Abstract
Diet-induced obesity is associated with fatty liver, insulin resistance, leptin resistance, and changes in plasma lipid profile. Endocannabinoids have been implicated in the development of these associated phenotypes, because mice deficient for the cannabinoid receptor CB1 (CB1–/–) do not display these changes in association with diet-induced obesity. The target tissues that mediate these effects, however, remain unknown. We therefore investigated the relative role of hepatic versus extrahepatic CB1 receptors in the metabolic consequences of a high-fat diet, using liver-specific CB1 knockout (LCB1–/–) mice. LCB1–/– mice fed a high-fat diet developed a similar degree of obesity as that of wild-type mice, but, similar to CB1–/– mice, had less steatosis, hyperglycemia, dyslipidemia, and insulin and leptin resistance than did wild-type mice fed a high-fat diet. CB1 agonist–induced increase in de novo hepatic lipogenesis and decrease in the activity of carnitine palmitoyltransferase–1 and total energy expenditure were absent in both CB1–/– and LCB1–/– mice. We conclude that endocannabinoid activation of hepatic CB1 receptors contributes to the diet-induced steatosis and associated hormonal and metabolic changes, but not to the increase in adiposity, observed with high-fat diet feeding. Theses studies suggest that peripheral CB1 receptors could be selectively targeted for the treatment of fatty liver, impaired glucose homeostasis, and dyslipidemia in order to minimize the neuropsychiatric side effects of nonselective CB1 blockade during treatment of obesity-associated conditions.
Introduction
Endocannabinoids increase food intake through their interaction with the leptin-regulated central neural appetitive circuitry (1, 2), and pharmacological blockade of CB1 receptors has shown promise in the treatment of visceral obesity and the metabolic syndrome (3–5). Mice exposed to high-fat diets develop obesity, steatosis, and insulin and leptin resistance as well as plasma lipid changes similar to those associated with the metabolic syndrome (6). Mice deficient in CB1 receptors have a lean phenotype (7) and are resistant to these diet-induced changes, even though their total caloric intake is not different from that of wild-type mice fed the same diet (8, 9). Similarly, chronic treatment of obese mice or rats with a CB1 antagonist induces sustained weight loss and protects rodents from this detrimental metabolic phenotype, even though it causes only a transient reduction in food intake (10–13). These findings indicate that endocannabinoids can directly affect peripheral energy metabolism by mechanisms unrelated to their effect on appetite. We recently reported that activation of CB1 receptors in wild-type mice stimulates de novo lipogenesis in the liver through induction of the lipogenic transcription factor SREBP1c and its target enzymes acetyl-CoA carboxylase–1 (ACC1) and fatty acid synthase (FAS) (9). Furthermore, a high-fat diet increased the hepatic levels of the endocannabinoid anandamide and CB1 receptors as well as the basal rate of de novo lipogenesis, and the latter could be attenuated by CB1 blockade (9). Although these findings strongly implicate hepatic CB1 receptors in the development of diet-induced obesity and related metabolic changes, they do not exclude the alternative possibility that endocannabinoids act on CB1 receptors in the CNS to influence peripheral energy metabolism indirectly or produce their effects via CB1 receptors at extrahepatic sites, such as the adipose tissue (14). For example, centrally acting leptin was previously shown to affect hepatic glucose fluxes (15) and hepatic lipid metabolism (16), possibly through neural input to the liver. Moreover, communication between the liver and adipose tissue may occur through neural pathways involving hepatic vagal afferents (17). In order to more directly test the role of hepatic CB1 receptors in diet-induced metabolic changes, we generated a mouse model with a hepatocyte-selective deletion of CB1 receptors and compared the metabolic effects of a high-fat diet as well as the effects of cannabinoid agonist and antagonist treatment with those observed in wild-type mice and in mice with global knockout of CB1 receptors.
The resistance of CB1 receptor–deficient mice to diet-induced obesity and steatosis despite their similar caloric intake indicates enhanced energy expenditure and fat elimination, but the mechanism by which CB1 receptor deficiency or blockade increases energy expenditure is not yet clear. Therefore, we also investigated the effects of pharmacological activation, inhibition, or genetic ablation of CB1 receptors on the gene expression and enzymatic activity of carnitine palmitoyltransferase–1 (CPT1), the rate-limiting enzyme in fatty acid β-oxidation. Overall, our results indicated that endocannabinoids acting at hepatic CB1 receptors mediated diet-induced steatosis through increasing de novo lipogenesis and inhibiting fatty acid oxidation in the liver. Endocannabinoids also played an essential role in diet-induced changes in plasma lipid profile and insulin and leptin resistance, but did not contribute to the parallel increase in fat deposition in adipose tissue and the resulting diet-induced obesity.
Results
High-fat diet feeding upregulates CB1 receptors in hepatocytes.
We previously demonstrated increased expression of CB1 receptors in whole liver tissue of mice fed a high-fat diet for 3 weeks (9). To further define the cellular localization of CB1 receptors regulated by the diet, we used Western blotting to analyze CB1 receptor protein levels in isolated pure fractions of hepatocytes from wild-type mice fed regular chow or high-fat diet for 3 weeks. As shown in Figure 1A, there was a marked increase in the level of CB1 receptor protein in cells from the latter group compared with controls.
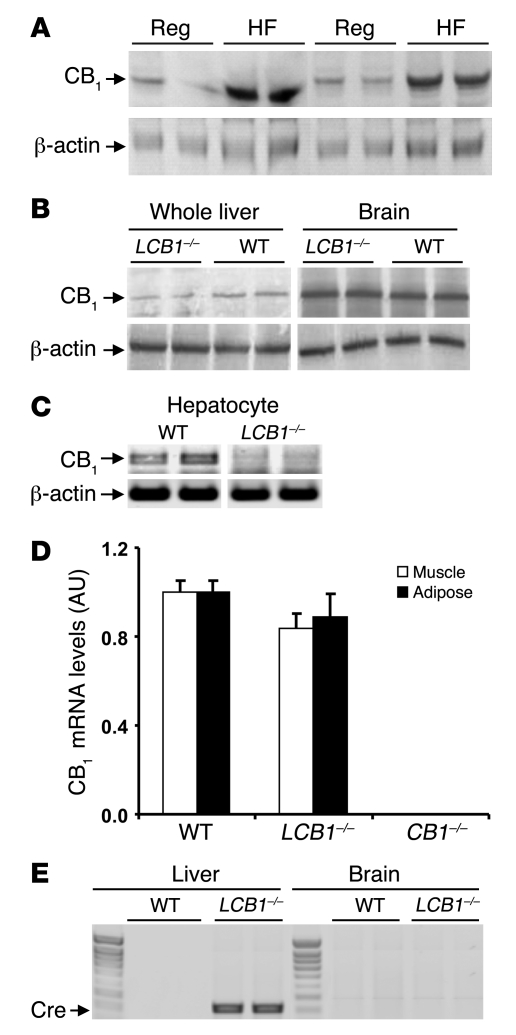
Figure 1. Upregulation of CB1 receptors by high-fat diet and selective deletion of CB1 receptors by hepatocyte-specific gene knockout in mouse hepatocytes.
