Abstract
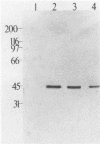
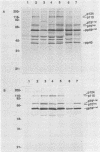
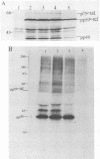
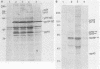
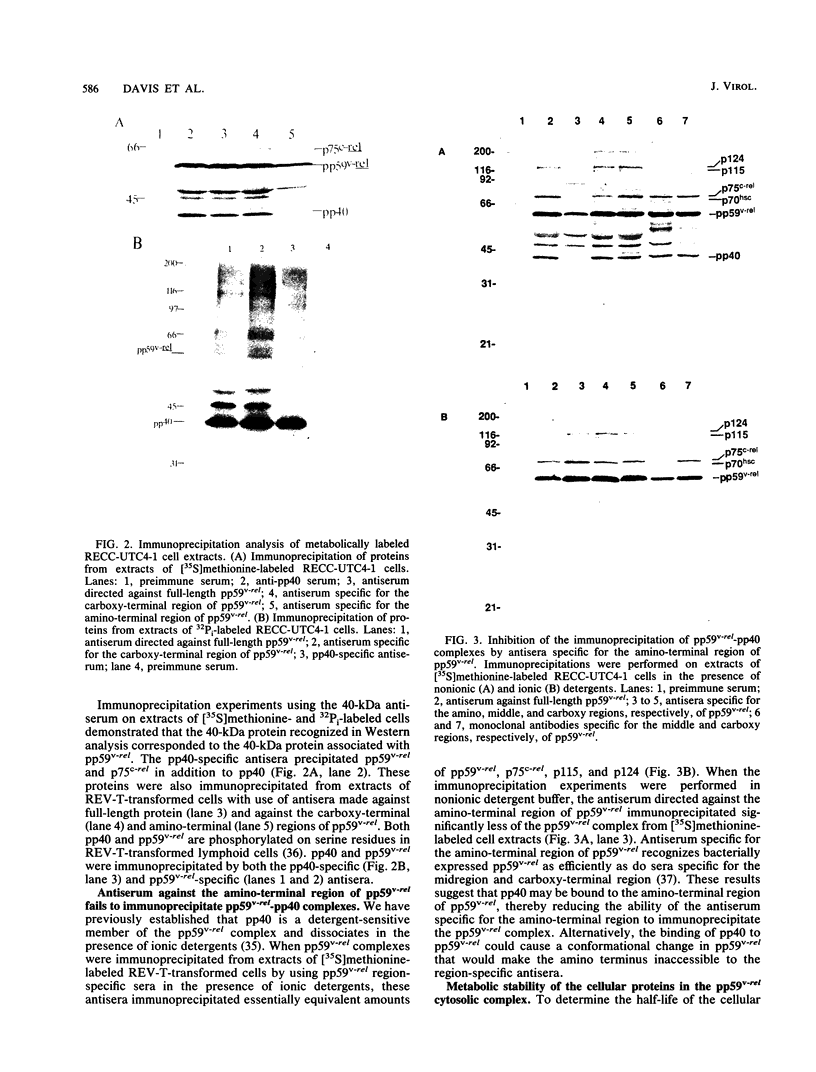
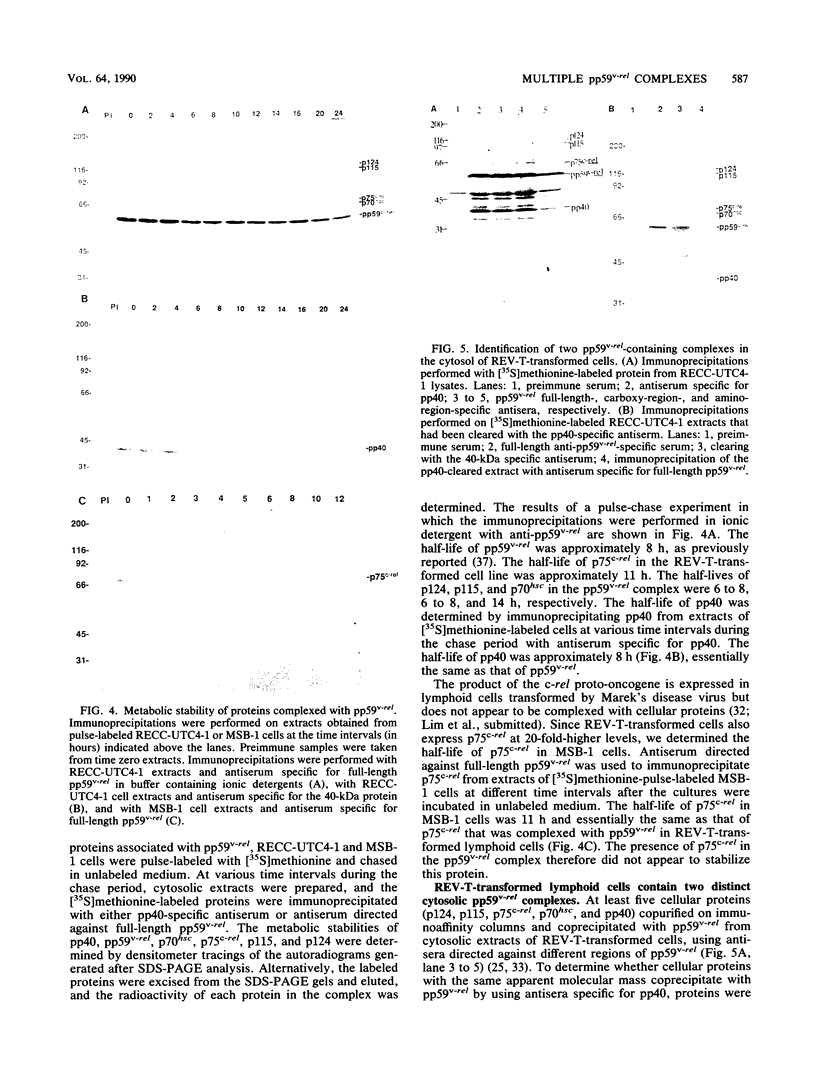
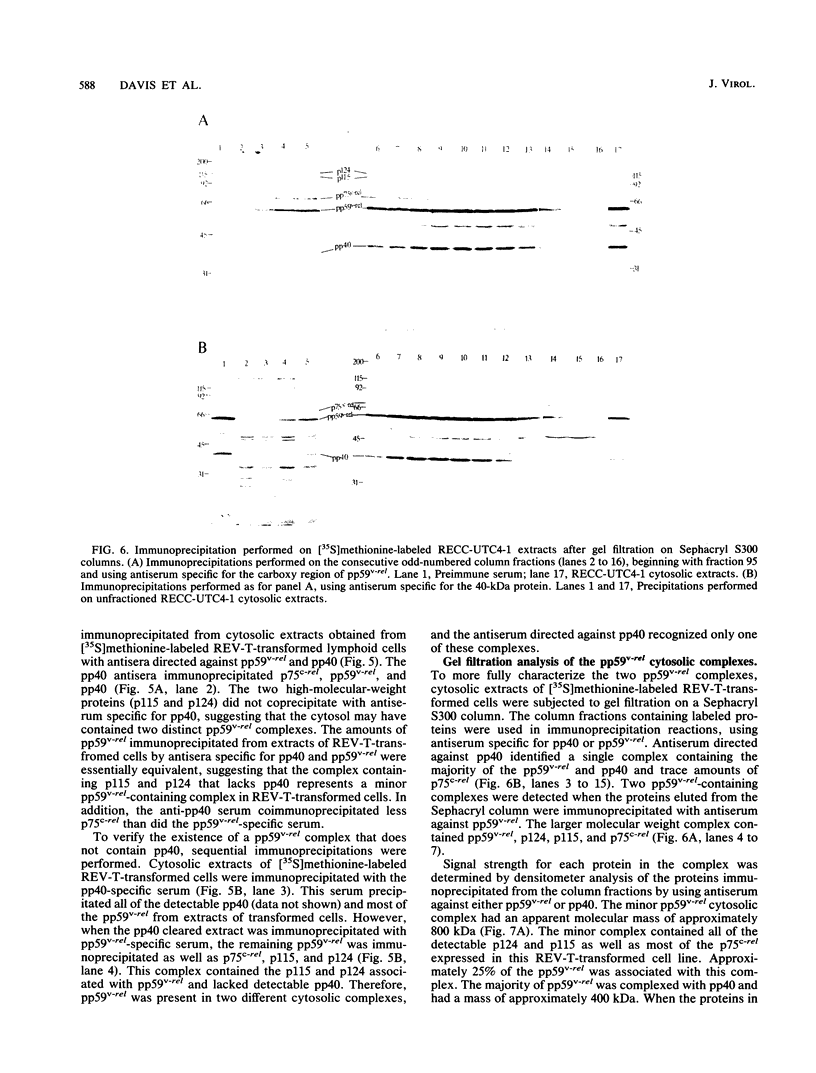
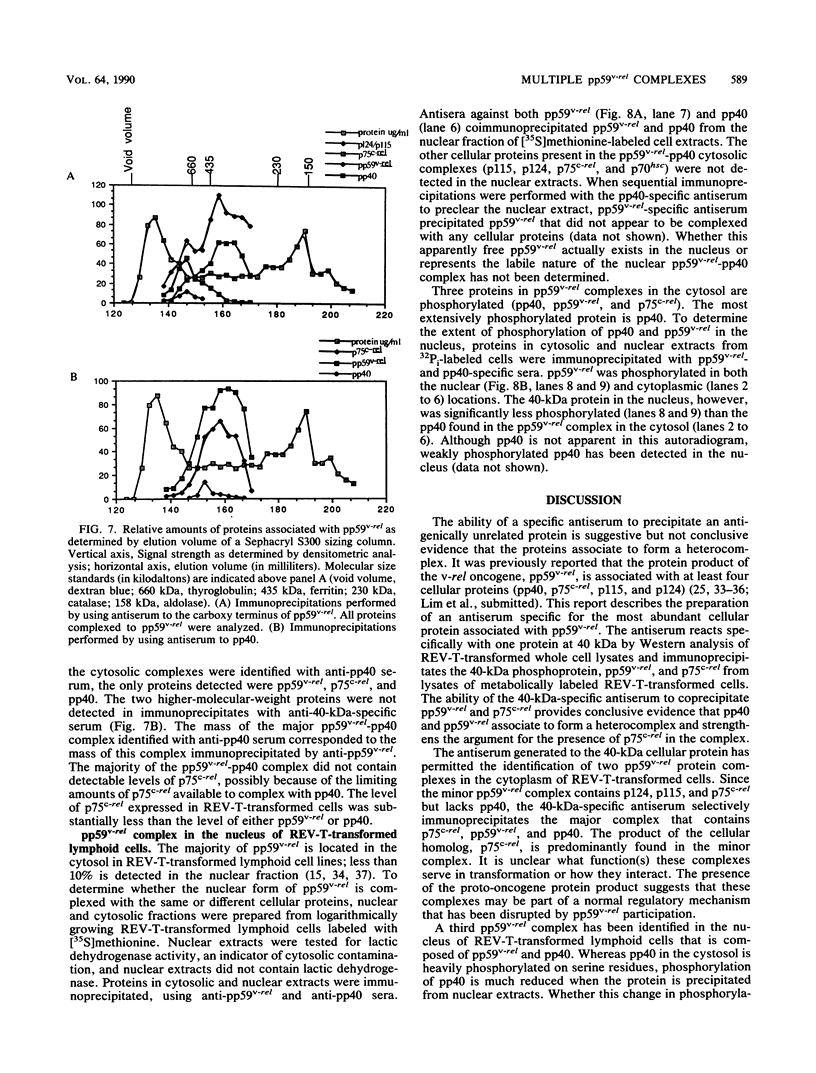
The v-rel oncogene of avian reticuloendotheliosis virus type T (REV-T) encodes a 59-kilodalton (kDa) phosphoprotein located principally in the cytosol of transformed lymphoid cells. All of the detectable pp59v-rel was present in high-molecular-weight complexes containing at least five cellular proteins (p124, p115, p75c-rel, p70hsc, and pp40). Antiserum was developed against the 40-kDa protein, the most abundant cellular protein associated with the complex. The 40-kDa phosphoprotein was complexed with pp59v-rel in REV-T-transformed lymphoid cell lines arrested at different stages of B-cell development as well as in lymphoid tumor cells and in fibrosarcomas. The half-life (8 h) of pp40 in REV-T-transformed lymphoid cells was the same as that of pp59v-rel. Antiserum against pp40 permitted the identification of two pp59v-rel complexes. The most abundant cytoplasmic complex contained approximately 75% of the pp59v-rel and all of the detectable pp40 in REV-T-transformed lymphoid cells. Twenty-five percent of the pp59v-rel was present in a minor complex that contained the majority of p75c-rel along with p115 and p124. In nuclear extracts of REV-T-transformed lymphoid cells, pp59v-rel was complexed with pp40. The two high-molecular-weight proteins (p115 and p124) and p75c-rel were not detected in the nuclear complex. In the cytosolic complexes, pp40 was heavily phosphorylated, whereas the nuclear form was much less extensively phosphorylated.