and locomotor activating effects [24] of nicotine. We therefore also determined what role β2* nAChRs play in nicotine’s effects on anxiety using the EPM.
MATERIAL AND METHODS
Animals
C57BL/6J (B6) mice were obtained from Jackson Laboratory (Bar Harbor, ME). β2 subunit knockout mice (β2KO) were backcrossed 12–20 generations to B6 mice. Mice were housed 4–5 to a cage in a colony room maintained at 22°C on a 12 hour light-dark cycle (lights on: 7AM). All animal experiments were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and the Yale Animal Care and Use Committee.
Nicotine Treatment: Elevated Plus Maze and Locomotor Activation
The hydrogen bitartrate salt of nicotine (Sigma, St. Louis, MO) was used and concentrations were calculated as free-base. Mice were treated with 2% saccharin (Sigma) in 2mM tartaric acid (Sigma) or 50, 100, or 200µg/ml nicotine in 2% saccharin (Sigma). The pH of all solutions was adjusted to the nicotine solution. All solutions were administered in drinking water as the sole source of fluid at concentrations based on previous studies of drinking water administration in mice [24,43].
Elevated Plus Maze
The EPM was constructed of white Plexiglas and consisted of two open arms (5 mm lip) and two closed arms (black Plexiglas, 15 cm walls). Arms were 30.5 cm long and 5.5 cm wide. Maze was positioned 31.5 cm above the ground in dim lighting.
The EPM test was performed as described [27]. Group-housed female and male wildtype (β2WT) and β2 subunit knockout mice (β2KO) were administered saccharin or 200µg/ml nicotine in drinking water for at least one month. Groups: SACCHARIN, male β2WT (n=8), male β2KO (n=9), female β2WT (n=11), female β2KO (n=10); NICOTINE, male β2WT (n=8), male β2KO (n=8), female β2WT (n=14), female β2KO (n=12). Mice were tested between 7AM and 9AM to assure nicotine was still on board during testing. Mice were placed at the intersection of the arms and allowed to explore for 5min. Two observers blind to genotype scored entries into the open and closed arms and time spent in open arms. Open arm entries were scored when all 4 paws crossed into the arm. Percent time spent in open arms (% time in open arms/time in open+closed arms) was used as a measure of anxiety-like behavior. Number of entries into the closed arms was used as a measure of locomotor activity.
Locomotor Activity
Group housed female and male mice were administered saccharin or nicotine (50, 100, or 200µg/ml) in drinking water for at least one month. Groups were SACCHARIN: females (n=17), males (n=15); 50µg/ml NICOTINE: females (n=17), males (n=15); 100µg/ml NICOTINE: females (n=15), males (n=14); 200µg/ml NICOTINE: females (n=16), males (n=15).
To measure locomotor activity, mice were singly housed in cages (19 × 29 × 13 cm) within the locomotor apparatus with food available at all times. Mice continued to drink nicotine or saccharin solutions for the duration of the test. The custom built locomotor apparatus (6 photocells, 4 cm apart, beam breaks collected in 1 hr blocks) was housed under the same lighting, temperature, and humidity conditions as the colony room. Mice acclimated to the cages housed within the apparatus for 1 day, and locomotor activity was recorded for 3 additional days.
Bottles were weighed and body weights were taken at the beginning and end of the experiment to compare nicotine intake between groups. To assess cotinine levels, blood was collected by decapitation between 7–8:30AM in mice that had nicotine in their drinking water.
Measurement of plasma cotinine levels
Blood was centrifuged, serum removed and stored at −80°C for later analysis. Serum nicotine and cotinine levels were measured by reversed-phase HPLC with ion pairing as described previously [15,16]. Internal standard was added, sample was alkalinized, extracted with dichloromethane and hexane, and back-extracted into 0.2 ml of 0.2M phosphoric acid. The aqueous phase was chromatographed on a C6 column (Alltech Associates). 100–300µl of serum was collected for each mouse. Serum levels <300µl were brought to volume with human lipid-stripped plasma (Scantibodies Laboratory) for chemical detection. Cotinine concentrations were corrected according to the dilution factor.
