Abstract
Study Objectives:
To investigate age and gender effects on the acute blood pressure (BP) and heart rate (HR) response to arousal from sleep in healthy adults.
Design:
Healthy young and older male and female adults were aroused from stage 2 sleep throughout the night using an auditory tone. The magnitude of the cardiovascular responses to arousal were assessed using 2 (young v older) by 2 (male v female) ANOVAs with repeated measures over time.
Setting:
Sleep laboratory at the Royal Brompton Hospital, London.
Patients or Participants:
25 healthy young (≤40 years, n = 15 males) and 20 healthy older adults (≥60 years, n = 11 males).
Interventions:
Arousals (>10 seconds) from undisturbed stage 2 sleep were induced by an auditory tone throughout the night.
Measurements and Results:
Overnight polysomnography (PSG) with HR, continuous beat-by-beat arterial BP and respiratory measurements was performed. Older adults had smaller and delayed initial mean BP and HR responses to arousal compared to young adults (both P < 0.001), whereas changes in ventilation and tidal volume responses to arousal were similar between age groups (P = 0.3 and P = 0.6 respectively).There were no differences between females and males in the cardiovascular or respiratory responses to arousal from sleep.
Conclusion:
The cause of the smaller and delayed response in healthy older adults is unknown; however, we speculate that for older people with sleep apnea, in whom nocturnal arousals occur frequently, the reduced cardiovascular response may be protective against the link between sleep apnea and hypertension.
Citation:
Goff EA; O'Driscoll DM; Simonds AK; Trinder J; Morrell MJ. The cardiovascular response to arousal from sleep decreases with age in healthy adults. SLEEP 2008;31(7):1009-1017.
Keywords: Age, sleep, arousal
AROUSALS FROM SLEEP LEAD TO ACUTE CHANGES IN AUTONOMIC CONTROL, RESULTING IN TRANSIENT INCREASES IN BLOOD PRESSURE (BP) AND HEART rate (HR).1–3 In patients with obstructive sleep apnea (OSA) these events may contribute to chronic hypertension.4–6 In older people the frequency of arousals from sleep increases as a consequence of increased sleep fragmentation and apneic events,7–9 which may in turn lead to a greater cardiovascular burden. However, older adults with sleep apnea do not have a greater risk of hypertension compared to age matched adults without apnea10; nor do they have an increased risk of mortality.11 Thus we speculated that the reduced cardiovascular morbidity and mortality in older adults with sleep apnea results from a smaller acute cardiovascular response to arousal from sleep, compared to younger people. Age-related reductions in the HR response to arousal from sleep supports this notion.12
The association between hypertension and sleep apnea may be more prominent in males compared to females5,13; for example it has been reported that male, but not female, OSA patients have a higher morning BP compared to evening BP.14 These gender differences may be accounted for by a reduced cardiovascular insult following arousal at the termination of an apneic event in females compared to males. However, interestingly the HR response to arousal from sleep has been reported to be greater in female OSA patients compared to males,15 although the BP response to arousal has not been previously investigated.
The overall aim of this study was to investigate the influence of age and gender on the cardiovascular response to arousal from sleep. Specifically we have investigated the cardiovascular response during the acute activation phase of the arousal, and the recovery phase to prearousal baseline levels. We hypothesized that the acute cardiovascular response to arousal from sleep would be smaller and delayed in older compared to young adults, and smaller and delayed in the females compared to males.
METHODS
Subjects
Twenty-five healthy young adults (≤ 40 years, n = 15 males) and 20 healthy older adults (≥ 60 years, n = 11 males) were studied. All were recruited from the general population and gave written informed consent. Subjects were medication free (including oral contraceptives or hormone replacement therapy); had no history of cardiovascular or respiratory disease; had an AHI < 15 events/h, confirmed by overnight polysomnography; and did not suffer from excessive daytime somnolence (Epworth Sleepiness Score < 10). All subjects had an office BP < 140/90 mm Hg; a BMI > 20 and < 35 kg/m2; normal lung function as determined by forced spirometry (ratio of forced expiratory volume in 1 s to forced vital capacity > 70%); and no hearing difficulties. All young females were studied during the earlier follicular phase of their menstrual cycle (days 4 to 10 following day 1 of their period) and all older females were postmenopausal. The study conformed to the standards set by the Declaration of Helsinki and was approved by The Brompton and Harefield Ethics Committee. Data from the 15 young male adults in the current study has been previously published as the control group16; both studies used the same methodology.
Protocol
Subjects were asked to arrive at the laboratory at 20:00, having restricted their previous night sleep to 4 h and having abstained from caffeine or alcohol intake during the day. Monitoring equipment was attached and subjects went to bed between 22:00 and 00:00; arousals were induced throughout the night until morning wake time (06:00).
Following at least 3 min of stable stage 2 sleep, subjects were aroused with frequency-modulated auditory tones (linear sweep from 500 Hz to 1000 Hz, 85 dB at subjects head, 1-s duration) sounded at the end of expiration. A minimum of 2 min stable stage 2 sleep was required before each subsequent induced arousal.
Measurements
Overnight polysomnography was performed. Physiological signals measured included electroencephalograms (EEG: C3/A2 and O1/A2); electrooculgrams (EOG: right and left); electromyograms (EMG: submentalis and anterior tibialis); electrocardiogram (3-lead; Lifetrak, HME); arterial oxygen saturation (SpO2) via finger pulse oximetry (model N-200E, Nellcor Inc.); airflow using a pneumotachometer (model 4700A, Hans Rudolf; flow range 0-160 L min−1) attached to a full face mask; and abdominal and thoracic respiratory effort via respiratory inductance plethysmography (RIP, Respitrace). Continuous beat-by-beat measurement of arterial BP was recorded via finger photoplethysmography (Finapres 2300, Ohmeda). BP recording commenced once stable sleep had been established and was stopped for short periods throughout the night when auditory arousals were not being induced to minimize discomfort to the finger.