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arion D., Meijer L., Brizuela L., Beach D. cdc2 is a component of the M phase-specific histone H1 kinase: evidence for identity with MPF. Cell. 1988 Oct 21;55(2):371–378. doi: 10.1016/0092-8674(88)90060-8. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Baltimore D. Activation of DNA-binding activity in an apparently cytoplasmic precursor of the NF-kappa B transcription factor. Cell. 1988 Apr 22;53(2):211–217. doi: 10.1016/0092-8674(88)90382-0. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Baltimore D. Activation of DNA-binding activity in an apparently cytoplasmic precursor of the NF-kappa B transcription factor. Cell. 1988 Apr 22;53(2):211–217. doi: 10.1016/0092-8674(88)90382-0. [DOI] [PubMed] [Google Scholar]
- Beug H., Müller H., Grieser S., Doederlein G., Graf T. Hematopoietic cells transformed in vitro by REVT avian reticuloendotheliosis virus express characteristics of very immature lymphoid cells. Virology. 1981 Dec;115(2):295–309. doi: 10.1016/0042-6822(81)90112-4. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chen I. S., Mak T. W., O'Rear J. J., Temin H. M. Characterization of reticuloendotheliosis virus strain T DNA and isolation of a novel variant of reticuloendotheliosis virus strain T by molecular cloning. J Virol. 1981 Dec;40(3):800–811. doi: 10.1128/jvi.40.3.800-811.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I. S., Wilhelmsen K. C., Temin H. M. Structure and expression of c-rel, the cellular homolog to the oncogene of reticuloendotheliosis virus strain T. J Virol. 1983 Jan;45(1):104–113. doi: 10.1128/jvi.45.1.104-113.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draetta G., Brizuela L., Potashkin J., Beach D. Identification of p34 and p13, human homologs of the cell cycle regulators of fission yeast encoded by cdc2+ and suc1+. Cell. 1987 Jul 17;50(2):319–325. doi: 10.1016/0092-8674(87)90227-3. [DOI] [PubMed] [Google Scholar]
