Abstract
Study Objective:
To determine OSA-related changes in variability of QT interval duration and in heart rate variability (HRV), and to evaluate the relationship of these parameters to disease severity.
Design:
Retrospective analysis of diagnostic sleep records.
Settings:
Clinical sleep laboratory in a hospital setting.
Patients:
Twenty patients (12 males and 8 females) without significant comorbidities who were undergoing polysomnography were studied.
Measurements and Results:
Standard heart rate variability measures and QT variability (Berger algorithm) were computed over consecutive 5-minute ECG epochs throughout the night. The effect of sleep stage and the relationship between these parameters and the severity of OSA as determined by the respiratory disturbance index (RDI) were explored. Further, a linear regression model of QT variability was developed. Severity of OSA (RDI) was 49 ± 28 (range from 17–107) events/hr. QT variability was the only ECG measure significantly correlated with RDI (both log-transformed; r = 0.6, P = 0.006). Further, QT variability was correlated with the minimum oxygen saturation (r = −0.55, P = 0.01). Sleep stage showed a significant effect on HRV, but not on QT variability. In the regression model, RDI was the strongest predictor of QT variability (R2 increase 38%), followed by high and low frequency power of HRV (R2 increase 10% each).
Conclusion:
Obstructive sleep apnea is associated with changes in QT interval variability during sleep. The variance of beat-to-beat QT intervals correlates more strongly with the severity of OSA (as determined by RDI) than standard measures of heart rate variability, and is correlated with blood oxygenation, but not sleep stage.
Citation:
Baumert M; Smith J; Catcheside P; McEvoy RD; Abbott D; Sanders P; Nalivaiko E. Variability of QT interval duration in obstructive sleep apnea: an indicator of disease severity. SLEEP 2008;31(7):959-966.
Keywords: QT variability, heart rate variability, arousal, sleep stage, respiratory disturbance index
OBSTRUCTIVE SLEEP APNEA (OSA) IS A COMMON DISORDER OF BREATHING THAT OCCURS DURING SLEEP AND AFFECTS OVER 4% OF MEN AND 2% OF WOMEN1 and is an independent risk factor for cardiovascular disease.2 Sudden cardiac death (SCD) amongst OSA patients occurs predominantly at night, in contrast to the general population.3 A study of 400 OSA patients revealed that nearly half of them develop cardiac arrhythmias of some kind during sleep.4 OSA is characterized by repetitive partial or complete closure of the upper airway. Obstructive episodes elicit visceral changes, including blood gas disturbances, large negative intrathoracic pressure changes that increase cardiac pre- and afterload, surges in sympathetic neural activity, alterations in heart rate, and surges in arterial blood pressure. These acute and repetitive events are thought to contribute to fatal nocturnal cardiovascular events.2
Cardiac autonomic activity in OSA patients has been studied mainly using heart rate variability (HRV) analysis. HRV refers to beat-to-beat fluctuations in the RR interval and provides a noninvasive means to assess autonomic heart control mediated by vagal and sympathetic efferents. HRV analysis is usually performed using a set of measures in the time and frequency domains (see Methods for details). Abnormal HRV is an independent predictor of cardiac mortality in different patient populations.5,6 It has been demonstrated that autonomic heart rate modulation is altered in OSA patients,7 and different indices of HRV have been proposed as a screening tool for OSA.8
Cardiac vulnerability is heightened during ventricular repolarization that could be indirectly assessed by measuring QT interval duration on the surface ECG. The few studies that have examined QT interval properties in OSA patients were based on the QT dispersion index that has, however, a questionable validity.9 A novel approach has been proposed to assess repolarization lability using the QT variability index (QTVi) to quantify beat-to-beat changes in the QT interval duration.10 The QTVi is increased in CHF patients11 and is a powerful predictor of arrhythmias and cardiac mortality.10,12 In the present study, we evaluated QT variability in OSA patients and characterized the relationship between the severity of OSA and QT variability.
METHODS
Study Population
The study conformed to the principles outlined in the Declaration of Helsinki, and was approved by the Research and Ethics Committee of the Repatriation General Hospital. The study comprised 20 patients (12 males, 8 females) who had already undergone diagnostic overnight polysomnography for suspected OSA in the last 6 months and who consented to detailed ECG analysis of their records. Twenty-eight patient records were initially randomly selected for inclusion. Seven patients refused participation and one ECG record subsequently proved to be technically inadequate. Inclusion criteria were patient age (30-75 years), absence of documented relevant comorbidities, BMI >25 kg/m2, RDI >15 events/hour. We excluded patients with documented craniofacial, metabolic, respiratory disorders, and cardiovascular disease, aiming to study the primary effects of OSA on heart control. None of the patients had sleep disorders other than OSA. All patients were nonsmokers and without any medication related to cardiovascular disease, diabetes, or sleep disorders. All patients provided written informed consent.
