Abstract
Study Objectives:
Chronic sleep deprivation of rats causes hyperphagia without body weight gain. Sleep deprivation hyperphagia is prompted by changes in pathways governing food intake; hyperphagia may be adaptive to sleep deprivation hypermetabolism. A recent paper suggested that sleep deprivation might inhibit ability of rats to increase food intake and that hyperphagia may be an artifact of uncorrected chow spillage. To resolve this, a palatable liquid diet (Ensure) was used where spillage is insignificant.
Design:
Sleep deprivation of male Sprague Dawley rats was enforced for 10 days by the flowerpot/platform paradigm. Daily food intake and body weight were measured. On day 10, rats were transcardially perfused for analysis of hypothalamic mRNA expression of the orexigen, neuropeptide Y (NPY).
Setting:
Morgan State University, sleep deprivation and transcardial perfusion; University of Maryland, NPY in situ hybridization and analysis.
Measurements and Results:
Using a liquid diet for accurate daily measurements, there was no change in food intake in the first 5 days of sleep deprivation. Importantly, from days 6–10 it increased significantly, peaking at 29% above baseline. Control rats steadily gained weight but sleep-deprived rats did not. Hypothalamic NPY mRNA levels were positively correlated to stimulation of food intake and negatively correlated with changes in body weight.
Conclusion:
Sleep deprivation hyperphagia may not be apparent over the short term (i.e., ≤5 days), but when extended beyond 6 days, it is readily observed. The timing of changes in body weight and food intake suggests that the negative energy balance induced by sleep deprivation prompts the neural changes that evoke hyperphagia.
Citation:
Koban M; Sita LV; Le WW; Hoffman GE. Sleep deprivation of rats: the hyperphagic response is real. SLEEP 2008;31(7):927-933.
Keywords: Sleep deprivation, hyperphagia, body weight, liquid diet, rats, NPY
IT IS WELL ESTABLISHED THAT CHRONIC SLEEP DEPRIVATION OF RATS RESULTS IN SIGNIFICANT MORBIDITY.1–3 A CLUSTER OF PATHOLOGIES OR syndromes develops that includes elevated energy metabolism, changes in circulating hormones, debilitation of physical appearance, loss of immune integrity, and many others. Adaptation to sleep deprivation is not possible because if unremitting, it is always fatal.4,5
A general feature of the rat sleep deprivation literature is that when applied over many days, it often results in two readily noticeable outcomes: hyperphagia with loss of body weight. These sleep deprivation effects are reliably observed with the chronic disc-over-water (DOW) paradigm4–10 developed by Rechtschaffen and Bergmann7; on the other hand, food intake patterns with the more commonly used flowerpot/platform11 paradigm and its variants12,13 have not been as consistent. If the time course is for 4 to 6 days, some studies show hyperphagia13–22 while others do not,23–26 but extending sleep deprivation to 10 or more days always leads to hyperphagia with declines in body weight.18–20
Some of the mechanisms driving increased food intake without gains in body weight are now better understood. The hyperphagic response is linked to decreases in circulating leptin,6,18 and within the hypothalamic arcuate nucleus, there is up-regulation of the orexigen, neuropeptide Y (NPY), and down-regulation of the anorexigen, α-melanocyte stimulating hormone (α-MSH).20 These changes are in agreement with the current understanding of how pathways prompting food intake are stimulated.27 The loss of body weight or the inability to gain it during sleep deprivation can be explained by development of increased energy expenditure4,5,9,28,29 and resting metabolic rate,18 mediated by a robust stimulation of gene expression of uncoupling protein 1 in mitochondria of brown adipose tissue.18
Recently, the precept of sleep deprivation hyperphagia in the rat was challenged experimentally. Martins et al. sleep-deprived rats for 5 days using the flowerpot/platform method.30 Standard chow was used to measure 24-h food intake and spilled crumbs were collected from the surrounding water, separated from waste, and dried to obtain a more accurate measure of food intake. Finding no substantial change, the authors concluded that food intake does not increase across 5 days of sleep deprivation. They suggest that reports of sleep deprivation hyperphagia may be artifacts of experimental conditions, and that despite increased energy expenditure, rats are unable to compensate by eating more, thus causing a loss in body weight.
Because there is a general lack of consensus in the literature with respect to hyperphagia and sleep deprivation using the flowerpot/platform method and its variants, we posit that the drive to eat is either inadequately met—perhaps because of spillage or insufficient caloric ingestion—or that longer periods of sleep deprivation are needed to fully promote increased food intake. To clarify this matter, we employed a palatable liquid diet (Ensure) and special feeding tubes where spillage is negligible. The flowerpot/platform method was used for 10 days compared to 4 to 5 days that most investigators use. We find that sleep deprivation causes a failure to gain body weight across 10 days while significantly increasing food intake beyond day 5 that is correlated to an increase in NPY mRNA in the hypothalamic arcuate nucleus.