(A) Short-term exposure to high-fat diet upregulates CB1 receptors in mouse hepatocytes. Mice were fed regular mouse chow (Reg) or a high-fat diet (HF) for 3 weeks, starting at 6–8 weeks of age. Hepatocytes from 4 mice per group were isolated, and cellular proteins were solubilized and fractionated by gel electrophoresis. CB1 receptor protein was visualized by Western blotting and quantified by densitometry (regular chow, 0.82 ± 0.09 AU; high-fat diet, 1.99 ± 0.41 AU; P < 0.05). Immunoblotting for β-actin was used as loading control. (B) CB1 receptor levels were normal in brain and greatly reduced in whole liver tissue from LCB1–/– mice compared with wild-type mice. Results of Western blots with 2 animals per group are shown. (C) CB1 receptor mRNA was absent in purified hepatocytes from LCB1–/– mice. Receptor mRNA was amplified using RT-PCR, with parallel amplification of β-actin mRNA as control. (D) Normal levels of CB1 receptor mRNA in adipose tissue and skeletal muscle of LCB1–/– mice. Receptor mRNA was quantified by real-time PCR from adipose tissue and soleus muscle of floxed/floxed, LCB1–/–, and CB1–/– mice. (E) Selective expression of cre in the liver of LCB1–/– mice. Cre mRNA expression was analyzed by RT-PCR in brain and liver of a wild-type mouse and an LCB1–/– mouse.
Liver-specific CB1 knockout mice are susceptible to diet-induced obesity.
Liver-specific CB1 knockout (LCB1–/–) mice lack CB1 receptors in hepatocytes, but have normal levels of CB1 receptors elsewhere in the body. Their phenotype is unremarkable; liver weight and the macroscopic and microscopic appearance of the liver are normal. As illustrated in Figure 1B, the level of CB1 receptor protein was similar in the brains of wild-type and LCB1–/– mice, whereas in the liver, where CB1 receptors are expressed at much lower levels than in the brain, there was a faint band detectable in extracts from LCB1–/– mice, probably representing CB1 receptors in nonhepatocyte liver cells. The complete lack of CB1 receptor expression in purified hepatocytes from LCB1–/– mice is illustrated in Figure 1C by the absence of CB1 mRNA, as documented by RT-PCR. In contrast, levels of CB1 receptor mRNA in adipose tissue and skeletal muscle were similar in floxed/floxed controls and LCB1–/– mice, as determined by real-time quantitative PCR (Figure 1D). The selectivity of the knockout for the LCB1–/– liver was further supported by the selective expression of Cre in the livers but not the brains of LCB1–/– mice, as documented by RT-PCR (Figure 1E).
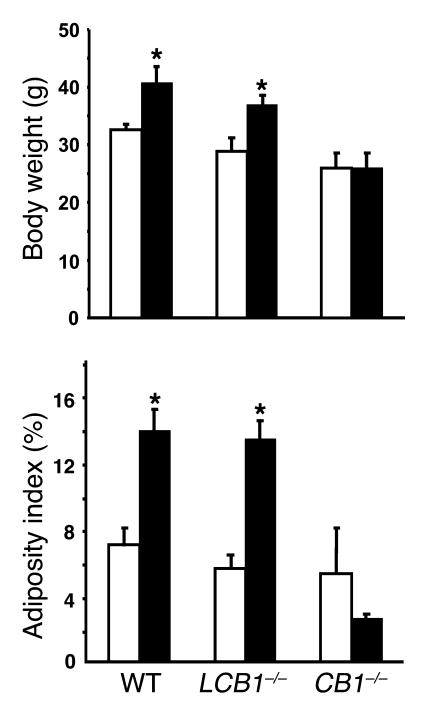
When maintained on a high-fat diet for 14 weeks, LCB1–/– mice developed obesity similar to their CB1 floxed/floxed control littermates, whereas mice with global CB1 receptor knockout (CB1–/– mice) were resistant to obesity when exposed to the same diet (Figure 2), in agreement with previous reports (8, 9). Total caloric intake over the 14-week period was not different among the 3 groups. The diet-induced increase in body weight in both control and LCB1–/– mice was associated with increased fat accumulation in peripheral adipose tissue, as indicated by the marked increase in adiposity index in both strains, whereas there were no significant changes in either parameter in the CB1–/– mice fed the high-fat diet (Figure 2).
Figure 2. LCB1–/– mice are susceptible to diet-induced obesity.
Male and female 6- to 8-week-old wild-type, CB1–/–, and LCB1–/– mice were fed regular chow (white bars) or high-fat diet (black bars) for 14 weeks, as described in Methods. Body weight and adiposity index at the end of the 14-week period are shown (mean ± SEM; n = 8–12 per group). Adiposity index was determined as the percentage of the combined weight of inguinal, retroperitoneal, epididymal, and subcutaneous fat pads relative to body weight. *P < 0.05 versus corresponding group fed regular chow.
LCB1–/– mice are resistant to diet-induced steatosis.
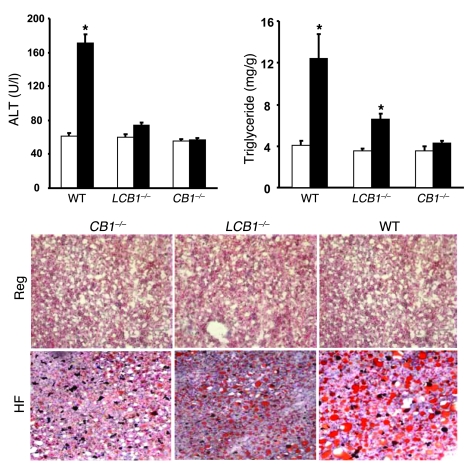
High-fat diet feeding also induced significant steatosis in wild-type mice, as documented histologically in Oil Red O–stained liver sections and by the significant increase in hepatic triglyceride content (Figure 3). These changes were associated with increased plasma alanine aminotransferase (ALT) levels, indicating hepatocellular damage. In contrast, CB1–/– mice were resistant to the diet-induced steatosis and hepatocellular damage. Although LCB1–/– mice on the high-fat diet did have somewhat more fat in their livers than did CB1–/– mice, their steatosis was significantly less than that observed in wild-type mice despite their similar susceptibility to diet-induced peripheral fat accumulation (Figure 3).
Figure 3. LCB1–/– mice are resistant to diet-induced steatosis.
Steatosis was assessed in the same groups of mice as in Figure 2 by measurement of hepatic triglyceride content of mice fed regular chow (white bars) or high-fat diet (black bars), and histologically by Oil Red O staining (representative sections). Hepatocellular damage was assessed by plasma ALT levels. *P < 0.05 versus corresponding group fed regular chow. n = 8 per group.
LCB1–/– mice are resistant to diet-induced changes in plasma lipid profile as well as leptin and insulin levels.