Data Analysis
EPM: Percent time in open arms was calculated as a measure of anxiety-like behavior and the number of crosses into the closed arms was calculated as a measure of general activity. An analysis of variance (ANOVA) was performed on each dependent measure with DRUG (saccharin, 200µg/ml nicotine), GENOTYPE (β2WT, β2KO), and SEX (female, male) as independent variables.
Locomotor activity: number of beam breaks in 1 hr blocks was collected across the 24 hr circadian cycle starting at 7AM. The maximum one hour of activity (averaged over 3 days) between the hours of 5–8AM was used as the measure of locomotor activation because this activity peak is sensitive to nicotine in both males and females [24]. ANOVA was performed on the peak locomotor activity dependent measure with NICOTINE DOSE (0, 50, 100, 200µg/ml) and SEX (female, male) as independent variables.
Nicotine intake and cotinine levels: ANOVA was used with NICOTINE DOSE (0, 50, 100, 200µg/ml) and SEX (female, male) as a between subjects factor. Significant main effects and interactions were followed with the Tukey test.
RESULTS
Elevated Plus Maze
Effects of nicotine, sex, and β2* nAChRs on anxiety-like behavior were examined using the EPM (Fig. 1). Chronic nicotine treatment was anxiogenic-like in the EPM in female, but not male β2WT and β2KO mice. No differences in closed arm entries were observed, consistent with the fact that nicotine-induced locomotor activation in mice is only seen when the animals are drinking non-stressfully in the home cage, and not in response to acute injection or movement into a novel environment
Figure 1.

Nicotine has anxiogenic-like effects in the EPM in female C57BL/6J mice. Female (A,C) and male (B,D) mice were treated chronically with saccharin or nicotine (200 µg/ml) in their drinking water and mean (±SEM) %time spent in open arms (A,B) and number of entries into closed arms (C,D) are shown. Time in open arms ANOVA: significant drug x sex interaction (F(1,72)=4.9, p<0.05). Females treated with nicotine spent significantly less time in the open arms than females treated with saccharin (Tukey post hoc test). Number of entries into closed arms ANOVA: significant sex x drug interaction (F(1,72)=4.5, p<0.05). No differences in closed arm entries between females treated with saccharin or nicotine (Tukey post hoc test). *p<0.05
Locomotor Activity
Sex differences in sensitivity to the locomotor stimulatory properties of nicotine were evaluated in male and female mice treated chronically with 0, 50, 100, or 200µg/ml of nicotine in drinking water (Fig. 2). At the highest dose of nicotine (200µg/ml), locomotor activation was seen in both male and female mice. At the middle dose (100µg/ml), males, but not females, showed locomotor activation, suggesting that male mice are more sensitive to the stimulant properties of chronic nicotine. The lowest dose of nicotine (50µg/ml) did not produce locomotor stimulant effects in either male or female mice.
Figure 2.