Overnight Polysomnography
Overnight polysomnography was performed using a Compumedics E series system (Compumedics, Australia). For sleep staging and arousal scoring, standard surface electrodes were applied to the face and scalp, including 2-channel electroencephalograms (C3-A2 and C4-A1), left and right electrooculograms, and a submental electromyogram. Leg movements were recorded from surface electrodes to the tibialis anterior muscle of both legs. Nasal pressure was recorded for airflow measurements. Respiratory effort was monitored from chest and abdominal respiratory bands. Sleep stages were assigned to consecutive 30-s epochs. Sleep scoring of all subjects was carried out by the same experienced sleep technician.
Apnea was defined as the absence of airflow >10 sec without (central apnea) or with the presence of persistent (obstructive apnea) or re-emerging (mixed apnea) respiratory efforts. Hypopnea was defined as a reduction of ≥50% in the amplitude of respiratory efforts lasting ≥10 sec and a fall in arterial oxyhemoglobin saturation ≥4%. Total sleep time, duration of REM and NREM periods, and number of respiratory and spontaneous arousals per hour were also measured. The mean and minimal arterial oxyhemoglobin saturations and cumulative time spent with an arterial oxyhemoglobin saturation <90% were also calculated.
Electrocardiographic Evaluation
The ECG signal (lead II) was amplified, band-pass filtered (0.3-30 Hz), and digitized at 512 Hz. The HRV and QT variability analyses were performed for all consecutive 5-min ECG segments throughout the night. Thus, quasi-stationary conditions of RR and QT time series, necessary for variability analyses from a signal processing point of view, were achieved. Segments where the beat-to-beat QT intervals could be detected for less than 95% of all R-waves due to artifacts or ectopic beats were excluded from the analysis.
Heart Rate Variability Measures
The following heart rate variability measures were computed in the time and frequency domain based on the measurement standards:13
A. Time domain:
meanNN–the mean normal-to-normal RR interval, in ms;
SDNN–the standard deviation of normal-to-normal RR interval, reflecting the overall heart rate variability, in ms;
RMSSD–root-mean-square of the beat-to-beat differences, in ms; reflecting the average magnitude of heart rate changes between consecutive beats (a marker of vagal heart rate modulation).
B. Frequency domain:
For HRV analysis in the frequency domain, RR time series were interpolated at 250 ms to obtain equidistant values. Subsequently, Fast Fourier transform was applied. We quantified HRV power in 3 frequency bands that have been associated with different physiological rhythms.
VLF–very low frequency power in the range of 0.003–0.04 Hz, in ms2; the origin of these oscillations is largely unknown but has been associated with thermoregulation and vagal modulations.
LF–low frequency power in the range of 0.04–0.15 Hz, in ms2; mainly reflecting a ∼10 s rhythm associated with Mayer waves in blood pressure and is influenced by vagal and sympathetic tone.
HF–high frequency power in the range 0.15–0.4 Hz, in ms2; mainly reflecting the magnitude of respiratory sinus arrhythmia; vagally mediated.
LF/HF–ratio of low-to-high frequency power. A unitless measure often computed as a marker of sympatho-vagal balance.
QT Interval Variability Analysis
To obtain beat-to-beat QT intervals, we applied the algorithm proposed by Berger et al.10 Here, the operator defines a template QT interval, and the algorithm then finds QT intervals of all other beats by determining how much each beat must be scaled in time to best match the template. In this way, a robust estimation of QT interval is achieved without determination of each individual T-wave end. The QTVi was defined as by Berger et al:10
QTVi = log ((QTvar/meanQT2)/(RRvar/meanRR2)),
where the numerator contains the variance of all QT intervals (QTvar) normalized to the square of the mean QT interval (meanQT). The denominator contains the variance of RR intervals (RRvar) normalized to the squared mean RR interval (meanRR). The logarithm is taken for purely statistical reasons, i.e., to ensure a normal distribution of the otherwise skewed QTVi distribution.
In addition, we determined:
QTvar–variance of beat-to-beat QT intervals without any normalization, in ms2; indicating the overall variability of beat-to-beat QT interval fluctuations around the mean QT interval.
QT/RR slope–slope of a linear regression function, fitted between RR and QT intervals; i.e., the factor by which the QT interval changes when the RR interval changes.
