Summary
During the elongation cycle, tRNA and mRNA undergo coupled translocation through the ribosome catalyzed by elongation factor EF-G. Cryo-EM reconstructions of certain EF-G-containing complexes led to the proposal that the mechanism of translocation involves rotational movement between the two ribosomal subunits. Here, using single-molecule FRET we observe that pre-translocation ribosomes undergo spontaneous intersubunit rotational movement in the absence of EF-G, fluctuating between two conformations corresponding to the classical and hybrid states of the translocational cycle. In contrast, post-translocation ribosomes are fixed predominantly in the classical, non-rotated state. Movement of the acceptor stem of deacylated tRNA into the 50S E site and EF-G binding to the ribosome both contribute to stabilization of the rotated, hybrid state. Furthermore, the acylation state of P-site tRNA has a dramatic effect on the frequency of intersubunit rotation. Our results provide direct evidence that the intersubunit rotation that underlies ribosomal translocation is thermally driven.
Introduction
Protein synthesis is a dynamic process carried out by the ribosome, an RNA-based molecular machine. During protein synthesis, tRNA and mRNA are translocated through the ribosome in a series of complex, large-scale molecular movements catalyzed by elongation factor G (EF-G) and GTP. However, translocation can occur, albeit very slowly, in the absence of EF-G and GTP (Cukras et al., 2003; Fredrick and Noller, 2003; Gavrilova et al., 1976; Gavrilova and Spirin, 1971; Pestka, 1969). Thus, translocation is a property of the ribosome itself, rather than of EF-G, and is thermodynamically favored even in the absence of GTP hydrolysis.
Chemical probing studies provided the first direct evidence that translocation takes place in two steps involving an intermediate hybrid state (Moazed and Noller, 1989b). In the first step, the acceptor ends of the tRNAs move relative to the 50S subunit, from their classical A/A and P/P binding states into hybrid A/P and P/E states (in which the peptidyl-tRNA is bound in the 30S A site and the 50S P site, and the deacylated tRNA is bound in the 30S P site and the 50S E site; Figure 1A). The specific affinity of the acceptor end of deacylated tRNA for the 50S E site (Lill et al., 1986) helps to account for the thermodynamic driving force for spontaneous formation of the hybrid state. In the second step, which strongly depends on participation of EF-G, their anticodon ends move on the 30S subunit, coupled with mRNA movement, into the post-translocational P/P and E/E states.
Figure 1. Experimental design.
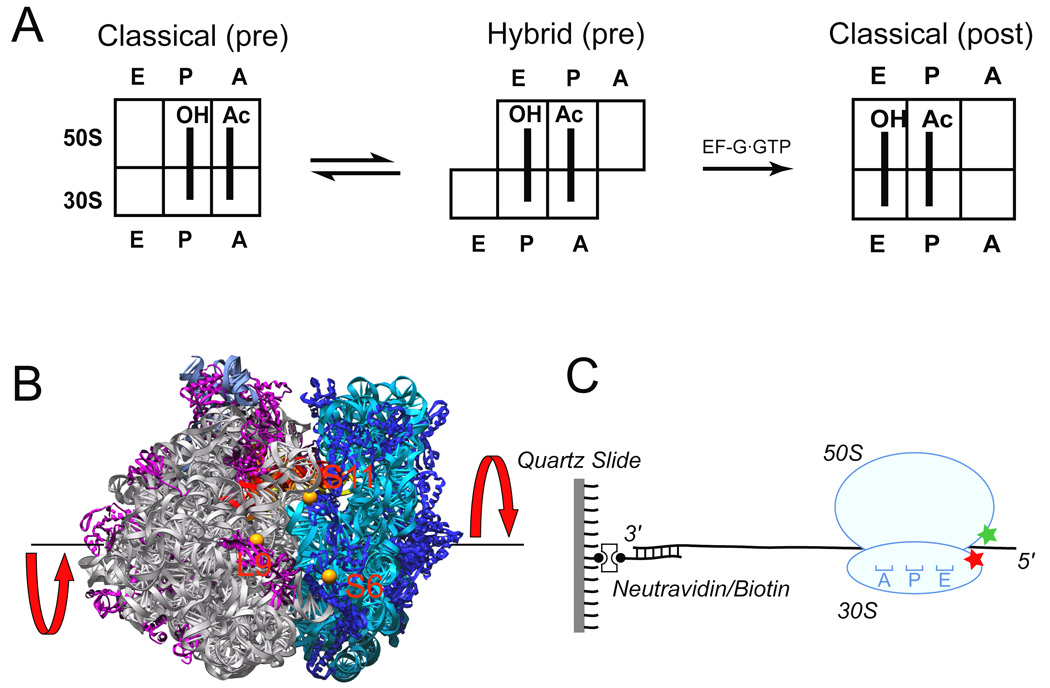
a. Cartoon showing the movement of deacylated tRNAfMet (initially in the P site) and peptidyl-tRNA analogue N-Ac-Phe-tRNAPhe (initially in A site) during translocation between classical pre-translocation, hybrid pre-translocation and classical post-translocation states. b. Positions of fluorescent dyes (orange spheres) coupled to proteins L9, S6 and S11 in the 70S ribosome, viewed from the E-site interface side of the crystal structure (Korostelev et al., 2006). The 50S subunit is on the left (23S and 5S rRNAs are in grey, proteins in magenta) and the 30S subunit is on the right (16S rRNA in cyan, proteins in blue). The E-site tRNA (red) can be seen spanning the interface. The red arrows indicate the direction of intersubunit rotation accompanying hybrid state formation. c. Ribosomes were immobilized by hybridization of the 3’ tail of the mRNA to a biotin-derivatized DNA strand that was bound via neutravidin to a quartz cover slip.
Cryo-EM studies have identified a conformation of the ribosome in which the 30S subunit is rotated by about 3°–10° counter-clockwise relative to the 50S subunit in complexes containing EF-G·GDPNP (a non-hydrolyzable analogue of GTP) or EF-G·GDP·fusidic acid (Frank and Agrawal, 2000; Gao et al., 2003; Valle et al., 2003). This finding led to the proposal of a ratchet-like mechanism, in which translocation of tRNA and mRNA is linked to intersubunit rotational movement (Frank and Agrawal, 2000; Tama et al., 2003; Valle et al., 2003). Recently, this model has been directly tested by formation of a disulfide bridge between ribosomal proteins S6 and L2 designed to restrict intersubunit movement, resulting in a specific block in translocation (Horan and Noller, 2007).
The hybrid-state and ratchet models have now converged. Recent bulk FRET measurements combined with chemical probing experiments show that the EF-G-induced rotation of the 30S subunit observed in cryo-EM reconstructions corresponds to formation of the hybrid state characterized by chemical probing studies (Ermolenko et al., 2007a; Ermolenko et al., 2007b). Although EF-G binding was found to stabilize the rotated, hybrid state (Spiegel et al., 2007), rotation of the 30S subunit was also observed in the absence of EF-G under conditions favoring the hybrid state (Ermolenko et al., 2007a) consistent with previous biochemical experiments with pre-translocation complexes (Sharma et al., 2004). Furthermore, spontaneous movement of two fluorescently-labeled tRNAs relative to each other, interpreted as movement of the tRNAs between the classical and hybrid states, was observed in individual pre-translocation ribosomes using single-molecule FRET (smFRET) (Blanchard et al., 2004b; Kim et al., 2007; Munro et al., 2007).
Although the above-mentioned evidence points to the role of ribosome structural dynamics in translocation, the underlying molecular mechanism of this process remains elusive. Intersubunit movements inferred from cryo-EM and static bulk FRET experiments have been performed at equilibrium and on the ensemble level and have yet to be observed in real time; moreover, there is so far no thermodynamic and kinetic description of ribosomal intersubunit movement. Finally, the proposal, based on cryo-EM (Frank and Agrawal, 2000; Gao et al., 2004) and FRET studies (Munro et al., 2007; Pan et al., 2007), that ribosomal subunits may occupy more than one intermediate conformational state has yet to be established. Here, we address these questions directly using smFRET (Ha et al., 1996) and total internal reflection microscopy (Zhuang et al., 2000). This method has been used previously to probe tRNA dynamics during and after tRNA accommodation (Blanchard et al., 2004a; Blanchard et al., 2004b; Gonzalez et al., 2007; Kim et al., 2007; Lee et al., 2007; Munro et al., 2007) and EF-G dynamics (Wang et al., 2007) on the ribosome. In our experiments, using fluorescently labeled ribosomal subunits, we use this approach to directly monitor the dynamics of the ribosome itself. We observe for the first time the hypothesized ratchet-like motions of individual ribosomes and characterize the determining factors of their dynamics. The ability of ribosomes to undergo spontaneous intersubunit rotation in the absence of EF-G or GTP has strong implications for the molecular mechanism of translocation.