In a subset of subjects (14 young males, and 9 older males) esophageal pressure (Pes) was recorded from a pressure sensor (model CTO-2, Gaeltec) connected to a pressure transducer (model S7b/2, Gaeltec). The pressure sensor was inserted nasally into the middle one-third of the esophagus, approximately 40 cm from the nares, after topical anesthesia was applied to the nose and the throat (lignocaine hydrochloride gel 2%, Biorex Laboratories). Pes measurements were made to confirm that during arousal from sleep older adults had similar changes in total inspiratory pulmonary resistance (RL) compared to younger people. To ensure this older adults breathed via low levels of continuous positive airway pressure (CPAP) manually titrated in order to reverse any airflow limitation.
All analogue data were digitized using an A/D converter (Micro 1401, Cambridge Electronic Design). The digitized signals were processed with Spike2 software (Cambridge Electronic Design).
Data Analysis
Sleep was scored from the EEG, EOG, and EMG using standard criteria.17 Arousals were categorized according to previously defined criteria3; only arousals with an abrupt shift in EEG activity for >10 s (including full awakenings) were analyzed; arousals during which body movements occurred were excluded. The scoring of sleep and arousals was performed by a researcher blinded to other physiological data.
For each induced arousal, beat-by-beat BP and RR interval responses were analyzed from 15 s before to 15 s after the auditory tone. These data were resampled at 1-s intervals via linear interpolation to enable comparisons between subject groups. Ventilation (VI), tidal volume (VT), and RL were also analyzed over the 6 breaths before and after the tone
A continuous on-line measurement of RL was calculated during the sleep study from the airflow and Pes signal. The continuous inspiratory resistance measurement for each breath was divided into 10 data points, the middle 8 of which were averaged to provide a single resistance measurement for each breath.16,18–20 CPAP was titrated in older adults using the online measurement RL, which was matched to values previously obtained in the younger adult group. For the older adults in whom Pes was not measured (n=11), CPAP was titrated to eliminate any flow limitation and upper airways resistance which was inferred from the shape of the inspiratory flow signal.21,22
The pre-tone average for BP and RR interval were calculated from the second-by-second data −15 s to −1 s pre-tone. Pre-tone averages for VI, VT, and RL were calculated from breath-by-breath data 6 breaths pre-tone. The magnitude of the responses for cardiovascular and respiratory variables were determined for each subject by subtracting the pre-tone mean values from the mean absolute values following the tone (second-by-second, 0 s to 15 s; breath-by-breath,1 to 6 breaths respectively). The time taken to initiate the EEG arousal, BP and RR interval response and the time taken to reach the maximum response following the tone were determined for each subject and group means were calculated. The response to arousal from sleep was then analyzed in 2 phases: the early and late response. For each variable the “early phase” was the time between tone onset and the peak response, and the “late phase” was the time between the peak response and 15 s post tone. The latency of the peak responses for each variable differed between subject groups, therefore the cut off time between the early and late response was defined by the subject group with the longest peak response. The early response for BP was from 0 s to 7 s post tone; for RR interval it was from 0 s to 5 s; and for VI, VT, and RL, it was breaths 1 to 2. The late response for BP was from 8 s to 15 s post tone; for RR interval it was from 6 s to 15 s; and for VI, VT, and RL, it was breaths 3 to 6.
Statistical Analysis
Demographic variables; pre-tone mean cardiovascular and respiratory data; arousal latency from tone onset; and the time taken to initiate and reach the maximum BP and HR response following the tone were compared between groups using 2 (young v older adults) by 2 (males v females) univariate ANOVAs.
To establish any differences between groups in the magnitude of the arousal response for each cardiovascular and respiratory variable, the group mean data were compared using 2 (young v older adults) by 2 (males v females) ANOVAs with repeated measures on a within group factor, i.e., “time” (magnitude of cardiovascular response each second post tone during the early phase) or “breath” (magnitude of respiratory response each breath post tone during the early phase). As older subjects had higher BMIs, this variable was included as a covariate. Post hoc analyses (Mann Whitney U tests) were then conducted on the variables that showed a statistically significant difference for between group factors. This analysis was repeated to determine the influence of arousal from sleep on late cardiovascular and respiratory responses. Statistical significance was defined as P < 0.05 with a Bonferroni correction applied for post hoc analyses.
RESULTS
Demographic data are given in Table 1. Older subjects had a higher BMI (P = 0.03), office diastolic BP (P < 0.001), and systolic BP (P < 0.001) than young subjects. Males had higher office systolic BP than females (P = 0.006). There were no significant age by gender interactions for any of the demographic variables.
Table 1.
Subject Demographic Data
| Young Males | Young Females | Older Males | Older Females | P value |
|||
|---|---|---|---|---|---|---|---|
| Age | Gender | Age x Gender | |||||
| N | 15 | 10 | 11 | 9 | n/a | n/a | n/a |
| Age (years) | 25 ± 1.4 | 23 ± 0.9 | 66 ± 1.7 | 65 ± 0.9 | n/a | 0.2 | 0.5 |
| BMI (kg/m2) | 23 ± 0.6 | 23 ± 0.7 | 26 ± 0.9 | 25 ± 1.8 | 0.03 | 0.8 | 0.6 |
| Systolic BP (mm Hg) | 122 ± 2.3 | 109 ± 3.2 | 131 ± 2.0 | 126 ± 4.3 | <0.001 | 0.006 | 0.2 |
| Diastolic BP (mm Hg) | 65 ± 1.6 | 66 ± 1.5 | 82 ± 1.3 | 77 ± 3.3 | <0.001 | 0.3 | 0.1 |
| AHI | 0.7 ± 0.3 | 0.9 ± 0.2 | 5.2 ± 1.6 | 3.6 ± 3.3 | 0.001 | 0.4 | 0.4 |
| ESS | 6 ± 0.6 | 6 ± 1.0 | 6 ± 0.9 | 6 ± 0.7 | 0.6 | 0.9 | 0.9 |
| FEV1 (% predicted) | 96 ± 2.6 | 98 ± 4.8 | 94 ± 5.5 | 99 ± 9.4 | 0.9 | 0.4 | 0.8 |
| FVC (% predicted) | 93 ± 2.7 | 94 ± 4.5 | 93 ± 5.4 | 103 ± 4.5 | 0.3 | 0.3 | 0.4 |
Values are mean ± SEM. BMI, body mass index; BP, blood pressure; ESS, Epworth Sleepiness Score; FEV1, force expiratory volume in 1 s; FVC, forced vital capacity.