MATERIALS AND METHODS
Animals
Two-month old male Sprague Dawley rats (Harlan, Indianapolis IN) were housed individually in standard shoebox plastic cages. Photoperiod was a 12:12 light-dark cycle with lights on at 09:00. During the first 2 weeks in the facility, rats were fed chow ad libitum (Teklad rodent diet 8604 [www.teklad.com], Madison WI). All of the rats were then adapted to a liquid diet (Ensure, Creamy Milk Chocolate Flavor, Abbott Laboratories [www.ensure.com]) using 150 mL-capacity feeding tubes (product 9007, Bio-Serv, Frenchtown, NJ; [www.bio-serv.com]). Caloric contents of chow and Ensure are as follows. Chow has 3.1 kcal/g; approximately 54% and 13% of kcal are obtained from carbohydrate (starches, simple sugars) and fat (soy, other vegetable oils), respectively. Ensure has 1.06 kcal/mL; approximately 35% and 20% of kcal come from carbohydrate (sucrose, corn syrup) and fat (soy, other vegetable oils), respectively. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Morgan State University, and they comply with National Institutes of Health guidelines.
Sleep Deprivation Paradigm
The flowerpot/platform paradigm was used to enforce sleep deprivation for 10 days. The method has been previously described in detail.18,20 Briefly, Plexiglas tanks are divided into 5 compartments, each 30 × 30 × 30 cm. In previous experiments,18,20 rats were 6 months of age; because of their larger size, 10-cm platforms were used, and compartment height was increased to 40 cm with an add-on lip. For the current study involving younger and smaller rats, each compartment had a 6.5-cm diameter platform atop a 10-cm high pedestal that is surrounded by water to about 1 cm below the platform surface. Warm water (∼ 30°C) flowed continuously through the tanks to carry away waste; the top of each compartment was closed with a perforated lid.
Stainless steel clamps held feeding tubes in place within easy access of the platform; drinking water was provided with a standard overhead water bottle. The design of the feeding tube requires the rat to lap the liquid diet, obviating any spillage. While residing on the platform, a rat can engage in grooming and exploratory behaviors. It can obtain some slow wave sleep but upon entering REM (i.e., paradoxical) sleep, the loss of muscle tone causes the rat to make contact with the water, and it awakens. Consequently, the flowerpot method is selective for abolishing REM sleep, but it also fragments slow wave sleep.31 For simplicity, we will refer to sleep deprivation while realizing that it is mostly deprivation of REM sleep.
Experimental Procedures
During the time rats were eating chow and then adapted to Ensure, they were accustomed to the novel environment of the water-filled sleep deprivation tanks by placing them on the 6.5-cm platforms for 1 h each day. Body weights and the amount of food eaten during the previous 24 h were obtained daily between 09:00–10:00. The study involved 8 rats undergoing sleep deprivation and 5 rats in their home cages as controls.
Tissue Preparation
On the final day of the experiment (i.e., day 10), rats were anesthetized with an overdose of sodium pentobarbital (100 mg/kg, ip), administered heparin (100 U) directly into the heart, and perfused transcardially with saline containing 2% sodium nitrite followed by 2.5% acrolein in buffered 4% paraformaldehyde.20 The brains were removed and sunk in 30% sucrose solution, frozen, and sectioned at 25 μm on a freezing sliding microtome into 1-in-12 series. The sections were collected in cryoprotectant antifreeze solution.32 Sections were stored at −20°C until they were processed.
NPY Probe Preparation, Hybridization, and Detection
The methods used were described in detail previously,20 but briefly, we used NPY cDNA (a generous gift of Dr. A. Sahu, University of Pittsburgh) that contains an insert with most of the cDNA of rat brain NPY ligated into the EcoRI site of the Bluescribe M13 vector.33 The antisense NPY riboprobe was made by linearizing plasmid with FspI and transcribing with T3 RNA polymerase to yield a probe of 511 bp; the sense-strand riboprobe was made by linearizing with SmaI and transcribing with T7 RNA polymerase.
In situ hybridization reactions involved hybridization with the biotinylated riboprobe and detection using the avidin-biotin complex (ABC) method initially described by Berghorn et al.,34 except that the concentration of the chromogen-peroxide solution was lowered as described in Koban et al.20 The method reveals clusters of specific mRNAs within cells whose level of expression is measured by quantifying the optical density (OD) of the arcuate nucleus NPY population. Cells on each side of the arcuate nucleus in a 1-in-12 series of sections were analyzed and the data expressed as the mean OD ± SEM.
Data Analysis
Data were analyzed using one- or two-way ANOVA followed by the Dunn or Bonferroni post-tests, respectively. For one-way ANOVA, if the Bartlett test for equal variances was significant, the nonparametric Kruskal-Wallis test was run. For comparisons of 2 sets of data, the t-test was used. Correlation analyses were determined using the Pearson correlation. In all tests P ≤ 0.05 was considered significant. Data are presented as mean ± standard error of the mean (SEM). Statistical analyses were done using GraphPad Prism, version 5.01 for Windows (GraphPad Software, San Diego, CA; www.graphpad.com).