Results
Intersubunit movement in individual pre-translocation ribosomes
We conjugated fluorescent labels to specific cysteine residues introduced by directed mutagenesis into ribosomal proteins S6 (D41C), S11 (E75C), and L9 (N11C) (Figure 1B) (Ermolenko et al., 2007a; Ermolenko et al., 2007b). Proteins S6 or S11 labeled with acceptor (Cy5) dye and protein L9 labeled with donor (Cy3) dye were incorporated into 30S and 50S subunits, respectively, by in vitro reconstitution as described previously (Ermolenko et al., 2007a). The labeled subunits were then associated to create two kinds of doubly-labeled 70S ribosomes, 70S:S6(Cy5)/L9(Cy3) and 70S:S11(Cy5)/L9(Cy3). In vitro assays showed that the at least 50–60% of purified reconstituted, labeled ribosomes were active in in vitro translocation (Ermolenko et al., 2007a), and 80–100% active in in vitro translation of a defined mRNA (L. Lancaster, unpublished results). In order to study the intrinsic structural dynamics of the pre-translocation ribosome, the peptidyl-tRNA analogue N-Ac-Phe-tRNAPhe was bound to the A site of ribosomes containing deacylated tRNAfMet bound to the P site in the presence of a defined mRNA. Pre-translocation ribosome complexes were then immobilized in polymer-passivated microscope slide/cover slip chambers via a biotin-derivatized DNA oligonucleotide annealed to the mRNA (Figure 1C) (Blanchard et al., 2004b) and were visualized using total internal reflection microscopy (Zhuang et al., 2000). This immobilization approach preserved the ribosome’s translocation activity (see below).
Time traces of individual S6-Cy5/L9-Cy3 pre-translocation complexes showed spontaneous, time-dependent anti-correlated changes in donor (Cy3) and acceptor (Cy5) fluorescence intensities (Figure 2A). Calculation of apparent FRET efficiency (FRET=ICy5/[ICy5+ICy3]) from donor (ICy3) and acceptor (ICy5) intensities revealed that pre-translocation ribosomes fluctuate between high (0.56) and low (0.40) FRET states. smFRET measurements performed with the Cy3 and Cy5 dyes reversed gave similar results (data not shown). Time traces recorded for S11-Cy5/L9-Cy3 ribosomes show a similar pattern of spontaneous fluctuations, but inverted from that of the S6/L9 construct (data not shown), because S11 moves closer to L9 in the hybrid state, whereas S6 moves away from L9 (Ermolenko et al., 2007a). Below, we present only data from the S6/L9 construct, because of the previously demonstrated strong anticorrelation between the FRET changes for the S6/L9 and S11/L9 dye pairs (Ermolenko et al., 2007a).
Figure 2. HMM analysis of FRET data obtained from S6-Cy5/L9-Cy3 pre-translocation ribosomes.
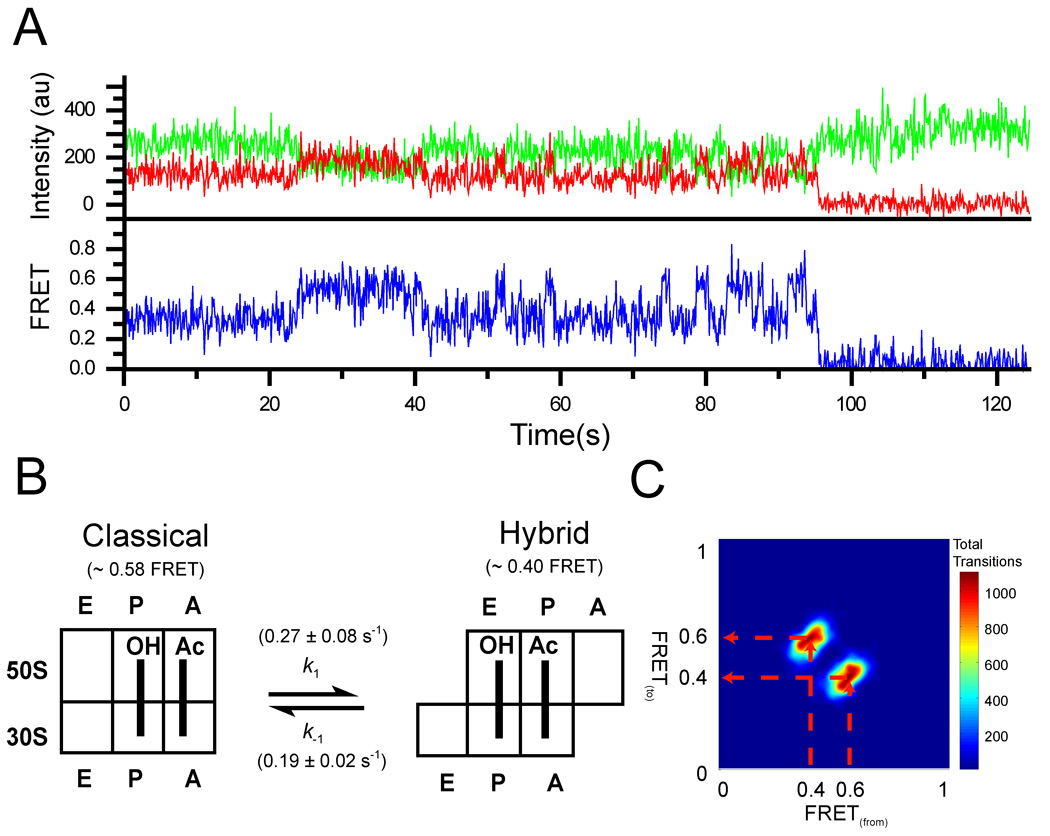
a. Representative trace showing fluorescence intensities observed for the Cy3 donor (green) attached to L9 and a Cy5 acceptor attached to S6 (red) in ribosomes containing tRNAfMet in the P site and N-Ac-Phe-tRNAPhe in the A site. b. Schematic showing the observed FRET values for the two states and the forward and reverse transition frequencies (k1 and k−1). c. Transition density plot (TDP) for the pre-translocation complex. The TDP is constructed by plotting values for each transition based upon the FRET value from which the transition originated (x-axis) and to which FRET value the transition ends (y-axis). The transition paths are indicated by the broken red arrows.
The high (0.56) FRET state for the S6/L9 pair corresponds to the non-rotated conformation of the ribosome, in which the tRNAs are bound in the classical state, whereas the low (0.4) FRET state corresponds to the conformation in which the small ribosomal subunit is rotated and the tRNAs are bound in the A/P and P/E hybrid states (Ermolenko et al., 2007a; Ermolenko et al., 2007b). The latter conformation is equivalent to the “ratcheted state” observed in cryo-EM studies of complexes of the ribosome bound with EF-G (Frank and Agrawal, 2000; Valle et al., 2003). Thus, our single-ribosome traces show that the pre-translocation ribosome, in the absence of EF-G or GTP, fluctuates spontaneously between the rotated and non-rotated conformations, corresponding to the hybrid and classical states, respectively.
To ask whether the ribosomal subunits also move through any additional, previously unobserved transient rotational states, the presence of which could be masked by noise in our FRET traces, we subjected our data to a hidden Markov modeling (HMM) algorithm (McKinney et al., 2006). This approach allows for determination of the number of states present in the system and the rates of exchange between them. HMM analysis of the S6-Cy5/L9-Cy3 pre-translocation complex (612 total molecules showing on average 30 transitions per molecule; Table 1) assuming three states (Figure 2C and Figure 3) showed that the pre-translocation complex fluctuates between just two distinct states, non-rotated and rotated, with a forward rotation (non-rotated to rotated) rate of 0.27 ± 0.08 s−1 and a reverse rotation (rotated to non-rotated) rate of 0.19 ± 0.02 s−1 at 25°C under our in vitro conditions (Figures 2B and 2C; Table 1).  The same analysis performed on ribosomes containing only a P-site tRNA (tRNAfMet or tRNAMet) also resulted in just two observed FRET states (Table 1 and Figure S1). In addition, dwell time analysis for all three complexes fit to a single exponential decay, consistent with two states (Figure S2).
The same analysis performed on ribosomes containing only a P-site tRNA (tRNAfMet or tRNAMet) also resulted in just two observed FRET states (Table 1 and Figure S1). In addition, dwell time analysis for all three complexes fit to a single exponential decay, consistent with two states (Figure S2).
Table 1.
Kinetic rates measured between 0.56 and 0.40 FRET states.
| P site tRNA / A site tRNA | Forward transitions (k1) | k1(s−1) | Reverse transitions (k−1) | k−1(s−1) | Transitions per-trace |
|---|---|---|---|---|---|
| tRNAfMet / Vacant | 9906 | 0.51 ± 0.03 | 9902 | 0.21 ± 0.03 | 35 |
| tRNAfMet / N-Ac-Phe-tRNAPhe | 9256 | 0.27 ± 0.08 | 9195 | 0.19 ± 0.02 | 30 |
| tRNAMet / Vacant | 3256 | 0.49 ± 0.12 | 3242 | 0.09 ± 0.03 | 17 |
Results of fitting FRET time trajectories with the HMM algorithm. Each data set was divided into three and analyzed separately. The reported number is an average from each of the three data sets with the standard deviation.