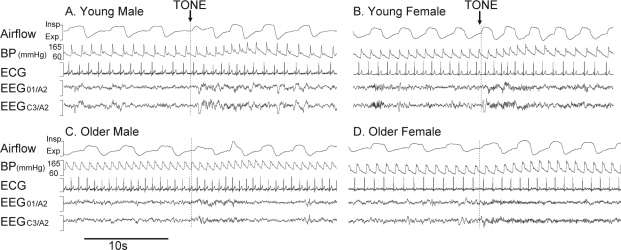
The mean (range) number of arousals >10 s achieved in each group was: young males 7 (3–16); young females 13 (9–16); older males 9 (4–13) and older females 10 (5–16). The percentage of these arousals that were classified as full awakenings (>15 s) was similar for each group: young males 48% (48 of 100), young females 50% (65 of 130), older males 62% (65 of 104) and older females 60% (56 of 93). The arousal latency from the tone initiation to the start of the EEG arousal was similar between older and young adults (both 0.4 ± 0.02 s, P = 0.3) and was delayed in males compared with females (0.4 ± 0.02 s v 0.3 ± 0.02 s, P = 0.01). Original recordings of arousals from sleep in a young male, young female, older male, and older female are shown in Figure 1.
Figure 1.
Original traces of auditory induced arousals (>10 s) from NREM stage 2 sleep in a young male (A), young female (B), older male (C) and older female (D). Dashed vertical line = start of 1-s auditory tone.
Baseline cardiorespiratory data prior to inducing arousal from sleep is presented in Table 2. Overall there were no differences between young and older adults. There was a significant main effect for gender for mean and systolic BPs (P < 0.001) due to a higher baseline measurement in males than females. There was also an unexpected trend for young males to have lower ventilation compared to young females, although the difference did not reach significance (P = 0.06). There were no significant age by gender interactions. Similar CPAP levels were delivered to older males and females (5 ± 0.4 cm H2O v 5 ± 0.3 cm H2O, respectively; P = 0.4) during the sleep studies.
Table 2.
Baseline Cardiorespiratory Data Prior to Inducing Arousal from Sleep
| Young Males | Young Females | Older Males | Older Females | P value |
|||
|---|---|---|---|---|---|---|---|
| Age | Gender | Age x Gender | |||||
| Mean BP (mm Hg) | 86 ± 2.8 | 69 ± 2.1 | 86 ± 3.6 | 77 ± 2.4 | 0.2 | < 0.001 | 0.2 |
| Diastolic BP (mm Hg) | 71 ± 2.4 | 53 ± 2.1 | 67 ± 3.6 | 58 ± 2.2 | 0.08 | 0.08 | 0.2 |
| Systolic BP (mm Hg) | 120 ± 4.9 | 107 ± 2.7 | 122 ± 3.5 | 120 ± 4.0 | 0.9 | < 0.001 | 0.2 |
| RR Interval (s) | 1.1 ± 0.03 | 1.1 ± 0.06 | 1.1 ± 0.05 | 1.0 ± 0.04 | 0.5 | 0.4 | 0.6 |
| Ventilation (Lmin−1) | 4.5 ± 0.3 | 5.5 ± 0.4 | 5.2 ± 0.4 | 5.0 ± 0.6 | 0.7 | 0.3 | 0.2 |
| Tidal Volume (L) | 0.3 ± 0.02 | 0.4 ± 0.02 | 0.4 ± 0.02 | 0.4 ± 0.04 | 0.08 | 0.4 | 0.7 |
| RL (cm H2O/l/s) | 11.9 ± 1.7 | – | 9.5 ± 1.7 | – | 0.4 | n/a | n/a |
Values are mean ± SEM. BP, blood pressure; RL, inspiratory pulmonary resistance. RL was measured in a subset of subjects: young males, n = 14; older males, n = 9.
Influence of Age on the Cardiovascular and Respiratory Responses to Arousal
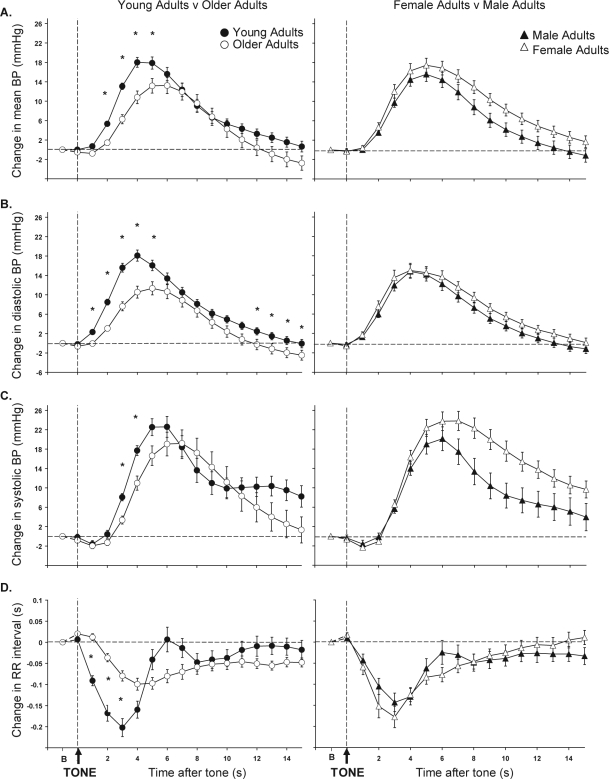
Figure 2 (left panels) shows the cardiovascular response to arousal from sleep in young and older adults. Older adults had a smaller early BP response to arousal from sleep compared to young adults (mean BP, P < 0.001; diastolic BP, P < 0.001; and systolic BP, P = 0.04). During the late response older adults' BP fell below baseline prearousal levels and was lower than young adults, although this was only significant for diastolic BP (mean BP, P = 0.06; diastolic BP, P = 0.03; systolic BP, P = 0.3). In addition, older adults had a smaller early, but not late, RR interval response to arousal from sleep compared to young adults (early response P < 0.001; late response P = 0.09). Significant differences in the magnitude of the cardiovascular responses between young and older adults (post hoc analysis) are depicted by stars on Figure 2. The times taken to initiate these cardiovascular responses and reach the peak responses following the tone were significantly delayed in older compared to young adults (Table 3).
