Abstract
Study Objective:
To examine the effects of fear extinction on subsequent sleep in rats and to compare it with the effects seen following contextual reminders of fear.
Design:
Habituation of the rats to handling and baseline recordings were obtained over 2 consecutive days. Afterward, the rats were subjected to shock training (ST; day 1), context reexposure (CR; either 30 or 60 min; day 2), and fear recall (R; day 3). Percentage time spent in freezing (FT%) was observed during ST, CR, and R exposures. Sleep was recorded for 20 h (8-h light and 12-h dark period) following ST, CR, and R.
Setting:
NA
Subjects:
The subjects were outbred Wistar rats randomly assigned to one of two groups: contextual fear (FR; n = 7) or contextual extinction (EXT; n = 7).
Interventions:
The rats were surgically implanted with electrodes for recording the electroencephalogram and electromyogram for determining arousal state.
Measurements and Results:
There were no differences between groups on FT% during ST or the first 30 min of CR; however, during R, the FR group had greater FT% than EXT. Sleep did not differ between groups following ST. Following CR, EXT exhibited significantly more total sleep, NREM, and REM than FR. After R, there were no differences between groups.
Conclusions:
Rats that exhibit extinction of contextual fear show significantly increased sleep compared to rats who continue to exhibit contextual fear. This suggests that sleep disturbances normally experienced in humans following traumatic events or reminders may be ameliorated by therapies that address and eliminate the associated fear.
Citation:
Wellman LL; Yang L; Tang X; Sanford LD. Contextual fear extinction ameliorates sleep disturbances found following fear conditioning in rats. SLEEP 2008;31(7):1035-1042.
Keywords: Extinction, contextual fear conditioning, sleep
FEARFUL EXPERIENCES CAN LEAD TO ENDURING ALTERATIONS IN EMOTION AND BEHAVIOR IN HUMANS1 AND ANIMALS.2–5 FEAR CONDITIONING ASSOCIATED with these experiences is thought to be important in the development of pathophysiology underlying anxiety disorders and posttraumatic stress disorder (PTSD).9–11 Moreover, disturbances in sleep often follow a stressful or traumatic event,6 and the persistence of sleep disturbances may be predictive for future psychiatric and physical pathology.6–8 In animals, fear conditioning associated with stressful footshock training also produces marked changes in subsequent sleep.10,12,13 Specifically, the original shock training is followed by a significant decrease in subsequent REM sleep.10,14,15 Conditioned “reminders” of the shock training also decrease subsequent REM.10,14
Experimental conditioned fear involves the use of specific cues such as tone or light that presage the occurrence of footshock or less specific contextual stimuli which become associated with shock. Through pairings of cue or contextual stimuli with the occurrence of shock, these previously neutral stimuli acquire the ability to elicit a conditioned fear response (behavioral and physiological responses indicative of fear and anxiety such as behavioral freezing) when presented alone. Interestingly, though, the conditioned fear response produced by fearful cues and contexts can typically be blocked through extended presentations of either type of stimuli without the reoccurrence of footshock. This fear “extinction” is considered a type of new learning that inhibits subsequent fear without erasing the original memory for fear conditioning.16
The failure of extinction is thought to be a significant factor in persisting fear responses and anxiety.17 However, even though disturbed sleep has been associated with the continuing effects of stress, and several studies have demonstrated the effects of conditioned fear on sleep, the effects of fear extinction on subsequent sleep have yet to be examined. Therefore, the goal of this study was to determine whether fear extinction was followed by different patterns of sleep compared to those after continued fear.
We trained 2 groups of rats in contextual fear using a footshock stressor and then re-exposed both groups to the fearful context alone. One group was removed from the context before extinction occurred and the other was allowed to remain in the fearful context until behavioral signs of fear (freezing) had completely subsided. Lastly, we recorded sleep in both groups after a second exposure to the fearful context without readministering footshock. This allowed us to compare the sleep of rats with extinguished fear behavior to that of rats which continued to show fear. We also examined both groups for similarities and differences in freezing during shock training and on reexposure to the context. These data demonstrate that fear extinction is associated with significantly improved sleep compared to continued fear, and may have implications for understanding the role of persisting sleep disruptions in the long-term effects of stress and trauma.
METHODS
Subjects
The subjects were 14 ninety-day-old Wistar rats obtained from Harlan (Indianapolis, IN). Upon arrival, the rats were individually housed in polycarbonate cages and given ad lib access to food and water. The rooms were kept on a 12:12 light: dark cycle with lights on from 07:00 to 19:00. Light intensity during the light period was 100–110 lux and less than 1 lux during the dark period. Ambient temperature was maintained at 24.5 ± 0.5 °C.
