Abstract
The aim of the study was to investigate the interaction between glutamate and capsaicin in inducing muscle pain and sensitization in humans. Fifteen male volunteers participated. Glutamate or capsaicin or isotonic saline, in a paired-sequence order, was injected randomly into the right or left masseter muscle. Two injections were given in a double-blinded design 25 minutes apart in 1 session/week over 4 weeks: saline (A1) followed by glutamate (A2), capsaicin (B1) followed by glutamate (B2), saline (C1) followed by capsaicin (C2), and glutamate (D1) followed by capsaicin (D2). The subjects drew the area of perceived pain and scored pain intensity on a 0–10 visual analogue scale (VAS). Pressure pain threshold (PPT) at the injection site, at a site 2-cm away, and on the contralateral side, as well as pressure pain tolerance (PPTol) at the injection site and contralateral site, were also measured before and after injection and subsequently at 5-minute intervals. Paired t-test analyses showed that the pain drawing area was significantly smaller in the B2 compared to the A2 condition (P = 0.028), and significantly larger in the D2 compared to the C2 condition (P = 0.027). It also revealed significantly lower VAS peak pain intensity (P = 0.008) and smaller VAS area under the curve (P=0.003) for the B2 compared to the A2 condition, and significantly higher VAS peak pain (P = 0.015) and larger VAS area under the curve (P = 0.037) for the D2 compared to the C2 condition. There was a significant PPT and PPTol decrease at the injection site after glutamate or capsaicin injection (ANOVA: P < 0.028). The percentage decrease in PPT or PPTol (at the injection site) was not significantly different for the B2 compared to the A2 condition (Paired t-test: P > 0.682) or for the D2 compared to the C2 condition (P > 0.133). Significant PPT changes were also observed at the site 2 cm away, but not on the contralateral side. In conclusion, these findings indicate that intramuscular administrations of glutamate and capsaicin interact and influence pain and sensitization of muscle nociceptors: glutamate causes a sensitization to subsequent administration of capsaicin whereas capsaicin is associated with a desensitization to subsequent injection of glutamate. These findings support previous animal data.
Keywords: Sensory Physiology, Trigeminal Pain, Allodynia, Human Experimental Pain Models
1. Introduction
Several lines of evidence suggest that elevated interstitial concentrations of glutamate may contribute to chronic deep tissue pain. The elevated concentrations of glutamate have been detected in human tendons and muscle in association with chronic non-inflammatory pain conditions (Alfredson et al., 2001; Rosendal et al., 2004). Further, artificial elevation of interstitial glutamate concentration by injection of glutamate into the human masseter muscle is associated with muscle pain and mechanical sensitization (Cairns et al., 2001, 2003a,b; Svensson et al., 2003, 2005). Animal studies also reveal that injection of glutamate into deep craniofacial tissues, such as the temporomandibular joint (TMJ), may sensitize deep craniofacial tissues to noxious chemical stimuli (Cairns et al., 2000a; Lam et al., 2004). However, it is not known whether elevated deep tissue concentrations of glutamate modify human pain responses to inflammatory algogens such as capsaicin in a manner similar to that observed for animals.
Capsaicin applied to skin and other tissues such as TMJ has been shown to produce inflammation, activate and sensitize spinal and trigeminal small-diameter nociceptive afferents as well as dorsal horn neurons, and also to evoke nociceptive behavior in animals and intense pain, hyperalgesia and referred pain in humans (Baumann et al., 1991; LaMotte et al., 1991, 1992; Simone et al., 1991, 1997; Raja et al., 1999; Ko et al., 2000; Arima et al., 2000, Witting et al, 2000a,b; Lam et al., 2004, 2005a,b; Gazerani et al., 2005; Hu et al., 2005). When compared with glutamate injections, injection of capsaicin into the human masseter muscle evokes muscle pain of a greater intensity and also induces mechanical sensitization (Arima et al., 2000; Wang et al., 2002). Animal research suggests that capsaicin may excite deep craniofacial tissue nociceptors in part through activation of peripheral NMDA receptors, which has been speculated to occur as a result of the neurogenic release of glutamate upon capsaicin administration (deGroot et al., 2000; Tang et al., 2004; Lam et al., 2005a, b; Hu et al., 2005). However, in animals, pre-administration of capsaicin into deep craniofacial tissue attenuates rather than enhances nociceptive responses to a subsequent injection of glutamate (Lam et al., 2004). While these findings in animal models may indicate a role for both peripheral and central sensitization mechanisms in capsaicin-evoked pain, it is not known whether a similar interaction between glutamate and capsaicin would occur in human subjects.
The aim of the present study was to investigate the interaction between glutamate and capsaicin in inducing pain and sensitization in human muscles. It was anticipated that in humans, prior elevation of glutamate levels would sensitize the masseter muscle to noxious chemical input produced by a subsequent injection of capsaicin. On the other hand, it was speculated that prior administration of capsaicin would attenuate the pain responses to glutamate injections.
2. Materials and methods
2.1. Subjects
Fifteen healthy male volunteers (mean age ± SD: 27 ± 5 years) participated in a double-blind, crossover experiment. The subjects had no signs or symptoms of temporomandibular disorders (Dworkin and LeResche, 1992) and were recruited from university students. The study protocol was approved by the local ethics committee in Denmark (County of Aarhus: 20040074) and followed the guidelines set out by the Helsinki Declaration. Informed consent was obtained from all subjects.
2.2. Experimental protocol
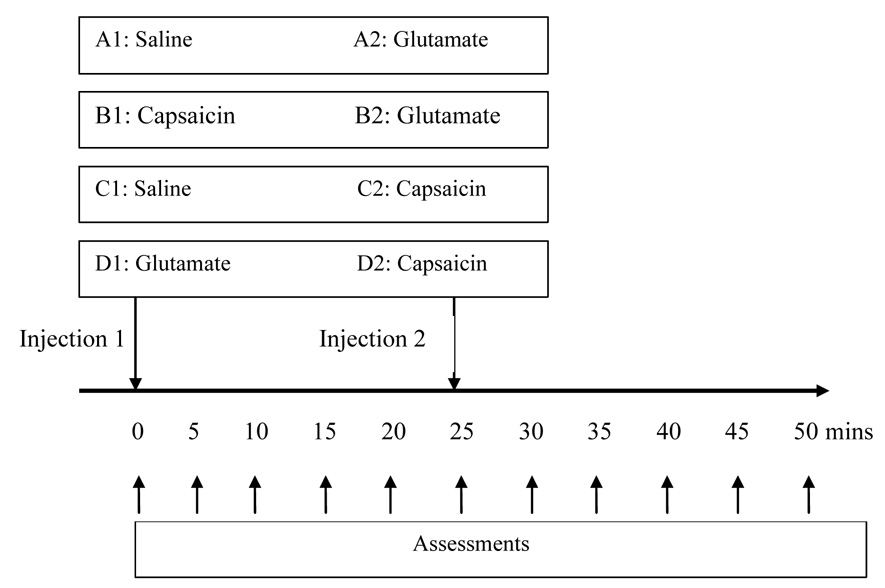
Glutamate, capsaicin or isotonic saline, in a paired-sequence order, was injected randomly into the right or left masseter muscle. Two injections were given in a double-blinded manner 25 minutes apart in 1 session. Four sessions were performed with an interval of 1 week: saline (A1) followed by glutamate (A2), capsaicin (B1) followed by glutamate (B2), saline (C1) followed by capsaicin (C2), and glutamate (D1) followed by capsaicin (D2) (see Fig. 1).