QT/RR r2 –residuals of the regression line fitted to QT vs. RR plots; reflecting the strength of the RR /QT dependence.
QT/RR coherence–average coherence between the RR and QT power spectra in the frequency range of 0–0.2 Hz; reflecting the similarity between slow oscillations in RR and in QT intervals. Coherence ranges between 0 (no similarity) and 1 (identical).
Statistical Analysis
HRV and QTV measures derived from the 5-min epochs were averaged for each patient and presented as group means and standard deviations. To investigate the possible relationship between HRV and QTV and the severity of OSA, we computed Pearson linear correlation coefficients (r) between RDI and the individually averaged HRV or QTV measures, respectively. Variables with a skewed distribution were transformed prior to the correlation analysis. To investigate the effect of sleep stage, measures from 5-min ECG segments with no change in sleep stage were individually averaged for the different sleep stages. One-way ANOVA was applied to test for differences in HRV and QTV between wake, stage 2 sleep (S2), slow wave sleep (SWS, sleep stages 3+4) and REM sleep, respectively. For post hoc analysis the Tukey multiple comparison test was used.
To identify those variables that are associated with QT variability, a linear regression model was employed. Here, the non-normalized QT variability measure (QTvar) was the dependent variable and age, gender, BMI, and overnight minimum oxygen saturation were entered into the model as independent measures. Subsequently, RDI and QT and HRV measures were allowed to be stepwise added in into the model, if they could significantly improve the predictive value.
RESULTS
Polysomnographic Findings
Demographic and polysomnographic data are presented in Table 1. RR and QT data are presented in Table 2. QT variability was significantly elevated in 5-min segments that had at least one hypopnea/apnea episode (group mean of logQTvar: 1.47 ± 0.44 for epochs with apneas vs. 1.22 ± 0.37 for epochs without apnea, P < 0.001). An example of RR and QT time series computed during a 5-min epoch with obstructions and during a 5-min epoch without any hypopnea/apnea is presented in Figure 1. The scatter plots in Figure 2 show the RR/QT dependence for the same 2 epochs.
Table 1.
Demographic Data and Overnight Polysomnography Findings in OSA Patients
| mean | SD | |
|---|---|---|
| Age (yr) | 44 | 10 |
| BMI (kg/m2) | 34 | 8 |
| Weight (kg) | 100 | 24 |
| Height (cm) | 172 | 13 |
| Heart rate (bpm) | 70 | 8 |
| RDI | 49 | 28 |
| Sleep time (min) | 347 | 58 |
| Wake time (min) | 63 | 48 |
| REM sleep (%) | 18.1 | 4.8 |
| Respiratory arousals (n/h) | 13.8 | 10.7 |
| Spontaneous arousals (n/h) | 9.2 | 4.0 |
| Limb movement arousals (n/h) | 2.8 | 2.1 |
| Total arousal (n/h) | 25.8 | 11.9 |
| Minimum oxygen saturation (%) | 83.3 | 9.2 |
| Average desaturation (%) | 3.8 | 1.8 |
Data from 20 patients (12 males, 8 females) are presented as mean and standard deviation (SD).
Table 2.
Heart Rate Variability and QT Variability Measures in OSA Patients
| Mean | SD | R | P | |
|---|---|---|---|---|
| RR measures | ||||
| meanNN (ms) | 873 | 110 | −0.07 | 0.78 |
| SDNN (ms) | 57 | 20 | 0.33 | 0.15 |
| RMSSD (ms) | 42 | 22 | 0.34 | 0.09 |
| logVLF (ms2) | 3.18 | 0.28 | 0.42 | 0.06 |
| logLF (ms2) | 2.97 | 0.30 | 0.18 | 0.44 |
| logHF (ms2) | 2.66 | 0.45 | 0.33 | 0.15 |
| logLF/logHF | 1.13 | 0.12 | −0.39 | 0.09 |
| QT measures | ||||
| logQTvar (log ms2) | 1.42 | 0.45 | 0.60 | 0.006 |
| QTVi (n.u.) | −1.34 | 0.47 | 0.35 | 0.13 |
| QT/RRslope (n.u.) | 0.030 | 0.028 | −0.30 | 0.21 |
| QT/RRr2 (n.u.) | 0.19 | 0.10 | −0.005 | 0.98 |
| QT/RRcoherence (n.u.) | 0.41 | 0.11 | −0.19 | 0.42 |
Data presented as mean and standard deviation (SD); n=20. R, Pearson linear correlation coefficients computed between respiratory disturbance index (log-transformed) and ECG measures; P, significance values; N.u., normalized units.