Surgery
Beginning one week following arrival, the rats were anesthetized with isoflurane (5% induction; 2% maintenance) and implanted with skull screw electrodes for recording their electroencephalogram (EEG) and stainless steel wire electrodes sutured to the dorsal neck musculature for recording their electromyogram (EMG). Leads from the recording electrodes were routed to a 9-pin miniature plug that was affixed to the skull with stainless steel screws and dental acrylic. Ibuprofen (15 mg/kg weight) was available in their water supply for relief of postoperative pain. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Experimental Animals and were approved by Eastern Virginia Medical School's Animal Care and Use Committee (Protocol # 07-005).
Procedures
Sleep Recording
For recording sleep, each animal, in its home cage, was placed in a chamber outfitted for electrophysiological recording and a lightweight, shielded cable was connected to the miniature plug on the rat's head. The cable was attached to a swivel that permitted free movement of the rat within its cage. EEG and EMG signals were processed by a Grass Model 12 polygraph equipped with model 12A5 amplifiers and routed to an A/D board (Eagle PC30) housed in a Pentium class PC. The signals were digitized at 128 Hz and collected in 10-s epochs using a custom sleep data collection program.
The rats were allowed a post surgery recovery period of 14 days prior to beginning the experiment. The rats were then habituated to the recording cable and chamber over 2 consecutive days and baseline sleep (BL) was recorded on the second day.
Fear Conditioning
The rats were randomly assigned to one of two groups (contextual fear, FR or contextual extinction, EXT). On experimental Day 1, both groups received a single shock-training session (ST) which lasted 30 min. During this procedure, individual rats were placed in shock chambers (Coulbourn Habitest cages equipped with grid floors (Model E10-18RF) that were housed in Coulbourn Isolation Cubicles (Model H10-23)) and were allowed to freely explore for 5 min. Over the next 20 min, they were presented with 20 footshocks (0.8 mA, 0.5-s duration) at 1.0-min intervals. Shock was produced by Coulbourn Precision Regulated Animal Shockers (Model E13-14) and presented via the grid floor of the shock chamber. Five min after the last shock, the rats were returned to their home cages. The following day (Day 2), the rats were placed back in the shock chambers (context reexposure, C) and allowed to explore freely for 30 min (FR group) or 1 h (EXT group) with no shock presented before being returned to their home cage. The next day (Day 3) the rats (both groups) were placed in the shock chambers for 30 min with no shock presented to test their recall of fear for the context (recall, R). The shock chamber was thoroughly cleaned with diluted alcohol following each session. Each session was videotaped using mini video cameras (Weldex, WDH-2500BS, 3.6-mm lens) attached to the center ceiling of the shock chamber for subsequent visual scoring of freezing.
All experimental manipulations were conducted during the forth h of the light period, such that sleep recording would begin at the start of the fifth hour. This resulted in 20 h of recording on each experimental day (8 h in the light period and 12 h in the dark period).
Home cages were changed at least 1 day prior to fear conditioning and were not otherwise disturbed for the remainder of the experiment. The same room was used for animal housing and sleep recording. The ST session and reexposure to the context on Days 2 and 3 were conducted in a separate room from that used for recording.
Determination of Freezing and Sleep
During the experimental sessions, freezing, defined as the absence of body movement except for respiration,18–20 was scored from videotape by a trained observer. Freezing was scored in 5-s intervals during 1.0-min observation periods at selected intervals over the course of the 30 min the rats were in the shock chamber. For ST, the preshock and postshock periods were scored for freezing at 1, 3, and 5 min. During shock presentation, freezing was scored following selected shock training trials (1, 2, 9, 10, 19, and 20). For day 2, freezing was scored every other min and processed in 30-min time periods giving one period (P1) for FR and 2 periods (P1 & P2) for EXT. For day 3, freezing was also scored every other minute and processed in a single 30-min time period (P1). The percentage time spent in freezing (freezing time%, FT%) was calculated (freezing time/observed time*100%) for each animal for each observation period.
Computerized EEG and EMG records were visually scored in 10-s epochs by trained observers to determine wakefulness, NREM, and REM. Wakefulness was scored based on the presence of low-voltage, fast EEG and high amplitude, tonic EMG levels. NREM was characterized by the presence of spindles interspersed with slow waves, lower muscle tone, and no gross body movements. REM was scored continuously during the presence of low voltage, fast EEG, theta rhythm, and muscle atonia. The following sleep parameters were examined in the data analyses: total NREM (min), total REM (min); total sleep (REM + NREM), latency to NREM or REM, number of NREM or REM episodes, and REM% (REM/total sleep*100).