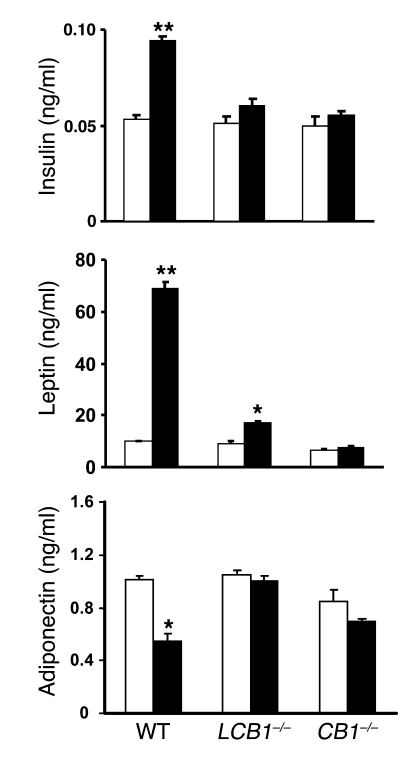
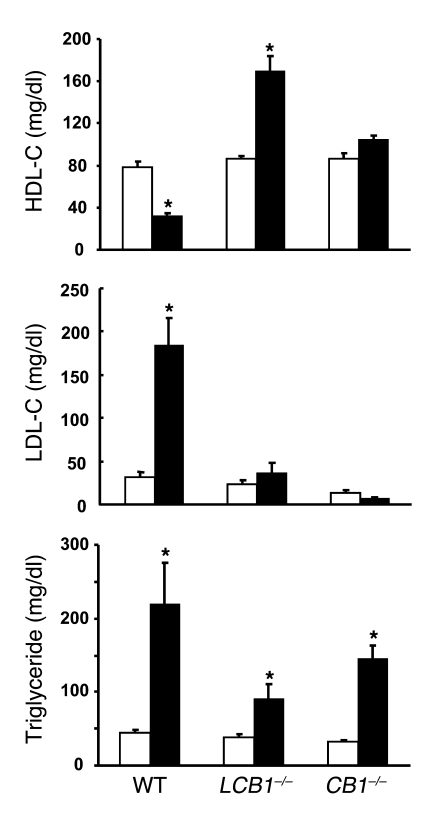
Obesity induced by high-fat diets in mice is associated with changes characteristic of the metabolic syndrome, i.e., altered plasma lipid profile as well as elevated serum insulin and leptin levels that most likely reflect insulin and leptin resistance (18). As illustrated in Figure 4, wild-type mice fed the high-fat diet developed hyperinsulinemia and hyperleptinemia, whereas their plasma adiponectin levels declined by nearly 50%. Strikingly, mice with either global or hepatocellular knockout of CB1 receptors displayed no significant changes in plasma insulin and adiponectin, whereas the hyperleptinemia was completely absent in CB1–/– mice and markedly reduced in LCB1–/– mice. Because the diet-induced obesity was similar in wild-type and LCB1–/– mice, these findings suggest that LCB1–/– mice are less leptin resistant than wild-type mice. This was tested by analyzing the anorexic effect of 200 μg leptin following a 24-h fast. In wild-type mice on regular diet, leptin reduced food intake by 38% ± 4% relative to vehicle treatment (2.54 versus 1.57 g/mouse, n = 4), whereas in wild-type mice fed the high-fat diet, leptin reduced food intake by 23% ± 5% (7.86 versus 6.07 g/mouse, n = 4; P < 0.05). In contrast, in LCB1–/– mice the leptin-induced reduction in food intake was similar with regular chow (37% ± 6%, from 2.18 to 1.37 g/mouse; n = 5) and high-fat diet (46% ± 5%, from 5.38 to 2.93 g/mouse; n = 5) feeding. Similarly, the marked increase in LDL cholesterol levels and parallel decrease in plasma HDL cholesterol detected in wild-type mice fed the high-fat diet versus regular chow were absent in both CB1 knockout strains, whereas the diet-induced hypertriglyceridemia remained statistically significant, suggesting the involvement of CB1-independent mechanisms in this latter parameter (Figure 5).
Figure 4. High-fat diet–induced hyperleptinemia, hyperinsulinemia, and decreased serum adiponectin levels are absent in CB1–/– and LCB1–/– mice fed the same high-fat diet.
Mice were fed regular chow (white bars) or a high-fat diet (black bars) for 14 weeks, at which time they were sacrificed and serum hormone levels were determined from trunk blood. *P < 0.05, **P < 0.01 versus corresponding group fed regular chow. n = 6–8 per group.
Figure 5. The high-fat diet–induced increases in serum triglyceride and LDL cholesterol (LDL-C) and decrease in HDL cholesterol (HDL-C) levels in wild-type mice are absent or attenuated in CB1–/– or LCB1–/– mice.
Serum lipid profile was determined in the same groups of mice as in Figure 4. White bars, regular chow; black bars, high-fat diet. *P < 0.05 versus corresponding control.
Hepatic CB1 receptors are involved in high-fat diet–induced glucose intolerance and insulin resistance.
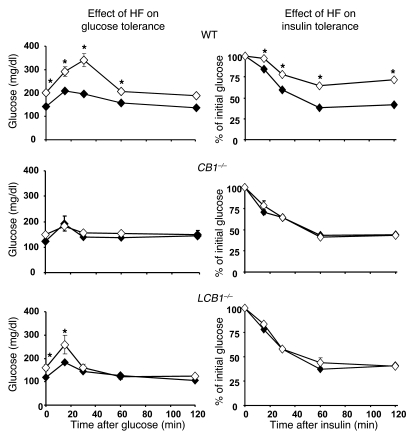
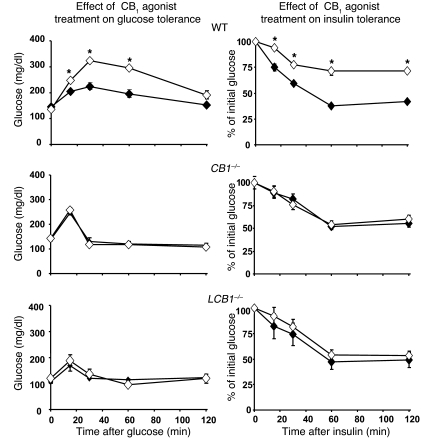
Wild-type mice fed the high-fat diet developed fasting hyperglycemia as well as glucose intolerance and insulin resistance, as reflected in the results of i.p. glucose tolerance testing followed by insulin tolerance testing (Figure 6). In contrast, high-fat diet feeding did not worsen glucose tolerance and insulin sensitivity in CB1–/– mice, which remained normoglycemic, and had a minor effect in LCB1–/– mice, which displayed a moderate elevation of baseline blood glucose (Figure 6). Because these findings suggested that CB1 receptor activation induces glucose intolerance and insulin resistance, we tested the effect of acute treatment of mice with a CB1 agonist on these parameters. As illustrated in Figure 7, i.p. injection of 20 ng/g HU-210 in wild-type mice resulted in glucose intolerance as well as decreased insulin sensitivity, whereas the agonist had no significant effect on these parameters in either CB1–/– or LCB1–/– mice.
Figure 6. High-fat diet–induced glucose intolerance and insulin resistance in wild-type mice, as revealed by intraperitoneal glucose tolerance and insulin resistance tests, is absent in CB1–/– mice and attenuated in LCB1–/– mice.
Filled symbols, regular chow; open symbols, high-fat diet. Values are mean ± SEM from 4 mice (for points without error bars, the SEM is within the size of the symbol). *P < 0.05 versus corresponding group fed regular chow.
Figure 7. Acute treatment of regular chow–fed mice with a CB1 agonist induces glucose intolerance and insulin resistance in wild-type mice, but not in CB1–/– or LCB1–/– mice.
Mice were injected with vehicle (filled symbols) or 20 ng/g HU-210 i.p. (open symbols) 10 min prior to the i.p. injection of glucose. Values are mean ± SEM from 4 mice (for points without error bars, the SEM is within the size of the symbol). *P < 0.05 versus corresponding vehicle-treated control.
Activation of hepatic CB1 receptors increases de novo lipogenesis.