Fig. 1.
Two different injections in four sessions: Glutamate, capsaicin or isotonic saline, in a paired-sequence order, was injected randomly into the right or left masseter muscle. Two injections were given in a double-blinded manner 25 minutes apart in each session. Four sessions were performed with an interval of 1 week: saline (A1) followed by glutamate (A2), capsaicin (B1) followed by glutamate (B2), saline (C1) followed by capsaicin (C2), and glutamate (D1) followed by capsaicin (D2).
After each injection, the subjects were asked to draw their perceived distribution of pain on a face-chart marking all areas of pain. The pain maps were then digitized (ACECAD, model D9000+digitizer, Taiwan) to calculate the area of perceived pain expressed in arbitrary units (a.u.) (Svensson et al., 2003; Cairns et al., 2003a,b). The induced pain was continuously scored on a 0–10 cm visual analogue scale (VAS) during each injection for over 15 minutes. Pressure pain threshold (PPT) at the injection site, at a site 2-cm away, and at a site on the contralateral side (analogous to the injection site) as well as pressure pain tolerance (PPTol) at the injection site and contralateral site, were measured before and after injection and subsequently at 5-minute intervals.
2.3. Experimental jaw-muscle pain
Glutamate (0.2 ml, 1 M) or capsaicin (0.2 ml, 100 µg/ml) or isotonic saline (0.2 ml) was injected into the deep mid-portion of the masseter muscle in accordance with previously described procedures (Svensson et al., 1995, 2003, 2005). A 27-G needle was used and the 0.2 ml volume was injected over 10 sec. The subjects were instructed to continuously rate the pain intensity evoked by each injection on an 0–10 cm electronic visual analogue scale (VAS). A computer sampled the VAS signals every 5 s. The lower endpoint of the VAS was labeled "no pain at all" and the upper endpoint labeled "the most pain imaginable". Peak pain intensity was measured as the peak VAS score (cm); the area under the VAS curve (cm × s) was used to obtain a measure of the overall amount of pain, and the onset to offset of pain was determined from the VAS profiles and used as a measure of pain duration (s). At the offset of the pain, the subjects described the quality of pain on a Danish version of the McGill Pain Questionnaire (MPQ) (Drewes et al., 1993).
2.4. Pressure pain threshold (PPT) and pressure pain tolerance (PPTol)
A pressure algometer (Somedic, Sweden) was used to measure the PPTs and PPTol. The PPT was defined as the amount of pressure (kPa) which the subjects first perceived to be painful and the PPtols was defined when pain becomes intolerable (tolerance) (Svensson et al., 1995). The algometer probe (1 cm2 area) was applied perpendicularly to the masseter at the 3 sites specified above. During the pressure stimulation, the subjects kept their teeth in the intercuspal position with minimum voluntary contraction and focused their attention on the experimental task. The subject pushed a button to stop the pressure stimulation when the threshold was reached. The PPTs were determined in triplicate at baseline (i.e. before any injection) and in duplicate after the injection at each of the 3 sites with approximately 1 minute between each repeated measurement.
The pressure was delivered with a constant application rate of 30 kPa/s, and PPT values were recorded every 5 minutes for 25 minutes following each injection. A single measurement of the PPTol at the injection site and at the contralateral site was also determined after 5 and after 25 minutes following each injection.
2.5. Statistic analyses
SigmaStat for Windows Version 2.03 was used for the data analysis. Pain drawing area, VAS peak, VAS area under curve produced by the injections were compared with paired t-tests between A2 and B2, as well as C2 and D2. The PPT and PPTol values were normalized to the baseline value. Percentage changes in PPT or PPTol were compared to the baseline by using one-way analysis of variance (ANOVA) with repeated measures (in time: 11 different time points). The percentage changes were also compared between injections with paired t-tests (in treatment: different injections). The significance level was set at P < 0.05. Mean values ± SEM are presented in the text and figures.
3. Results
3.1. Pain drawing area
The injections were performed randomly on the right or left side of the masseter. Eight subjects were injected into the right masseter and 7 subjects were injected into the left masseter. The pain areas for all 8 injections are shown in Fig 2. The pain drawing area was smaller in the condition where glutamate was preceded by capsaicin (B2), compared to the condition where glutamate was preceded by isotonic saline (A2) (paired t-test: P = 0.028), and significantly larger when capsaicin was preceded by glutamate (D2) than when capsaicin was preceded by isotonic saline (C2) (P= 0.027).
Fig. 2.
Pain drawing areas for all 8 masseter injections in 15 subjects. Individual pain drawings are superimposed to demonstrate the typical location of pain. The * indicates significant difference between the two treatments (P<0.05) (N = 15).
3.2. VAS pain
The VAS pain scores revealed a lower peak pain intensity in the B2 condition (4.4 ± 0.8 cm) compared to the A2 condition (6.1 ± 0.8 cm) (paired t-test: P = 0.008) and a smaller VAS area under the curve in the B2 (825 ± 172 cm × s) compared to the A2 condition (1406 ± 298 cm × s) (P = 0.003) (Fig. 3 A, B). There was also a higher VAS peak pain intensity in the D2 (6.3 ± 0.8 cm) compared to the C2 condition (5.5 ± 0.8 cm) (paired t-test: P = 0.015), and a larger VAS area under the curve in the D2 (2649 ± 612 cm × s) compared to the C2 condition (1830 ± 0.8 cm× s) (P = 0.037) (Fig. 3 C, D).
Fig. 3.
Continues (0–900 s) VAS scores of perceived pain intensity for all 8 masseter injections (Mean + SEM) (N=15). The * indicates significant difference between the two treatments (P<0.05) (N = 15).