Figure 2.
The group mean ± SEM mean blood pressure (BP) (A), diastolic BP (B), systolic BP (C) and RR interval (D) responses to auditory induced arousals from sleep are shown for young adults (filled circles) v older adults (open circles) (left) and female adults (open triangles) v males adults (closed triangles) (right). *P < 0.05 with Bonferroni correction for young adults v older adults. There were no significant differences in responses for female adults v male adults. Horizontal dashed line represents baseline (B) cardiovascular level. Vertical dashed line represents start of 1-s auditory tone (TONE).
Table 3.
Time Taken to Initiate and Reach Peak Cardiovascular Responses Following Tone Onset
| Young Males | Young Females | Older Males | Older Females | P value |
|||
|---|---|---|---|---|---|---|---|
| Age | Gender | Age x Gender | |||||
| Time to initiate response (s) | |||||||
| Mean BP | 0.8 ± 0.1 | 0.45 ± 0.1 | 1.5 ± 0.1 | 1.5 ± 0.2 | <0.001 | 0.3 | 0.2 |
| RR Interval | 0.1 ± 0.09 | 0.05 ± 0.05 | 1.7 ± 0.7 | 0.6 ± 0.2 | 0.009 | 0.1 | 0.2 |
| Time to reach peak response (s) | |||||||
| Mean BP | 4.5 ± 1.2 | 5.1 ± 1.1 | 5.6 ± 0.8 | 5.8 ± 1.4 | 0.02 | 0.3 | 0.6 |
| RR Interval | 3.8 ± 1.5 | 3.1 ± 1.1 | 5.3 ± 1.4 | 5.0 ± 1.5 | <0.001 | 0.3 | 0.6 |
Values are mean ± SEM. BP, blood pressure.
Young and older adults had similar VI and VT responses to arousal from sleep (Table 4. Early response: VI P = 0.3, VT P = 0.6; late response: VI P = 0.6, VT P = 0.6). In the subgroup of subjects in whom RL was measured young males had a greater reduction in RL on arousal from sleep compared to older males (Table 4. Early P = 0.005; late P = 0.03), however post hoc analysis revealed no significant differences between groups on a breath by breath basis when multiple comparisons were accounted for.
Table 4.
Respiratory Responses to Arousal from Sleep
| Respiratory Variable | Group | Breath Post Tone |
|||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Change in VI (Lmin−1) | Young Males | 0.02 ± 0.2 | 2.3 ± 0.4 | 2.2 ± 0.4 | 1.5 ± 0.3 | 1.0 ± 0.3 | 0.8 ± 0.3 |
| Young Females | 0.7 ± 0.5 | 2.5 ± 0.3 | 1.5 ± 0.3 | 0.8 ± 0.2 | 0.4 ± 0.2 | 0.3 ± 0.2 | |
| Older Males | −0.8 ± 0.5 | 1.8 ± 0.7 | 1.4 ± 0.5 | 1.3 ± 0.5 | 0.9 ± 0.3 | 0.7 ± 0.3 | |
| Older Females | 0.5 ± 0.4 | 2.0 ± 0.5 | 1.4 ± 0.2 | 0.8 ± 0.2 | 0.4 ± 0.2 | 0.3 ± 0.2 | |
| Change in VT (L) | Young Males | −0.03 ± 0.02 | 0.1 ± 0.03 | 0.1 ± 0.03 | 0.1 ± 0.04 | 0.07 ± 0.02 | 0.06 ± 0.02 |
| Young Females | 0.03 ± 0.03 | 0.1 ± 0.02 | 0.07 ± 0.02 | 0.04 ± 0.01 | 0.02 ± 0.02 | 0.01 ± 0.01 | |
| Older Males | −0.05 ± 0.07 | 0.09 ± 0.05 | 0.08 ± 0.03 | 0.07 ± 0.03 | 0.05 ± 0.02 | 0.04 ± 0.03 | |
| Older Females | 0.03 ± 0.05 | 0.2 ± 0.09 | 0.1 ± 0.03 | 0.09 ± 0.03 | 0.06 ± 0.03 | 0.05 ± 0.03 | |
| Change in RL * (cm H2O.l−1s−1) | Young Males not on CPAP (n=14) | −2.93 ± 1.05 | −3.61 ± 0.81 | −4.4 ± 1.42 | −3.91 ± 1.35 | −3.32 ± 0.99 | −3.63 ± 0.81 |
| Older Males on CPAP (n=14) | −0.77 ± 0.57 | −1.51 ± 0.89 | −1.85 ± 1.03 | −1.60 ± 0.90 | −1.81 ± 0.93 | −1.48 ± 0.80 | |
Values are mean ± SEM. VI, minute ventilation; VT, tidal volume; RL, inspiratory pulmonary resistance.
Influence of Gender on the Cardiovascular and Respiratory Responses to Arousal
Figure 2 (right panels) shows the cardiovascular response to arousal from sleep in male and females. Both groups had similar mean BP (early response, P = 0.1; late response, P = 0.06) and diastolic BP (early response, P = 0.3; late response, P = 0.2) responses to arousal from sleep. There were also no gender differences for the early systolic BP response (P = 0.2). During the late response, systolic BP was larger in females than males (P = 0.03); however, post hoc analyses revealed that for any given time point there were no gender differences. Furthermore, there was no difference between males and females in the magnitude of the RR interval response (early response, P = 0.2; late response, P = 0.8), the VI response (Early response, P = 0.1; late response, P = 0.1 [Table 4]), or the VT response (early response, P = 0.2; late response, P = 0.5). The times taken to initiate and reach the peak cardiovascular responses following the tone were also similar for males and females (Table 3).