RESULTS
Food Consumption
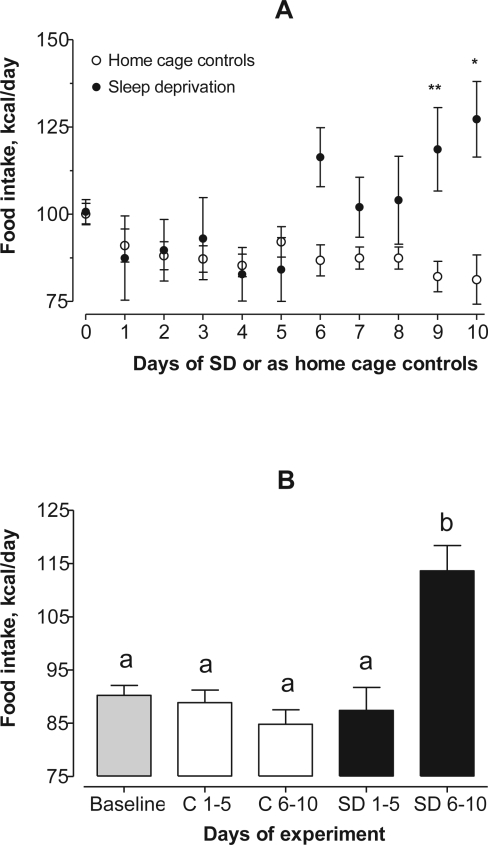
With a liquid diet, accurate measurements of daily food intake were possible with practically no waste. The amount of Ensure consumed each day as kcal is shown in Figure 1A. Food intake was first analyzed over the entire experimental time course. Two-way ANOVA revealed significant effects of interaction (F10, 149 = 2.72; P = 0.0042) and treatment (F1, 149 = 19.04; P < 0.0001), but not for day of experiment (P = 0.0884). The Bonferroni post test showed significant differences between sleep-deprived and control rats on days 9 and 10.
Figure 1.
Panel A: Food intake (Ensure) as kcal/day. Two-way ANOVA showed significant effects of interaction (F10, 149 = 2.72; P = 0.0042) and treatment (F1, 149 = 19.04; P < 0.0001), but not for day of experiment (P = 0.0884). The Bonferroni post test showed significant differences between sleep-deprived and control rats on days 9 and 10. *P < 0.05, **P < 0.01
Panel B: Ten days of experiments were divided into equal halves. There was an overall difference in food intake (KW = 20.3, P = 0.0004). The Dunn post test found a significant increase (P < 0.001) of 29% more daily consumption of Ensure for sleep-deprived rats over days 6–10 compared to the other groups. Groups not sharing a common lower-case letter are statistically different from one another at P < 0.05 or less.
Next, the 10 days of sleep deprivation were divided into equal halves of 5 days each. This was done because the majority of flowerpot studies are terminated at 4 or 5 days. There was a significant difference in food intake using one-way nonparametric ANOVA (KW = 20.3, P = 0.0004). The Dunn multiple comparison test showed that during the first half of the experiment, there were no differences in average food intake across baseline, during days 1–5 and 6–10 for home cage control rats, and for rats sleep-deprived during days 1–5. But for rats sleep-deprived during days 6–10, there was an increase in average consumption of Ensure of 29% compared to the other groups (P < 0.001; Figure 1B).
Body Weight
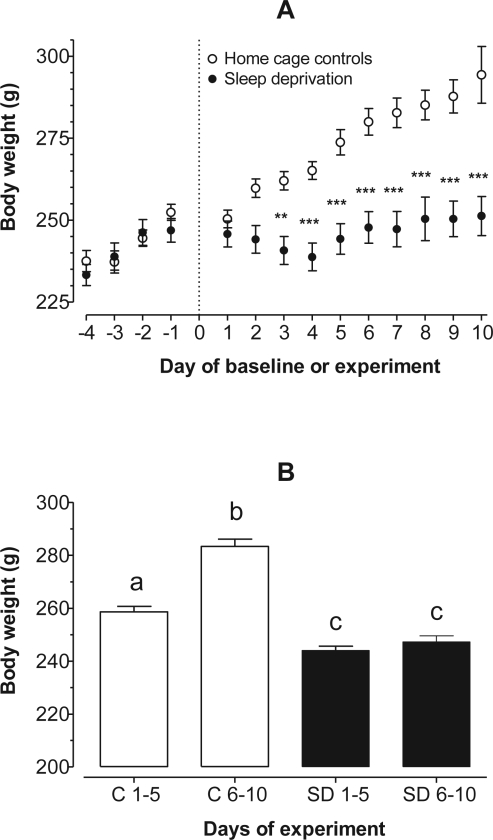
Daily changes in average body weight while consuming Ensure during baseline for all rats, and over 10 days of sleep deprivation or as controls are shown in Figure 2A. There was a steady daily gain of body weight for all rats during baseline and the 5 rats kept as controls for the remainder of the experiment. Two-way ANOVA indicated significant effects of interaction (F13, 191 = 6.55; P < 0.0001), treatment (F1, 191 = 157.91; P < 0.0001), and day of experiment (F13, 191 = 13.99; P < 0.0001). Post-testing showed significant differences in body weights between controls and sleep-deprived rats beginning with day 3 until day 10.
Figure 2.
Panel A: Daily changes in body weight (g). Two-way ANOVA indicated significant effects of interaction (F13, 191 = 6.55; P < 0.0001), treatment (F1, 191 = 157.91; P < 0.0001), and day of experiment (F13, 191 = 13.99; P < 0.0001). Post testing showed significant differences in body weights between controls and sleep-deprived rats beginning with day 3 until day 10. **P < 0.01, ***P < 0.001. Panel B: Ten days of experiments were divided into equal halves. By day 10, control rats gained an average of 38.4 ± 4 g compared to only 4.4 ± 5.5 g for sleep-deprived rats (t = 4.35, P = 0.049). Note that sleep-deprived rats had significantly lower body weights throughout the experiment compared to controls (KW = 62.88, P < 0.0001). Groups not sharing a common lower-case letter are statistically different from one another at P < 0.05 or less.