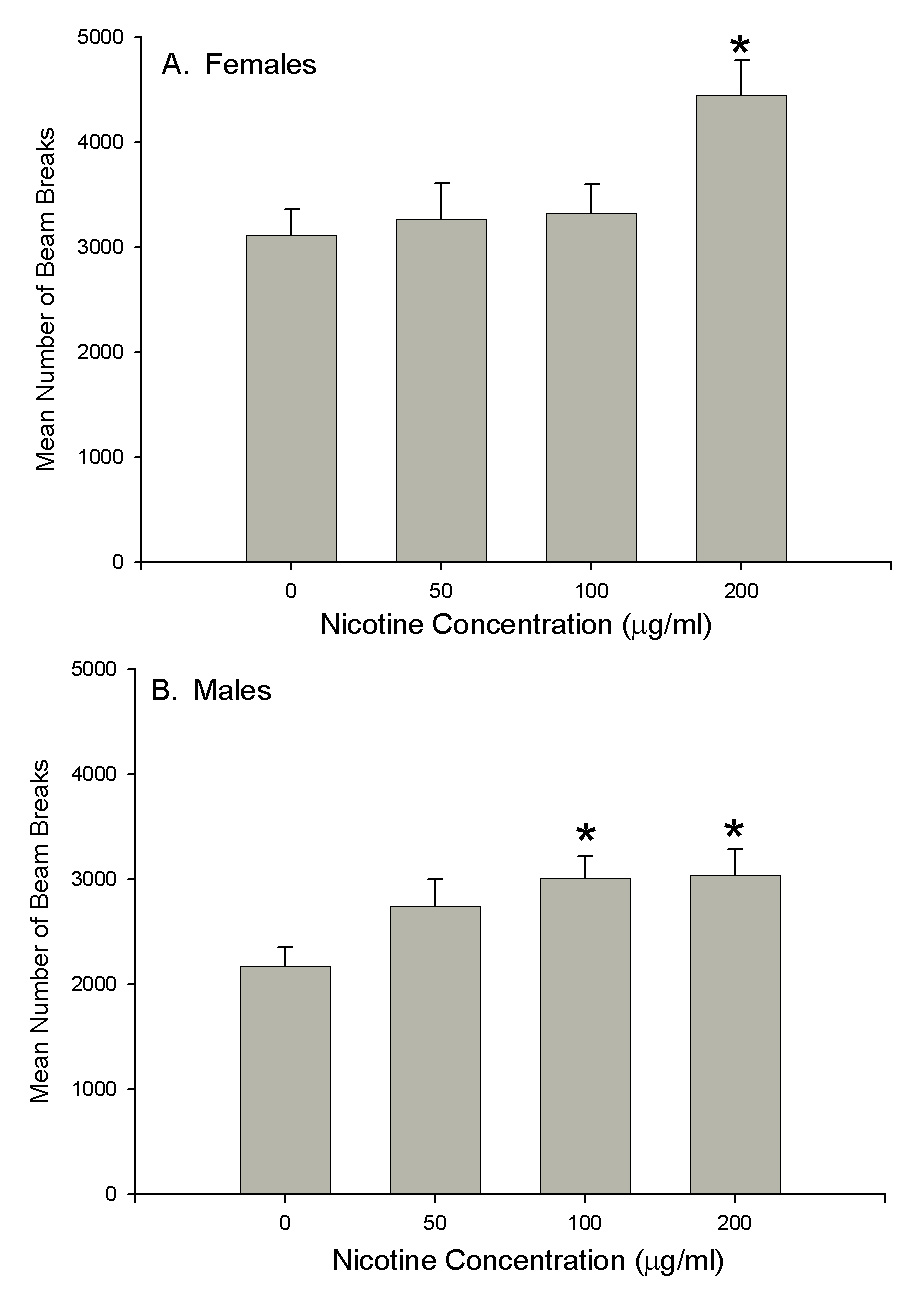
Male C57BL/6J mice are more sensitive to locomotor stimulatory effects of nicotine than female mice. Mice received saccharin, 50, 100, or 200 mg/ml nicotine chronically in their drinking water. Mean (±SEM) beam breaks for female (A) and male (B) mice for one hour during the second burst of homecage activity is shown. The maximum hour of activity between 5–8am was averaged across 3 days. ANOVA: significant main effect of sex (F(1,116)=17.5, p<0.0001) and nicotine dose (F(3,116)=5.8, p<0.001). Female mice treated with 200 mg/ml nicotine were more active than the female saccharin control group. Male mice treated with both 100 and 200 mg/ml of nicotine were more active than male saccharin treated controls (*p<0.05 vs. vehicle, Tukey post hoc tests).