- Dunphy W. G., Newport J. W. Unraveling of mitotic control mechanisms. Cell. 1988 Dec 23;55(6):925–928. doi: 10.1016/0092-8674(88)90234-6. [DOI] [PubMed] [Google Scholar]
- Franklin R. B., Kang C. Y., Min-Min Wan K., Bose H. R., Jr Transformation of chick embryo fibroblasts by reticuloendotheliosis virus. Virology. 1977 Dec;83(2):313–321. doi: 10.1016/0042-6822(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Garson K., Kang C. Y. Identification of the v-rel protein in REV-T transformed chicken bone marrow cells and expression in Cos1 cells. Biochem Biophys Res Commun. 1986 Jan 29;134(2):716–722. doi: 10.1016/s0006-291x(86)80479-x. [DOI] [PubMed] [Google Scholar]
- Gautier J., Norbury C., Lohka M., Nurse P., Maller J. Purified maturation-promoting factor contains the product of a Xenopus homolog of the fission yeast cell cycle control gene cdc2+. Cell. 1988 Jul 29;54(3):433–439. doi: 10.1016/0092-8674(88)90206-1. [DOI] [PubMed] [Google Scholar]
- Gilmore T. D., Temin H. M. Different localization of the product of the v-rel oncogene in chicken fibroblasts and spleen cells correlates with transformation by REV-T. Cell. 1986 Mar 14;44(5):791–800. doi: 10.1016/0092-8674(86)90845-7. [DOI] [PubMed] [Google Scholar]
- Gonda M. A., Rice N. R., Gilden R. V. Avian reticuloendotheliosis virus: characterization of the high-molecular-weight viral RNA in transforming and helper virus populations. J Virol. 1980 Jun;34(3):743–751. doi: 10.1128/jvi.34.3.743-751.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog N. K., Bargmann W. J., Bose H. R., Jr Oncogene expression in reticuloendotheliosis virus-transformed lymphoid cell lines and avian tissues. J Virol. 