The MPQ pain rating indices (PRI) of the sensory, affective, evaluative, and miscellaneous dimensions of pain, and the most frequently selected words from the MPQ during the 4 test conditions, were analyzed (data are not shown). There was no significant difference in the PRI between the A2 and B2 conditions, or between the C2 and D2 conditions (paired t-test: P > 0.07).
3.3. PPT changes
The baseline measurements of the PPT at the injected site were 303 ± 16 kPa in the A, 286 ± 18 kPa in the B, 303 ± 21 kPa in the C, and 286 ± 16 kPa in the D conditions. There were no significant differences between the 4 conditions for the baseline PPT values (ANOVA: F (3, 14) = 0.670, P = 0.575).
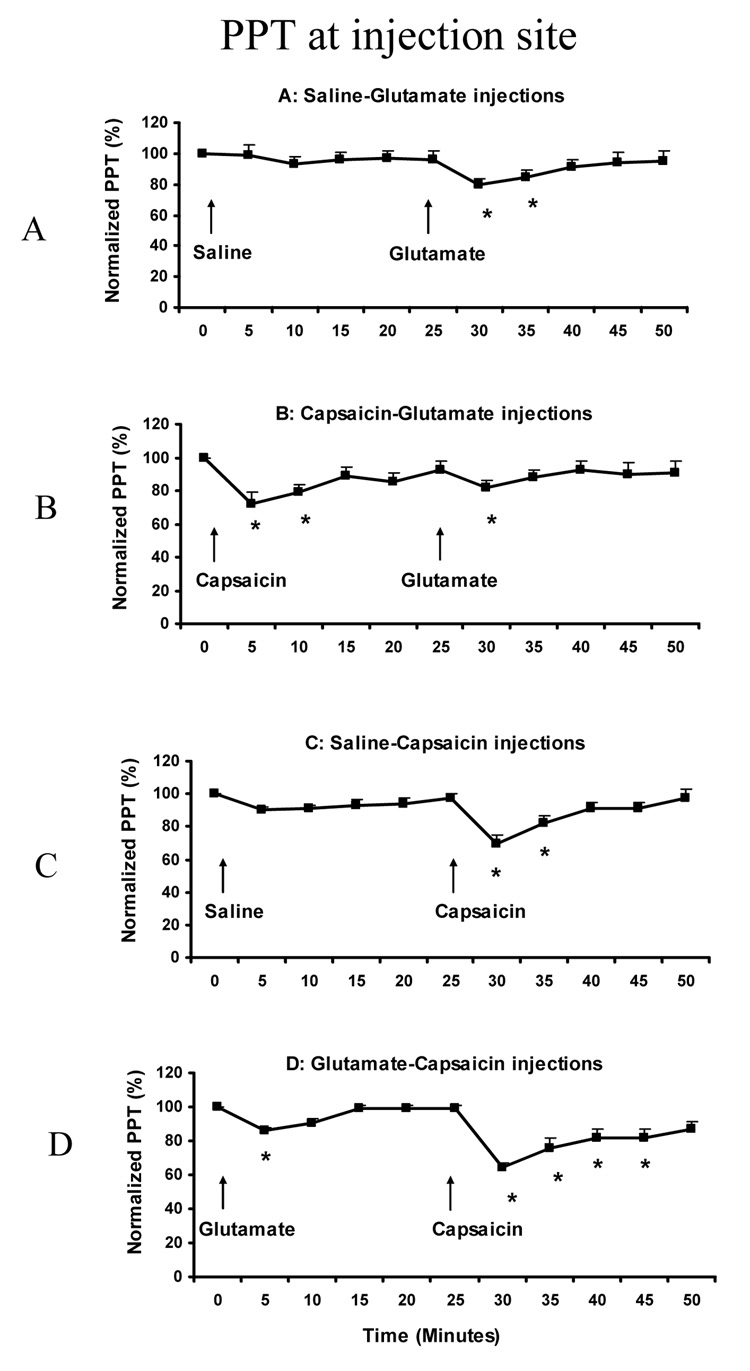
The PPT values were normalized to the baseline values and the percentage changes during the injection compared between the A2 and B2 conditions, and between the C2 and D2 conditions. Both the both normality test and equal variance test were passed. At the injection site, the normalized PPT value was significantly changed in all 4 sessions (ANOVA: F (10, 14) = 4.150, P < 0.001). Post-hoc tests indicated that normalized PPTs were significantly decreased at 30 min (80 ± 3.4%) and 35 min (85 ± 4.0%) compared to the baseline value (100%) in the A session (P< 0.003) (Fig 4A); at 5 min (72 ± 6.8%), 10 min (79 ± 4.1%) and 30 min (82 ± 4.2%) compared to the baseline value in the B session (P < 0.028) (Fig 4B); at 30 min (69 ± 5.6%) and 35 min (82 ± 4.3%) compared to the baseline value in the C session (P < 0.003) (Fig 4C); and at 5 min (86 ± 1.7%), 30 min (64 ± 3.4%), 35 min (76 ± 5.9%), 40 min (82 ± 5.5%) and 45 min (82 ± 5.0) compared to the baseline value in the D session (P < 0.028) (Fig 4D).
Fig. 4.
Normalized PPTs at masseter injection site (Mean + SEM). The arrows show the time point of two injections in each session. The * indicates significant decrease at the time point compared to baseline (P<0.05) (N = 15).
There was no significant difference in the PPT decease between the A2 and B2 conditions at 30 min (paired t-test: P = 0.682) or at 35 min (P = 0.604). Similarly, there was no significant difference between the C2 and D2 conditions at 30 min (P = 0.345), at 35 min (P = 0.133), at 40 min (P = 0.147), or at 45 min (P = 0.142).