Figure 3. Representative time traces from HMM analysis of S6-Cy5/L9-Cy3 ribosomes containing deacylated tRNAfMet in the P site and N-Ac-Phe-tRNAPhe in the A site.
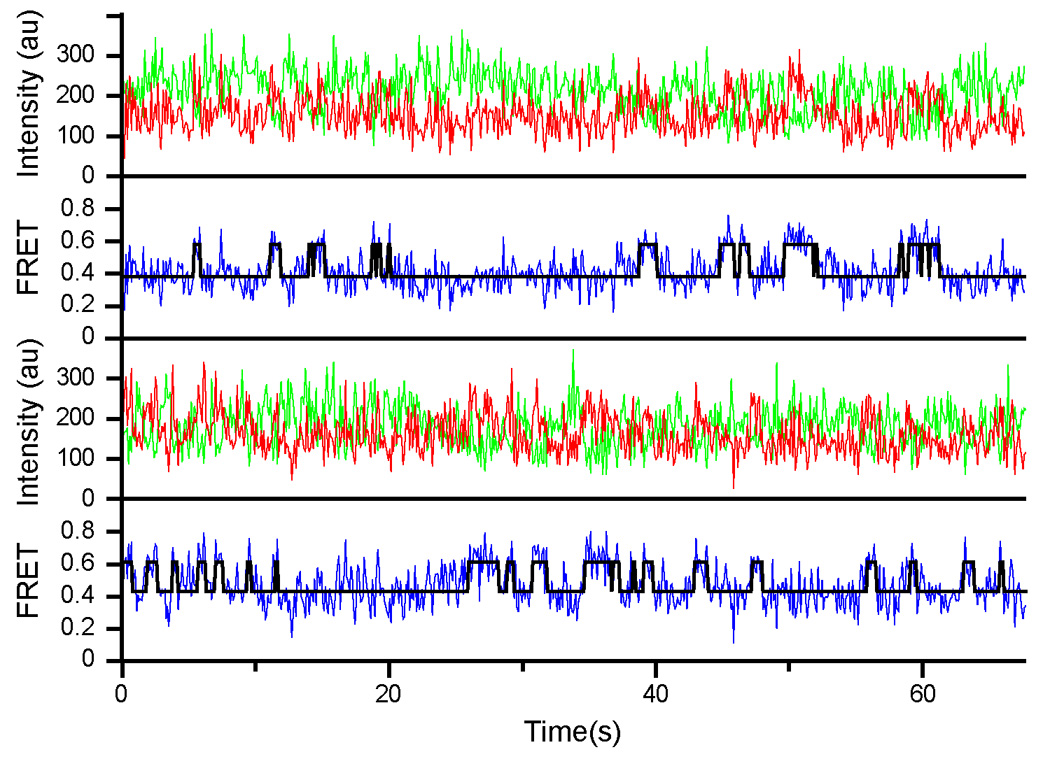
Cy3 and Cy5 intensities are shown as green and red traces, respectively. The calculated FRET curve is shown in blue with the HMM-determined fit overlaid in black.
Effects of the acylation state of the P-site tRNA and EF-G on intersubunit rotation
We next asked how the acylation state of P-site tRNA and EF-G binding affect the non-rotated/rotated states equilibrium. The equilibrium constant, Keq=[%roated]/[%non-roated] is determined from double Gaussian fits to smFRET histograms (Dahan et al., 1999) built from several hundred molecules for each construct (Figure 4 and Figure S3; Table 2). A majority (75%) of post-translocation ribosomes, containing the peptidyl-tRNA analogue N-Ac-Phe-tRNAPhe bound in the P site with a vacant A site, exhibited a high FRET value (Figure 4A), corresponding to the classical, non-rotated conformation, in agreement with chemical probing (Moazed and Noller, 1989b) and bulk FRET (Ermolenko et al., 2007a) experiments. In a complex containing a different peptidyl tRNA, fMet-tRNAfMet, bound to the P site, 66% of ribosomes were also in the non-rotated conformation (Figure 4B). Likewise, 52% and 79% of authentic post-translocation complexes obtained by incubation of the pre-translocation complex (the former containing the N-Ac-Phe-tRNAPhe bound to the A site and deacylated tRNAfMet bound to the P site and the latter containing N-Ac-Phe-tRNAPhe bound to the A site and deacylated tRNATyr bound to the P site) with EF-G·GTP were found in the non-rotated conformation (Figures 4C and 4D). 61% of vacant ribosomes (i.e., ones lacking tRNA), also exhibited a high FRET value (Figure 4K).
Figure 4. Histograms compiled from several hundred time traces showing distributions of FRET values for different ribosome complexes.
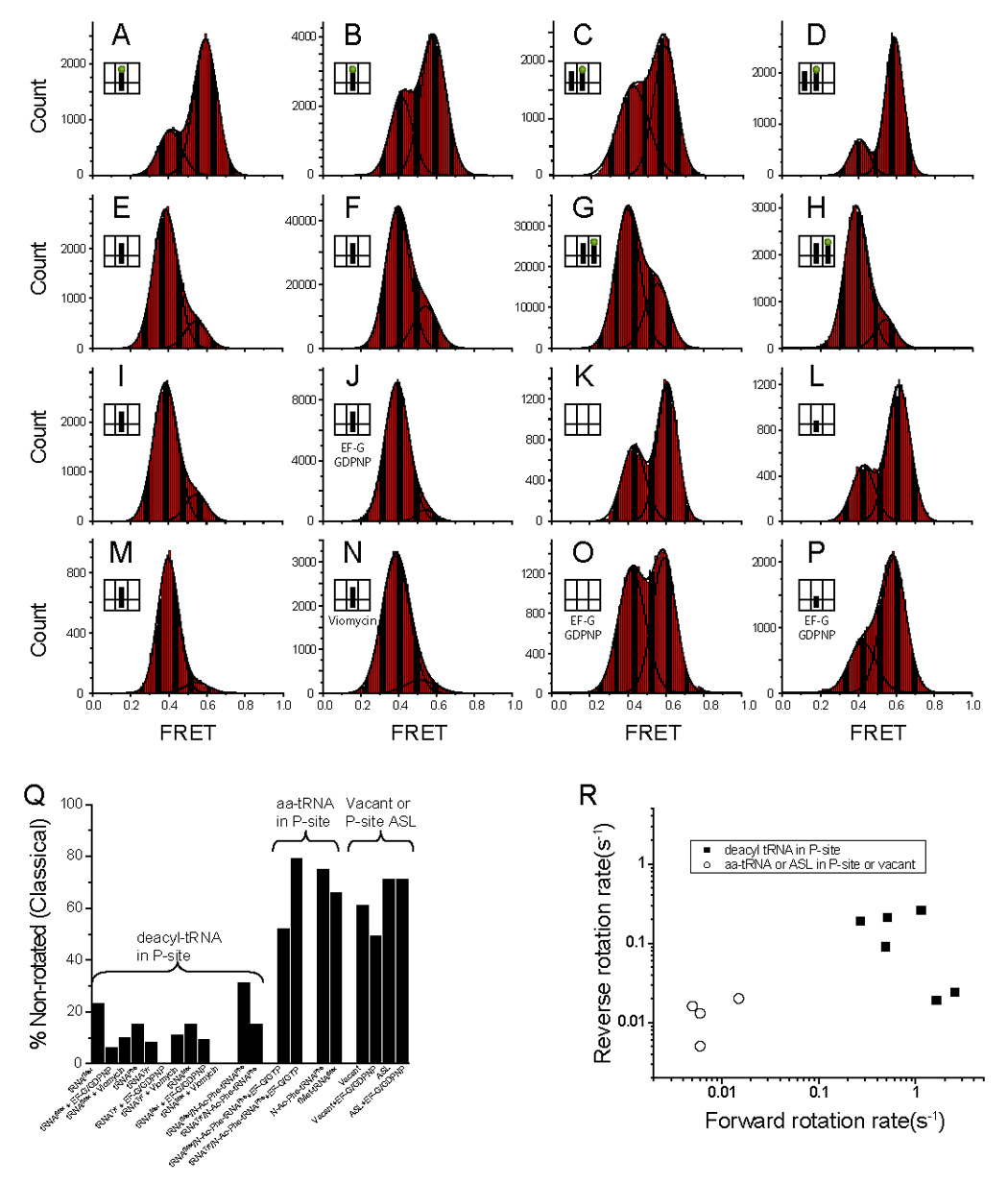
S6-Cy5/L9-Cy3 ribosomes were assembled with mRNA and (a) N-Ac-Phe-tRNAPhe in the P-site, (b) fMet-tRNAfMet in the P-site, (c) tRNAfMet in the P-site and N-Ac-Phe-tRNAPhe in the A-site translocated with EF-G·GTP, (d) tRNATyr in the P-site and N-Ac-Phe-tRNAPhe in the A-site translocated with EF-G·GTP, (e) tRNAPhe in the P-site due to deacylation of the complex in panel (a) with puromycin, (f) tRNAfMet in the P-site, (g) tRNAfMet in the P-site and N-Ac-Phe-tRNAPhe in the A-site, (h) tRNATyr in the P-site and N-Ac-Phe-tRNAPhe in the A-site, (i) tRNAMet in the P-site (j) tRNAfMet with EF-G·GDPNP, (k) no tRNA, (l) Anticodon Stem Loop (ASL) from tRNAfMet, (m) tRNATyr in the P-site, (n) tRNAfMet with viomycin, (o) no tRNA with EF-G·GDPNP, and (p) Anticodon Stem Loop (ASL) from tRNAfMet with EF-G·GDPNP. The FRET data was smoothed with a 5 point window and can be fitted to two Gaussians. (q) A graphical depiction of the percent of each complex in the non-rotated state (Table 2). It is clear that the non-rotated state is weakly populated if the P-site tRNA is deacylated in comparison to other constructs. (r) A scatter plot showing the forward vs. reverse rotation rates for various ribosomal complexes (Table 2). The ribosomes with a deacylated tRNA have much higher rates of forward and reverse rotation. The data with EF-G are not included in the plot.