1986 Jan;57(1):371–375. doi: 10.1128/jvi.57.1.371-375.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog N. K., Bose H. R., Jr Expression of the oncogene of avian reticuloendotheliosis virus in Escherichia coli and identification of the transforming protein in reticuloendotheliosis virus T-transformed cells. Proc Natl Acad Sci U S A. 1986 Feb;83(3):812–816. doi: 10.1073/pnas.83.3.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbe J. C., Lee M. G., Nurse P., Picard A., Doree M. Activation at M-phase of a protein kinase encoded by a starfish homologue of the cell cycle control gene cdc2+. Nature. 1988 Sep 15;335(6187):251–254. doi: 10.1038/335251a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis R. B., McClure J., Rup B., Niesel D. W., Garry R. F., Hoelzer J. D., Nazerian K., Bose H. R., Jr Avian reticuloendotheliosis virus: identification of the hematopoietic target cell for transformation. Cell. 1981 Aug;25(2):421–431. doi: 10.1016/0092-8674(81)90060-x. [DOI] [PubMed] [Google Scholar]
- Lohka M. J., Hayes M. K., Maller J. L. Purification of maturation-promoting factor, an intracellular regulator of early mitotic events. Proc Natl Acad Sci U S A. 1988 May;85(9):3009–3013. doi: 10.1073/pnas.85.9.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A. A new solid-state reagent to iodinate proteins. I. Conditions for the efficient labeling of antiserum. Anal Biochem. 1982 Sep 15;125(2):427–432. doi: 10.1016/0003-2697(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Moore B. E., Bose H. R., Jr Expression of the v-rel oncogene in reticuloendotheliosis virus-transformed fibroblasts. Virology. 1988 Feb;162(2):377–387. doi: 10.1016/0042-6822(88)90478-3. [DOI] [PubMed] [Google Scholar]