Early during sleep deprivation, the average body weight of rats showed a decreasing trend, but by day 5 it appeared to level off (Figure 2A). Overall, the body weight of sleep-deprived rats did not change beyond the last day of baseline. Nevertheless, an important observation is that they failed to gain body weight during the 10 days of the experiment, despite significantly higher daily intake of a palatable and calorie-dense food compared to controls during the latter half of the experiment. As expected, control rats had a steady increase in body weight of about 4 g per day. By day 10, control rats gained an average of 38.4 ± 4 g compared to only 4.4 ± 5.5 g for sleep-deprived rats (t = 4.35, P = 0.049). The high SEM of rats undergoing deprivation of sleep (i.e., 5.5 g) is explained by the variation in body weight we observed during the 10-day regimen. One rat gained 33 g while another lost 15 g; the remainder showed only small changes from baseline. Similar to the food intake data, we separated changes in body weight for days 1–5 and days 6–10 (Figure 2B). The principal finding is that on the whole, sleep-deprived rats had significantly lower body weights throughout the experiment compared with controls (KW = 62.88, P < 0.0001).
Hypothalamic NPY mRNA
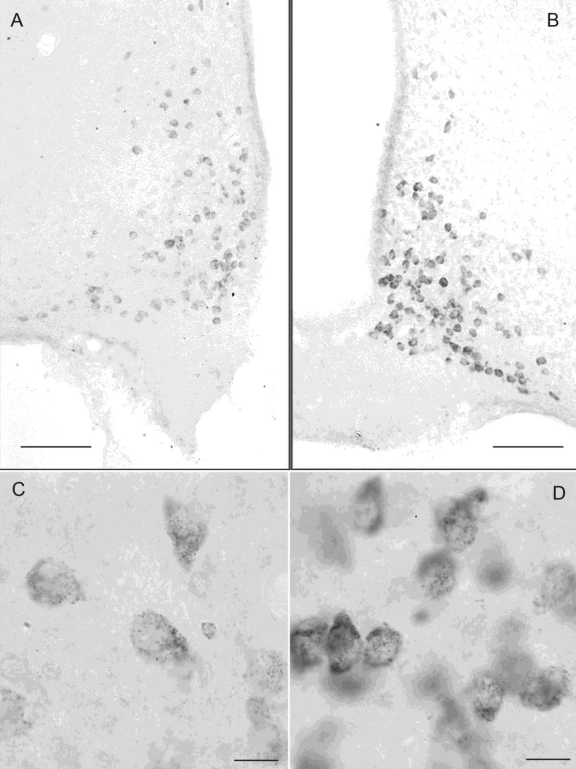
Specific clusters of NPY mRNA are seen in neurons of the cortex, hippocampus, and arcuate nucleus of the hypothalamus with antisense, but not sense probes. We focused our attention on levels of NPY mRNA in the neurons of the hypothalamic arcuate nucleus because these cells are among the most important central regulators of food intake.27 In Figure 3 are micrographs showing NPY mRNA expression in the arcuate nucleus of representative control (panels A and C) and 10-day sleep-deprived (panels B and D) rats. As is apparent when the neurons were examined under high magnification (panels C [control] and D [sleep deprivation]), the difference in cell intensity arose from the increased number of mRNA clusters within the cytoplasm, estimated as optical density (OD) units. After 10 days of sleep deprivation, NPY mRNA was about 35% higher compared to the same neuron population of control rats (Figure 4; 838.6 ± 45 and 619.8 ± 56 OD units, respectively; t = 3.038, P = 0.0113).
Figure 3.
NPY mRNA was visualized within neurons of the hypothalamic arcuate nucleus and was analyzed as optical density units from representative rats. Panels A and C are of a home cage control rat; panels B and D are of a rat sleep-deprived for 10 days. The low power micrograph in panel A (control) shows that NPY mRNA within the cells is less intense than that in arcuate cells of the sleep-deprived rat (panel B). At high magnification (panels C and D), the increase in cell intensity is apparent as increased number of mRNA clusters within the cytoplasm. Calibration bars – low power, 100 μm; high power, 10 μm.
Figure 4.
Overall mean NPY mRNA optical density (OD) of NPY mRNA within the arcuate nuclei was approximately 35% higher in 10-day sleep-deprived rats compared to home cage controls (838.6 ± 45 and 619.8 ± 56 OD units, respectively; t = 3.038, P = 0.0113).