Table 2.
Statistical data for all tested complexes
| Figure | % NRa | % Rb | Keqc | k1 (s−1)d | k−1 (s−1)e | Methodf | % transg | |
|---|---|---|---|---|---|---|---|---|
| Vacant and ASL in P-site | ||||||||
| Vacant | 4K | 61 | 39 | 0.63 | 0.015 | 0.020 | 1, 1 | 4 |
| Vacant with EF-G·GDPNP | 4O | 49 | 51 | 1.1 | 0.004 | 0.003 | 1, 1 | 5 |
| ASLfMet | 4L | 71 | 29 | 0.41 | 0.006 | 0.005 | 1, 1 | 3 |
| ASLfMet with EF-G·GDPNP | 4P | 71 | 29 | 0.41 | 0.004 | 0.009 | 1, 1 | 2 |
| Peptidyl-tRNA in P site | ||||||||
| N-Ac-Phe-tRNAPhe | 4A | 75 | 25 | 0.33 | 0.005 | 0.016 | 1, 1 | 4 |
| fMet-tRNAfMet | 4B | 66 | 34 | 0.50 | 0.006 | 0.013 | 1, 1 | 2 |
| Pre-translocation Complexes | ||||||||
| tRNAfMet / N-Ac-Phe-tRNAPhe | 4G | 31 | 69 | 2.2 | 0.27±0.08 | 0.19±0.02 | 2, 2 | 59 |
| tRNATyr / N-Ac-Phe-tRNAPhe | 4H | 15 | 85 | 5.7 | 1.65±0.12 | 0.019 | 3, 1 | 14 |
| Post-translocation Complexes | ||||||||
| tRNAfMet / N-Ac-Phe-tRNAPhe with EF-G·GTP | 4C | 52 | 48 | 0.92 | 0.034 | 0.031 | 1, 1 | 8 |
| tRNATyr / N-Ac-Phe-tRNAPhe with EF-G·GTP | 4D | 79 | 21 | 0.27 | 0.020 | 0.074 | 1, 1 | 9 |
| Deacyl-tRNA in P site | ||||||||
| tRNAfMet | 4F | 23 | 77 | 3.3 | 0.51±0.03 | 0.21±0.03 | 2, 2 | 71 |
| tRNAfMet with EF-G·GDPNP | 4J | 6 | 94 | 17 | 1.19±0.04 | 0.021 | 3, 1 | 7 |
| tRNAfMet Viomycin | 4N | 10 | 90 | 9.2 | nd | nd | 3 | |
| tRNAPhe | 4E | 15 | 85 | 5.6 | 1.14±0.01 | 0.26±0.01 | 3, 3 | 45 |
| tRNATyr | 4M | 8 | 92 | 12 | 2.53±0.34 | 0.024 | 3, 1 | 12 |
| tRNATyr with EF-G·GDPNP | S3B | 0 | 100 | - | nd | nd | 1 | |
| tRNATyr with Viomycin | S3D | 11 | 89 | 8.5 | nd | nd | 0 | |
| tRNAMet | 4I | 15 | 85 | 5.6 | 0.49±0.12 | 0.09±0.03 | 2, 2 | 51 |
| tRNAMet with EF-G·GDPNP | S3A | 9 | 91 | 10 | nd | nd | 3 | |
| tRNAMet with Viomycin | S3C | 0 | 100 | - | nd | nd | 0 |
These values are derived from fitting the histograms providing the percent of the molecules in the non-rotated (NR) or high FRET state and the rotated (R) or low FRET state.
Equilibrium constant calculated from the relative populations of non-rotated and rotated ribosomes(see text).
These rates were calculated as described in the text and in the methods section.
Method used to calculate the rates where 1 is #transitions/dwell time, 2 is HMM analysis, and 3 is dwell time analysis. A more detailed description is in the Experimental procedures section.
This percentage represents the total number of traces that contain at least one unambiguous FRET transition between the fitted high and low FRET states divided by the total number of traces.
Insufficient data to calculate rate information. For complexes stabilized in the rotated conformation (e.g., complexes containing EF-G or viomycin), equilibrium constants calculated from the relative populations of non-rotated and rotated ribosomes deviate significantly from constants calculated from the ratios of rates for forward and reverse rotation. This discrepancy is likely due to the presence of a fraction of inactive ribosomes that skews the value of the equilibrium constant calculated from the distribution.
In contrast to post-translocation complexes, ribosomes containing deacylated tRNAfMet in the P site without an A-site tRNA showed a majority of ribosomes (77%) in the low-FRET rotated state (Figure 4F). An even more pronounced effect was observed in the case of deacylated tRNAPhe, which shifted 85% of the ribosome population to the rotated state (Figure 4E), consistent with the higher propensity of tRNAPhe to occupy the hybrid P/E state, compared with tRNAfMet (Dorner et al., 2006; Spiegel et al., 2007). A similar difference was also observed between tRNAfMet and tRNAMet (Studer et al., 2003). In contrast, only 29% of ribosomes containing only a tRNA anticodon stem-loop (ASL) bound to the P site were found in the hybrid, rotated state (Figure 4L), indicating that interactions between the elbow and/or acceptor end of a deacylated tRNA with the 50S E site promotes stabilization of the P/E hybrid state.
When EF-G·GDPNP was bound to the complex containing deacylated tRNAfMet 94% of the ribosomes were observed in the low-FRET, rotated state (Figure 4J; compare with Figure 4F), demonstrating that EF-G·GDPNP converts almost the entire ribosome population to the rotated, hybrid-state conformation. The translocation inhibitor viomycin also converted 90% of tRNAfMet-containing ribosomes into the rotated state (Figure 4N) (Ermolenko et al., 2007b; Spiegel et al., 2007). In contrast, a significantly lower number of ribosomes containing only an ASL in the P site (29%) or vacant ribosomes (51%) were observed in the rotated, hybrid-state conformation in the presence of EF-G·GDPNP (Figures 4O and 4P). These results show that a deacylated tRNA in the P site, with or without EF-G bound to the ribosome, contributes to stabilization of the rotated, hybrid state and that EF-G alone is insufficient to convert all ribosomes to the hybrid state (Figure 4Q).
Deacylation of P-site tRNA has a dramatic effect on ratcheting kinetics
A significant portion of vacant ribosomes and ones containing an ASL or peptidyl-tRNA exist in the rotated conformation (Figures 4A, 4B, 4K, and 4L) yet only 5% or less of the time traces exhibit any transition between FRET states (Table 2). For example, only 2% of the traces for complexes containing fMet-tRNA show any transitions, as compared to 71% of traces for deacylated tRNAfMet. To estimate the interconversion rate where transitions are very rare, for which HMM analysis is not applicable, we divided the total number of observed transitions by the total observation time from hundreds of molecules and obtained an estimate for the forward rotation (non-rotated to rotated) and reverse rotation (rotated to non-rotated) rates in the range of 0.02 – 0.005 s−1 (Table 2 and Experimental procedures). These calculated rates potentially represent an upper limit due to the paucity of observed transitions and may be further complicated by the presence of inactive ribosomes. However, these estimates are sufficient to clearly demonstrate that while there is at most a 5-fold difference in the equilibrium constants between these complexes and their deacyl counterparts, there is as much as a 100-fold difference in kinetic rates (Table 2). These differences are solely due to the presence of a deacylated tRNA in the P site of the ribosome, which significantly affects both the forward and reverse rotation rates of the ribosome (Figure 4R).
Addition of N-Ac-Phe-tRNAPhe to the A site of ribosomes that contained a single deacylated tRNA in the P site had only a modest effect on the equilibrium and kinetic rates of inter-subunit rotation (Table 2 and Figures 4F and 4M compared to Figures 4G and 4H, respectively). In the case of tRNAfMet, for example, there was only a 1.5-fold increase in the equilibrium constant upon adding N-Ac-Phe-tRNAPhe to the A site and a 1.9-fold increase in the forward rotation rate with no change in the reverse rotation rate.