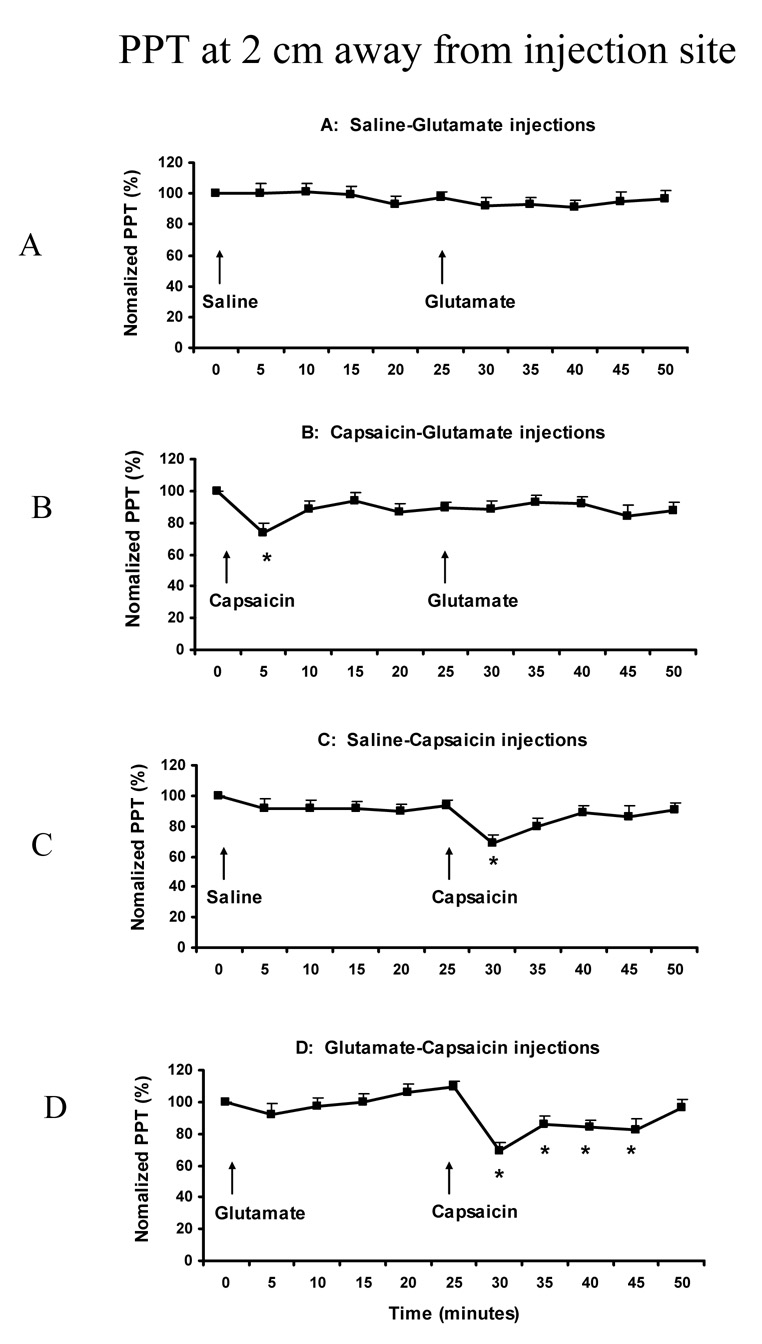
At the site 2-cm away from the injection site, the normalized PPT value was not significantly changed in the A session (F (10,14) = 0.466, P = 0.150), but was significantly changed in B, C and D sessions (F (10,14) = 2.296, P < 0.016). It was decreased at 5 min (72 ± 5.3%) compared to the baseline (100%) in the B session (P < 0.028) (Fig 5B); at 30 min (69 ± 3.8%) and 35 min (81 ± 4.6%) compared to the baseline in the C session (P < 0.004) (Fig 5C); and at 30 min (70 ± 5.1%), 35 min (87 ± 4.8%), 40 min (84 ± 4.5%), and 45 min (82 ± 5.0%) compared to the baseline in the D session (P < 0.003) (Fig 5D). There was no significant difference in the PPT decease between the A2 and B2 conditions (there was no significant PPT decrease in either the A2 or B2 condition compared with baseline) (paired t-test: P > 0.506). Similarly, there was no significant difference between the C2 and D2 conditions at any time point: 69% vs 70% at 30 min, 87% vs 81% at 35 min, 89% vs 84% at 40 min, and 86% vs 82% at 45 min (paired t-test: P > 0.317).
Fig. 5.
Normalized PPTs at the site 2 cm away from masseter injection site. The arrows show the time point of two injections in each session (Mean + SEM). The * indicates significant decrease at the time point compared to baseline (P<0.05) (N = 15).
The baseline measurements of the PPT at the contralateral site were 312 ± 13 kPa in the A, 296 ± 17 kPa in the B, 306 ± 20 kPa in the C, and 302 ± 16 kPa in the D sessions. There were no significant differences between the 4 sessions for the baseline PPT values (ANOVA: F (10, 14) = 0.576, P = 0.778) and there were no significant PPT changes at the contralateral site following any injection ((ANOVA: F (10, 14) < 0.114, P > 0.323).
3.4. PPTol changes
The baseline measurements of the PPTol at the injection site were 488 ± 27 kPa in the A, 508 ± 30 kPa in the B, 497 ± 29 kPa in the C, and 507 ± 38 kPa in the D conditions. There were no significant differences between the 4 sessions for the baseline PPTol values (ANOVA: F (4, 14) =1.459, P = 0.168).
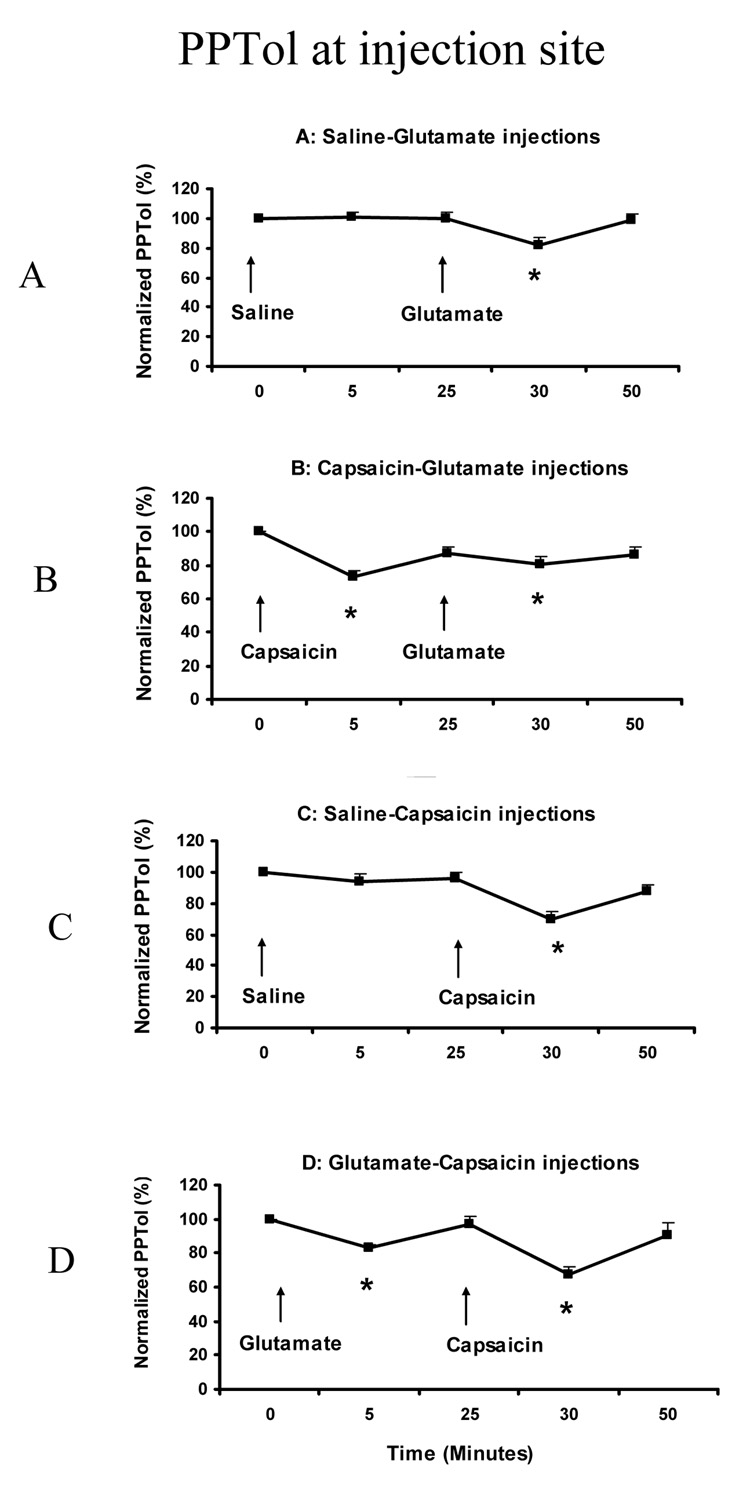
The PPTol values were normalized to the baseline values and the percentage changes during the injection compared between the A2 and B2 conditions, and between the C2 and D2 conditions. Both the normality test and equal variance test were passed. The normalized PPTol value was significantly changed in all 4 sessions (ANOVA: F (4, 14) = 7.904, P < 0.001). Post-hoc comparisons indicated that the normalized PPTol was significantly decreased at 30 min (82 ± 4.9%) compared to the baseline value (100%) in the A ession (P < 0.001) (Fig 6A); at 5 min (73 ± 4.2%) and 30 min (81 ± 3.9%) compared to the baseline value in the B session (P < 0.001) (Fig 6B); at 30 min (69 ± 5.3%) compared to the baseline value in the C session (P < 0.001) (Fig 6C); and at 5 min (83 ± 2.2%) and 30 min (68 ± 4.7%) compared to the baseline value in the D session (P < 0.033) (Fig 6D).
Fig. 6.
Normalized PPTols at masseter injection site. The arrows show the time point of two injections in each session (Mean + SEM). The * indicates significant decrease at the time point compared to baseline (P<0.05) (N = 15).
No significant differences in the degree of PPTol decrease was detected between the A2 (82 ± 4.9%) and B2 (81 ± 3.9%) conditions at 30 min (Paired t-test: P = 0.865), or between the C2 (69 ± 5.3%) and the D2 (68 ± 4.7%) conditions at 30 min (P = 0.808).
The baseline measurements of the PPTol at the contralateral site were 498 ± 27 kPa in the A, 502 ± 29 kPa in the B, 494 ± 31 kPa in the C, and 511 ± 29 kPa in the D conditions. There were no significant differences between the 4 sessions for the baseline PPTol values (ANOVA: F (4, 14) = 1.012, P = 0.868), and there were no significant PPTol changes at the contralateral site following any injection (ANOVA: F (4, 14) < 1.857, P > 0.403).
4. Discussion