Data Analyses
Statistical analyses were conducted using SigmaStat (SPSS, Inc). Comparisons involving the entire light or dark periods within groups and across days were conducted using one-way repeated measures analysis of variance (ANOVA) procedures. Comparisons between groups across days were conducted using one-way between factors ANOVAs. Comparisons of 4-h blocks of time (block1 and block 2) within treatment days were conducted using two-way (day x block) ANOVAs with repeated measures on both factors. Comparisons between FR and EXT groups were conducted using two-way (condition x time) mixed factor ANOVAs with repeated measures on time. The Tukey test was used when all pairwise comparisons among means were considered. For freezing, two-tailed t-tests or paired t-tests were performed.
RESULTS
Freezing
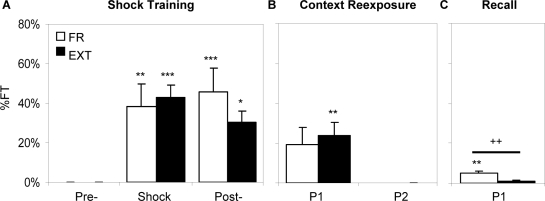
Figure 1 presents comparisons of FT% during ST, C, and R. For ST, the data were divided into 3 distinct periods: preshock, shock, and postshock. For C, the data were grouped into 30-min blocks, with period P1 (30 min) for FR and periods P1–P2 (60 min) for EXT. For R, the data were considered as a single 30-min block (for both FR and EXT, P1). Each bar in Figure 1 represents the mean FT% per min averaged across the number of 1-min observations obtained in that period. Measures during the pre-shock period, in which naïve animals were initially placed in the shock chamber, were used as a baseline to determine whether the animals exhibited significant fearful behaviors in the shock and post-shock periods as well as during C and R. Data within and between groups were compared using two-tailed t-tests.
Figure 1.
Percent time spent in freezing behavior (FT%) plotted in the time block used for analysis. Note that the plot for shock training (A) shows the freezing during the pre-shock, shock, and post-shock period. Context reexposure (B) and Recall (C) are plotted in 30-min time blocks. Values are mean ± SEM. Differences relative to pre-shock period of ST: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. Differences between the two groups FR and EXT: ++ P ≤ 0.01.
Contextual Fear (FR) Group
On day 1 (ST), FT% during the shock trials as well as postshock was significantly higher than that observed preshock (Figure 1A; P = 0.002 and P < 0.001 respectively). On day 2 (C), FT% showed a trend of being greater than that observed preshock that just missed significance (Figure 1B; P = 0.058). This lack of significance is likely due to variability, since all animals exhibited some level of freezing behavior during C, however, none of the animals froze during the preshock period. Finally, on day 3 (R), FT% was significantly greater than that observed preshock (Figure 1C; P = 0.005).
Contextual Extinction (EXT) Group
On day 1 (ST), FT% during the shock trials as well as postshock was significantly higher than that observed preshock (Figure 1A; P < 0.001 and P = 0.016 respectively). On day 2 (C), FT% during P1 (the first 30 min) was significantly greater than that observed preshock (Figure 1B; P = 0.01), however, FT% during P2 (the second 30 min) was not significantly different from that of preshock (P = 1.0). This is indicative of the loss of fear by the end of the context reexposure in the EXT group. Finally, on day 3 (R), FT% was again not significantly greater than that observed preshock (Figure 1C; P = 0.25).
FR Group vs EXT Group
There were no significant differences between FR and EXT when analyzing FT% during preshock (P = 1.0), ST (P = 0.676), postshock (P = 0.163), nor P1 of CR (P = 1.0). However, on day 3 (R), FR exhibited significantly greater FT% than did EXT (P = 0.008).
Sleep
Contextual Fear (FR) Group
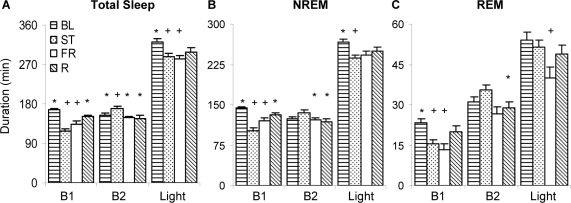
Significant changes in sleep were only observed during the light period in the FR group. There was a significant interaction of condition x 8 h light period for total sleep duration (F3,17=5.168, P = 0.01; Figure 2A) due to a decrease in total sleep following ST and CR compared to BL (P = 0.025 and P = 0.01 respectively). Moreover, there was a significant interaction of condition x 4 h block for total sleep duration (F3,15 = 16.694, P = 0.01; Figure 2A). In the first 4 h, total sleep duration was decreased following ST compared to BL (P < 0.001) and R (P = 0.001) and decreased following CR compared to BL (P < 0.001). In the second 4 h, total sleep duration was increased following ST compared to BL (P = 0.048), CR (P = 0.013), and R (P = 0.004).
Figure 2.