Overall, a total of 6 complexes were tested that contained a deacylated tRNA in the P site. For the complexes that contained tRNAfMet in the P-site, ~71% and ~59% (the latter with N-Ac-Phe-tRNAPhe in the A site) of traces showed FRET transitions between states. The fraction of traces showing FRET transitions was lower for other P-site tRNAs, with 51%, 45%, 12%, and 14% for tRNAMet, tRNAPhe, tRNATyr and tRNATyr with N-Ac-Phe-tRNAPhe in the A site, respectively (Table 2). This indicates that the identity of the P-site tRNA can also influence ribosome dynamics. The different propensities of the various tRNAs to favor intersubunit rotation is consistent with previous results (Dorner et al., 2006; Spiegel et al., 2007).
EF-G facilitates ratcheting mildly and stabilizes the rotated state
The kinetic rates for movement from non-rotated to rotated conformations of the ribosome, for complexes containing deacylated tRNA bound to the P site, range from 0.27 to 2.53 s−1 (Table 1, 2). As these rates are reduced relative to known in vitro translocation rates measured at room temperature of between 1 and 10 s−1 (Dorner et al., 2006; Pan et al., 2007; Studer et al., 2003), we asked whether the additional rotational rate enhancement is due to EF-G. As mentioned above, various complexes were prepared with EF-G·GDPNP or viomycin present in solution providing near-complete conversion of ribosomes containing deacyl-tRNA in the P-site to the rotated conformation (Figure 4 and Figure S3). In all of these cases, less than 8% of the time traces exhibited transitions between FRET states and the equilibrium constants are 9 and higher (Table 2). The reduced occurrence of transitions between the two states can be primarily attributed to a significant reduction in the reverse rotation rate by stabilization of the rotated state with EF-G·GDPNP. To test whether EF-G has any effect on the forward rotation rate, we calculated a dwell-time histogram of the non-rotated state from the time traces that showed one or more transient excursions to the non-rotated state (Figure S4 and Figure S5). The dwell-time histogram of the non-rotated state for ribosomes containing tRNAfMet in the P-site with EF-G·GDPNP were fit to a single exponential resulting in a kinetic rate of 1.2 s−1. This corresponds to a rate enhancement of only ~2-fold compared to the forward rotation rate for the same complex in the absence of EF-G·GDPNP calculated by HMM (0.5 s−1) or dwell-time analysis (0.6 s−1) (Table 1; Supp. Figure 2B). An accurate determination of the forward rotation rates for other samples is not possible for the current dataset as the dwell times are consistently shorter than 1s and transitions are infrequent (Table 2). For vacant ribosomes or for ribosomes with an ASL in the P site, EF-G·GDPNP had minimal effect on ribosome dynamics (Table 2).
Real-time observation of intersubunit movement triggered by deacylation, EF-G binding and translocation
smFRET measurements also allowed us to visualize the real-time dynamics of hybrid state formation caused by in situ deacylation of peptidyl-tRNA or binding of EF-G. When puromycin was flowed into a sample cell containing immobilized ribosomes occupied with fMet-tRNAfMet, a decrease in FRET was observed within 3 s (Figure 5A), indicating that deacylation of fMet-tRNAfMet triggered movement into the rotated, hybrid-state conformation. When EF-G·GDPNP was added to ribosomes containing deacylated tRNAfMet bound to the P site, spontaneous fluctuations ceased after ~20 s on average and the ribosomes became stabilized in the rotated, hybrid-state conformation (Figure 5B). Translocation induced by adding EF-G·GTP to a pre-translocation complex in which tRNAfMet was bound to the P site and N-Ac-Phe-tRNAPhe to the A site converted ribosomes into the non-rotated, classical state, accompanied by disappearance of fluctuations after ~22s on average (Figure 5C). These single-molecule data further support our previous assignment of conformational states of the ribosome and demonstrate that our immobilization scheme preserves ribosome function. As such, the results presented here serve as a basis for future single-molecule measurements of the ribosome’s conformational dynamics during protein synthesis.
Figure 5. Single-molecule time traces of S6-Cy5/L9-Cy3 ribosome complexes showing intersubunit movement induced by (a) reaction with puromycin, (b) EF-G binding or (c) translocation.

(a) Ribosomes containing fMet-tRNAfMet bound to the P site were reacted with puromycin (1 mM) in buffer B (see Experimental procedures), flowed into the microscope slide ~20 s after beginning the fluorescence recording (arrow). Intersubunit movement is seen as a sharp decrease in the FRET signal (indicated by the vertical dashed line). 13 of 24 molecules that did not photobleach before addition of puromycin showed similar behavior with an average deacylation time of ~20s. (b) EFG-GDPNP (300 nM, 250 µM) in buffer B (see Experimental procedures) was introduced at ~40s (arrow) into complexes containing deacylated tRNAfMet bound to the P site and a vacant A site. EF-G-induced intersubunit rotation is observed as stabilization of the low FRET state (vertical dashed line). 42 of 51 molecules showed similar behavior with an average time between addition of EFG·GDPNP and the last FRET fluctuation of ~15s (c) EF-G·GTP (300 nM, 250 µM) in buffer B (see Experimental procedures) was introduced at ~20 s (arrow) to pre-translocation complexes containing deacylated tRNAfMet bound to the P site and N-Ac-Phe-tRNAPhe bound to the A site. Translocation is observed as the transition to the high FRET state (vertical dashed line). 40 of 76 molecules showed similar behavior with an average translocation time of ~22s after addition of EF-G·GTP. For further information regarding the flow experiments, see Experimental Procedures.
Discussion
The intersubunit rotational states are in dynamic equilibrium
Our smFRET experiments have allowed direct observation of the long-hypothesized ribosomal intersubunit movements in real time. Remarkably, spontaneous forward and reverse rotation between the non-rotated and rotated states was observed in the absence of EF-G and GTP. Consistent with earlier reports (Ermolenko et al., 2007a; Ermolenko et al., 2007b; Spiegel et al., 2007; Valle et al., 2003), the forward rotation to the rotated state appeared to be coupled to the transition of deacylated tRNA from the P/P state into the hybrid P/E state. Indeed, significant fluctuations between the two major conformational states were observed only when a deacylated tRNA was bound to the P site, regardless of A-site occupancy. Conversely, ribosomes containing either peptidyl-tRNA or an ASL in the P site were fixed predominantly in the classical, non-rotated state (Figures 4A, 4B, and 4L). Since stable interaction between tRNA and the 50S E site has a stringent requirement for a deacylated acceptor end (Lill et al., 1986), the presence of an amino acid or an elongating peptide on the P-site tRNA is expected to block its movement into the P/E hybrid state (Korostelev et al., 2006; Selmer et al., 2006).
Unexpectedly, a significant fraction of post-translocation ribosomes occupied the rotated state (Figures 4A and 4B), although intersubunit fluctuations were nearly absent and the majority of ribosomes were in the non-rotated state. Possible explanations are (i) spontaneous deacylation of tRNA, leading to formation of the P/E state; (ii) incomplete saturation of the P site by peptidyl-tRNA; (iii) reverse translocation, as recently reported (Konevega et al., 2007; Shoji et al., 2006), leading to formation of the A/P hybrid state; (iv) the presence of inactive ribosomes unable to bind tRNA but adopting the rotated conformation; (v) an unprecedented movement of a fraction of peptidyl-tRNA into the P/E hybrid state. Our previous probing, filter binding and toe-printing experiments (Spiegel et al., 2007) indicate that significant spontaneous deacylation of peptidyl-tRNA is unlikely under our experimental conditions. Moreover, this explanation is inconsistent with the lack of rotational fluctuations observed for post-translocation ribosomes (Table 2). Increasing the concentration of peptidyl-tRNA in the imaging buffer by two orders of magnitude did not markedly shift the distribution between classical and hybrid states (Figure S6), ruling out partial occupancy of the P site. Finally, the results of biochemical experiments do not support reverse translocation under these conditions (data not shown). Future smFRET experiments using fluorescently labeled peptidyl-tRNA should help to test the remaining possibilities, that a fraction of ribosomes are unable to bind tRNA and so is able to occupy the rotated conformation or whether our results are, in fact, indicative of peptidyl-tRNA bound in the hybrid P/E state.