There were no significant age by gender interaction effects for any of the early and late cardiovascular or respiratory responses or for the time taken to initiate and reach the maximum cardiovascular response following the tone.
DISCUSSION
The aim of the present study was to investigate the effect of age and gender on the cardiovascular response to arousals from sleep. The key findings were that the acute increases in BP and HR associated with arousals were smaller and delayed in older compared to young adults; an effect that occurred in both males and females. The smaller cardiovascular response was independent of changes in the respiratory responses to arousal. Gender had no influence on the cardiovascular or respiratory responses to arousal from sleep.
Influence of Age on the Cardiovascular Response to Arousal
Our data support the hypothesis that the acute cardiovascular response to arousal from sleep is smaller in older compared to younger adults. The age-related reduction in the HR response is consistent with a previous study carried out in older adults where HR was measured in response to spontaneous arousals12; in younger adults the magnitude of the response was also consistent with that previously reported.2,12,23,24
The acute surges in BP and HR associated with arousal at the termination of an apneic event have been implicated in the increased prevalence of hypertension in patients with sleep apnea.5,6 In older adults (≥ 60 years) with sleep apnea, the risk of having hypertension is no greater than for older people without the disorder.10 This may be a consequence of a shift in characteristics of sleep apnea with age, such as a shift from compromised anatomy to impaired neuromuscular/respiratory control.25 Alternatively, it may be a survival bias with older sleep apnea patients who develop hypertension not surviving. While our study was not designed to investigate these factors, our data suggest the lack of association between sleep apnea and hypertension in older adults may be a consequence of reduced HR and BP arousal responses. Thus the poorer cardiovascular reactivity of older adults may, paradoxically, reduce the impact of arousals from sleep.
Previous evidence indicating that older adults had a smaller HR response to arousal from sleep12 raised the possibility that baroreflex sensitivity may be lower in older adults which would produce larger surges in BP during arousal from sleep, compared to those in younger adults. The reduced BP response seen in our study is inconsistent with this suggestion. Further, in healthy young adults, arousal from sleep is not associated with a change in cardiac output and consequently transient surges in BP are considered to be a result of increased peripheral resistance.24 Thus age related structural and functional changes to the vasculature26–29 contributing to decreased compliance and increased arterial stiffness28 may partly account for the smaller BP response to arousal from sleep in older adults. Consistent with this notion, the older adults in our study had a slightly widened pulse pressure prearousal compared with young adults (i.e., prearousal baseline systolic BP was higher and diastolic BP was lower in older adults) indicating subtle decreases in arterial compliance may have occurred in our older adults.30 These data are in agreement with data from the Framingham Heart Study cohort.31
We investigated the early and late cardiovascular responses to arousal from sleep. By considering these two phases, we were able to examine immediate responses directly associated with arousal, as well as the return of cardiovascular regulation to baseline conditions. EEG arousal latency from tone initiation was not different between young and older subjects, but the initiation of the immediate cardiovascular response and the time taken to reach the maximum cardiovascular response was delayed in older compared with young adults. Furthermore, all immediate cardiovascular responses were smaller in older adults. The late diastolic BP was significantly higher in young compared to older adults; young adults returned to levels close to prearousal baseline conditions, whereas older adults experienced a drop in BP below baseline conditions. This may reflect age related changes in homeostatic mechanisms involved in resetting cardiovascular activity post sympathetic activation.32
Longer EEG arousals from sleep are associated with larger increases in cardiovascular activity.2 In the present study arousals lasting ≥ 10 s were analyzed in order to identify the largest cardiovascular activation possible in all subjects. The older adults experienced a greater number of full awakenings (>15 s) that may have been a consequence of increased arousability in this group due to aging per se or due to the effect of using CPAP. However, we do not believe that this would have influenced the reduced cardiovascular response to arousal observed in the older adults; indeed it is likely that the longer arousals would have increased the response of this group.
Influence of Gender on the Cardiovascular Response to Arousal
Our data are inconsistent with the hypothesis that the acute cardiovascular response to arousal from sleep is reduced in female compared with age matched male adults; specifically we found no difference in the acute blood pressure, RR interval, or respiratory response between males and females. This finding is unlikely to be the consequence of a type II error, as the peak change in RR interval associated with arousal from sleep in males was 0.14 ± 0.11 s compared with 0.18 ± 0.11 s in females; to detect a significant difference between genders in this study, it was calculated that a minimum of 130 subjects in each group would have been required (power, 0.8; rejection rule α = 0.05).
In contrast to the results of the present study, a previous investigation has shown males to have greater ventilatory responses on arousal from sleep compared to females in the follicular menstrual phase.33 We suggest that the inclusion of longer arousals (including full awakenings) in the present study, and the use of a shorter analysis period, may account for the inconsistencies between the two studies. Longer arousals were chosen in the present study as we wanted to ensure that the maximum cardiovascular response possible was achieved in all subjects.2,34
Premenopausal females have a lower incidence of sleep apnea compared to males.35,36 However, females of all ages do experience sleep apnea and it is important to determine whether the risks associated with the disorder are gender specific. Analysis of data from the Sleep Heart Health Cohort showed a stronger association between sleep apnea and hypertension in males compared to females.5 Further evidence from an animal model exposing male and female rats to intermittent hypoxia, an event that occurs during an apnea, suggests males with sleep apnea have a greater risk of developing hypertension.37 Thus, females with sleep apnea may be protected from cardiovascular insult associated with acute sympathetic activation on arousal at the termination of an apnea. Indeed, it has been found that increasing AHI in males, but not females, is associated with larger differences in evening-morning BP.14
Contradictory to the above, investigations into the association between sleep apnea and hypertension within the Wisconsin Sleep Cohort reported no significant effect for gender,38 although additional analysis of these data suggested that females with sleep apnea had a greater mortality risk than males of similar age and AHI.39 Furthermore, two clinical studies reported increased prevalence of hypertension in female compared to male patients40,41; the females in these populations had a higher BMI and this was only accounted for in the later study.40 Considering these inconsistent reports, and given that the magnitude of the cardiovascular response does not appear to differ as a function of gender in healthy adults, further investigation is required to establish the magnitude and cause of the cardiovascular risk associated with sleep apnea in males and females.