Average total sleep (A), NREM sleep (NREM, B), or REM sleep (REM, C) broken into two 4-h blocks (B1 & B2) or the entire 8-h light period (LIGHT) during baseline (BL) sleep or following shock training (ST), 30-min context reexposure (FR), or retest (R). Values are mean ± SEM. Differences relative to BL: +P ≤ 0.05; differences relative to ST: *P ≤ 0.05.
Similarly there was a significant interaction of condition x 8 h light period as well as condition x 4 h block for NREM duration (F3,17 = 3.967, P = 0.026 and F3,15 = 14.685, P < 0.001, respectively Figure 2B). During the entire light period, NREM following ST was significantly less than BL (P = 0.02). Furthermore, in the first 4 h following ST, there was a decrease in NREM duration compared to BL (P < 0.001) and R (P = 0.001), as well as a decrease in number of episodes (ST: 25.4 ± 1.1) compared to BL (34 ± 1, P = 0.009) and R (32.1 ± 3, P = 0.03). A decrease in NREM duration was also observed following CR compared to BL (P = 0.002). In the second 4 h following ST, NREM duration was increased compared to CR (P = 0.036) and R (P = 0.012); latency to NREM following ST (69 ± 9.4) was significantly greater than BL (7.4 ± 1.5, P< 0.001), CR (36.4 ± 5.2, P = 0.048) and R (17.5 ± 3.6, P < 0.001).
Finally, there was a significant interaction of condition x 8 h light period (F3,17 = 3.248, P = 0.048) as well as condition x 4 h block for duration (F3,15 = 6.637, P = 0.005; Figure 2C) and episodes (F3,15 = 3.550, P = 0.04) of REM. This was evident during the entire light period with a decrease in REM duration following CR compared to BL (P = 0.044). In the first 4 h, there was a decrease in REM duration following ST and CR compared to BL (P = 0.018 and P = 0.012 respectively). In the second 4 h following ST, REM duration was increased compared to R (P = 0.045). Furthermore, latency to REM following ST (117.6 ± 13.9) was significantly greater than BL (31.6 ± 8.4, P < 0.001), CR (69.2 ± 7.4, P < 0.001) and R (52.9 ± 8.6, P < 0.001) and following CR was significantly greater than BL (P = 0.016).
Contextual Extinction (EXT) Group
Significant changes in sleep were observed during both the light period and dark period. There was a significant interaction of condition x 4 h block for total sleep duration (F3,18 = 7.190, P = 0.002) during the light period (Figure 3A). Total sleep duration was increased following CR and R compared to ST in the first 4h (P = 0.001 and P = 0.011 respectively). During the dark period, there was a significant interaction of condition x 12 h dark period (F3,18 = 4.816, P = 0.012) due to increased total sleep duration following ST (267 ± 27) and CR (258 ± 25) compared to BL (188 ± 32, P = 0.013 and P = 0.032 respectively).
Figure 3.
Average total sleep (A) NREM sleep (NREM, B) or REM sleep (REM, C) broken into two 4-h blocks (B1 & B2) or the entire 8-h light period (LIGHT) during baseline (BL) sleep or following shock training (ST), 60-min context reexposure (EXT), or retest (R). Values are mean ± SEM. Differences relative to BL: +P ≤ 0.05; differences relative to ST: *P ≤ 0.05.
There was also a significant interaction of condition x 4 h block for NREM duration (F3,18 = 5.942, P = 0.005) during the light period (see Figure 3B). In the first 4 h, there was an increase in CR and R NREM duration compared to ST (P = 0.004 and P = 0.031 respectively) and NREM number of episodes (ST: 26 ± 1.86; CR: 34.6 ± 1.46, P = 0.015; R: 37.6 ± 1.69, P< 0.001). Furthermore, latency to NREM was significantly shorter following CR (11.1 ± 3.5) and R (R: 4.1 ± 1.2) than ST (58.5 ± 16; P = 0.026 and P = 0.009 respectively). During the dark period, there was a significant interaction of condition x 12 h dark period (F3,18 = 3.522, P = 0.036) due to increased NREM duration following ST (212 ± 21) compared to BL (155 ± 29, P = 0.032).