Nicotine Intake and Cotinine Levels
A subset of mice was tested to identify any sex-based differences in nicotine intake or plasma levels of nicotine. Both male and female mice show the expected increase in dose with increasing concentrations of nicotine (values in mg/kg/day for males: 50µg/ml, 7.8±0.5; 100µg/ml, 14.3±0.8; 200µg/ml, 28.3±1.3; females: 50µg/ml, 10.8±0.6; 100µg/ml 18.5±1.2; 200µg/ml, 31.5±1.3). There were no significant effects of sex or nicotine dose on mean plasma cotinine levels (values in ng/ml for males: 50µg/ml, 298±50; 100µg/ml, 720±109; 200µg/ml, 1289±399; females: 50µg/ml, 575±161; 100µg/ml, 489±66; 200µg/ml, 611±159). Males tended to show a dose-dependent increase in plasma cotinine (significantly higher plasma levels of cotinine in males at 200 vs. 50µg/ml). In contrast, female mice showed no relationship between nicotine dose and plasma cotinine.
DISCUSSION
This study demonstrates sex differences in C57BL/6J mice in response to chronic nicotine in measures of anxiety and locomotor activity. Chronic nicotine was anxiogenic in females in the EPM, but showed no effect in males. Females were also less sensitive to the locomotor stimulatory effects of nicotine, with locomotor activation exhibited only at the highest dose in females (200µg/ml) and at the middle and high (100, 200µg/ml) doses in males.
Chronic nicotine was anxiogenic in female mice, with decreases in time spent in open arms of the EPM in nicotine-treated females. Anxiogenic effects of chronic nicotine could not be explained by general decreases in locomotor activity, as measured be entries into the closed arms of the EPM. In contrast, males showed no effect of chronic nicotine in the EPM. Because males and females treated with the high dose of nicotine (200µg/ml) showed equivalent nicotine intake and plasma cotinine, sex differences in dose or metabolism of nicotine did not account for sex differences in behavioral responses. Both β2* knockout and wildtype females showed similar reductions in the percent time in the open arms in the EPM, suggesting that these anxiogenic effects are not mediated by β2* nAChRs. Finally, no overall differences in anxiety-like behavior were noted between β2* knockout and wildtype mice, confirming that β2* receptors do not mediate baseline anxiety levels in mice [37].
Previous studies have shown that nicotine can be anxiolytic [6,30], anxiogenic [31], inactive, or can antagonize the anxiogenic effect of other drugs [21] in the EPM. Nicotine dose, length and timing of nicotine administration, strain, or baseline levels of anxiety have been well-characterized [35], few experiments have examined how sex may impact nicotine’s effects on anxiety. In one study in adolescent rats, nicotine was anxiogenic in the EPM in females but anxiolytic in males [12]. Sex differences were also reported in the social interaction test, with adolescent female rats showing greater sensitivity to the anxiolytic actions of nicotine [8]. In adult rats, however, no sex differences were found in acute effects of nicotine in the EPM, and nicotine was anxiogenic in both males and females [12]. In contrast, in adult mice, acute nicotine had anxiolytic effects in the EPM, with females showing less sensitivity than males [9]. In the present experiment, mice treated with chronic nicotine showed a unique pattern of response, with females exhibiting an anxiogenic response, and males showing no effect. Future studies examining nicotine and anxiety should determine how sex interacts with the other variables described here.
The present study tested whether β2* nAChRs contribute to chronic nicotine’s effects on anxiety-like behavior. The magnitude of response to chronic nicotine was equivalent in female β2* receptor knockout and wildtype mice, suggesting that β2* receptors are not involved in anxiogenic-like effects of chronic nicotine in the EPM, although β2* receptors may be involved in other aspects of anxiety. Promising candidates for mediating anxiety like behavior in the EPM are β4* and β3* subunits. Knockout mice lacking β4* [42] or β3* [3] subunits showed less anxiety-like behavior than wildtype mice in the EPM, suggesting that endogenous ACh may produce anxiogenic effects under conditions of stress mediated by β3* and β4* nAChRs.