To the best of our knowledge, this is the first human study to demonstrate that capsaicin-evoked pain responses can be facilitated by the peripheral application of glutamate, and more interestingly that glutamate-evoked pain responses can be inhibited by the peripheral application of capsaicin to deep craniofacial tissue. We propose that these results reflect, at least in part, an interaction between capsaicin and glutamate receptor mechanisms within the masseter muscle, however, it is possible that central mechanisms, such as central sensitization and/or the recruitment of diffuse noxious inhibitory control, could also contribute to the observed interaction between these substances. In contrast, although the PPT and PPTol values were significantly decreased after glutamate or capsaicin injection, there were no significant interactions between the 2 injections. We interpret these findings to suggest that peripheral glutamate and capsaicin receptor mechanisms may interact to modulate the activation of nociceptive processes in muscle tissue.
4.1. Glutamate-evoked muscle pain and sensitization
Consistent with our previous studies, injection of glutamate into the masseter muscle evoked tonic muscle pain and significantly decreased PPT and PPTol (Cairns et al., 2003 a,b, 2006; Castrillon et al., 2007; Svensson et al., 2003, 2005). Animal studies suggest that glutamate excites slowly-conducting masseter afferent fibers principally through activation of NR2B subunit-containing peripheral NMDA receptors which are expressed by ~40–60% of trigeminal ganglion neurons that innervate the masseter muscle (masseter ganglion neurons) (Cairns et al., 2003a; Dong et al 2007). Glutamate can also induce a period of prolonged afferent mechanical sensitization which appears to be mediated through activation of NMDA, non-NMDA and metabotropic glutamate (mGluR) receptors (Cairns et al., 2002b, 2007; Lee and Ro et al., 2007). Our parallel findings in humans that local administration of ketamine attenuates both glutamate-evoked pain and glutamate-induced mechanical sensitization (Cairns et al., 2003a, 2006), suggest that activation of peripheral NMDA receptors may excite and sensitize slowly conducting masseter muscle nociceptors and could contribute to pain responses in humans.
Injection of glutamate into the rat TMJ has been shown to sensitize nociceptive afferent responses to a subsequent injection of capsaicin into the TMJ, which suggests that a peripheral mechanism may underlie our observation of enhanced human pain responses to capsaicin after glutamate injection (Lam et al., 2004). The effects of capsaicin are mediated through activation of the transient receptor potential vanilloid 1 (TRPV1) receptors that has been found on trigeminal afferents and trigeminal ganglion neurons innervating the rat TMJ or tooth pulp (e.g. Ichikawa et al., 2004; Morgan et al., 2005). Further, in vitro experiments indicate that ~50% of masseter ganglion neurons respond to capsaicin with rapidly desensitizing currents (Tominaga and Tominaga, 2005). As mentioned, a similar percentage of masseter ganglion neurons express NMDA receptors and at least some masseter nociceptors also express the mGluR5 receptor (Dong et al., 2007; Lee and Ro, 2007), which suggests that TRPV1 and peripheral glutamate receptors are likely co-localized on a subpopulation of masseter nociceptors. Although the molecular pathways that might mediate an interaction between TRPV1 and peripheral glutamate receptors have yet to be elucidated, it bears mentioning that activation of the protein kinase C (PKC) pathway appears to enhance currents mediated through the TRPV1 receptor and that this same pathway plays a role in mechanical sensitization induced by activation of peripheral mGluR5 receptors (Lee and Ro, 2007; Sikand and Premkumar, 2007). However, injection of glutamate into the rat TMJ also induces central sensitization of trigeminal nociceptive neurons (Lam et al. 2003), which may mean central mechanisms could also contribute to the effect of glutamate on capsaicin-induced muscle pain.