Finally, during the light period there was a significant interaction of condition x 4 h block for REM duration (F3,18 = 7.820, P = 0.002; Figure 3C) and REM episodes (F3,18 = 4.202, P = 0.02) as well as a significant interaction of condition x 8 h light period for REM duration (F3,18 = 4.254, P = 0.019). This was evident during the entire light period with an increase in REM duration following CR compared to ST (P = 0.021) and a trend towards a decrease in REM duration following ST compared to BL (P = 0.054). In the first 4 h, a significant decrease following ST compared to BL, CR, and R was observed in REM duration ( P < 0.001, P < 0.001, and P = 0.012 respectively), and number of REM episodes (ST: 6.29 ± 1.54; BL: 12.7 ± 2, P = 0.002; CR: 12 ± 1.02, P = 0.003; R: 11.1 ±1.06, P = 0.011). Moreover, REM% was significantly decreased following ST (8.8% ± 1.7%) compared to BL (15.9% ± 2%, P < 0.001) and CR (13.1% ± 1.1%, P = 0.028) during the first 4 h and compared to BL during the light period (ST: 14.6% ± 1.2%; BL: 17.9% ± 1%, P = 0.019). In addition, latency to REM was significantly longer following ST (132.5 ± 23.3) than BL (51.7 ± 7.6, P < 0.001), CR (63.6 ± 9.4, P < 0.001), and R (52.2 ± 7.8, P < 0.001). During the dark period there was a significant interaction of condition x 12 h dark period for REM duration (F3,18 = 7.281, P = 0.002) due to a significant increase in REM following ST (55.5 ± 6.1) and CR (56.3 ± 5.6) compared to BL (33.2 ± 3.4, P = 0.005 and P = 0.003 respectively). There was also a significant interaction of condition x 12 h dark period for REM episodes (F3,18 = 5.031, P = 0.01) due to a significant increase in episodes following ST (36 ± 4.6) and CR (33 ± 3.5) compared to BL (22.3 ± 2.6, P = 0.008 and P = 0.045 respectively).
FR Group vs EXT Group
There were no significant differences between FR and EXT groups for duration of total sleep or REM in BL during the 8 h light period or 12 h dark period. There was however a significant difference (P = 0.046) in light period NREM between FR (267 ± 4.6) and EXT (234 ± 13). There were no significant differences between FR and EXT groups for duration of total sleep, NREM, or REM following ST during the 8 h light period or 12 h dark period. Moreover there were no significant differences for these parameters following R during the 8 h light period or 12 h dark period.
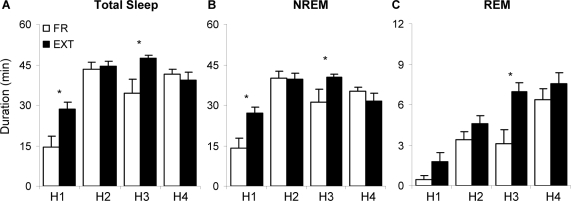
When comparing sleep following CR, there was a significant difference in duration of total sleep during the light period (P = 0.006). This can be further seen when analyzing condition x hour interactions (F7,96 = 2.367, P = 0.028). Specifically, total sleep duration for EXT compared to FR (Figure 4A) was significantly greater during H1 (Tukey, P < 0.001) and H3 (P = 0.001).
Figure 4.
Average total sleep (A) NREM sleep (NREM, B) or REM sleep (REM, C) for each hour over the first 4 h (H1&H4) following context reexposure of either 30 min (FR) or 60 min (EXT). Values are mean ± SEM. *P ≤ 0.05.
During the light period, NREM duration (P = 0.049) was significantly greater for EXT than for FR. An interaction of condition x hour was also present for NREM duration (F7,96 = 2.438, P = 0.024) which can be further explained by a greater NREM duration during H1 & H3 following extinction versus fear (Figure 4B; P < 0.001 and P = 0.013 respectively) as well as a greater number of NREM episodes in H1 (EXT: 8.3 ± 1.4; FR: 4 ± 1; Tukey, P = 0.003). Finally, latency to NREM was significantly shorter following CR for EXT compared to FR (P = 0.01).
During the light period, REM duration was also significantly greater for EXT than for FR (P = 0.041). A greater REM duration during H3 (Figure 4C; Tukey, P = 0.004) and a greater REM% during H3 (EXT: 14.5 ± 1.4; FR: 8.1 ± 2.7; Tukey, P = 0.037) were also observed EXT versus FR animals following CR.
DISCUSSION
Similar alterations in sleep were observed following shock training in both FR and EXT groups including a decrease in REM duration in the first 4 h of sleep. However when reexposed to the shock context, the subsequent sleep of FR and EXT animals was drastically different. In the FR group, total sleep, NREM and REM continued to be decreased in the first 4 h following context exposure similar to that observed after shock training. Conversely, the EXT group showed levels of total sleep, NREM, and REM in the first 4 h following context exposure that did not differ from baseline sleep levels.