Sex differences were observed in the psychostimulant effects of nicotine in response to chronic exposure. This finding expands on our previous report [24], showing that the high (200µg/ml) dose of chronic nicotine resulted in locomotor activation in both females and males. In the present study, only males showed activation at the middle dose (100µg/ml). It is unlikely that this difference in sensitivity can be explained by sex differences in nicotine intake or plasma levels of nicotine. At the 100µg/ml concentration, nicotine intake was actually higher in females than males, resulting in equivalent plasma levels of cotinine for both sexes. The current results differ from previous reports showing greater sensitivity in female rats to the locomotor stimulant effects of chronic nicotine [4,14,17,20]. Future studies will determine if the nature of these differences are due to species (mouse vs. rat) or route of administration (drinking water in the present study vs. iv administration [4,17,20] and minipump [14]).
Several potential mechanisms may explain sex differences in response to chronic nicotine. First, pharmacokinetic variations in plasma or brain levels of nicotine could explain sex differences. One study found that C57BL/6 females eliminated nicotine faster than males [18]. Studies in humans have found that women metabolize nicotine more rapidly, an effect related to estrogen [1] Second, chronic nicotine differentially regulates nicotinic receptors in males and females, with males showing more pronounced nAChR upregulation than females [26, 29]. Third, steroid hormones modulate responses to nicotine and both progesterone and estradiol blocked nicotine-induced analgesia in one study [9]. Estrogen also enhances dopamine release from striatal slices in females, but decreases dopamine release in males [10]. As nicotine-induced increased locomotor in the current model is dopamine-mediated [24], estrogen-induced modulation of dopamine release could mediate sex differences observed in this test.
It should be emphasized that sex differences in the present study do not reflect general increases or decreases in sensitivity for a particular sex, but rather different sensitivities dependent upon the behavioral measure. This is consistent with other reports. Females were more sensitive to nicotine in oral self-administration [25,28], intravenous self-administration [11], operant response to visual cues [7], and reductions in body weight and feeding [13]. Females were less sensitive than males to the effect of nicotine in analgesia [9] and cognition (active avoidance) [46] tests, and were less sensitive to the anxiogenic effects of nicotine during ethanol withdrawal [19].
The pattern of behavioral response in the current mouse model, with females showing greater anxiety-like behavior but less sensitivity to dopamine-mediated locomotor activation, may represent an appropriate model of female smokers. It has been proposed that women are less sensitive than men to dopamine-dependent positive reinforcing effects of nicotine (see [34]). Furthermore, nicotine may contribute to negative mood states and anxiety in smokers (see [32]), an effect that may be more pronounced in women, who have higher rates of anxiety and depressive disorders than men [22]. Future investigation into the hormonal and neurochemical mechanisms underlying sex differences in behavioral responsiveness to nicotine will further aid in understanding the relationship between smoking and anxiety disorders.
In summary, female mice were less sensitive to the anxiogenic and locomotor activating effects of nicotine. These data suggest that non-nicotine therapeutics may be more useful for smoking cessation in women as compared to men. Future studies will determine whether similar sex differences are important for the lower cessation rates in women.