- Morrison L. E., Kabrun N., Mudri S., Hayman M. J., Enrietto P. J. Viral rel and cellular rel associate with cellular proteins in transformed and normal cells. Oncogene. 1989 Jun;4(6):677–683. [PubMed] [Google Scholar]
- Rice N. R., Copeland T. D., Simek S., Oroszlan S., Gilden R. V. Detection and characterization of the protein encoded by the v-rel oncogene. Virology. 1986 Mar;149(2):217–229. doi: 10.1016/0042-6822(86)90123-6. [DOI] [PubMed] [Google Scholar]
- Rice N. R., Hiebsch R. R., Gonda M. A., Bose H. R., Jr, Gilden R. V. Genome of reticuloendotheliosis virus: characterization by use of cloned proviral DNA. J Virol. 1982 Apr;42(1):237–252. doi: 10.1128/jvi.42.1.237-252.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986 Dec 26;47(6):921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986 Aug 29;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Shibuya T., Chen I., Howatson A., Mak T. W. Morphological, immunological, and biochemical analyses of chicken spleen cells transformed in vitro by reticuloendotheliosis virus strain T. Cancer Res. 1982 Jul;42(7):2722–2728. [PubMed] [Google Scholar]
- Simek S. L., Stephens R. M., Rice N. R. Localization of the v-rel protein in reticuloendotheliosis virus strain T-transformed lymphoid cells. J Virol. 1986 Jul;59(1):120–126. doi: 10.1128/jvi.59.1.120-126.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simek S., Rice N. R. Detection and characterization of the protein encoded by the chicken c-rel protooncogene. Oncogene Res. 1988;2(2):103–119. [PubMed] [Google Scholar]
- Simek S., Rice N. R. p59v-rel, the transforming protein of reticuloendotheliosis virus, is complexed with at least four other proteins in transformed chicken lymphoid cells. J Virol. 1988 Dec;62(12):4730–4736. doi: 10.1128/jvi.62.12.4730-4736.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung H. Y., Bargmann W. J., Bose H. R., Jr Serine phosphorylation of the v-rel oncogene product/pp40 complex. Biochem Biophys Res Commun. 1988 Apr 15;152(1):441–448. doi: 10.1016/s0006-291x(88)80733-2. [DOI] [PubMed] [Google Scholar]