Fear-Induced Changes in Behavior
The behavioral measure most often used to assess fear during shock training or context reexposure has been freezing, with greater freezing percentage interpreted as indicating stronger fear reactions.18–20 Freezing during the shock and postshock period was significantly more than the preshock period in all animals, indicating that both groups acquired conditioned fear to the shock. Moreover, FT% was initially high during context reexposure in both groups and decreased over the duration in the chamber with the EXT group showing a complete cessation of freezing by the end of the hour long session indicating that extinction occurred. On the recall day, there was a significant difference between FR and EXT freezing indicating that the FR group was more fearful than the EXT group when presented with the fearful context on day 3. Furthermore, the difference in freezing observed on day 3 signifies that the FR group did not experience extinction to the context during the 30-min CR on day 2. Unfortunately there was a large amount of variability between animals and on Day 2, the FR group freezing was not significant compared to pre-shock levels (P = 0.058) which may be thought to suggest that the FR group was not fearful on during context reexposure on day 2. However, despite the lack of significance, the plots demonstrate that no freezing observed in the FR animals during the preshock period whereas freezing was evident in all animals (2%-66%) in the CR period. Jha et al.21 suggest that REM may be a more sensitive measure of fear than freezing, a suggestion supported by our data in this study as well as others from our lab.14 Future studies using physiological indices such as the EKG or respiratory measures to assess fear responses could provide additional parametric measures to address this issue.
Fear-Induced Changes in Sleep
Previous findings10,14,22 indicate that changes in REM found following context reexposure are similar to those observed following shock training in rats. In the FR group there was a significant reduction in REM sleep during the first 4 h of light period sleep following shock training and context reexposure compared to baseline sleep. Moreover, during the entire light period a decrease in REM duration was observed following context versus baseline. This is consistent with previous findings in rats and mice14,23 that the decrease in REM seen initially following fear conditioning or context reexposure is not recovered later in sleep, and in this case, following context reexposure, was further decreased. This finding is especially interesting as the animals showed a further decrease in REM during a time when there should be greater homeostatic pressure for a return to normal REM levels.
In contrast to the findings for the FR group, the EXT group showed an increase in REM duration and number of episodes as well as an increase in REM% during the first 4 h following extinction compared to shock training. Since extinction is a form of learning,16 this finding may be seen as a complement to other studies which found an increase in REM following various learning paradigms.21,24–28 It is important to note however that fear conditioning itself is a learning paradigm which induces a predictable reduction in REM. Most likely the type of learning (and the characteristics of stress response accompanying it) determines the ultimate change found in subsequent REM.
Additionally, the increase in REM, indicating more normal sleep, following context reexposure for the EXT group supports our notion that extinction did readily occur and that the animals are no longer fearful of the context. The longer duration of context reexposure may have impacted the stress response of these animals as well (as REM is a very sensitive indicator for stress21), however, we did not collect other indices of the stress response in this study. Further studies will be necessary to utilize physiological measures of stress to assess the stress levels of these animals following extinction.
While reductions in REM after shock training and reexposure to fearful contexts have been consistent findings in both rats10,14,22 and mice,23 the changes in NREM across studies have been more variable. Previously our lab showed a significant decrease in NREM following shock and context exposure relative to baseline in Wistar rats.14 In the current study, the contextual fear group also showed a significant decrease in NREM duration following shock training during the light period and a trend towards a decrease (P = 0.078) in NREM duration following context reexposure compared to baseline sleep. The slight discrepancy between the two studies could be due to differences in the experimental procedure. In the present study rats were placed in the shock context 24 h after shock training for CR whereas the previous study waited 4-5 days between training and context reexposure. Interestingly, we found an increase in NREM in the first 4 h following extinction and recall versus shock training in our EXT group.
Overall, the EXT group exhibited more total sleep, NREM, and REM following CR than did FR. Moreover, there was a shorter latency to NREM following extinction than CR. Taken together, this further suggests a reduced/eliminated fear in the extinguished animals compared to their fearful counterparts. It also supports the notion that 30-min context reexposure is not sufficient for extinguishing the fear response.
A potential concern in the experimental design we used was that there was a difference in the amount of time the FR and EXT groups experienced CR. The most obvious issue is the difference in the amount of time the animals are potentially awake across groups due to the FR group being exposed to CR 30 min and the EXT group being exposed to 60 min. Unfortunately, it was not possible to control for time or arousal without introducing additional variables and potentially more stress to the CR animals. We did however examine sleep in a small number of animals that experienced identical handling and the context (mock condition [CRM]), but never received shock (FRM n = 5, EXTM n = 4) and there were no significant between group differences following CRM in total sleep (FRM: 318.8 ± 5.2; EXTM: 309.5 ± 5.9; P = 0.391), NREM (FRM: 263.1 ± 3.7; EXTM: 251.5 ± 4.1; P = 0.105), or REM (FRM: 55.7 ± 8.7; EXTM: 57.7 ± 5.1; P = 0.866) sleep during the light period. Thus, we are reasonably confident that our results are indicative of the effects of CR and not are due to differences in total waking time. Moreover, there was potential for the FR group to begin to extinguish during the 30-min CR. Yet despite the potential for extinction, the animals continued to show changes in sleep similar to those observed following shock training, again supporting our contention that 30-min context is insufficient for behavior altering extinction to occur.