ACKNOWLEDGEMENTS
We would like to thank Haleh Nadim and Dr. Peter Jatlow for help with the cotinine measurements. These studies were supported by grants DA00436, DA10455, DA14241 and AA15632 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Benowitz NL, Lessov-Schlaggar CN, Swan GE, Jacob P., 3rd Female sex and oral contraceptive use accelerate nicotine metabolism. Clin Pharmacol Ther. 2006;79:480–488. doi: 10.1016/j.clpt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Bjornson W, Rand C, Connett JE, Lindgren P, Nides M, Pope F, Buist AS, Hoppe-Ryan C, O'Hara P. Gender differences in smoking cessation after 3 years in the Lung Health Study. Am J Public Health. 1995;85:223–230. doi: 10.2105/ajph.85.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Booker TK, Butt CM, Wehner JM, Heinemann SF, Collins AC. Decreased anxiety-like behavior in beta3 nicotinic receptor subunit knockout mice. Pharmacol Biochem Behav. 2007;87:146–157. doi: 10.1016/j.pbb.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Booze RM, Welch MA, Wood ML, Billings KA, Apple SR, Mactutus CF. Behavioral sensitization following repeated intravenous nicotine administration: gender differences and gonadal hormones. Pharmacol Biochem Behav. 1999;64:827–839. doi: 10.1016/s0091-3057(99)00169-0. [DOI] [PubMed] [Google Scholar]
- 5.Borland R. Slip-ups and relapse in attempts to quit smoking. Addict Behav. 1990;15:235–245. doi: 10.1016/0306-4603(90)90066-7. [DOI] [PubMed] [Google Scholar]
- 6.Brioni JD, O'Neill AB, Kim DJ, Buckley MJ, Decker MW, Arneric SP. Anxiolytic-like effects of the novel cholinergic channel activator ABT-418. J Pharmacol Exp Ther. 1994;271:353–361. [PubMed] [Google Scholar]
- 7.Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Craven LA, Allen SS, Sved AF, Perkins KA. Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats. Psychopharmacology (Berl) 2005;180:258–266. doi: 10.1007/s00213-005-2152-3. [DOI] [PubMed] [Google Scholar]
- 8.Cheeta S, Irvine EE, Tucci S, Sandhu J, File SE. In adolescence, female rats are more sensitive to the anxiolytic effect of nicotine than are male rats. Neuropsychopharmacology. 2001;25:601–607. doi: 10.1016/S0893-133X(01)00258-5. [DOI] [PubMed] [Google Scholar]
- 9.Damaj MI. Influence of gender and sex hormones on nicotine acute pharmacological effects in mice. J Pharmacol Exp Ther. 2001;296:132–140. [PubMed] [Google Scholar]
- 10.Dluzen DE, Anderson LI. Estrogen differentially modulates nicotine-evoked dopamine release from the striatum of male and female rats. Neurosci Lett. 1997;230:140–142. doi: 10.1016/s0304-3940(97)00487-4. [DOI] [PubMed] [Google Scholar]
- 11.Donny EC, Caggiula AR, Rowell PP, Gharib MA, Maldovan V, Booth S, Mielke MM, Hoffman A, McCallum S. Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology (Berl) 2000;151:392–405. doi: 10.1007/s002130000497. [DOI] [PubMed] [Google Scholar]
- 12.Elliott BM, Faraday MM, Phillips JM, Grunberg NE. Effects of nicotine on elevated plus maze and locomotor activity in male and female adolescent and adult rats. Pharmacol Biochem Behav. 2004;77:21–28. doi: 10.1016/j.pbb.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Faraday MM, Blakeman KH, Grunberg NE. Strain and sex alter effects of stress and nicotine on feeding, body weight, and HPA axis hormones. Pharmacol Biochem Behav. 2005;80:577–589. doi: 10.1016/j.pbb.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 14.Faraday MM, O'Donoghue VA, Grunberg NE. Effects of nicotine and stress on locomotion in Sprague-Dawley and Long-Evans male and female rats. Pharmacol Biochem Behav. 2003;74:325–333. doi: 10.1016/s0091-3057(02)00999-1. [DOI] [PubMed] [Google Scholar]