Comparing ribosome dynamics and tRNA dynamics at the single-molecule level
Under our conditions, even in the absence of EF-G the hybrid state appears to be thermodynamically favored over the classical state by about 0.5–1 kcal/mol for pre-translocation ribosomes (Figure 2 and Table 1). The frequencies of oscillation of fluorescently-labeled tRNA molecules between states that are believed to correspond to the classical and hybrid states (1–5 s−1) measured in recent smFRET experiments (Kim et al., 2007; Munro et al., 2007) are about an order of magnitude higher than the frequencies of intersubunit motion observed here with tRNAfMet in the P site (0.1–0.5 s−1, Table 2). In addition, the tRNA dynamics data showed that the classical state is favored over the hybrid state under most conditions. These differences are most likely due to the different experimental conditions and constructs used in the two studies. The reconstituted ribosomes used in our experiments, fluorescent labeling of tRNAs in the studies by Kim et al. (2007) and Munro et al. (2007), and the different ionic conditions may all affect the observed frequencies. For example, differences in magnesium ion concentrations can increase the propensity of the ribosome for the classical state by up to 5-fold (Kim et al., 2007). However, because no one has measured the single-molecule dynamics of the ribosome and the tRNAs simultaneously, we can not presently rule out the possibility that the tRNAs may undergo movement independently of intersubunit rotation, at a faster rate. Rates for the overall step of translocation in the presence of EF-G·GTP have been reported as between 1 and 10 s−1 at 25°C, depending on experimental conditions (Dorner et al., 2006; Pan et al., 2007; Studer et al., 2003). The differences between previously reported rates of translocation and the rates of intersubunit rotation determined here may be at least partially accounted for by an acceleration of the rate of hybrid state formation by EF-G (Spiegel et al., 2007) as suggested by experiments performed in the presence of EF-G·GDPNP (Figure 4 and Figure S3 and Table 2).
Recently, the existence of an additional hybrid-state intermediate, containing tRNAs bound in the A/A-P/E state, was proposed based on smFRET (Munro et al., 2007) and stopped-flow (Pan et al., 2007) experiments using fluorescently labeled tRNAs. This proposal implies independent movement of A- and P-site tRNAs into the A/P and P/E hybrid states. Moreover, different degrees of small ribosomal subunit rotation (3–10°) have been observed in different cryo-EM reconstructions (Frank and Agrawal, 2000; Gao et al., 2003; Gao et al., 2004; Valle et al., 2003) and bulk FRET experiments (Ermolenko et al., 2007a), also raising the possibility of intermediate rotational states. However, in our experiments only a single hybrid-state intermediate was found. This is consistent with a recent maximum likelihood classification of cryo-EM data that was able to only identify two genuine classes of ribosomes in the data set (Scheres et al., 2007). Analysis of our single-molecule data shows that the different magnitudes of FRET changes observed in ensemble experiments (Ermolenko et al., 2007a) are caused by shifts in the population of two major conformations rather than by the existence of additional conformations with intermediate degrees of rotation. If any undetected rotational intermediates exist, their lifetimes must be lower than the time-resolution of our experiments (25–100 ms) or the magnitude of rotation accompanying formation of these intermediates must be too small for detection by FRET. Although we found no evidence for existence of an additional A/A-P/E hybrid-state intermediate, it is possible that movement of tRNA from the A/A to A/P state would result in a change in intersubunit orientation below the limit of detection of our method. This would not be surprising, since, in contrast to the transition from the P/P to P/E state, which entails movement of the CCA-end of tRNA by about 50Å, transition from the A/A to A/P states involves a much smaller movement (Korostelev et al., 2006; Noller et al., 2002; Valle et al., 2003).
The P-site tRNA significantly impacts ribosome dynamics
One of the most striking observations is that deacylation of the P-site tRNA dramatically enhances both the forward and reverse rotation rates of intersubunit rotation, in addition to shifting the equilibrium toward the rotated state. A nearly 100-fold increase in the forward rotation rate was observed upon deacylation of the P-site tRNA (fMet-tRNAfMet vs. tRNAfMet in the P-site; Table 2). This result supports previous proposals suggesting that deacylation of peptidyl-tRNA plays a decisive role in triggering both tRNA and intersubunit movement (Ermolenko et al., 2007a; Moazed and Noller, 1989a; Valle et al., 2003; Zavialov and Ehrenberg, 2003). It is remarkable that a single formyl-methionyl or acetyl-phenylalanyl acyl group can critically affect the dynamic behavior of a 2.4 MD macromolecular complex.
The identity of the deacylated tRNA in the P site conferred modest, but measurable differences in intersubunit rotational rates for the four different deacylated tRNAs used. The largest differences were observed between the tRNAfMet and tRNATyr complexes where the forward rotation rate for tRNATyr was ~5-fold faster than for tRNAfMet (Figures 4F and 4M). A similar effect was observed for these two tRNAs when N-Ac-Phe-tRNAPhe was present in the A site (Figures 4C and 4D). Because the elbow and/or acceptor stem moieties facilitate tRNA movement into the hybrid state (compare Figures 4F and 4L; Table 2), these differences may be influenced by the differential affinities of these features of the respective tRNAs for the P and E sites. Addition of N-Ac-Phe-tRNAPhe to the A site had less than a two-fold effect on the forward rotation rate while leaving the reverse rotation rate unchanged. Other constituents on the A-site tRNA were not investigated in this study, but have been investigated biochemically and at the single-molecule level in other studies (Kim et al., 2007; Munro et al., 2007; Sharma et al., 2004; Shoji et al., 2006). These reports suggest that the identity of the A-site tRNA constituent can influence the dynamics of ribosomal movement. Nevertheless, more systematic studies will be needed to fully understand the contributions of the A-site tRNA to intersubunit dynamics.
Intersubunit movement and the mechanism of translocation
Thermal Brownian motion has been shown to play a key role in the mechanical movement of macromolecular machines (Astumian, 1997; Cordova et al., 1992) and may also underlie the mechanism of ribosomal translocation (Spirin, 2004). Although the time-resolved smFRET trajectories (Figure 2, Figure 3, Figure 6 and Figure S4) show relatively long (1–10 s) dwell times, the actual transit time between the hybrid, rotated state and the classical, non-rotated states are faster than the time resolution (~25–100 ms) of our measurements. The rapid transit times, which are consistent with a correlation time for rotational Brownian diffusion of the 30S ribosomal subunit of 1–2 µs (Amand et al., 1977) and the fact that intersubunit movement can occur spontaneously in the absence of EF-G (Figure 2 and Figure 3) provide evidence that thermal energy is sufficient to drive the ribosomal movements associated with translocation, obviating the need for a “power stroke” from GTP hydrolysis for the first step of translocation. Although EF-G binding contributes to stabilization of the hybrid state (Figure 4 and Figure S3), a more critical role for EF-G may be to uncouple movement of the small subunit from that of the mRNA-tRNA complex, to promote the second step of translocation.
In this study, we investigated 20 different ribosomal complexes, only one of which has previously been studied, using an alternative approach of labeling two tRNA molecules (Blanchard et al., 2004b; Kim et al., 2007; Munro et al., 2007). This comprehensive analysis of intersubunit dynamics in pre- and post-translocation ribosomes represents a first step toward a description of the internal movements of the ribosome during the complete process of protein synthesis at the single-molecule level.
EXPERIMENTAL PROCEDURES
Buffers
Buffer A: 20 mM Hepes•KOH (pH 7.5), 6 mM MgCl2, 150 mM NH4Cl, 6 mM β–mercaptoethanol, 2 mM spermidine and 0.1 mM spermine. Buffer B: 20 mM Hepes•KOH (pH 7.5), 6 mM MgCl2, 150 mM NH4Cl, 6 mM β–mercaptoethanol, 2 mM spermidine, 0.1 mM spermine, 0.8 mg/ml glucose oxidase, 0.625% glucose, ~1.5 mM 6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic (Trolox) and 0.03 mg/ml catalase.
Materials and sample preparations
tRNAfMet was purchased from MP Biomedicals; GTP, GDPNP, puromycin, tRNAPhe, tRNATyr and tRNAMet were purchased from Sigma. Defined mRNA m291 and m301 were transcribed in vitro and further purified as described previously (Fredrick and Noller, 2002). The biotin-labeled DNA primer (5’ biotin – CTTTATCTTCAGAAGAAAAACC-3’) was synthesized by Integrated DNA Technologies. NeutrAvidin used for sample immobilization at a final concentration of 0.2 mg/ml was purchased from Pierce. fMet-tRNAfMet, N-Ac-Phe-tRNAPhe, and EF-G with a 6-histidine tag were prepared and purified as previously described (Dorner et al., 2006; Moazed and Noller, 1989b; Wilson and Noller, 1998). The components of the oxygen scavenging system (glucose oxidase from Aspergillus Niger, glucose and 6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid) were purchased from Sigma. Catalase from beef liver was from Roche.
Ribosomal subunits were prepared from E. coli strains MRE600 (wild-type) and CSH142/ΔL9K (containing a chromosomal deletion of the gene encoding protein L9) as described (Hickerson et al., 2005). Mutant variants of ribosomal proteins S6 (D41C), S11 (E75C), and L9 (N11C) were created by site-directed mutagenesis, expressed, purified, and labeled separately with maleimide derivatives of Cy3 (donor) or Cy5 (acceptor) dyes (Amersham Biosciences) as described previously (Hickerson et al., 2005; Lieberman et al., 2000). Labeled proteins S6 or S11 were incorporated into 30S subunits by in vitro reconstitution from purified 16S rRNA and the other 19 individually purified ribosomal proteins according to published procedures (Culver and Noller, 1999; Hickerson et al., 2005). Labeled protein L9 was incorporated into 50S subunits by partial reconstitution from 50S subunits carrying an L9 deletion and doubly-labeled 70S ribosomes were isolated using previously described procedures (Ermolenko et al., 2007a).