In the present study, a reduction in PPT and/or PPTol was taken as an indication of allodynia or hyperalgesia of the muscle to mechanical stimuli. There was, however, no effect of pre-injections of glutamate on capsaicin-induced mechanical sensitization when compared with the effect of pre-injection of saline at the site of injection. The lack of interaction effects on PPT and PPTol following glutamate and capsaicin injections may have been due to the small sample size, but another possible explanation is that PPT and PPTol were already significantly reduced following either injection, and so it was difficult to show a further reduction when comparisons were made. The lack of significant changes in PPT and PPTol on the contralateral (control) side in the present study supports a localized peripheral mechanism of action for glutamate- and capsaicin-induced mechanical sensitization.
4.2. Capsaicin-evoked muscle pain and sensitization
Capsaicin is a widely used algogenic agent that excites nociceptive afferents and causes local neurogenic inflammation, primary heat and mechanical hyperalgesia, and secondary mechanical hyperalgesia in humans and rats (Baumann et al., 1991; LaMotte et al., 1991, 1992; Simone et al., 1991, 1997; Raja et al, 1999; Ko et al., 2000; Arima te a., 2000; Witting et al, 2000a, b; Lam et al., 2004; Gazerani et al., 2005; Hu et al., 2005). The vanilloid type 1 receptor TRPV1, which is activated by noxious heat, protons or irritants such as the small-fiber excitant capsaicin, is found on small-diameter afferent nerve fibers and dorsal root ganglion neurons (Caterina et al. 1997; Tominaga et al., 1998; Jordt et al., 2003). TRPV1 receptors have also recently been described on trigeminal afferents and trigeminal ganglion neurons innervating the rat TMJ or tooth pulp (e.g. Ichikawa et al., 2004; Morgan et al., 2005). Furthermore, capsaicin injected into the rat TMJ reflexly evokes a dose-dependent increase in jaw muscle EMG activity (Tang et al., 2004; Lam et al., 2005a), produces an inflammatory response (Hu et al., 2005) and induces activation and sensitization of brainstem nociceptive neurons (Lam et al., 2003, 2004, 2005b).
In the present study, capsaicin injected into the masseter muscle evoked tonic muscle pain that was described as “sharp”, “pulsing” and “shooting” and significantly decreased the PPT and PPTol values. The results are in accordance with previous studies (LaMotte et al., 1991; Arima et al., 2000; Witting et al., 2000b; Sluka, 2002; Gazerani et al., 2005) demonstrating sensitization to mechanical stimuli after intramuscular injection of capsaicin.
The injection of glutamate following capsaicin evoked significantly less pain compared to its injection following saline, which suggests that pre-injection of capsaicin can desensitize masseter muscle pain pathways. Again, this appears to be due to peripheral mechanisms since activation of nociceptive TMJ afferents by glutamate following capsaicin injection was abolished or significantly reduced (Lam et al., 2004). The mechanisms which mediate capsaicin-induced chemical desensitization of afferent fibers are not yet completely understood, however, evidence suggests that capsaicin can act to decrease both voltage-gated sodium and calcium currents (Docherty et al., 1991; Balla et al. 2001; Liu et al., 2001; Hagenacker et al., 2005). It has been reported that capsaicin only desensitizes trigeminal ganglion neurons that are sensitive to capsaicin, i.e. ganglion neurons in which capsaicin induces depolarizing currents and a burst of action potentials through activation of TRPV1 receptors (Liu et al., 2001). This suggests that the capsaicin-induced reduction in glutamate-evoked muscle pain observed in the present study could be mediated by an interaction between peripheral TRPV1 and glutamate-receptor mechanisms in a subgroup of masseter muscle afferent fibers.
In addition to its ability to induce afferent desensitization, capsaicin has also been demonstrated to cause significant inflammation and edema in deep craniofacial tissues (Tang et al., 2004; Hu et al., 2005), which could dilute the injected glutamate and decrease glutamate-evoked muscle pain. We have previously demonstrated that the magnitude of glutamate-evoked masseter muscle pain in humans is concentration-dependent (Svensson et al., 2003). Thus, a physical mechanism involving dilution of the injected glutamate could also contribute to our observations of decreased glutamate-evoked muscle pain after capsaicin.
4.3. Conclusions
The present study has revealed that there is, indeed, an interaction between glutamate and capsaicin injections into human masseter muscle. The glutamate injection following capsaicin evoked significantly less pain compared to its injection following isotonic saline, and the capsaicin injection following glutamate evoked a significantly stronger pain compared to its injection following isotonic saline. Peripheral interactions between EEA and TRPV1 receptors may thus present important modulatory mechanisms influencing the magnitude of musculoskeletal pain, and further studies addressing such biological interactions are needed to help clarify the mechanisms underlying clinical pain conditions manifested in musculoskeletal tissues.