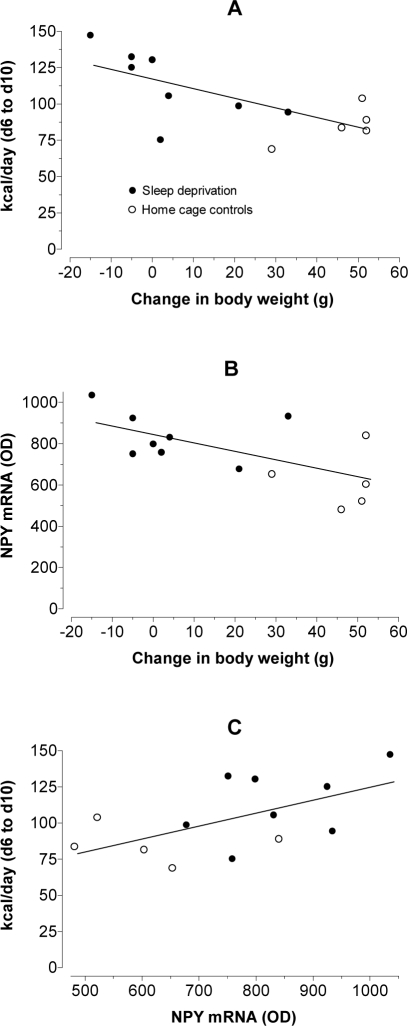
We found correlations between changes in body weight over 5 days of sleep deprivation, food consumption over the last 5 days of sleep deprivation, and NPY mRNA expression on day 10. These data are illustrated in Figure 5. The data points for sleep-deprived and control rats generally grouped into 2 populations. In panel A, control and sleep-deprived rats were analyzed for changes in body weight over the entire time course of the experiment against food intake (kcal/day) during the last 5 days. The Pearson correlation (r2 = 0.4672, P = 0.01) suggests that when less weight is gained (as is seen during sleep deprivation), rats consume more food. Next, the same changes in body weight over 10 days were plotted against expression of NPY mRNA within the hypothalamic arcuate nucleus for control and sleep-deprived rats (panel B). Similar to the previous correlation, these data (r2 = 0.4088, P = 0.0186) indicate that during sleep deprivation, as body weight is lost or less is gained, there is neuroendocrinological evidence pointing to an increased drive to consume more food, presumably because of dysregulation of energy homeostasis. Finally, a plot was constructed of NPY mRNA expression on day 10 against food consumed during the final 5 days of sleep deprivation (panel C). The predictive value of the data is that there was a positive correlation between the parameters (r2 = 0.3102, P = 0.048), so that as expression of NPY mRNA increased, more food was consumed.
Figure 5.
Pearson correlation analyses between changes in body weight (i.e., after 10 days of experiments), food consumption over the last 5 days, and NPY mRNA expression on day 10 (-●-, sleep deprivation; -○-, home cage controls).
Panel A: Control and sleep-deprived rats were analyzed for changes in body weight against food intake (kcal/day). The correlation (r2 = 0.4672, P = 0.01) suggests that when less weight is gained, as is seen during sleep deprivation, rats consume more food.
Panel B: Changes in body weight were plotted against expression of NPY mRNA within the hypothalamic arcuate nucleus for control and sleep-deprived rats. The correlation (r2 = 0.4088, P = 0.0186) indicates that the reduced body weight gain seen upon sleep deprivation prompts an increased central nervous system drive to consume more food via elevated NPY gene expression.
Panel C: A plot of NPY mRNA expression against food consumption illustrates a positive correlation between the parameters (r2 = 0.3102, P = 0.048), showing that as expression of NPY increased, more food was consumed.
DISCUSSION
The purpose of this experiment was to more definitively investigate the tenet of sleep deprivation hyperphagia of rats. As has been pointed out, hyperphagia is the outcome observed in studies of chronically enforced sleep deprivation4–8,10,18–20; however, in many instances when the sleep deprivation regimen is shorter (i.e., 4 to 6 days), hyperphagia is not found.23–26 Of particular interest is that Martins et al. recently questioned if hyperphagia even occurs when sleep deprivation is held to 5 days.30 We will attempt to explain that some of the differences may be due to the methodology by which sleep deprivation is enforced and the manner of food delivery. But more importantly, we believe that the critical factor is the time course of sleep deprivation.
Regardless of whether one employs the simple flowerpot/platform or the technically sophisticated DOW method, both paradigms involve the rat making contact with water to maintain wakefulness. It is therefore not surprising that chow can be scattered with spillage into the surrounding water. Indeed, in another recent study by Tufik's group, evidence suggested that sleep-deprived rats become more “sloppy” in consuming food because they increasingly engage in gnawing behaviors.35 In our previous studies, we noted that chow crumbs were found in the water daily.18–20 Crumbs could not all be recovered to correct for spillage because the tanks were designed with a water flow-through system. Admittedly, food intake measurements for 10 to 20 days of sleep deprivation were overestimates, but how much could not easily be determined with our experimental conditions; however, upon euthanasia our animals had greatly distended stomachs, suggesting that even with increased spillage they were eating much more than normal.
Martins et al. have submitted an alternative viewpoint, and there is no reason to question their results because the study appears to be well designed and executed.30 At the same time, we are not in agreement with their suggestion that sleep deprivation in rats somehow causes an inability to increase food intake as energy expenditure rises. Instead, we propose that within the first few days of sleep deprivation, it is the decrease in body weight and changes in hypothalamic neuropeptides (e.g., NPY) and peripheral hormones (e.g., leptin) regulating food intake that later stimulate hyperphagia. To support our perspective, several lines of evidence and reasoning are offered.
First, a liquid diet results in negligible waste; we show that beyond 5 days of sleep deprivation, food intake increases almost linearly. Insofar as we know, there are only 2 other published rat studies of sleep deprivation involving a liquid diet. In one, a 5-day time course by Hanlon et al. found a small but nevertheless significant increase in food intake using a plain liquid diet (Bio-Serve).21 Another study of 4 days of sleep deprivation with rats on a more calorie-dense liquid diet (Research Diets) resulted in no change in food intake.25 Although food palatability and other experimental conditions such as the ratio of the body size of the rat to the diameter of the platform36 may be important determinants of how much food is eaten, it is probably the length of sleep deprivation that establishes onset of noticeable hyperphagia. From our results, at least 6 days appear to be necessary.