- 15.Hariharan M, VanNoord T. Liquid-chromatographic determination of nicotine and cotinine in urine from passive smokers: comparison with gas chromatography with a nitrogen-specific detector. Clin Chem. 1991;37:1276–1280. [PubMed] [Google Scholar]
- 16.Hariharan M, VanNoord T, Greden JF. A high-performance liquid-chromatographic method for routine simultaneous determination of nicotine and cotinine in plasma. Clin Chem. 1988;34:724–729. [PubMed] [Google Scholar]
- 17.Harrod SB, Mactutus CF, Bennett K, Hasselrot U, Wu G, Welch M, Booze RM. Sex differences and repeated intravenous nicotine: behavioral sensitization and dopamine receptors. Pharmacol Biochem Behav. 2004;78:581–592. doi: 10.1016/j.pbb.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 18.Hatchell PC, Collins AC. The influence of genotype and sex on behavioral sensitivity to nicotine in mice. Psychopharmacology (Berl) 1980;71:45–49. doi: 10.1007/BF00433251. [DOI] [PubMed] [Google Scholar]
- 19.Jung ME, Wallis CJ, Gatch MB, Lal H. Sex differences in nicotine substitution to a pentylenetetrazol discriminative stimulus during ethanol withdrawal in rats. Psychopharmacology (Berl) 2000;149:235–240. doi: 10.1007/s002130000392. [DOI] [PubMed] [Google Scholar]
- 20.Kanyt L, Stolerman IP, Chandler CJ, Saigusa T, Pogun S. Influence of sex and female hormones on nicotine-induced changes in locomotor activity in rats. Pharmacol Biochem Behav. 1999;62:179–187. doi: 10.1016/s0091-3057(98)00140-3. [DOI] [PubMed] [Google Scholar]
- 21.Kayir H, Uzbay IT. Nicotine antagonizes caffeine- but not pentylenetetrazole-induced anxiogenic effect in mice. Psychopharmacology (Berl) 2006;184:464–469. doi: 10.1007/s00213-005-0036-1. [DOI] [PubMed] [Google Scholar]
- 22.Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 23.Killen JD, Fortmann SP, Newman B, Varady A. Evaluation of a treatment approach combining nicotine gum with self-guided behavioral treatments for smoking relapse prevention. J Consult Clin Psychol. 1990;58:85–92. doi: 10.1037//0022-006x.58.1.85. [DOI] [PubMed] [Google Scholar]
- 24.King SL, Caldarone BJ, Picciotto MR. Beta2-subunit-containing nicotinic acetylcholine receptors are critical for dopamine-dependent locomotor activation following repeated nicotine administration. Neuropharmacology. 2004;47 Suppl 1:132–139. doi: 10.1016/j.neuropharm.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 25.Klein LC, Stine MM, Vandenbergh DJ, Whetzel CA, Kamens HM. Sex differences in voluntary oral nicotine consumption by adolescent mice: a dose-response experiment. Pharmacol Biochem Behav. 2004;78:13–25. doi: 10.1016/j.pbb.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Koylu E, Demirgoren S, London ED, Pogun S. Sex difference in up-regulation of nicotinic acetylcholine receptors in rat brain. Life Sci. 1997;61:PL 185–PL 190. doi: 10.1016/s0024-3205(97)00665-6. [DOI] [PubMed] [Google Scholar]
- 27.Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- 28.Meliska CJ, Bartke A, McGlacken G, Jensen RA. Ethanol, nicotine, amphetamine, and aspartame consumption and preferences in C57BL/6 and DBA/2 mice. Pharmacol Biochem Behav. 1995;50:619–626. doi: 10.1016/0091-3057(94)00354-8. [DOI] [PubMed] [Google Scholar]
- 29.Mochizuki T, Villemagne VL, Scheffel U, Dannals RF, Finley P, Zhan Y, Wagner HN, Jr, Musachio JL. Nicotine induced up-regulation of nicotinic receptors in CD-1 mice demonstrated with an in vivo radiotracer: gender differences. Synapse. 1998;30:116–118. doi: 10.1002/(SICI)1098-2396(199809)30:1<116::AID-SYN15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 30.Olausson P, Engel JA, Soderpalm B. Behavioral sensitization to nicotine is associated with behavioral disinhibition; counteraction by citalopram. Psychopharmacology (Berl) 1999;142:111–119. doi: 10.1007/s002130050869. [DOI] [PubMed] [Google Scholar]