Stressful and traumatic events are often followed by disturbances in sleep and the persistence of sleep disturbances may be predictive for future psychiatric pathology. PTSD is one such pathology in which a core feature (and common grievance) is insomnia.29 Because fear conditioning resembles PTSD,4,10 the repeated presentation of the CS is often used to treat PTSD patients by extinguishing the conditioned fear responses.30,31 However, to our knowledge, this is the first study examining the effects of extinction on subsequent sleep. Our data clearly show increased sleep (both NREM and REM) following an extinction paradigm to levels indicative of normal sleep. While this issue has not been examined in humans, our data suggest that exposure therapy in PTSD patients may not only decrease their behavioral symptoms but could also alleviate their sleep disturbances as well.
Although we did not look at long-term spontaneous recovery in this study, we did examine short-term spontaneous recovery on the recall day of the EXT group. In this setting we did not find evidence of spontaneous recovery of fear in the EXT group. A future study is necessary to look at further time points to evaluate if or when fear returns in the EXT group. Evaluating whether CR animals spontaneously remit to being fearful in a shorter period of time than EXT animals could potentially help answer questions concerning the importance of timing and length of exposure therapy for successfully treating PTSD patients.
Conclusion
Our data indicate that contextual fear in rats decreases subsequent REM sleep (a potentially good indicator of stress). Furthermore, contextual extinction in rats increases subsequent sleep (both NREM and REM) to normal levels. When compared, the two groups showed significantly different amounts of total sleep, NREM, and REM. These findings suggest that extinction does produce a sleep pattern that is distinctive from that observed after fearful reminders that are not extinguished. Moreover, our results suggest that sleep disturbances normally experienced following traumatic events or reminders may be ameliorated by therapies that address and eliminate the associated fear.
ACKNOWLEDGMENTS
This work was supported by NIH research grants MH64827 and MH61716 and EVMS institutional funds.
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
References
- 1.Shalev AY. Biological responses to disasters. Psychiatr Q. 2000;71:277–88. doi: 10.1023/a:1004686211979. [DOI] [PubMed] [Google Scholar]
- 2.Van Dijken H, Mos J, van der Heyden J, Tilders F. Characterization of stress-induced long-term behavioural changes in rats: evidence in favor of anxiety. Physiol Behav. 1992;52:945–51. doi: 10.1016/0031-9384(92)90375-c. [DOI] [PubMed] [Google Scholar]
- 3.Adamac R, Shallow T. Lasting effects on rodent anxiety of a single exposure to a cat. Physiol Behav. 1993;54:101–09. doi: 10.1016/0031-9384(93)90050-p. [DOI] [PubMed] [Google Scholar]
- 4.Pynoos R, Ritzmann R, Steinberg A, Goenjian A, Prisecaru I. A behavioral animal model of posttraumatic stress disorder featuring repeated exposure to situational reminders Biol Psychiatry. 1996;39:129–34. doi: 10.1016/0006-3223(95)00088-7. [DOI] [PubMed] [Google Scholar]
- 5.Van den Berg C, Lamberts R, Wolterink G, Wiegant V, Van Ree J. Emotional and footshock stimuli induce differential long-lasting behavioural effects in rats; involvement of opioids. Brain Res. 1998;799:6–15. doi: 10.1016/s0006-8993(98)00397-7. [DOI] [PubMed] [Google Scholar]
- 6.Lavie P. Sleep disturbances in the wake of traumatic events. N Engl J Med. 2001;345:1825–32. doi: 10.1056/NEJMra012893. [DOI] [PubMed] [Google Scholar]
- 7.Korte SM. Corticosteroids in relation to fear, anxiety and psychopathology. Neurosci Biobehav Rev. 2001;25:117–42. doi: 10.1016/s0149-7634(01)00002-1. [DOI] [PubMed] [Google Scholar]
- 8.Koren D, Arnon I, Lavie P, Klein E. Sleep complaints as early predictors of posttraumatic stress disorder: a 1-year prospective study of injured survivors of motor vehicle accidents. Am J Psychiatry. 2002;159:855–57. doi: 10.1176/appi.ajp.159.5.855. [DOI] [PubMed] [Google Scholar]
- 9.Charney D, Deutch A. A functional neuroanatomy of anxiety and fear: implications for the pathophysiology and treatment of anxiety disorders. Crit Rev Neurobiol. 1996;10:419–46. doi: 10.1615/critrevneurobiol.v10.i3-4.70. [DOI] [PubMed] [Google Scholar]