Acknowledgments
This study was Supported by US National Institutes of Health (NIH), Grant 1 R01DE015420.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alfredson H, Forsgren S, Thorsen K, Lorentzon RJ. In vivo microdialysis and immunohistochemical analyses of tendon tissue demonstrated high amounts of free glutamate and glutamate NMDAR1 receptors, but no signs of inflammation, in Jumper's knee. Orthop Res. 2001;19:881–886. doi: 10.1016/S0736-0266(01)00016-X. [DOI] [PubMed] [Google Scholar]
- Arima T, Svensson P, Arendt-Nielsen L. Capsaicin-induced muscle hyperalgesia in the exercised and non-exercised human masseter muscle. J Orofac Pain. 2000;14:213–223. [PubMed] [Google Scholar]
- Balla Z, Szoke E, Czeh G, Szolcsanyi J. Effect of capsaicin on voltage-gated currents of trigeminal neurones in cell culture and slice preparations. Acta Physiol Hung. 2001;88:173–196. doi: 10.1556/APhysiol.88.2001.3-4.1. [DOI] [PubMed] [Google Scholar]
- Baumann TK, Simone DA, Shain CN, LaMotte RH. Neurogenic hyperalgesia: the search for the primary cutaneous afferent fibers that contribute to capsaicin-induced pain and hyperalgesia. J Neurophysiol. 1991;66:212–227. doi: 10.1152/jn.1991.66.1.212. [DOI] [PubMed] [Google Scholar]
- Cairns BE, Hu JW, Arendt-Nielsen L, Sessle BJ, Svensson P. Sex-related differences in human pain and rat afferent discharge evoked by injection of glutamate into the masseter muscle. J Neurophysiol. 2001;86:782–791. doi: 10.1152/jn.2001.86.2.782. [DOI] [PubMed] [Google Scholar]
- Cairns BE, Sim Y-L, Bereiter DA, Sessle BJ, Hu JW. Influence of sex on reflex jaw muscle activity evoked from the rat temporomandibular joint. Brain Res. 2002a;957:338–344. doi: 10.1016/s0006-8993(02)03671-5. [DOI] [PubMed] [Google Scholar]
- Cairns BE, Gambarota G, Svensson P, Arendt-Nielson L, Berde CB. Glutamate-induced sensitization of rat masseter muscle fibers. Neurosci. 2002b;109:389–399. doi: 10.1016/s0306-4522(01)00489-4. [DOI] [PubMed] [Google Scholar]
- Cairns BE, Svensson P, Wang K, Hupfeld S, Graven-Nielsen T, Sessle BJ, Berde CB, Arendt-Nielsen Lars. Activation of peripheral NMDA receptors contributes to human pain and rat afferent discharges evoked by injection of glutamate into the masseter muscle. J Neurophys. 2003a;90:2098–2105. doi: 10.1152/jn.00353.2003. [DOI] [PubMed] [Google Scholar]
- Cairns BE, Wang K, Hu JW, Sessle BJ, Arendt-Nielsen L, Svensson P. The effect of glutamate-evoked masseter muscle pain on the human jaw-stretch reflex differs in men and women. J Orofac Pain. 2003b;17:317–325. [PubMed] [Google Scholar]
- Cairns BE, Svensson P, Wang K, Castrillon E, Hupfeld S, Sessle BJ, Arendt-Nielsen L. Ketamine attenuates glutamate-induced mechanical sensitization of the masseter muscle in human males. Exp Brain Res. 2006;169:467–472. doi: 10.1007/s00221-005-0158-z. [DOI] [PubMed] [Google Scholar]
- Cairns BE, Dong XD, Mann MK, Svensson P, Sessle BJ, Arendt-Nielsen L, McErlane KM. Systemic administration of monosodium glutamate elevates intramuscular glutamate levels and sensitizes rat masseter muscle afferent fibers. Pain. 2007 doi: 10.1016/j.pain.2007.01.023. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillon E, Cairns B, Ernberg M, Wang K, Sessle BJ, Arendt-Nielsen Lars, Svensson Peter. Effect of a Peripheral NMDA Receptor Antagonist on Glutamate-Evoked Masseter Muscle Pain and Mechanical Sensitization in Women. J Orofac Pain. 2007;21:216–224. [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- deGroot JF, Zhou S, Carlton SM. Peripheral glutamate release in the hindpaw following low and high intensity sciatic stimulation. Neuroreport. 2000;11:497–502. doi: 10.1097/00001756-200002280-00014. [DOI] [PubMed] [Google Scholar]
- Docherty RJ, Robertson B, Bevan S. Capsaicin causes prolonged inhibition of voltage-activated calcium currents in adult rat dorsal root ganglion neurons in culture. Neuroscience. 1991;40:513–521. doi: 10.1016/0306-4522(91)90137-d. [DOI] [PubMed] [Google Scholar]
- Dong XD, Mann MK, Kumar U, Svensson P, Arendt-Nielsen L, Hu JW, Sessle BJ, Cairns BE. Sex-related differences in NMDA-evoked rat masseter muscle afferent discharge result from estrogen-mediated modulation of peripheral NMDA receptor activity. Neuroscience. 2007;146:822–832. doi: 10.1016/j.neuroscience.2007.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewes AM, Helweg-Larsen S, Petersen P, Brennum J, Andreasen A, Poulsen LH, Jensen TS. McGill Pain Questionnaire translated into Danish: experimental and clinical findings. Clin J Pain. 1993;9:80–87. doi: 10.1097/00002508-199306000-00002. [DOI] [PubMed] [Google Scholar]
- Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders. Review, criteria, examinations and specifications, critique. J Craniomandib Disord Facial Oral Pain. 1992;6:301–355. [PubMed] [Google Scholar]
- Gazerani P, Andersen OK, Arendt-Nielsen L. A human experimental capsaicin model for trigeminal sensitization. Gender-specific differences. Pain. 2005;118:155–163. doi: 10.1016/j.pain.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Hagenacker T, Splettstoesser F, Greffrath W, Treede RD, Busselberg D. Capsaicin differentially modulates voltage-activated calcium channel currents in dorsal root ganglion neurones of rats. Brain Res. 2005;1062:74–85. doi: 10.1016/j.brainres.2005.09.033. [DOI] [PubMed] [Google Scholar]
- Hu JW, Fiorentino PM, Cairns BE, Sessle BJ. Capsaicin-induced inflammation within temporomandibular joint involves VR-1 receptor mechanisms. Oral Biosci Med. 2005;4:241–248. [Google Scholar]
- Ichikawa H, Fukunaga T, Jin HW, Fujita M, Takano-Yamamoto T, Sugimoto T. VR1-, VRL-1- and P2X3 receptor-immunoreactive innervation of the rat temporomandibular joint. Brain Res. 2004;1008:131–136. doi: 10.1016/j.brainres.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Jordt SE, McKemy DD, Julius D. Lessons from peppers and peppermint: the molecular logic of thermosensation. Curr Opin Neurobiol. 2003;13:487–492. doi: 10.1016/s0959-4388(03)00101-6. [DOI] [PubMed] [Google Scholar]
- Keast JR, Stephensen TM. Glutamate and aspartate immunoreactivity in dorsal root ganglion cells supplying visceral and somatic targets and evidence for peripheral axonal transport. J Comp Neurol. 2000;424:577–587. [PubMed] [Google Scholar]