Methodological Considerations
Our protocol specified an AHI cut off of <15 events/h for all subjects. This was verified by an additional nocturnal polysomnography without CPAP. The relatively high threshold of 15 events/h was chosen as “normal” to accommodate for the increased prevalence of sleep related respiratory events that often occurs in older adults without evidence of increased daytime sleepiness and hypertension.10,42–45 Indeed, the older adults in the present study did have greater AHIs compared to the young group but this is unlikely to have affected the cardiovascular responses to arousal as older adults breathed via low levels of CPAP and auditory arousals were only induced after 2 minutes of stable stage 2 with no respiratory disturbances. The baseline BP was higher in the older adults but all subjects had BP within normal limits.46
Use of CPAP increases thoracic pressure and is likely to decrease cardiac preload and afterload47; theoretically this could explain the dampened cardiovascular response observed in the older adults studied on CPAP compared to young adults not on CPAP. Indeed, a previous study has demonstrated that the HR response to arousal from sleep is smaller in subjects on optimal compared to suboptimal CPAP.15 However, this is unlikely to be the main explanation for the findings of the present study because at prearousal baseline, there were minimal differences in RL between young and older male adults, and following arousal the reduction in RL was not significantly different between groups for any specific breath post arousal. However, given the reduced sample size we can not rule out a type II error. We suggest that the inconsistency in the findings of our study, compared to those of Jordan et al15 may be due to the fact that they reported upper airways resistance to be significantly higher in the suboptimal CPAP group, resulting in a significantly larger reduction in resistance in the suboptimal group on arousal from sleep. We attribute our findings to either an age-related increase in peripheral vascular stiffness or reduced cardiac output.
RL measurements were made during the initial phase of our experimental protocol to establish that we could abolish age related differences in RL using CPAP. When the protocol was widened to include female subjects (to investigate gender differences), we found it difficult to recruit subjects who were willing to tolerate the esophageal pressure probe during sleep. Therefore gender differences in RL during arousal from sleep cannot be excluded from the interpretation of our data. However, the use of CPAP sufficient to eliminate visual evidence of airflow limitation is likely to have been adequate to reduce RL in older females to similar levels as young adults. Furthermore, it is unlikely differences in airways resistance between genders would affect the results of the present study, since it has been reported that the change in upper airways resistance from wakefulness to sleep, during stage 2 sleep, and on arousal from sleep is similar between males and females.33,48,49
In the present study we did not measure hormone levels, however we did study our young females in early follicular phase of their menstrual cycle. Accordingly reproductive hormone levels are likely to have been at their lowest levels.
Sleep deprivation the night prior to the study was employed to promote sleep in the laboratory. Previous studies have shown that acute sleep deprivation does not affect cardiac responses to arousal from sleep in healthy young men,50 but other subject groups included in the present study may have varied in their susceptibility to sleep deprivation.
BP was recorded via finger photoplethysmography, using a device that does not have a height correction unit to compensate for hydrostatic blood pressure changes in the finger (Finapres 2300, Ohmeda). Considering this, all subjects were studied in the supine position, and data were screened to ensure that no body movements occurred during analysis periods selected. Furthermore, the change in BP from the prearousal baseline in response to arousal from sleep was determined for each arousal rather than using absolute measures of BP, thus we believe changes in hand position between arousals would not have affected the results obtained in the present study.
A higher BMI in the older compared to young adults could have potentially influenced the reduced cardiovascular response to arousal from sleep observed in the older adults. Indeed, a higher BMI is associated with higher resting BP,51 decreased parasympathetic and sympathetic activation,52–54 and a reduction in baroreflex function.52 For this reason, adjustment for between group differences in BMI was included in the statistical analysis of the present study.
Conclusions
This study has shown for the first time that the BP and HR responses to arousal from sleep were smaller and delayed in healthy older adults compared to young adults, independent of any differences in the ventilatory response. The cause of the age related reduction is unknown; however, we speculate that for older people with sleep apnea, in whom nocturnal arousals frequently occur, the reduced cardiovascular response may be protective against the link between sleep apnea and hypertension.