Assembly and immobilization of ribosomal complexes
Ribosomal complexes were constructed in buffer A. Ribosome·P-site-tRNA·mRNA complexes were constructed by incubation of 70S ribosomes (0.3 µM) with mRNA m291 or m301 (0.6 µM) preannealed to biotin-labeled primer (0.8 µM) and tRNA (tRNAfMet, tRNAMet, tRNATyr, N-Ac-Phe-tRNAPhe or fMet-tRNAfMet ), depending on the experiment, (0.7 µM) for 20 minutes at 37°C. Pre-translocation complexes were made by binding N-Ac-Phe-tRNAPhe (0.6 µM) to a P site complex containing deacylated tRNAfMet and m291 or tRNATyr and m301, for 30 minutes at 37°C. All complexes were subsequently diluted with buffer A to a final concentration of ~2 nM and were immobilized on the quartz surface of microscope slides prepared for these experiments using an established protocol (Zhuang et al., 2000). To prevent photobleaching during data acquisition the sample buffer was exchanged for imaging buffer B, containing an oxygen scavenging system (Rasnik et al., 2006). For the flow experiments (Figure 6), the average event time is an estimated difference between when the solution was added to the sample cell and the appearance of the expected event.
smFRET data acquisition and analysis
For single-molecule measurements, an Olympus IX-70 or IX-71 microscope with a UPlanApo 60x/1.20w objective lens was used. A 532nm laser (CrystaLaser or Meshtel) was used for excitation of Cy3. Total internal reflection was obtained by using prism-type TIR (Cornish and Ha, 2007). The fluorescence emission was split in two (Cy3, Cy5 emission) with a 630nm dichroic mirror (Chroma). For visualizing the fluorescence signal, an Andor iXon or iXon+ EMCCD camera was used. Various optical components were purchased from Thorlabs, Newport, or Edmund Optics.
TIRF movies were obtained using in-house software. For all constructs, the time binning of the data was set at 100ms with some of the data acquired for histogram analysis at 200 ms. The resultant image files were processed using IDL software and analyzed using Matlab. Time traces were selected from the data set by choosing only traces that contained single photobleaching steps for Cy3 and Cy5. Thus, data that contains multiple dyes or only a single donor were not included in subsequent analysis. Furthermore, fluorescence blinking events, although extremely rare, were removed by truncating the traces prior to the blinking event. Histograms were created from the selected traces and smoothed with a 5 point window. Histograms were fit to Gaussian distributions using Origin. The peak position was left unrestrained. Minor deviations in peak position was observed for complexes that existed predominately in one state and therefore represents an inability to accurately fit this small portion of the distribution as opposed to an actual change in the FRET value. For figure 4 and figure S3 the data were best fit to two Gaussians in which the resultant widths were self-consistent and the residuals were random except for figure S3 panels b and c which were best fit to a single Gaussian. HaMMy was used for Hidden Markov Modeling (HMM) analysis of the FRET data (McKinney et al., 2006). A total of 572, 612, and 385 molecules were analyzed for S6-Cy5/L9-Cy3 ribosomes containing tRNAfMet, tRNAfMet/N-Ac-Phe-tRNAPhe, and tRNAMet, respectively. Fitting to three FRET states resulted in just two observable FRET states with a total of 19808, 18451, and 6498 transitions, respectively. Kinetic rates were determined by transition density plot analysis (McKinney et al., 2006). The data were fit to three FRET states, which is more than the expected FRET states, to determine if hidden intermediate states existed. The data were also fit to two and five different states each resulting in the same two states as observed in a transition density plot (data not shown). Markov analysis was not performed on the remainder of the data since the time traces failed to contain at least 5 transitions in 50% of the total data set. To estimate the kinetic rates for all of other complexes one of the following two methods was performed. In the first, the kinetic rates were calculated by dividing the total number of transitions from one state to another by the sum of the dwell times from which the transitions originated ( k =# transitions1→2/∑τ1, where τ is the dwell time in state 1). This analysis was performed on the vacant, ASL, and acyl-tRNA containing ribosomes including these constructs in the presence of EF-G·GDPNP. Also, this analysis was performed on the rotated to non-rotated state transition for all constructs that had less than 15% of the data in the non-rotated state (Table 2). To calculate the non-rotated to rotated state transitions for these complexes a second method was employed. For this method, the dwell times in the non-rotated state were tabulated, plotted as a histogram, and fit to an exponential decay (Figure S5). This dwell time analysis was also performed on the constructs analyzed by HMM analysis (Figure S3). For several constructs, kinetic rates could not be determined since it was determined that the rate was on the order of or faster than the time resolution of the experiments.
Supplementary Material
Acknowledgments
These studies were supported by grant no. GM-17129 from the NIH to H.F.N., an NSF CAREER award to T.H, postdoctoral fellowship PF-07-123-01-GMC from the American Cancer Society to P.V.C., and a NATO-NSF postdoctoral fellowship to D.N.E. The authors thank Robyn Hickerson for small ribosomal subunit proteins used in reconstitution; Robyn Hickerson, Zigurts Majumdar and Michelle Nahas for their early contributions to the project and Andrei Korostelev and Laura Lancaster for discussions. T.H. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Amand B, Pochon F, Lavalette D. Rotational diffusion of Escherichia coli ribosomes. I. - Free 70 S, 50 S and 30 S particles. Biochimie. 1977;59:779–784. doi: 10.1016/s0300-9084(77)80207-1. [DOI] [PubMed] [Google Scholar]
- Astumian RD. Thermodynamics and kinetics of a Brownian motor. Science. 1997;276:917–922. doi: 10.1126/science.276.5314.917. [DOI] [PubMed] [Google Scholar]
- Blanchard SC, Gonzalez RL, Kim HD, Chu S, Puglisi JD. tRNA selection and kinetic proofreading in translation. Nat Struct Mol Biol. 2004a;11:1008–1014. doi: 10.1038/nsmb831. [DOI] [PubMed] [Google Scholar]
- Blanchard SC, Kim HD, Gonzalez RL, Jr, Puglisi JD, Chu S. tRNA dynamics on the ribosome during translation. Proceedings of the National Academy of Sciences of the United States of America. 2004b;101:12893–12898. doi: 10.1073/pnas.0403884101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordova NJ, Ermentrout B, Oster GF. Dynamics of single-motor molecules: the thermal ratchet model. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:339–343. doi: 10.1073/pnas.89.1.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish PV, Ha T. A survey of single-molecule techniques in chemical biology. ACS chemical biology. 2007;2:53–61. doi: 10.1021/cb600342a. [DOI] [PubMed] [Google Scholar]
- Cukras AR, Southworth DR, Brunelle JL, Culver GM, Green R. Ribosomal proteins S12 and S13 function as control elements for translocation of the mRNA:tRNA complex. Mol Cell. 2003;12:321–328. doi: 10.1016/s1097-2765(03)00275-2. [DOI] [PubMed] [Google Scholar]
- Culver GM, Noller HF. Efficient reconstitution of functional Escherichia coli 30S ribosomal subunits from a complete set of recombinant small subunit ribosomal proteins. RNA. 1999;5:832–843. doi: 10.1017/s1355838299990714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan M, Deniz AA, Ha T, Chemla DS, Schultz PG, Weiss S. Ratiometric measurement and identification of single diffusing molecules. Chem Phys. 1999;247:85–106. [Google Scholar]
- Dorner S, Brunelle JL, Sharma D, Green R. The hybrid state of tRNA binding is an authentic translation elongation intermediate. Nat Struct Mol Biol. 2006;13:234–241. doi: 10.1038/nsmb1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermolenko DN, Majumdar ZK, Hickerson RP, Spiegel PC, Clegg RM, Noller HF. Observation of Intersubunit Movement of the Ribosome in Solution Using FRET. J Mol Biol. 2007a;370:530–540. doi: 10.1016/j.jmb.2007.04.042. [DOI] [PubMed] [Google Scholar]
- Ermolenko DN, Spiegel PC, Majumdar ZK, Hickerson RP, Clegg RM, Noller HF. The antibiotic viomycin traps the ribosome in an intermediate state of translocation. Nat Struct Mol Biol. 2007b;14:493–497. doi: 10.1038/nsmb1243. [DOI] [PubMed] [Google Scholar]
- Frank J, Agrawal RK. A ratchet-like inter-subunit reorganization of the ribosome during translocation. Nature. 2000;406:318–322. doi: 10.1038/35018597. [DOI] [PubMed] [Google Scholar]