Second, the behavioral drive of rats to consume more food during sleep deprivation is borne out by appropriate changes in mRNA and immunoreactive peptides of hypothalamic NPY and POMC20 and of circulating leptin.6,18 We showed earlier that these changes were statistically significant by day 5 of sleep deprivation.18,20 In studies of human sleep restriction, stimulation of feelings of hunger or the desire to eat calorie-dense foods are consistent with changes in serum leptin and ghrelin that occur within a short period of the experimental time course.37,38
Third, description of food intake with the DOW apparatus mentions in detail how feeders were designed to minimize waste.8,39 Moreover, it has been brought to our attention that in many of Rechtschaffen's studies, scattered crumbs were collected from the water to correct for the actual amount of food eaten (Dr. Paul Shaw, Washington University, personal communication). We note also that even with the DOW method and using ordinary chow, the first 5 days of sleep deprivation suggests a trend toward increased food intake, but this does not become significant until at least 10 days had elapsed.10
Finally, we should mention that making direct comparisons between chow and Ensure, a highly palatable and calorie-dense human food supplement, is a complicated one. Even though Ensure provides more immediate caloric energy than chow because of its higher disaccharide and fat content per unit volume or mass, the important outcome was that all of the rats were consuming Ensure, but sleep-deprived rats ate more than the controls. This helps strengthens our assertion that sleep deprivation hyperphagia is a real phenomenon.
The intricacies of food intake, sleep deprivation, and stress are complex issues that have yet to be satisfactorily resolved. To begin, consider the matter of food palatability and caloric density in the context of sleep deprivation and hyperphagia. When flowerpot/platform rats were given chow, water, and solutions of saccharin or sucrose (5%), they consumed more chow plus sucrose solution during sleep deprivation compared to baseline, and yet lost body weight.17 A similar pattern was found with sleep deprivation enforced by the DOW method. With regular chow, high protein, or high calorie (from fat) diets, sleep deprivation resulted in increased consumption of the high calorie food compared to other foods but with failure to gain weight.8 As previously pointed out, sleep-restricted humans report increased appetite for carbohydrate-rich, calorie-dense foods.37,38 Perhaps the simplest rationale for stimulation for sleep deprivation hyperphagia, irrespective of enforcement method or caloric density or palatability of the food, is that there is a critical need to fuel the ensuing increase in metabolism.4,5,9,18,28,29 Clearly, since sleep deprivation results in loss of body weight or a failure to gain it, caloric input is not met by caloric output. This is reinforced by substantial mobilization of liver and skeletal muscle glycogen even by day 5 (Koban, unpublished observations) and the emaciated conditions of rats following chronic sleep deprivation.3 Another explanation is that palatable foods rich in fats or carbohydrates are preferred because they somehow mitigate feelings of stress.17,37,40
Analysis of the interactions among the various measures taken in the present study and their timing shed some light as to the sequence of events that leads to hyperphagia during chronic sleep deprivation. The earliest sign of dysregulation was the failure to gain weight. By day 3 of sleep deprivation, rats weighed significantly less than the controls. From earlier studies, the reduced body weight represented stimulation of metabolism,4,5,9,18,28,29 likely resulting from high sympathetic tone.28,41,42 The extent to which weight gain was reduced correlated with the eventual magnitude of up-regulation of NPY mRNA. Within the next 2 days, increased food intake of sleep-deprived rats reached significance. It is at this same time that NPY mRNA increased and POMC mRNA showed a significant decline,20 as did circulating leptin.18 The fact that the amount of food consumed in the last 5 days of the present experiment was predicted by the magnitude of NPY mRNA expression suggests that synthesized NPY is effective in prompting feeding.
Several examples from the literature were pointed out where no change in food intake was found with rats experiencing 4 or 5 days of sleep deprivation.23–26 Data from the present study suggest that sleep deprivation hyperphagia emerges only after 5 days. While the exact timing of when food intake will rise during sleep deprivation will vary with the caloric content of the diet and the ease and efficiency by which it can be eaten, our findings point clearly to an eventual increase in food intake that follows when there is failure to gain body weight. Indeed, stimulation of hyperphagia is a consequence of chronic sleep deprivation, but there is a lag before the level of food intake increases.
ACKNOWLEDGMENTS
This study was supported by NIH grant 2-SO6-GM-51971-04 to MK and to a Pilot and Feasibility grant from the Clinical Nutrition Research Unit of Maryland, P30DK072488 to GEH. Dr. L. V. Sita was supported by FAPESP grants 04/13850-3 and 06/06289-9. We thank Dr. Susan K. Fried (Division of Endocrinology, Diabetes, and Nutrition, Department of Medicine, University of Maryland School of Medicine) for advice and discussions, and Mr. Ziqiang Zhang for valuable technical assistance.
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
Authorship Responsibility
Dr. Michael Koban helped design the study, conducted the sleep deprivation experiments, analyzed the physiological data, and wrote the paper. Dr. Luciane V. Sita perfused the rats, prepared brains for in situ hybridization, and assisted in writing the paper. Dr. Wei Wei Le performed the in situ hybridization experiments and analyzed the anatomical data. Dr. Gloria E. Hoffman helped design the study, supervised the in situ hybridization experiments, analyzed the anatomical data, and wrote the paper.