ABBREVIATIONS
| Symbol/Abbreviation | Explanation |
|---|---|
| AHI | Apnea hypopnea index |
| BP | Blood pressure |
| CPAP | Continuous positive airway pressure |
| EEG | Electroencephalogram |
| EMG | Electromyogram |
| EOG | Electrooculgram |
| HR | Heart rate |
| OSA | Obstructive sleep apnea |
| Pes | Esophageal pressure |
| RL | Total inspiratory pulmonary resistance |
| VI | Minute ventilation |
| VT | Tidal volume |
ACKNOWLEDGMENTS
Source of Support: Wellcome Trust
Footnotes
Disclosure Statement
This was not an industry supported study. Dr. Morrell has participated in research funded by ResMed. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Trinder J, Padula M, Berlowitz D, et al. Cardiac and respiratory activity at arousal from sleep under controlled ventilation conditions. J Appl Physiol. 2001;90:1455–63. doi: 10.1152/jappl.2001.90.4.1455. [DOI] [PubMed] [Google Scholar]
- 2.Davies RJ, Belt PJ, Roberts SJ, Ali NJ, Stradling JR. Arterial blood pressure responses to graded transient arousal from sleep in normal humans. J Appl Physiol. 1993;74:1123–30. doi: 10.1152/jappl.1993.74.3.1123. [DOI] [PubMed] [Google Scholar]
- 3.O'Driscoll DM, Meadows GE, Corfield DR, Simonds AK, Morrell MJ. Cardiovascular response to arousal from sleep under controlled conditions of central and peripheral chemoreceptor stimulation in humans. J Appl Physiol. 2004;96:865–70. doi: 10.1152/japplphysiol.00749.2003. [DOI] [PubMed] [Google Scholar]
- 4.Davies CW, Crosby JH, Mullins RL, Barbour C, Davies RJ, Stradling JR. Case-control study of 24 hour ambulatory blood pressure in patients with obstructive sleep apnoea and normal matched control subjects. Thorax. 2000;55:736–40. doi: 10.1136/thorax.55.9.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 6.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 7.Mathur R, Douglas NJ. Frequency of EEG arousals from nocturnal sleep in normal subjects. Sleep. 1995;18:330–3. doi: 10.1093/sleep/18.5.330. [DOI] [PubMed] [Google Scholar]
- 8.Boselli M, Parrino L, Smerieri A, Terzano MG. Effect of age on EEG arousals in normal sleep. Sleep. 1998;21:351–7. [PubMed] [Google Scholar]
- 9.Browne HA, Adams L, Simonds AK, Morrell MJ. Sleep apnoea and daytime function in the elderly--what is the impact of arousal frequency? Respir Med. 2003;97:1102–8. doi: 10.1016/s0954-6111(03)00142-2. [DOI] [PubMed] [Google Scholar]
- 10.Haas DC, Foster GL, Nieto FJ, et al. Age-dependent associations between sleep-disordered breathing and hypertension: importance of discriminating between systolic/diastolic hypertension and isolated systolic hypertension in the Sleep Heart Health Study. Circulation. 2005;111:614–21. doi: 10.1161/01.CIR.0000154540.62381.CF. [DOI] [PubMed] [Google Scholar]
- 11.Lavie P, Lavie L, Herer P. All-cause mortality in males with sleep apnoea syndrome: declining mortality rates with age. Eur Respir J. 2005;25:514–20. doi: 10.1183/09031936.05.00051504. [DOI] [PubMed] [Google Scholar]
- 12.Gosselin N, Michaud M, Carrier J, Lavigne G, Montplaisir J. Age difference in heart rate changes associated with micro-arousals in humans. Clin Neurophysiol. 2002;113:1517–21. doi: 10.1016/s1388-2457(02)00189-x. [DOI] [PubMed] [Google Scholar]
- 13.Hedner J, Bengtsson-Bostrom K, Peker Y, Grote L, Rastam L, Lindblad U. Hypertension prevalence in obstructive sleep apnoea and sex: a population-based case-control study. Eur Respir J. 2006;27:564–70. doi: 10.1183/09031936.06.00042105. [DOI] [PubMed] [Google Scholar]
- 14.Lavie-Nevo K, Pillar G. Evening-morning differences in blood pressure in sleep apnea syndrome: effect of gender. Am J Hypertens. 2006;19:1064–9. doi: 10.1016/j.amjhyper.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 15.Jordan AJ, McEvoy RD, Edwards JK, et al. The influence of gender and upper airway resistance on the ventilatory response to arousal in obstructive sleep apnoea in humans. J Physiol. 2004;558:993–1004. doi: 10.1113/jphysiol.2004.064238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Driscoll DM, Kostikas K, Simonds AK, Morrell MJ. Occlusion of the upper airway does not augment the cardiovascular response to arousal from sleep in humans. J Appl Physiol. 2005;98:1349–55. doi: 10.1152/japplphysiol.00706.2004. [DOI] [PubMed] [Google Scholar]
- 17.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring systems for sleep stages of human subjects. Washington DC: US Government printing Office; 1968. [Google Scholar]
- 18.Badr MS, Skatrud JB, Simon PM, Dempsey JA. Effect of hypercapnia on total pulmonary resistance during wakefulness and during NREM sleep. Am Rev Respir Dis. 1991;144:406–14. doi: 10.1164/ajrccm/144.2.406. [DOI] [PubMed] [Google Scholar]
- 19.Morrell MJ, Badr MS, Harms CA, Dempsey JA. The assessment of upper airway patency during apnea using cardiogenic oscillations in the airflow signal. Sleep. 1995;18:651–8. doi: 10.1093/sleep/18.8.651. [DOI] [PubMed] [Google Scholar]
- 20.Browne HA, Adams L, Simonds AK, Morrell MJ. Ageing does not influence the sleep-related decrease in the hypercapnic ventilatory response. Eur Respir J. 2003;21:523–9. doi: 10.1183/09031936.03.00039002. [DOI] [PubMed] [Google Scholar]
- 21.Condos R, Norman RG, Krishnasamy I, Peduzzi N, Goldring RM, Rapoport DM. Flow limitation as a noninvasive assessment of residual upper-airway resistance during continuous positive airway pressure therapy of obstructive sleep apnea. Am J Respir Crit Care Med. 1994;150:475–80. doi: 10.1164/ajrccm.150.2.8049832. [DOI] [PubMed] [Google Scholar]
- 22.Montserrat JM, Ballester E, Olivi H, et al. Time-course of stepwise CPAP titration. Behavior of respiratory and neurological variables. Am J Respir Crit Care Med. 1995;152:1854–9. doi: 10.1164/ajrccm.152.6.8520746. [DOI] [PubMed] [Google Scholar]
- 23.Trinder J, Allen N, Kleiman J, et al. On the nature of cardiovascular activation at an arousal from sleep. Sleep. 2003;26:543–51. [PubMed] [Google Scholar]