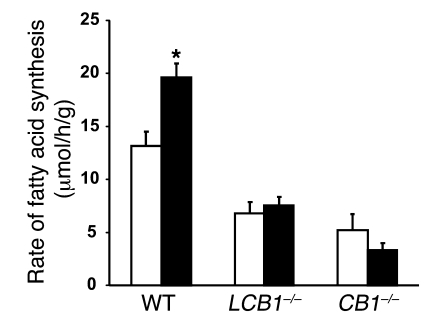
We previously reported that treatment of wild-type mice with the potent synthetic cannabinoid HU-210 (20 ng/g i.p.) increased the incorporation of 3H20 into hepatic fatty acids, a measure of de novo lipogenesis. This effect was inhibited by pretreatment of mice with the CB1 antagonist rimonabant and was absent in CB1–/– mice, confirming the role of CB1 receptors (9). In order to distinguish between a direct hepatic effect and an indirect, centrally mediated effect, we measured de novo hepatic lipogenesis in LCB1–/– mice. As illustrated in Figure 8, the basal rate of de novo lipogenesis was lower in the livers of LCB1–/– mice than in their controls and remained unaffected by HU-210 treatment, similar to our observations in CB1–/– mice. This finding confirms the role of hepatic CB1 receptors in this effect.
Figure 8. Effects of a CB1 agonist on the rate of de novo hepatic lipogenesis in mice fed regular diet.
Mice were injected with vehicle (white bars) or 20 ng/g HU-210 i.p. (black bars), and de novo lipogenesis was measured by the rate of incorporation of 3H2O into hepatic fatty acids, as described in Methods. Note that HU-210 increased lipogenesis in wild-type mice, but not in CB1–/– or LCB1–/– mice. *P < 0.05 versus corresponding vehicle-treated control.
CB1 regulation of hepatic fatty acid oxidation.
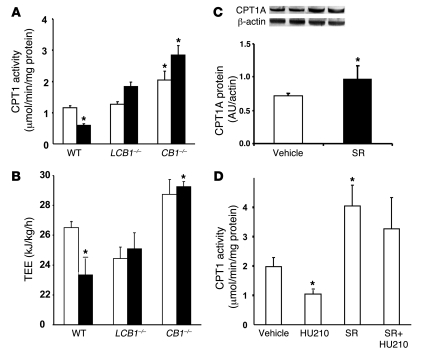
In wild-type mice with high-fat diet–induced obesity, the activity of hepatic CPT1, the rate-limiting enzyme in fatty acid β-oxidation, was significantly reduced compared with regular chow–fed controls (Figure 9A), in agreement with previously published observations (19). However, high-fat diet feeding failed to reduce CPT1 activity in either CB1–/– or LCB1–/– mice, which may account for their resistance to diet-induced steatosis. These changes in enzyme activity were likely caused, at least in part, by parallel changes in CPT1 gene expression: CPT1 mRNA levels were markedly reduced in wild-type mice fed the high-fat diet versus regular diet, whereas in CB1–/– mice basal CPT1 mRNA levels tended to be higher than in wild-type mice and were unaffected by the high-fat diet, as detected by real-time PCR (data not shown). Furthermore, chronic treatment of high-fat diet–fed mice with 3 μg/g/d rimonabant for 7 d resulted in elevated protein levels of CPT1A, the hepatic isoform of CPT1, as documented by Western blotting (Figure 9C). Together, these findings suggest that, similar to the involvement of CB1 receptors in the diet-induced increase in de novo lipogenesis (Figure 8 and ref. 9), the accompanying decrease in fatty acid oxidation is also CB1 receptor mediated. To further test this, we examined the effects of pharmacological activation and/or inhibition of CB1 receptors on hepatic CPT1 activity. Acute treatment of wild-type mice with 20 ng/g HU-210 i.p. decreased hepatic CPT1 activity, and this effect was inhibited by pretreatment with 3 μg/g rimonabant i.p. Treatment with rimonabant alone significantly increased CPT1 activity and prevented the inhibitory effect of subsequently administered HU-210 (Figure 9D).
Figure 9. CB1 receptor regulation of hepatic fatty acid oxidation.
(A) Effect of diet on hepatic CPT1 activity. Mice fed regular chow (white bars) or high-fat diet for 14 weeks (black bars) were sacrificed, their livers were removed, and mitochondria were isolated for measurement of hepatic CPT1 activity, as described in Methods. (B) TEE of wild-type, CB1–/–, and LCB1–/– mice fed regular chow (white bars) or high-fat diet (black bars), measured by indirect calorimetry (n = 4–7 per group). (C) Chronic treatment of wild-type high-fat diet–fed mice with rimonabant (SR; 3 μg/g/d for 7 d, right 2 lanes) upregulated hepatic CPT1A protein levels, as shown by Western blotting. (D) Effect of the CB1 agonist HU-210, the CB1 antagonist rimonabant, or their combination on hepatic CPT1 activity in wild-type mice fed regular chow. n = 4 per group. *P < 0.05 versus regular chow–fed wild-type control or versus vehicle-treated control as appropriate.
CB1 regulation of energy expenditure and substrate use.
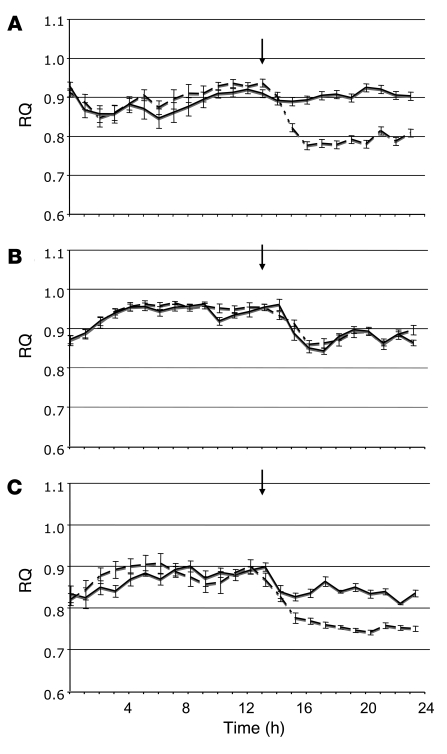
The CB1-mediated effects on hepatic fatty acid synthesis and oxidation suggest that endocannabinoids may also be involved in the control of substrate use and whole-body energy expenditure, which we examined using indirect calorimetry. Oxygen consumption (VO2) and carbon dioxide production (VCO2) were continuously monitored in individually housed mice. The mice had free access to regular chow and water during the dark phase, and food was removed at the beginning of the light phase (postprandial state), after i.p. injection of vehicle or 10 mg/kg rimonabant, so that secondary effects of changes in food intake could be excluded. As shown in Figure 10A, in wild-type mice rimonabant induced an immediate reduction in the respiratory quotient (RQ) that lasted up to 12 h, which indicates a shift from carbohydrate to lipid oxidation. A similar postprandial shift to lipid oxidation was apparent in vehicle-treated CB1–/– mice, in which rimonabant did not cause any further change in RQ (Figure 10B). Responses in LCB1–/– mice were intermediate between wild-type and CB1–/– mice, in that a small reduction in RQ in vehicle-treated mice was enhanced when the mice were treated with rimonabant (Figure 10C). These findings therefore indicate that pharmacological or genetic ablation of CB1 receptors results in increased lipid oxidation.
Figure 10. The effect of rimonabant on RQ in regular chow–fed mice, as monitored by indirect calorimetry.
In wild-type (A), CB1–/– (B), and LCB1–/– (C) mice, rimonabant (10 mg/kg i.p., dashed lines) or vehicle (solid lines) was injected at the end of the dark period (indicated by arrows), followed by removal of food from the cage. n = 8 per group. Values are mean ± SEM.