- 31.Ouagazzal AM, Kenny PJ, File SE. Modulation of behaviour on trials 1 and 2 in the elevated plus-maze test of anxiety after systemic and hippocampal administration of nicotine. Psychopharmacology (Berl) 1999;144:54–60. doi: 10.1007/s002130050976. [DOI] [PubMed] [Google Scholar]
- 32.Parrott AC. Does cigarette smoking cause stress? Am Psychol. 1999;54:817–820. doi: 10.1037//0003-066x.54.10.817. [DOI] [PubMed] [Google Scholar]
- 33.Perkins KA. Smoking cessation in women. Special considerations. CNS Drugs. 2001;15:391–411. doi: 10.2165/00023210-200115050-00005. [DOI] [PubMed] [Google Scholar]
- 34.Perkins KA, Donny E, Caggiula AR. Sex differences in nicotine effects and self-administration: review of human and animal evidence. Nicotine Tob Res. 1999;1:301–315. doi: 10.1080/14622299050011431. [DOI] [PubMed] [Google Scholar]
- 35.Picciotto MR, Brunzell DH, Caldarone BJ. Effect of nicotine and nicotinic receptors on anxiety and depression. Neuroreport. 2002;13:1097–1106. doi: 10.1097/00001756-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 36.Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- 37.Picciotto MR, Zoli M, Zachariou V, Changeux JP. Contribution of nicotinic acetylcholine receptors containing the beta 2-subunit to the behavioural effects of nicotine. Biochem Soc Trans. 1997;25:824–829. doi: 10.1042/bst0250824. [DOI] [PubMed] [Google Scholar]
- 38.Pomerleau CS, Pomerleau OF, Garcia AW. Biobehavioral research on nicotine use in women. Br J Addict. 1991;86:527–531. doi: 10.1111/j.1360-0443.1991.tb01802.x. [DOI] [PubMed] [Google Scholar]
- 39.Rosecrans JA. Brain area nicotine levels in male and female rats with different levels of spontaneous activity. Neuropharmacology. 1972;11:863–870. doi: 10.1016/0028-3908(72)90045-7. [DOI] [PubMed] [Google Scholar]
- 40.Rosecrans JA. Effects of nicotine on brain area 5-hydroxytryptamine function in male and female rats separated for differences of activity. Eur J Pharmacol. 1971;16:123–127. doi: 10.1016/0014-2999(71)90067-7. [DOI] [PubMed] [Google Scholar]
- 41.Rosecrans JA, Schechter MD. Brain area nicotine levels in male and female rats of two strains. Arch Int Pharmacodyn Ther. 1972;196:46–54. [PubMed] [Google Scholar]
- 42.Salas R, Pieri F, Fung B, Dani JA, De Biasi M. Altered anxiety-related responses in mutant mice lacking the beta4 subunit of the nicotinic receptor. J Neurosci. 2003;23:6255–6263. doi: 10.1523/JNEUROSCI.23-15-06255.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sparks JA, Pauly JR. Effects of continuous oral nicotine administration on brain nicotinic receptors and responsiveness to nicotine in C57Bl/6 mice. Psychopharmacology (Berl) 1999;141:145–153. doi: 10.1007/s002130050818. [DOI] [PubMed] [Google Scholar]
- 44.USDHHS, U.S.D.o.H.a.H.S. Washington, DC: U.S. Government Printing Office Number 0-16-051576-2. Atlanta: Centers for Disease Control and Prevention, Office of Smoking and Health; The Health Consequences of Smoking: A Report of the Surgeon General. 2004 [PubMed]
- 45.Wetter DW, Fiore MC, Young TB, McClure JB, de Moor CA, Baker TB. Gender differences in response to nicotine replacement therapy: objective and subjective indexes of tobacco withdrawal. Exp Clin Psychopharmacol. 1999;7:135–144. doi: 10.1037//1064-1297.7.2.135. [DOI] [PubMed] [Google Scholar]
- 46.Yilmaz O, Kanit L, Okur BE, Pogun S. Effects of nicotine on active avoidance learning in rats: sex differences. Behav Pharmacol. 1997;8:253–260. [PubMed] [Google Scholar]