- 24.Morgan BJ, Crabtree DC, Puleo DS, Badr MS, Toiber F, Skatrud JB. Neurocirculatory consequences of abrupt change in sleep state in humans. J Appl Physiol. 1996;80:1627–36. doi: 10.1152/jappl.1996.80.5.1627. [DOI] [PubMed] [Google Scholar]
- 25.Arens R, Marcus CL. Pathophysiology of upper airway obstruction: a developmental perspective. Sleep. 2004;27:997–1019. doi: 10.1093/sleep/27.5.997. [DOI] [PubMed] [Google Scholar]
- 26.Al-Shaer MH, Choueiri NE, Correia ML, Sinkey CA, Barenz TA, Haynes WG. Effects of aging and atherosclerosis on endothelial and vascular smooth muscle function in humans. Int J Cardiol. 2006;109:201–6. doi: 10.1016/j.ijcard.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Dinenno FA, Jones PP, Seals DR, Tanaka H. Age-associated arterial wall thickening is related to elevations in sympathetic activity in healthy humans. Am J Physiol Heart Circ Physiol. 2000;278:H1205–10. doi: 10.1152/ajpheart.2000.278.4.H1205. [DOI] [PubMed] [Google Scholar]
- 28.Gaballa MA, Jacob CT, Raya TE, Liu J, Simon B, Goldman S. Large artery remodeling during aging: biaxial passive and active stiffness. Hypertension. 1998;32:437–43. doi: 10.1161/01.hyp.32.3.437. [DOI] [PubMed] [Google Scholar]
- 29.Lidman D. Histopathology of human extremital arteries throughout life. Including measurements of cystolic pressures in ankle and arm. Acta Chir Scand. 1982;148:575–80. [PubMed] [Google Scholar]
- 30.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–7. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 31.Franklin SS, Gustin Wt, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96:308–15. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- 32.Gribbin B, Pickering TG, Sleight P, Peto R. Effect of age and high blood pressure on baroreflex sensitivity in man. Circ Res. 1971;29:424–31. doi: 10.1161/01.res.29.4.424. [DOI] [PubMed] [Google Scholar]
- 33.Jordan AS, Eckberg DJ, Catcheside PG, McEvoy RD. Ventilatory response to brief arousal from non-rapid eye movement sleep is greater in men than in women. Am J Respir Crit Care Med. 2003;168:1512–9. doi: 10.1164/rccm.200302-150OC. [DOI] [PubMed] [Google Scholar]
- 34.Catcheside PG, Chiong SC, Mercer J, Saunders NA, McEvoy RD. Noninvasive cardiovascular markers of acoustically induced arousal from non-rapid-eye-movement sleep. Sleep. 2002;25:797–804. doi: 10.1093/sleep/25.7.797. [DOI] [PubMed] [Google Scholar]
- 35.Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163:608–13. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 36.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 37.Hinojosa-Laborde C, Mifflin SW. Sex differences in blood pressure response to intermittent hypoxia in rats. Hypertension. 2005;46:1016–21. doi: 10.1161/01.HYP.0000175477.33816.f3. [DOI] [PubMed] [Google Scholar]
- 38.Young T, Peppard P, Palta M, et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157:1746–52. [PubMed] [Google Scholar]
- 39.Young T, Finn L. Epidemiological insights into the public health burden of sleep disordered breathing: sex differences in survival among sleep clinic patients. Thorax. 1998;53(Suppl 3):S16–9. doi: 10.1136/thx.53.2008.s16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drager LF, Pereira AC, Barreto-Filho JA, et al. Phenotypic characteristics associated with hypertension in patients with obstructive sleep apnea. J Hum Hypertens. 2006;20:523–8. doi: 10.1038/sj.jhh.1002012. [DOI] [PubMed] [Google Scholar]
- 41.Quintana-Gallego E, Carmona-Bernal C, Capote F, et al. Gender differences in obstructive sleep apnea syndrome: a clinical study of 1166 patients. Respir Med. 2004;98:984–9. doi: 10.1016/j.rmed.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 42.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14:486–95. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Young T, Shahar E, Nieto FJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 44.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–8. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 45.Ancoli-Israel S, Coy T. Are breathing disturbances in elderly equivalent to sleep apnea syndrome? Sleep. 1994;17:77–83. doi: 10.1093/sleep/17.1.77. [DOI] [PubMed] [Google Scholar]
- 46.JBS 2: Joint British Societies' guidelines on prevention of cardiovascular disease in clinical practice. Heart. 2005;91(Suppl 5):v1–52. doi: 10.1136/hrt.2005.079988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shekerdemian L, Bohn D. Cardiovascular effects of mechanical ventilation. Arch Dis Child. 1999;80:475–80. doi: 10.1136/adc.80.5.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thurnheer R, Wraith PK, Douglas NJ. Influence of age and gender on upper airway resistance in NREM and REM sleep. J Appl Physiol. 2001;90:981–8. doi: 10.1152/jappl.2001.90.3.981. [DOI] [PubMed] [Google Scholar]
- 49.Rowley JA, Zhou X, Vergine I, Shkoukani MA, Badr MS. Influence of gender on upper airway mechanics: upper airway resistance and Pcrit. J Appl Physiol. 2001;91:2248–54. doi: 10.1152/jappl.2001.91.5.2248. [DOI] [PubMed] [Google Scholar]
- 50.Sforza E, Chapotot F, Pigeau R, Paul PN, Buguet A. Effects of sleep deprivation on spontaneous arousals in humans. Sleep. 2004;27:1068–75. doi: 10.1093/sleep/27.6.1068. [DOI] [PubMed] [Google Scholar]
- 51.Wilsgaard T, Schirmer H, Arnesen E. Impact of body weight on blood pressure with a focus on sex differences: the Tromso Study, 1986-1995. Arch Intern Med. 2000;160:2847–53. doi: 10.1001/archinte.160.18.2847. [DOI] [PubMed] [Google Scholar]
- 52.Laederach-Hofmann K, Mussgay L, Ruddel H. Autonomic cardiovascular regulation in obesity. J Endocrinol. 2000;164:59–66. doi: 10.1677/joe.0.1640059. [DOI] [PubMed] [Google Scholar]
- 53.Piccirillo G, Vetta F, Fimognari FL, et al. Power spectral analysis of heart rate variability in obese subjects: evidence of decreased cardiac sympathetic responsiveness. Int J Obes Relat Metab Disord. 1996;20:825–9. [PubMed] [Google Scholar]
- 54.Rossi M, Marti G, Ricordi L, et al. Cardiac autonomic dysfunction in obese subjects. Clin Sci (Lond) 1989;76:567–72. doi: 10.1042/cs0760567. [DOI] [PubMed] [Google Scholar]