Total energy expenditure (TEE) was also calculated in mice fed either regular chow or the high-fat diet. As shown in Figure 9B, wild-type mice fed the high-fat diet had significantly lower TEE than did mice fed regular chow. TEE of CB1–/– mice fed regular chow was elevated compared with wild-type control mice and remained unchanged with high-fat diet feeding. The TEE of LCB1–/– mice fed regular chow was similar to that of wild-type mice, but, similar to CB1–/– mice, it remained unchanged with high-fat diet feeding. The pattern of diet- and strain-related differences in TEE was very similar to the differences in hepatic CPT1 activity shown in Figure 9A.
Discussion
The present findings delineate the unique contribution of the hepatic endocannabinoid/CB1 receptor system to specific components of the metabolic syndrome induced in mice by a high-fat diet. In contrast to the resistance of CB1–/– mice to high-fat diet–induced obesity (9, 10), LCB1–/– mice developed obesity similar to that of wild-type mice when maintained on a high-fat diet. This observation suggests that the well-documented ability of CB1 antagonist treatment to reduce fat mass in adipose tissue is mediated via CB1 receptors at extrahepatic sites. CB1 antagonists may act at receptors in the CNS to affect peripheral fat metabolism indirectly through neural or humoral signals (16), or their action may be the result of inhibition of CB1 receptors on adipocytes, which was previously shown to cause increased adiponectin production and release (20). Our present findings do not identify the specific site or sites of the antiobesity action of CB1 antagonism, which may be better defined through the targeted deletion of CB1 receptors at those locations.
In contrast to the obesity induced by high-fat diet feeding, the associated steatosis and hepatocellular damage were prevented by global absence — and significantly attenuated by hepatocyte-specific absence — of CB1 receptors, which indicates that activation of hepatic CB1 receptors is necessary to produce these latter effects but does not exclude the possibility that extrahepatic CB1 receptors may also contribute. Whether hepatic CB1 receptor activation is sufficient to cause steatosis and hormonal/metabolic changes remains to be determined.
The relevance of CB1-mediated hepatic steatosis to public health is illustrated by recent epidemiological findings that daily marijuana use predisposes to hepatic steatosis in subjects with hepatitis C viral infection (21). Chronic, heavy marijuana use has also been recently found to increase the plasma level of apolipoprotein C-III (22), a liver-derived plasma constituent that correlates with dyslipidemia and cardiovascular risk in the metabolic syndrome (23).
Because caloric intake on the high-fat diet was similar in wild-type and CB1–/– mice (9), the resistance of the latter to obesity means that these animals must have increased energy expenditure. Two lines of evidence support this conclusion. First, basal CPT1 expression and activity increased in CB1–/– mice compared with both wild-type and LCB1–/– mice, whereas the diet-induced suppression of CPT1 activity seen in controls was absent in both CB1–/– and LCB1–/– mice (Figure 9). Second, parallel changes in TEE were evident in vivo, as measured by indirect calorimetry. Again, TEE increased in CB1–/– mice versus wild-type and LCB1–/– mice, whereas the high-fat diet–induced suppression of TEE observed in wild-type mice was absent in both CB1–/– and LCB1–/– mice. This pattern of responses is compatible with the possibility that under control conditions, fatty acid oxidation and TEE are tonically suppressed by activation of mostly extrahepatic CB1 receptors and that the absence of this mechanism in CB1–/– mice accounts for their lean phenotype (7) as well as their resistance to diet-induced obesity (8, 9). On the other hand, activation of hepatic CB1 receptors substantially contributed to the ectopic accumulation of fat in the liver, which manifested in the diet-induced suppression of hepatic CPT1 activity and contributed to the decline in TEE in wild-type mice. Endocannabinoid activation of hepatic CB1 receptors may be a common mechanism of steatosis of different etiologies, as indicated by our recent finding that LCB1–/– mice are also resistant to fatty liver induced by chronic ethanol feeding (24).
The rimonabant-induced postprandial decrease in RQ in wild-type mice (Figure 10A), which is similar to the recently reported effect of rimonabant in rats (25), reflects increased lipid oxidation, the likely mechanism of both the antiobesity effect of rimonabant treatment (26) and its ability to reverse hepatic steatosis (12, 13). In CB1–/– mice, a postprandial decrease in RQ similar to that induced by rimonabant in wild-type mice was observed, and it remained unchanged following rimonabant treatment (Figure 10B), which further proves that the effect of rimonabant is caused by CB1 inactivation.
Interestingly, LCB1–/– mice not only are resistant to diet-induced steatosis, but also do not develop elevated LDL cholesterol and reduced HDL cholesterol levels, which are important cardiovascular risk factors of the metabolic syndrome (27). In humans, the role of CB1 receptors in the regulation of these parameters has been suggested by the results of several multicenter phase III studies, which documented substantial improvements of the plasma lipid profile of obese individuals chronically treated with rimonabant (3–5). The finding of a similar improved lipid profile in LCB1–/– mice compared with wild-type mice fed the high-fat diet implicates hepatic CB1 receptors in the regulation of these parameters. Further studies are required to define the mechanisms by which activation of hepatic CB1 receptors increases LDL cholesterol levels and decreases HDL cholesterol levels.
High-fat diets induce insulin and leptin resistance (18), which are predisposing factors for type II diabetes. Pharmacological activation of CB1 receptors was also previously reported to induce glucose intolerance both in humans (28) and in rats (29). A major finding of the present study is that deletion of hepatic CB1 receptors led to a dissociation of obesity from insulin and leptin resistance induced by a high-fat diet. The marked diet-induced elevation of plasma insulin and leptin levels (Figure 4) and the accompanying hyperglycemia in wild-type mice were significantly attenuated in LCB1–/– mice (Figure 6), which indicates that endocannabinoids acting at hepatic CB1 receptors contribute to high-fat diet–induced insulin and leptin resistance. The role of hepatic CB1 receptors in the diet-induced glucose intolerance and insulin resistance is further supported by the ability of CB1 agonist treatment to elicit similar changes in wild-type mice (Figure 7) and by the near absence of both the diet- and CB1 agonist–induced changes in LCB1–/– mice (Figures 6 and 7). Diet-induced insulin resistance involves multiple target organs, including adipose tissue, skeletal muscle, and liver, and complex interactions among the 3 that may involve neurogenic mechanisms (17) and/or circulating factors (30), which were not explored in the present experiments and will require further study. High-fat diet feeding in mice was previously reported to induce the expression of CB1 receptors in skeletal muscle (31), and CB1 blockade was shown to increase muscle insulin sensitivity in genetically obese mice (32). As-yet unidentified circulating factors may explain how endocannabinoid action in the liver may influence insulin sensitivity in muscle.
Leptin is an adipocyte-derived hormone, and its elevated levels in obesity may be a reflection of the increased fat mass. However, the increase in plasma leptin induced by the high-fat diet was significantly smaller in LCB1–/– than in wild-type mice, even though these 2 groups developed similar obesity. This means that the striking, approximately 7-fold increase in plasma leptin in wild-type mice must be predominantly related to leptin resistance, which was also reflected by the reduced anorexic effect of leptin in wild-type compared with LCB1–/– mice fed the high-fat diet. Multiple mechanisms have been invoked to account for the leptin resistance induced by high-fat diets (18, 33). Our earlier studies demonstrated that hypothalamic endocannabinoids are negatively regulated by leptin (1). The present findings point to the existence of a bidirectional relationship whereby endocannabinoids in turn induce leptin resistance. Further studies are needed to explore the mechanism by which hepatic CB1 receptor activation may have a permissive effect on the development of diet-induced leptin resistance.