- Ko MC, Tuchman JE, Johnson MD, Wiesenauer K, Woods JH. Local administration of mu or kappa opioid agonists attenuates capsaicin-induced thermal hyperalgesia via peripheral opioid receptors in rats. Psychopharmacol. 2000;148:180–185. doi: 10.1007/s002130050040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam DK, Sessle BJ, Hu JW. Program No. 1178. 2003 Abstract Viewer/Itinerary Planner. International Association for Dental Research; 2003. Trigeminal Nociceptive Neuronal Activity Modulated by Glutamate and Capsaicin Application to Rat TMJ. [Google Scholar]
- Lam DK, Sessle BJ, Hu JW. Glutamate and capsaicin-induced activation and peripheral sensitisation in deep craniofacial trigeminal nociceptive primary afferents. Program No. 294.6. 2004 Abstract Viewer/Itinerary Planner; Washington, DC. Society for Neuroscience; 2004. Online. [Google Scholar]
- Lam DK, Sessle BJ, Cairns BE, Hu JW. Peripheral NMDA receptor modulation of jaw muscle electromyographic activity induced by capsaicin injection into the temporomandibular joint of rats. Brain Res. 2005a;1046:68–76. doi: 10.1016/j.brainres.2005.03.040. [DOI] [PubMed] [Google Scholar]
- Lam DK, Sessle BJ, Cairns BE, Hu JW. Neural mechanisms of temporomandibular joint and masticatory muscle pain: a possible role for peripheral glutamate receptor mechanisms. Pain Res Manag. 2005b;10:145–152. doi: 10.1155/2005/860354. [DOI] [PubMed] [Google Scholar]
- LaMotte R, Shain CN, Simone DA, Tsai EF. Neurogenic hyperalgesia: psychophysical studies of underlying mechanisms. J Neurophysiol. 1991;66:190–211. doi: 10.1152/jn.1991.66.1.190. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Lundberg LE, Torebjork HE. Pain, hyperalgesia and activity in nociceptive C units in humans after intradermal injection of capsaicin. J Physiol. 1992;448:749–764. doi: 10.1113/jphysiol.1992.sp019068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawand NB, McNearney T, Westlund KN. Amino acid release into the knee joint: key role in nociception and inflammation. Pain. 2000;86:69–74. doi: 10.1016/s0304-3959(99)00311-5. [DOI] [PubMed] [Google Scholar]
- Lee JS, Ro JY. Peripheral metabotropic glutamate receptor 5 mediates mechanical hypersensitivity in craniofacial muscle via protein kinase C dependent mechanisms. Neuroscience. 2007;146:375–383. doi: 10.1016/j.neuroscience.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Liu L, Oortgiesen M, Li L, Simon SA. Capsaicin inhibits activation of voltage-gated sodium currents in capsaicin-sensitive trigeminal ganglion neurons. J Neurophysiol. 2001;85:745–758. doi: 10.1152/jn.2001.85.2.745. [DOI] [PubMed] [Google Scholar]
- Marchettini P, Simone DA, Caputi G, Ochoa JL. Pain from excitation of identified muscle nociceptors in human. Brain Res. 1996;740:109–116. doi: 10.1016/s0006-8993(96)00851-7. [DOI] [PubMed] [Google Scholar]
- Morgan CR, Rodd HD, Clayton N, Davis JB, Boissonade FM. Vanilloid receptor 1 expression in human tooth pulp in relation to caries and pain. J Orofac Pain. 2005;19:248–260. [PubMed] [Google Scholar]
- Petersen M, LaMotte RH. Relationships between capsaicin sensitivity of mammalian sensory neurons, cell size and type of voltage gated Ca-currents. Brain Res. 1991;561:20–26. doi: 10.1016/0006-8993(91)90744-g. [DOI] [PubMed] [Google Scholar]
- Raja SN, Meyer RA, Ringkamp M, Campbell JN. Textbook of Pain. Fourth Edition. Toronto: Churchill Livingstone; 1999. pp. 11–57. [Google Scholar]
- Rosendal L, Larsson B, Kristiansen J, Peolsson M, Sogaard K, Kjaer M, Sorensen J, Gerdle B. Increase in muscle nociceptive substances and anaerobic metabolism in patients with trapezius myalgia: microdialysis in rest and during exercise. Pain. 2004;112:324–334. doi: 10.1016/j.pain.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Sikand P, Premkumar LS. Potentiation of glutamatergic synaptic transmission by protein kinase C mediated sensitization of TRPV1 at the first sensory synapse. J Physiol. 2007 doi: 10.1113/jphysiol.2006.118620. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone DA, Sorkin LA, Oh U, Chung JM, Owens C, LaMotte RH, Willis WD. Neurogenic hyperalgesia: central correlates in responses of spinothalamic tract neurons. J Neurophysiol. 1991;66:228–246. doi: 10.1152/jn.1991.66.1.228. [DOI] [PubMed] [Google Scholar]
- Simone DA, Marchettini P, Ochoa JL. Primary afferent nerve fibers that contribute to muscle pain sensation in human. Pain Forum. 1997;6:207–212. [Google Scholar]
- Sluka KA. Stimulation of deep somatic tissue with capsaicin produces long-lasting mechanical allodynia and heat hypoalgesia that depends on early activation of the cAMP pathway. J Neurosci. 2002;22 doi: 10.1523/JNEUROSCI.22-13-05687.2002. 56875693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson P, Arendt-Nielsen L, Nielsen H, Larsen JK. Effect of chronic and experimental jaw muscle pain on pain-pressure thresholds and stimulus-response curves. J Orofac Pain. 1995;9:347–356. [PubMed] [Google Scholar]
- Svensson P, Cairns BE, Wang K, Hu JW, Graven-Nielsen T, Arendt-Nielsen L, Sessle BJ. Glutamate-evoked pain and mechanical allodynia in the human masseter muscle. Pain. 2003;101:221–227. doi: 10.1016/S0304-3959(02)00079-9. [DOI] [PubMed] [Google Scholar]
- Svensson P, Wang K, Arendt-Nielsen L, Cairns BE, Sessle BJ. Pain effects of glutamate injections into human jaw or neck muscles. J Orofac Pain. 2005;19:109–118. [PubMed] [Google Scholar]
- Tang ML, Haas DA, Hu JW. Capsaicin-induced joint inflammation is not blocked by local anesthesia. Anesth Prog. 2004;51:2–9. [PMC free article] [PubMed] [Google Scholar]
- Tominaga M, Tominaga T. Structure and function of TRPV1. Pflugers Arch. 2005;451:143–150. doi: 10.1007/s00424-005-1457-8. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-modulating stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Wang K, Arendt-Nielsen L, Svensson P. Capsaicin-induced muscle pain alters the excitability of human jaw-stretch reflex. J Dent Res. 2002;81:650–654. doi: 10.1177/154405910208100915. [DOI] [PubMed] [Google Scholar]
- Witting N, Svensson P, Arendt-Nielsen L, Jensen TS. Repetitive intradermal capsaicin: differential effect on pain and areas of allodynia and punctate hyperalgesia. Somatosens Mot Res. 2000a;17:5–12. doi: 10.1080/08990220070256. [DOI] [PubMed] [Google Scholar]
- Witting N, Svensson P, Gottrup H, Arendt-Nielsen L, Jensen TS. Intramuscular and intradermal injection of capsaicin: a comparison of local and referred pain. Pain. 2000b;84:407–412. doi: 10.1016/s0304-3959(99)00231-6. [DOI] [PubMed] [Google Scholar]