- Frank J, Gao H, Sengupta J, Gao N, Taylor DJ. The process of mRNA-tRNA translocation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19671–19678. doi: 10.1073/pnas.0708517104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrick K, Noller HF. Accurate translocation of mRNA by the ribosome requires a peptidyl group or its analog on the tRNA moving into the 30S P site. Mol Cell. 2002;9:1125–1131. doi: 10.1016/s1097-2765(02)00523-3. [DOI] [PubMed] [Google Scholar]
- Fredrick K, Noller HF. Catalysis of ribosomal translocation by sparsomycin. Science. 2003;300:1159–1162. doi: 10.1126/science.1084571. [DOI] [PubMed] [Google Scholar]
- Gao H, Sengupta J, Valle M, Korostelev A, Eswar N, Stagg SM, Van Roey P, Agrawal RK, Harvey SC, Sali A, et al. Study of the structural dynamics of the E coli 70S ribosome using real-space refinement. Cell. 2003;113:789–801. doi: 10.1016/s0092-8674(03)00427-6. [DOI] [PubMed] [Google Scholar]
- Gao H, Valle M, Ehrenberg M, Frank J. Dynamics of EF-G interaction with the ribosome explored by classification of a heterogeneous cryo-EM dataset. J Struct Biol. 2004;147:283–290. doi: 10.1016/j.jsb.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Gavrilova LP, Kostiashkina OE, Koteliansky VE, Rutkevitch NM, Spirin AS. Factor-free ("non-enzymic") and factor-dependent systems of translation of polyuridylic acid by Escherichia coli ribosomes. J Mol Biol. 1976;101:537–552. doi: 10.1016/0022-2836(76)90243-6. [DOI] [PubMed] [Google Scholar]
- Gavrilova LP, Spirin AS. Stimulation of "non-enzymic" translocation in ribosomes by p-chloromercuribenzoate. FEBS Lett. 1971;17:324–326. doi: 10.1016/0014-5793(71)80177-1. [DOI] [PubMed] [Google Scholar]
- Gonzalez RL, Jr, Chu S, Puglisi JD. Thiostrepton inhibition of tRNA delivery to the ribosome. Rna. 2007;13:2091–2097. doi: 10.1261/rna.499407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha T, Enderle T, Ogletree DF, Chemla DS, Selvin PR, Weiss S. Probing the interaction between two single molecules: fluorescence resonance energy transfer between a single donor and a single acceptor. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:6264–6268. doi: 10.1073/pnas.93.13.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickerson R, Majumdar ZK, Baucom A, Clegg RM, Noller HF. Measurement of internal movements within the 30 S ribosomal subunit using Forster resonance energy transfer. J Mol Biol. 2005;354:459–472. doi: 10.1016/j.jmb.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Horan LH, Noller HF. Intersubunit movement is required for ribosomal translocation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:4881–4885. doi: 10.1073/pnas.0700762104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HD, Puglisi J, Chu S. Fluctuations of tRNAs between classical and hybrid states. Biophys J. 2007;104:13661–13665. doi: 10.1529/biophysj.107.109884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konevega AL, Fischer N, Semenkov YP, Stark H, Wintermeyer W, Rodnina MV. Spontaneous reverse movement of mRNA-bound tRNA through the ribosome. Nat Struct Mol Biol. 2007;14:318–324. doi: 10.1038/nsmb1221. [DOI] [PubMed] [Google Scholar]
- Korostelev A, Trakhanov S, Laurberg M, Noller HF. Crystal structure of a 70S ribosome-tRNA complex reveals functional interactions and rearrangements. Cell. 2006;126:1065–1077. doi: 10.1016/j.cell.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Lee TH, Blanchard SC, Kim HD, Puglisi JD, Chu S. The role of fluctuations in tRNA selection by the ribosome. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13661–13665. doi: 10.1073/pnas.0705988104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman KR, Firpo MA, Herr AJ, Nguyenle T, Atkins JF, Gesteland RF, Noller HF. The 23 S rRNA environment of ribosomal protein L9 in the 50 S ribosomal subunit. J Mol Biol. 2000;297:1129–1143. doi: 10.1006/jmbi.2000.3621. [DOI] [PubMed] [Google Scholar]
- Lill R, Robertson JM, Wintermeyer W. Affinities of tRNA binding sites of ribosomes from Escherichia coli. Biochemistry. 1986;25:3245–3255. doi: 10.1021/bi00359a025. [DOI] [PubMed] [Google Scholar]
- McKinney SA, Joo C, Ha T. Analysis of single-molecule FRET trajectories using hidden Markov modeling. Biophys J. 2006;91:1941–1951. doi: 10.1529/biophysj.106.082487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazed D, Noller HF. Interaction of tRNA with 23S rRNA in the ribosomal A, P, and E sites. Cell. 1989a;57:585–597. doi: 10.1016/0092-8674(89)90128-1. [DOI] [PubMed] [Google Scholar]
- Moazed D, Noller HF. Intermediate states in the movement of transfer RNA in the ribosome. Nature. 1989b;342:142–148. doi: 10.1038/342142a0. [DOI] [PubMed] [Google Scholar]
- Munro JB, Altman RB, O'Connor N, Blanchard SC. Identification of two distinct hybrid state intermediates on the ribosome. Mol Cell. 2007;25:505–517. doi: 10.1016/j.molcel.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noller HF, Yusupov MM, Yusupova GZ, Baucom A, Cate JH. Translocation of tRNA during protein synthesis. FEBS Lett. 2002;514:11–16. doi: 10.1016/s0014-5793(02)02327-x. [DOI] [PubMed] [Google Scholar]
- Pan D, Kirillov SV, Cooperman BS. Kinetically competent intermediates in the translocation step of protein synthesis. Mol Cell. 2007;25:519–529. doi: 10.1016/j.molcel.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S. Studies on the formation of transfer ribonucleic acid-ribosome complexes. VI. Oligopeptide synthesis and translocation on ribosomes in the presence and absence of soluble transfer factors. J Biol Chem. 1969;244:1533–1539. [PubMed] [Google Scholar]
- Rasnik I, McKinney SA, Ha T. Nonblinking and long-lasting single-molecule fluorescence imaging. Nature methods. 2006;3:891–893. doi: 10.1038/nmeth934. [DOI] [PubMed] [Google Scholar]
- Scheres SH, Gao H, Valle M, Herman GT, Eggermont PP, Frank J, Carazo JM. Disentangling conformational states of macromolecules in 3D-EM through likelihood optimization. Nature methods. 2007;4:27–29. doi: 10.1038/nmeth992. [DOI] [PubMed] [Google Scholar]
- Selmer M, Dunham CM, Murphy FVt, Weixlbaumer A, Petry S, Kelley AC, Weir JR, Ramakrishnan V. Structure of the 70S ribosome complexed with mRNA and tRNA. Science. 2006;313:1935–1942. doi: 10.1126/science.1131127. [DOI] [PubMed] [Google Scholar]
- Sharma D, Southworth DR, Green R. EF-G-independent reactivity of a pre-translocation-state ribosome complex with the aminoacyl tRNA substrate puromycin supports an intermediate (hybrid) state of tRNA binding. RNA. 2004;10:102–113. doi: 10.1261/rna.5148704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji S, Walker SE, Fredrick K. Reverse translocation of tRNA in the ribosome. Mol Cell. 2006;24:931–942. doi: 10.1016/j.molcel.2006.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel PC, Ermolenko DN, Noller HF. Elongation factor G stabilizes the hybrid-state conformation of the 70S ribosome. RNA. 2007;13:1473–1482. doi: 10.1261/rna.601507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spirin AS. The ribosome as an RNA-based molecular machine. RNA Biol. 2004;1:3–9. [PubMed] [Google Scholar]
- Studer SM, Feinberg JS, Joseph S. Rapid kinetic analysis of EF-G-dependent mRNA translocation in the ribosome. J Mol Biol. 2003;327:369–381. doi: 10.1016/s0022-2836(03)00146-3. [DOI] [PubMed] [Google Scholar]
- Tama F, Valle M, Frank J, Brooks CL., 3rd Dynamic reorganization of the functionally active ribosome explored by normal mode analysis and cryo-electron microscopy. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9319–9323. doi: 10.1073/pnas.1632476100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle M, Zavialov A, Sengupta J, Rawat U, Ehrenberg M, Frank J. Locking and unlocking of ribosomal motions. Cell. 2003;114:123–134. doi: 10.1016/s0092-8674(03)00476-8. [DOI] [PubMed] [Google Scholar]
- Wang Y, Qin H, Kudaravalli RD, Kirillov SV, Dempsey GT, Pan D, Cooperman BS, Goldman YE. Single-molecule structural dynamics of EF-G--ribosome interaction during translocation. Biochemistry. 2007;46:10767–10775. doi: 10.1021/bi700657d. [DOI] [PubMed] [Google Scholar]
- Wilson KS, Noller HF. Mapping the position of translational elongation factor EF-G in the ribosome by directed hydroxyl radical probing. Cell. 1998;92:131–139. doi: 10.1016/s0092-8674(00)80905-8. [DOI] [PubMed] [Google Scholar]
- Zavialov AV, Ehrenberg M. Peptidyl-tRNA regulates the GTPase activity of translation factors. Cell. 2003;114:113–122. doi: 10.1016/s0092-8674(03)00478-1. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Bartley LE, Babcock HP, Russell R, Ha T, Herschlag D, Chu S. A single-molecule study of RNA catalysis and folding. Science. 2000;288:2048–2051. doi: 10.1126/science.288.5473.2048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