- 10.Pawlyk AC, Jha SK, Brennan FX, Morrison AR, Ross RJ. A rodent model of sleep disturbances in posttraumatic stress disorder: the role of context after fear conditioning. Biol Psychiatry. 2005;57:268–77. doi: 10.1016/j.biopsych.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry. 2006;60:376–82. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Sanford LD, Fang J, Tang X. Sleep after differing amounts of conditioned fear training in BALB/cJ mice. Behav Brain Res. 2003;147:193–202. doi: 10.1016/s0166-4328(03)00180-3. [DOI] [PubMed] [Google Scholar]
- 13.Tang X, Liu X, Yang L, Sanford LD. Rat strain differences in sleep after acute mild stressors and short-term sleep loss. Behav Brain Res. 2005;160:60–71. doi: 10.1016/j.bbr.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Tang X, Yang L, Sanford LD. Rat strain differences in freezing and sleep alterations associated with contextual fear. Sleep. 2005;28:1235–44. doi: 10.1093/sleep/28.10.1235. [DOI] [PubMed] [Google Scholar]
- 15.Sanford LD, Tang X, Ross RJ, Morrison AR. Influence of shock training and explicit fear-conditioned cues on sleep architecture in mice: strain comparison. Behav Genet. 2003;33:43–58. doi: 10.1023/a:1021051516829. [DOI] [PubMed] [Google Scholar]
- 16.Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11:485–94. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- 17.Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–50. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- 18.Blanchard RJ, Blanchard DC. Crouching as an index of fear. J Comp Physiol Psychol. 1969;67:370–5. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- 19.Blanchard RJ, Blanchard DC. Passive and active reactions to fear-eliciting stimuli. J Comp Physiol Psychol. 1969;68:129–35. doi: 10.1037/h0027676. [DOI] [PubMed] [Google Scholar]
- 20.Doyere V, Gisquet-Verrier P, de Marsanich B, Ammassari-Teule M. Age-related modifications of contextual information processing in rats: role of emotional reactivity, arousal and testing procedure. Behav Brain Res. 2000;114:153–65. doi: 10.1016/s0166-4328(00)00223-0. [DOI] [PubMed] [Google Scholar]
- 21.Jha SK, Brennan FX, Pawlyk AC, Ross RJ, Morrison AR. REM sleep: a sensitive index of fear conditioning in rats. Eur J Neurosci. 2005;21:1077–80. doi: 10.1111/j.1460-9568.2005.03920.x. [DOI] [PubMed] [Google Scholar]
- 22.Morrison AR, Sanford LD, Ross RJ. The amygdala: a critical modulator of sensory influence on sleep. Biol Signals Recept. 2000;9:283–96. doi: 10.1159/000014652. [DOI] [PubMed] [Google Scholar]
- 23.Sanford LD, Yang L, Tang X. Influence of contextual fear on sleep in mice: a strain comparison. Sleep. 2003;26:527–40. doi: 10.1093/sleep/26.5.527. [DOI] [PubMed] [Google Scholar]
- 24.Smith C, Rose GM. Posttraining paradoxical sleep in rats is increased after spatial learning in the Morris water maze. Behav Neurosci. 1997;111:1197–204. doi: 10.1037//0735-7044.111.6.1197. [DOI] [PubMed] [Google Scholar]
- 25.Smith C, Young J, Young W. Prolonged increases in paradoxical sleep during and after avoidance-task acquisition. Sleep. 1980;3:67–81. [PubMed] [Google Scholar]
- 26.Smith C, Wong PT. Paradoxical sleep increases predict successful learning in a complex operant task. Behav Neurosci. 1991;105:282–8. doi: 10.1037//0735-7044.105.2.282. [DOI] [PubMed] [Google Scholar]
- 27.Portell-Cortes I, Marti-Nicolovius M, Segura-Torres P, Morgado-Bernal I. Correlations between paradoxical sleep and shuttle-box conditioning in rats. Behav Neurosci. 1989;103:984–90. doi: 10.1037//0735-7044.103.5.984. [DOI] [PubMed] [Google Scholar]
- 28.Datta S. Avoidance task training potentiates phasic pontine-wave density in the rat: a mechanism for sleep-dependent plasticity. J Neurosci. 2000;20:8607–13. doi: 10.1523/JNEUROSCI.20-22-08607.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harvey AG, Jones C, Schmidt DA. Sleep and posttraumatic stress disorder: a review. Clin Psychol Rev. 2003;23:377–407. doi: 10.1016/s0272-7358(03)00032-1. [DOI] [PubMed] [Google Scholar]
- 30.Pitman RK, Shin LM, Rauch SL. Investigating the pathogenesis of posttraumatic stress disorder with neuroimaging. J Clin Psychiatry. 2001;62(Suppl 17):47–54. [PubMed] [Google Scholar]
- 31.Hermans D, Craske MG, Mineka S, Lovibond PF. Extinction in human fear conditioning. Biol Psychiatry. 2006;60:361–68. doi: 10.1016/j.biopsych.2005.10.006. [DOI] [PubMed] [Google Scholar]