- Tung H. Y., Bargmann W. J., Lim M. Y., Bose H. R., Jr The v-rel oncogene product is complexed to a 40-kDa phosphoprotein in transformed lymphoid cells. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2479–2483. doi: 10.1073/pnas.85.8.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walro D. S., Herzog N. K., Zhang J., Lim M. Y., Bose H. R., Jr The transforming protein of avian reticuloendotheliosis virus is a soluble cytoplasmic protein which is associated with a protein kinase activity. Virology. 1987 Oct;160(2):433–444. doi: 10.1016/0042-6822(87)90015-8. [DOI] [PubMed] [Google Scholar]
- Wittenberg C., Reed S. I. Control of the yeast cell cycle is associated with assembly/disassembly of the Cdc28 protein kinase complex. Cell. 1988 Sep 23;54(7):1061–1072. doi: 10.1016/0092-8674(88)90121-3. [DOI] [PubMed] [Google Scholar]
- Wong T. C., Lai M. M. Avian reticuloendotheliosis virus contains a new class of oncogene of turkey origin. Virology. 1981 May;111(1):289–293. doi: 10.1016/0042-6822(81)90674-7. [DOI] [PubMed] [Google Scholar]
- Zhang J. Y., Bargmann W., Bose H. R., Jr Rearrangement and diversification of immunoglobulin light-chain genes in lymphoid cells transformed by reticuloendotheliosis virus. Mol Cell Biol. 1989 Nov;9(11):4970–4976. doi: 10.1128/mcb.9.11.4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. Y., Bose H. R., Jr Acquisition of new proviral copies in avian lymphoid cells transformed by reticuloendotheliosis virus. J Virol. 1989 Mar;63(3):1107–1115. doi: 10.1128/jvi.63.3.1107-1115.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]