The metabolic consequences of hepatic CB1 receptor inactivation or ablation raise the question of the nature and source of the endogenous ligand responsible for its tonic activation in obesity. Both anandamide and 2-arachidonoylglycerol (2-AG) are present in the liver at concentrations comparable to that in the brain (9, 24), and they have been detected in different types of liver cells, including hepatocytes and stellate cells (24, 34). A selective increase in hepatic anandamide levels, but not 2-AG levels, caused by high-fat diet could be attributed to the suppression of the hepatic activity of fatty acid amidohydrolase (9), the enzyme responsible for the in vivo degradation of anandamide. Anandamide may thus act as an autocrine ligand of hepatocyte CB1 receptors to induce lipogenesis and inhibit fatty acid oxidation. Interestingly, steatosis induced by chronic ethanol feeding resulted in a selective increase in 2-AG in hepatic stellate cells, and there is evidence for a CB1-mediated paracrine activation of hepatocyte lipogenesis by activated stellate cells (24). Thus, the nature and cellular source of the endogenous CB1 ligand may depend on the primary lipogenic stimulus.
The albumin-cre mice used to generate the LCB1–/– mice have been widely used for the hepatocyte-selective ablation of numerous other genes, and the conclusions in all these studies, including the present one, are heavily dependent on the hepatocyte specificity of gene deletion. Although trace-level expression of albumin mRNA has been detected in the anterior olfactory nucleus, tegmental reticular nucleus, and cerebral cortex of rats (see http://mouse.brain-map.org/brain/Alb1.html), here we found no detectable cre expression in the LCB1–/– brain using RT-PCR, whereas the expected expression of cre in the liver was clearly observed (Figure 1E).
The unique metabolic profile of hepatic CB1 receptor activation has potentially important therapeutic implications. Chronic treatment with a CB1 antagonist was recently shown to dramatically reduce steatosis in genetically obese rats (13). This finding points to the potential usefulness of CB1 blockade in the treatment of fatty liver of various etiologies, which are predisposing factors for both type II diabetes and liver cirrhosis. The therapeutic potential of CB1 antagonists in obesity/metabolic syndrome appears to be limited by CNS-mediated side effects, including anxiety and depression (35). The present findings that the favorable effects of CB1 blockade on steatosis and cardiovascular risk factors, such as insulin resistance and changes in plasma lipid profile, may be mediated at the level of the liver offers the impetus to develop peripherally restricted CB1 antagonists, thus minimizing CNS side effects. A further reduction of the risk of side effects may come from combination therapies taking advantage of potential synergisms between CB1 antagonists and statins on cholesterol metabolism or CB1 antagonists and antidiabetic agents in reversing insulin resistance and the associated cardiometabolic complications.
Methods
Animals.
All animal study protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the National Institute on Alcohol Abuse and Alcoholism, NIH. CB1+/+ and CB1–/– littermate mice were obtained by breeding of heterozygotes that had been backcrosssed to a C57BL/6J background, as described previously (36). LCB1–/– mice were generated by crossing mice homozygous for the CB1-floxed allele (CB1f/f), which were on a predominantly C57BL/6N background (7–8 crossings; ref. 37), with mice expressing the bacterial Cre recombinase driven by the mouse albumin promoter (TG[Alb-cre]21Mgn) that had been backcrossed 7 times to a C57BL/6J background, from Jackson Laboratory (38), to obtain CB1f/f × CB1f/f,AlbCre breeding pairs. The littermates obtained were on a mixed C57BL/6 J×N background. In each experimental paradigm, littermates were used as controls. Genotyping by PCR for the Cre transgene was done as described previously (37).
Diet-induced obesity.
Male and female mice (6–8 weeks old) were fed either regular mouse chow or a high-fat diet for 14 weeks. The high-fat diet contained 33.8% fat (59.9% of calories), 27.1% carbohydrate (21.3% of calories), and 23.9% protein (18.8% of calories) as well as 5.8% fiber, 6.2% moisture, and 3.2% vitamins and minerals (TD.97070; Harlan Teklad). The fatty acid profile was 45% saturated, 24% trans, 24% monounsaturated (cis), and 7% polyunsaturated (cis). The mice had free access to food and water and were kept on a 12-hour light/12-hour dark cycle. Food intake and body weight were measured daily. Mice were sacrificed by cervical dislocation. The liver and combined adipose tissue (inguinal, retroperitoneal, subcutaneous, and epididymal) were removed, weighed, and snap-frozen, and the combined adipose tissue weight expressed as percent of total body weight was defined as adiposity index. Trunk blood was collected for measuring endocrine and biochemical parameters.
Preparation of pure hepatocyte fractions.
Mouse hepatocytes were isolated via in situ collagenase perfusion and differential centrifugation on Percoll density gradients as described previously (39).
Leptin resistance.
To test the sensitivity to the anorexic effect of leptin, wild-type and LCB1–/– mice fed regular chow or high-fat diet were fasted for 24 h, after which they were injected i.p. with either vehicle or 200 μg leptin. Food was then presented to each animal, and cumulative food intake was measured for 3 h at the beginning of the dark period. Each mouse was tested with vehicle and leptin on different days, and percent reduction in food intake after leptin versus vehicle was calculated.
Histology.
For analysis of fat accumulation in the liver, the tissue was fixed in 10% formalin, and 10-μm-thick frozen sections were stained with Oil Red O (Vector Laboratories) and visualized by light microscopy.
Blood chemistry.
Plasma ALT levels were quantified using a kit from Drew Scientific. Plasma triglycerides and cholesterol were measured using a clinical chemistry analyzer system (PROCHEM-V; Drew Scientific). Hepatic triglyceride contents were also quantified using the PROCHEM-V system. Plasma leptin (Quantikine M; R&D Systems) and adiponectin levels (B-Bridge International) were determined using commercial sandwich ELISA assays in accordance with the manufacturers’ instructions. Plasma insulin was determined using the Ultrasensitive Mouse Insulin EIA kit (ALPCO Diagnostics).
Glucose tolerance and insulin resistance tests.
Glucose tolerance tests were performed i.p. in mice fasted overnight and subsequently injected with glucose (2 g/kg i.p.) Tail blood was collected at –1, 15, 30, 60, and 120 minutes relative to glucose injection (40). Blood glucose levels were determined using the Elite glucometer (Bayer). In some animals, CB1 receptor agonist or antagonist or vehicle control was injected i.p. 1 h prior to glucose tolerance test. Once blood glucose levels returned to baseline, the animals received an i.p. injection of insulin (0.75 U/kg; Eli Lilly), and blood glucose levels were determined at the same intervals as indicated above.
Indirect calorimetry.
VO2 and VCO2 were monitored using a 4-chamber Oxymax system (Columbus Instruments) with 1 mouse per chamber. The animals were accustomed to the chambers for 1 d, and VO2 and VCO2 measurements every 30 min were then collected and recorded on a computer over the next 24 h at room temperature. During the 12-h dark phase, mice had free access to food and water. Food was removed at the beginning of the light phase (postprandial state), at which point mice received an i.p. injection of vehicle or 10 mg/kg rimonabant and were monitored for an additional 12 h. RQ was measured as VCO2/VO2, and TEE was calculated as VO2 • (3.815 + 1.232 • RQ), normalized to (body mass)0.75, and expressed as kJ/kg/h.
Hepatic de novo fatty acid synthesis.