REFERENCES
- 1.Everson CA. Functional consequences of sustained sleep deprivation in the rat. Behav Brain Res. 1995;69:43–54. doi: 10.1016/0166-4328(95)00009-i. [DOI] [PubMed] [Google Scholar]
- 2.Rechtschaffen A, Bergmann BM. Sleep deprivation in the rat by the disk-over-water method. Behav Brain Res. 1995;69:55–63. doi: 10.1016/0166-4328(95)00020-t. [DOI] [PubMed] [Google Scholar]
- 3.Rechtschaffen A, Bergmann BM. Sleep deprivation in the rat: an update of the 1989 paper. Sleep. 2002;25:18–24. doi: 10.1093/sleep/25.1.18. [DOI] [PubMed] [Google Scholar]
- 4.Everson CA, Bergmann BM, Rechtschaffen A. Sleep deprivation in the rat: III. Total sleep deprivation. Sleep. 1989;12:13–21. doi: 10.1093/sleep/12.1.13. [DOI] [PubMed] [Google Scholar]
- 5.Kushida CA, Bergmann BM, Rechtschaffen A. Sleep deprivation in the rat: IV. Paradoxical sleep deprivation. Sleep. 1989;12:22–30. doi: 10.1093/sleep/12.1.22. [DOI] [PubMed] [Google Scholar]
- 6.Everson CA, Crowley WR. Reductions in circulating anabolic hormones induced by sustained sleep deprivation in rats. Am J Physiol Endocrinol Metab Physiol. 2004;286:E1060–70. doi: 10.1152/ajpendo.00553.2003. [DOI] [PubMed] [Google Scholar]
- 7.Rechtschaffen A, Gilliland MA, Bergmann BM, Winter JB. Physiological correlates of prolonged sleep deprivation in rats. Science. 1983;221:182–4. doi: 10.1126/science.6857280. [DOI] [PubMed] [Google Scholar]
- 8.Everson CA, Wehr TA. Nutritional and metabolic adaptations to prolonged sleep deprivation in the rat. Am J Physiol Regul Integr Comp Physiol. 1993;264:R376–87. doi: 10.1152/ajpregu.1993.264.2.R376. [DOI] [PubMed] [Google Scholar]
- 9.Balzano S, Bergmann BM, Gilliland MA, Silva JE, Rechtschaffen A, Refetoff S. Effect of total sleep deprivation on 5'-deiodinase activity of rat brown adipose tissue. Endocrinology. 1990;127:882–90. doi: 10.1210/endo-127-2-882. [DOI] [PubMed] [Google Scholar]
- 10.Everson CA, Laatsch CD, Hogg N. Antioxidant defense responses to sleep loss and sleep recovery. Am J Physiol Regul Integr Comp Physiol. 2005;288:R374–83. doi: 10.1152/ajpregu.00565.2004. [DOI] [PubMed] [Google Scholar]
- 11.Cohen HB, Dement WC. Sleep: changes in threshold to electroconvulsive shock in rats after deprivation of “paradoxical” phase. Science. 1965;150:1318–9. doi: 10.1126/science.150.3701.1318. [DOI] [PubMed] [Google Scholar]
- 12.van Hulzen ZJ, Coenen AM. Paradoxical sleep deprivation and locomotor activity in rats. Physiol Behav. 1981;27:741–4. doi: 10.1016/0031-9384(81)90250-x. [DOI] [PubMed] [Google Scholar]
- 13.Elomaa E. The cuff pedestal: an alternative to flowerpots? Physiol Behav. 1979;23:669–72. doi: 10.1016/0031-9384(79)90158-6. [DOI] [PubMed] [Google Scholar]
- 14.Bhanot JL, Chhina GS, Singh B, Sachdeva U, Kumar VM. REM sleep deprivation and food intake. Indian J Physiol Pharmacol. 1989;33:139–45. [PubMed] [Google Scholar]
- 15.Brock JW, Farooqui SM, Ross KD, Payne S, Prasad C. Stress-related behavior and central norepinephrine concentrations in the REM sleep-deprived rat. Physiol Behav. 1994;55:997–1003. doi: 10.1016/0031-9384(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 16.Suchecki D, Tufik S. Social stability attenuates the stress in the modified multiple platform method for paradoxical sleep deprivation in the rat. Physiol Behav. 2000;68:309–16. doi: 10.1016/s0031-9384(99)00181-x. [DOI] [PubMed] [Google Scholar]
- 17.Suchecki D, Antunes J, Tufik S. Palatable solutions during paradoxical sleep deprivation: reduction of hypothalamic-pituitary-adrenal axis activity and lack of effect on energy imbalance. J Neuroendocrinol. 2003;15:815–21. doi: 10.1046/j.1365-2826.2003.01067.x. [DOI] [PubMed] [Google Scholar]
- 18.Koban M, Swinson KL. Chronic REM-sleep deprivation of rats elevates metabolic rate and increases UCP1 gene expression in brown adipose tissue. Am J Physiol Endocrinol Metab Physiol. 2005;289:E68–74. doi: 10.1152/ajpendo.00543.2004. [DOI] [PubMed] [Google Scholar]
- 19.Koban M, Stewart CV. Effects of age on recovery of body weight following REM sleep deprivation of rats. Physiol Behav. 2006;87:1–6. doi: 10.1016/j.physbeh.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Koban M, Le WW, Hoffman GE. Changes in hypothalamic corticotropin-releasing hormone, neuropeptide Y, and proopiomelanocortin gene expression during chronic rapid eye movement sleep deprivation of rats. Endocrinology. 2006;147:421–31. doi: 10.1210/en.2005-0695. [DOI] [PubMed] [Google Scholar]