Rates of in vivo hepatic fatty acid synthesis were measured by the incorporation of 3H2O into hepatic fatty acids, as described previously (41). Briefly, following an overnight fast, mice were injected i.p. with vehicle, 20 ng/g HU-210, 3 μg/g rimonabant, or HU-210 and rimonabant combined. After 1 h, each animal received an intrahepatic injection of 250 μCi 3H2O in 50 μl saline. An additional hour later, the animals were sacrificed, the liver was removed and homogenized, and fatty acids were extracted with petroleum ether and quantified by liquid scintillation spectrometry. The rate of lipogenesis was expressed as micromole 3H2O incorporated into fatty acids per milligram tissue per minute.
Isolation of mitochondria and CPT1 activity assay.
Mitochondria were isolated using previously established methods (42). Briefly, 1 g liver tissue was placed in the homogenization buffer (250 mM mannitol, 5 mM HEPES, 1 mM EGTA, pH 7.2), homogenized, and diluted to 20 ml. The homogenate was centrifuged at 800 g for 6 min, the pellet was discarded, and the supernatant was collected into a fresh tube and recentrifuged at 8,000 g for 10 min. The mitochondrial pellet was reconstituted in a small amount of medium, and protein concentration was determined using the Bradford method. About 100 μg mitochondrial protein was resuspended in 100 μl of the assay buffer (25 mM HEPES, 50 mM mannitol, 75 mM KCl, 2 mM NaCN, 1% fat-free BSA, 0.2 mM EGTA, 1 mM DTT, 70 μM palmitoyl-CoA; and 0, 1, 3, 5, or 10 μM malonyl-CoA at pH 7.3) and incubated at 30°C for 3 min. We added l-carnitine containing 0.1 μCi 3H-methylcarnitine (Amersham Bioscience) to a final concentration of 200 μM and incubated at 30°C for 6 min. The reaction was stopped by the addition of 0.5 ml of 4M ice-cold perchloric acid and centrifuged at 8,000 g. The mitochondrial pellet was washed with 500 μl of 2 mM perchloric acid and centrifuged, and the resulting pellet was resuspended in 800 μl H2O and extracted with 600 μl butanol. For liquid scintillation counting, 300 μl butanol extract was used.
Western blot analysis.
The relative tissue content of CPT1A protein was assessed by Western blot analysis using a CPT1A antibody (Alpha Diagnostic International). Western blotting was performed was performed as described previously (9). The CB1 receptor antibody used for Western analyses was raised against the C-terminal (aa 461–472) intracellular region of the human CB1 receptor (Cayman Chemical).
RT-PCR.
mRNA expression under different experimental conditions was assessed by RT-PCR and normalized with β-actin as described previously (9). Total RNA was isolated from liver tissue using TRIzol and reverse transcribed using the SuperScript First-Strand Synthesis System in accordance with the manufacturer’s instructions (Invitrogen). The resulting single-stranded cDNA (5 μl) was denatured at 94°C for 5 min and — after the addition of the polymerase — subjected to 35 cycles of amplification, each consisting of 15 s at 94°C, 30 s at 58°C, and 1 min at 68°C, with a 30-s final extension at 68°C during the last cycle. Each 100-μl PCR reaction mixture contained the cDNA template, 1 μM of the primers, 200 μM of dNTPs, 1.5 mM MgCl2, 10 mM Tris/HCl (pH 9.0), 50 mM KCl, 0.1% Triton X-100, and 2.5 μM Taq polymerase (Invitrogen). The following forward and reverse primers were used: CPT1A (NM 013495.1), 5′-TCCATGCATACCAAAGTGGA-3′ and 5′-TGGTAGGAGAGAGCAGCACCTT-3′; CB1 (NM 007726.3), 5′-GTACCATCACCACAGACCTCCT-3′ and 5′-GGATTCAGAATCATGAAGCACTCCA-3′; Cre, 5′-GTGAAACAGCATTGCTGTCACTT-3′ and 5′-GCGGTCTGGCAGTAAAAACTATC-3′. The mouse β-actin gene was amplified as a control. The PCR products were separated by electrophoresis on a 1% agarose gel. RNA without reverse transcriptions did not yield any amplicons, indicating the absence of genomic DNA contamination.
Quantitative real-time PCR.
Real-time quantification of gene expression was performed according to previously established methods (9), except that the Opticon real-time detector system with a PTC-2000 Peltier base (MJ Research) and the iTaq SYBR Green Supermix with ROX (BioRad) were used. Amplification and reaction protocols were as described previously (9).
Statistics.
Values shown are expressed as mean ± SEM. Statistical analysis was performed by unpaired 2-tailed Student’s t test or by 1-way ANOVA, as appropriate. A P value less than 0.05 was considered significant.
Acknowledgments
We thank Keming Xiong for expert technical assistance. This study was supported by funds from the intramural research program of the National Institute on Alcohol Abuse and Alcoholism, NIH. G. Marsicano is supported by an AVENIR grant of INSERM in partnership with Foundation Bettencourt-Schueller.
Footnotes
Nonstandard abbreviations used: ALT, alanine aminotransferase; CPT1, carnitine palmitoyltransferase–1; LCB1–/–, liver-specific CB1 knockout; RQ, respiratory quotient; TEE, total energy expenditure; VO2, oxygen consumption; VCO2, carbon dioxide production.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 118:3160–3169 (2008). doi:10.1172/JCI34827
References
- 1.Di Marzo V., et al. Leptin-regulated hypothalamic endocannabinoids acting at CB1 receptors are involved in maintaining food intake. . Nature. . 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- 2.Jo Y.H., et al. Integration of endocannabinoid and leptin signaling in an appetite-related neural circuit. Neuron. . 2005;48:1055–1066. doi: 10.1016/j.neuron.2005.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Gaal L.F., et al. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients:1-year experience from the RIO-Europe study. Lancet. . 2005;365:1389–1397. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- 4.Despres J.-P., et al. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N. Engl. J. Med. . 2005;353:2121–2134. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- 5.Pi-Sunyer F.X., et al. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients. RIO-North America: a randomized controlled trial. JAMA. . 2006;295:761–775. doi: 10.1001/jama.295.7.761. [DOI] [PubMed] [Google Scholar]
- 6.Poirier B., et al. The anti-obesity effect of rimonabant is associated with an improved serum lipid profile. Diabetes Obes. Metab. . 2005;7:65–72. doi: 10.1111/j.1463-1326.2004.00374.x. [DOI] [PubMed] [Google Scholar]
- 7.Cota D., et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J. Clin. Invest. . 2003;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ravinet-Trillou C., Delgorge C., Menet C., Arnone M., Soubrié P. CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int. J. Obes. Relat. Metab. Disord. . 2004;28:640–648. doi: 10.1038/sj.ijo.0802583. [DOI] [PubMed] [Google Scholar]
- 9.Osei-Hyiaman D., et al. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. . J. Clin. Invest. . 2005;115:1298–1305. doi: 10.1172/JCI23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ravinet Trillou C., et al. Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. . 2003;284:R345–R353. doi: 10.1152/ajpregu.00545.2002. [DOI] [PubMed] [Google Scholar]
- 11.Doyon C., et al. Effects of rimonabant (SR141716) on fasting-induced hypothalamic-pituitary-adrenal axis and neuronal activation in lean and obese Zucker rats. Diabetes. 2006;55:3403–3410. doi: 10.2337/db06-0504. [DOI] [PubMed] [Google Scholar]
- 12.Serrano A., et al. The cannabinoid CB1 receptor antagonist SR141716A (Rimonabant) enhances the metabolic benefits of long-term treatment with oleoylethanolamide in Zucker rats. Neuropharmacology. 2008;54:226–234. doi: 10.1016/j.neuropharm.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Gary-Bobo M., et al. Rimonabant reduces obesity-associated hepatic steatosis and features of metabolic syndrome in obese Zucker fa/fa rats. Hepatology. . 2007;46:122–129. doi: 10.1002/hep.21641. [DOI] [PubMed] [Google Scholar]
- 14.Tedesco, L., et al. 2008. Cannabinoid type 1 receptor blockade promotes mitochondrial lipogenesis through eNOS expression in white adipocytes. Diabetes. Online publication ahead of print. doi:10.2337/db07-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buettner C., et al. Critical role of STAT3 in leptin’s metabolic action. Cell Metab. . 2006;4:49–60. doi: 10.1016/j.cmet.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aslimaz E., et al. Site and mechanism of leptin action in a rodent form of congenital lipodystrophy. J. Clin. Invest. 2004;113:414–424. doi: 10.1172/JCI19511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uno K., et al. Neuronal pathway from ther liver modulates energy expenditure and systemic insulin sensitivity. Science. . 2006;312:1656–1659. doi: 10.1126/science.1126010. [DOI] [PubMed] [Google Scholar]
- 18.Wang J., et al. Overfeeding rapidly induces leptin and insulin resistance. Diabetes. . 2001;50:2786–2791. doi: 10.2337/diabetes.50.12.2786. [DOI] [PubMed] [Google Scholar]
- 19.Ji H., Friedman M.I. reduced capacity for fatty acid oxidation in rats with inherited susceptibility to diet-induced obesity. Metabolism. . 2007;56:1124–1130. doi: 10.1016/j.metabol.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bensaid M., et al. The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. . Mol. Pharmacol. . 2003;63:908–914. doi: 10.1124/mol.63.4.908. [DOI] [PubMed] [Google Scholar]
- 21.Hezode C., et al. Daily cannabis use, a novel risk factor of steatosis severity in patients with chronic hepatitis C. Gastroenterology. . 2008;134:432–439. doi: 10.1053/j.gastro.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 22.Jayanthi, S., et al. 2008. Heavy marijuana users show increased serum apolipoprotein C–III levels: evidence from proteomic analyses. Mol. Psychiatry. Online publication ahead of print. doi:10.1038/mp.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ooi E.M.M., Barrett H.R., Chan D.C., Watts G.F. Apolipoprotein C-III: understanding an emerging cardiovascuular risk factor. Clin. Sci. . 2008;114:611–624. doi: 10.1042/CS20070308. [DOI] [PubMed] [Google Scholar]
- 24.Jeong W-I., et al. Paracrine activation of hepatic CB1 receptors by stellate cell-derived endocanabinoids mediates alcoholic fatty liver. . Cell Metab. . 2008;7:227–235. doi: 10.1016/j.cmet.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Herling A.W., et al. CB1 receptor antagonist AVE1625 affects primarily metabolic parameters independently of reduced food intake in Wistar rats. Am. J. Physiol. Endocrinol. Metab. . 2007;293:E826–E832. doi: 10.1152/ajpendo.00264.2007. [DOI] [PubMed] [Google Scholar]
- 26.Herling A.W., Kilp S., Elvert R., Haschke G., Kramer W. Increased energy expenditure contributes more to the body weight-reducing effect of rimonabant than reduced food intake in candy-fed Wistar rats. Endocrinology. 2008;149:2557–2566. doi: 10.1210/en.2007-1515. [DOI] [PubMed] [Google Scholar]
- 27.Cannon C.P. High-density lipoprotein cholesterol and residual cardiometabolic risk in metabolic syndrome. Clin. Cornerstone. . 2007;8(Suppl. 6):S14–S23. doi: 10.1016/s1098-3597(07)80011-1. [DOI] [PubMed] [Google Scholar]
- 28.Hollister L.E., Reaven G.M. Delta-9-tetrahydrocannabinol and glucose tolerance. Clin. Pharmacol. Ther. . 1974;16:297–302. doi: 10.1002/cpt1974162297. [DOI] [PubMed] [Google Scholar]
- 29.Bermúdez-Siva F.J., et al. Activation of cannabinoid CB1 receptors induces glucose intolerance in rats. . Eur. J. Pharmacol. . 2006;531:282–284. doi: 10.1016/j.ejphar.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 30.Yang Q., et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. . 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 31.Pagotto U., Marsicano G., Cota D., Lutz B., Pasquali R. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr. Rev. . 2006;27:73–100. doi: 10.1210/er.2005-0009. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y.L., Connoley I.P., Wilson C.A., Stock M.J. Effects of the cannabinoid CB1 receptor antagonist SR141716 on oxygen consumption and soleus muscle glucose uptake in Lep(ob)/Lep(ob) mice. Int. J. Obes. (Lond.) . 2005;29:183–187. doi: 10.1038/sj.ijo.0802847. [DOI] [PubMed] [Google Scholar]
- 33.El-Haschimi K., Pierroz D.D., Hileman S.M., Bjørbaek C., Flier J.S. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J. Clin. Invest. . 2000;105:1827–1832. doi: 10.1172/JCI9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siegmund S.V., et al. The endocannabinoid 2-arachidonoyl glycerol induces death of hepatic stellate cells via mitochondrial reactive oxygen species. FASEB J. . 2007;21:2798–2806. doi: 10.1096/fj.06-7717com. [DOI] [PubMed] [Google Scholar]
- 35.Christensen R., Kristensen P.K., Bartels E.M., Bliddal H., Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. . 2007;370:1706–1713. doi: 10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- 36.Zimmer A., Zimmer A.M., Hohmann A.G., Herkenham M., Bonner T.I. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc. Natl. Acad. Sci. U. S. A. . 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marsicano G., et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. . Science. . 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- 38.Postic C., et al. Dual roles for glukokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J. Biol. Chem. . 1999;274:305–315. doi: 10.1074/jbc.274.1.305. [DOI] [PubMed] [Google Scholar]
- 39.Sun R., Jaruga B., Kulkarni S., Sun H., Gao B. IL-6 modulates hepatocyte proliferation via induction of HGF/p21cip1: regulation by SOCS3. Biochem. Biophys. Res. Commun. . 2005;338:1943–1949. doi: 10.1016/j.bbrc.2005.10.171. [DOI] [PubMed] [Google Scholar]
- 40.Jeon J.Y., et al. MCH–/– mice are resistant to aging-associated increases in body weight and insulin resistance. . Diabetes. . 2006;55:428–434. doi: 10.2337/diabetes.55.02.06.db05-0203. [DOI] [PubMed] [Google Scholar]
- 41.Shimano H., et al. Overproduction of cholesterol and fatty acids causes massive liver enlargement in transgenic mice expressing truncated SREBP-1a. J. Clin. Invest. . 1996;98:1575–1584. doi: 10.1172/JCI118951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kudo N., Barr A.J., Barr R.L., Desai S., Lopaschuk G.D. High rates of fatty acid oxidation during reperfusion of ischemic hearts are associated with a decrease in malonyl-CoA levels due to an increase in 5′-AMP-activated protein kinase inhibition of acetyl-CoA carboxylase. J. Biol. Chem. . 1995;270:17513–17520. doi: 10.1074/jbc.270.29.17513. [DOI] [PubMed] [Google Scholar]