- 21.Hanlon EC, Andrzejewski ME, Harder BK, Kelley AE, Benca RM. The effect of REM sleep deprivation on motivation for food reward. Behav Brain Res. 2005;163:58–69. doi: 10.1016/j.bbr.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 22.Patchev V, Felszeghy K, Koranyi L. Neuroendocrine and neurochemical consequences of long-term sleep deprivation in rats: similarities to some features of depression. Homeost Health Dis. 1991;33:97–108. [PubMed] [Google Scholar]
- 23.Mendelson WB, Guthrie RD, Frederick G, Wyatt RJ. The flower pot technique of rapid eye movement (REM) sleep deprivation. Pharmacol Biochem Behav. 1974;2:553–6. doi: 10.1016/0091-3057(74)90018-5. [DOI] [PubMed] [Google Scholar]
- 24.Balestrieri S, D'Onofrio G, Giuditta A. Deprivation of paradoxical sleep. Effect on weight and nucleic acid content of liver and brain. Neurochem Res. 1980;5:1251–64. doi: 10.1007/BF00964961. [DOI] [PubMed] [Google Scholar]
- 25.Youngblood BD, Smagin GN, Elkins PD, Ryan DH, Harris RB. The effects of paradoxical sleep deprivation and valine on spatial learning and brain 5-HT metabolism. Physiol Behav. 1999;67:643–9. doi: 10.1016/s0031-9384(99)00120-1. [DOI] [PubMed] [Google Scholar]
- 26.Elomaa E. The light/dark difference in meal size in the laboratory rat on a standard diet is abolished during REM sleep deprivation. Physiol Behav. 1981;26:487–93. doi: 10.1016/0031-9384(81)90178-5. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–71. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 28.Bergmann BM, Everson CA, Kushida CA, et al. Sleep deprivation in the rat: V. Energy use and mediation. Sleep. 1989;12:31–41. doi: 10.1093/sleep/12.1.31. [DOI] [PubMed] [Google Scholar]
- 29.Hipolide DC, Suchecki D, Pimentel de Carvalho Pinto A, Chiconelli Faria E, Tufik S, Luz J. Paradoxical sleep deprivation and sleep recovery: effects on the hypothalamic-pituitary-adrenal axis activity, energy balance and body composition of rats. J Neuroendocrinol. 2006;18:231–8. doi: 10.1111/j.1365-2826.2006.01412.x. [DOI] [PubMed] [Google Scholar]
- 30.Martins PJ, D'Almeida V, Nobrega JN, Tufik S. A reassessment of the hyperphagia/weight-loss paradox during sleep deprivation. Sleep. 2006;29:1233–8. doi: 10.1093/sleep/29.9.1233. [DOI] [PubMed] [Google Scholar]
- 31.Machado RB, Hipolide DC, Benedito-Silva AA, Tufik S. Sleep deprivation induced by the modified multiple platform technique: quantification of sleep loss and recovery. Brain Res. 2004;1004:45–51. doi: 10.1016/j.brainres.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 32.Watson RE, Jr, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–9. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- 33.Higuchi H, Yang HY, Sabol SL. Rat neuropeptide Y precursor gene expression. mRNA structure, tissue distribution, and regulation by glucocorticoids, cyclic AMP, and phorbol ester. J Biol Chem. 1988;263:6288–95. [PubMed] [Google Scholar]
- 34.Berghorn KA, Bonnett JH, Hoffman GE. cFos immunoreactivity is enhanced with biotin amplification. J Histochem Cytochem. 1994;42:1635–42. doi: 10.1177/42.12.7983364. [DOI] [PubMed] [Google Scholar]
- 35.Martins PJ, Nobrega JN, Tufik S, D'Almeida V. Sleep deprivation-induced gnawing-relationship to changes in feeding behavior in rats. Physiol Behav. 2007;93:229–34. doi: 10.1016/j.physbeh.2007.08.026. [DOI] [PubMed] [Google Scholar]
- 36.Hicks RA, Okuda A, Thomsen D. Depriving rats of REM sleep: the identification of a methodological problem. Am J Psychol. 1977;90:95–102. [PubMed] [Google Scholar]
- 37.Spiegel K, Tasali E, Penev P, Van Cauter E. Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–50. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 38.Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11:163–78. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bergmann BM, Kushida CA, Everson CA, Gilliland MA, Obermeyer W, Rechtschaffen A. Sleep deprivation in the rat II. Methodology. Sleep. 1989:5–12. doi: 10.1093/sleep/12.1.5. [DOI] [PubMed] [Google Scholar]
- 40.Dallman MF, Pecoraro N, Akana SF, et al. Chronic stress and obesity: a new view of “comfort food”. Proc Natl Acad Sci. 2003;100:11696–701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pilcher JJ, Bergmann BM, Fang VS, Refetoff S, Rechtschaffen A. Sleep deprivation in the rat: XI. The effect of guanethidine-induced sympathetic blockade on the sleep deprivation syndrome. Sleep. 1990;13:218–31. doi: 10.1093/sleep/13.3.218. [DOI] [PubMed] [Google Scholar]
- 42.Andersen ML, Martins PJ, D'Almeida V, Bignotto M, Tufik S. Endocrinological and catecholaminergic alterations during sleep deprivation and recovery in male rats. J Sleep Res. 2005;14:83–90. doi: 10.1111/j.1365-2869.2004.00428.x. [DOI] [PubMed] [Google Scholar]