Figure 1.
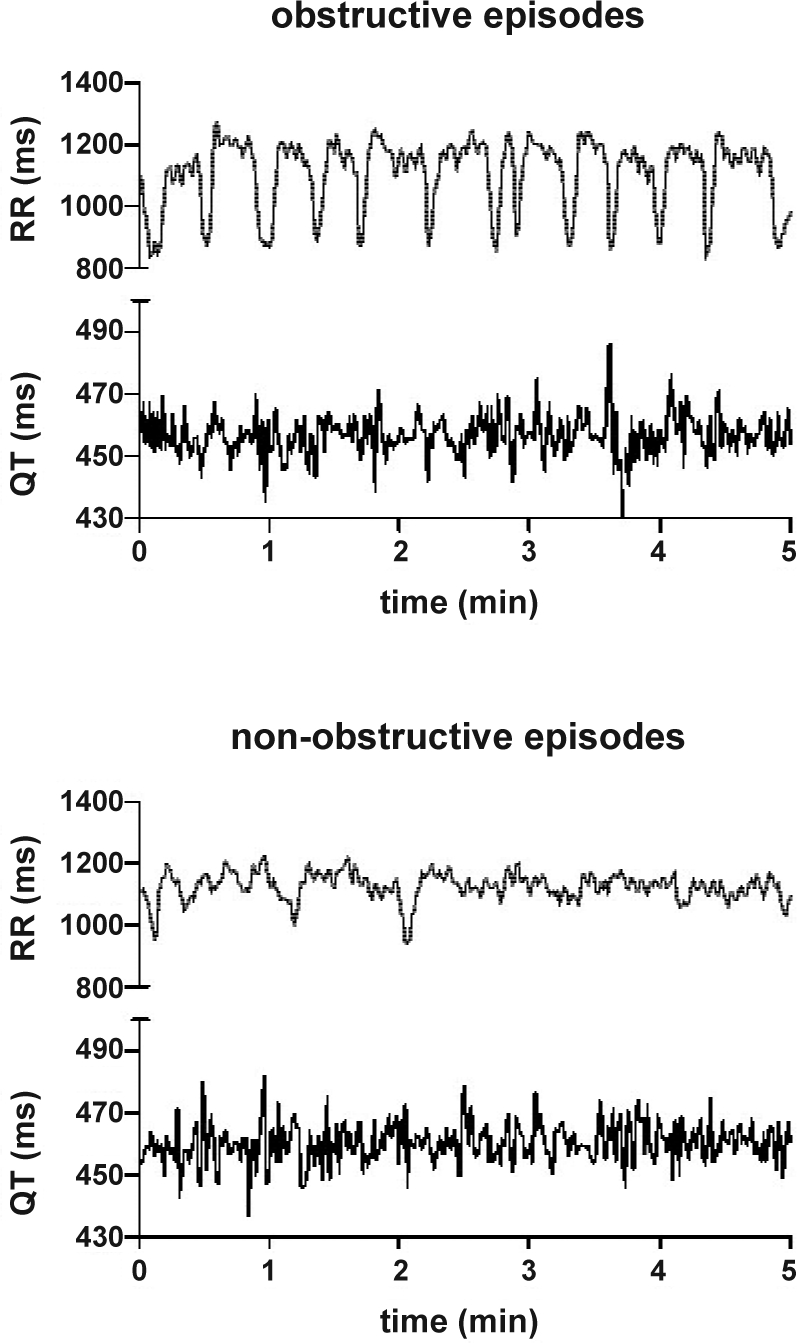
Example of time series of RR and QT intervals in an OSA patient over 5 min during an episode of repetitive upper airway obstructions (top) and during an epoch without apnea/hypopnea (bottom), both recorded during NREM sleep. The mean values of RR (1081 vs. 1122 ms) and QT (457 vs. 460 ms) are very similar for both epochs. The sequence of repetitive obstructive events causes a cyclical heart rate pattern. This is reflected in all HRV measures, particularly in the low (7403 ms2 vs. 748 ms2) and very low frequency power (5393 ms2 vs. 693 ms2), with values around ten times greater than those obtained during the non-obstructive epoch. The QT variability (QTvar) itself is increased during the obstructive episode (51 ms2 vs. 37 ms2), but the variability index QTVi is reduced (−1.74 vs. −0.96). This paradoxical effect is caused by the normalization of QTvar to HRV, which is relatively more increased during the repetitive obstructive episodes than QTvar.
Figure 2.
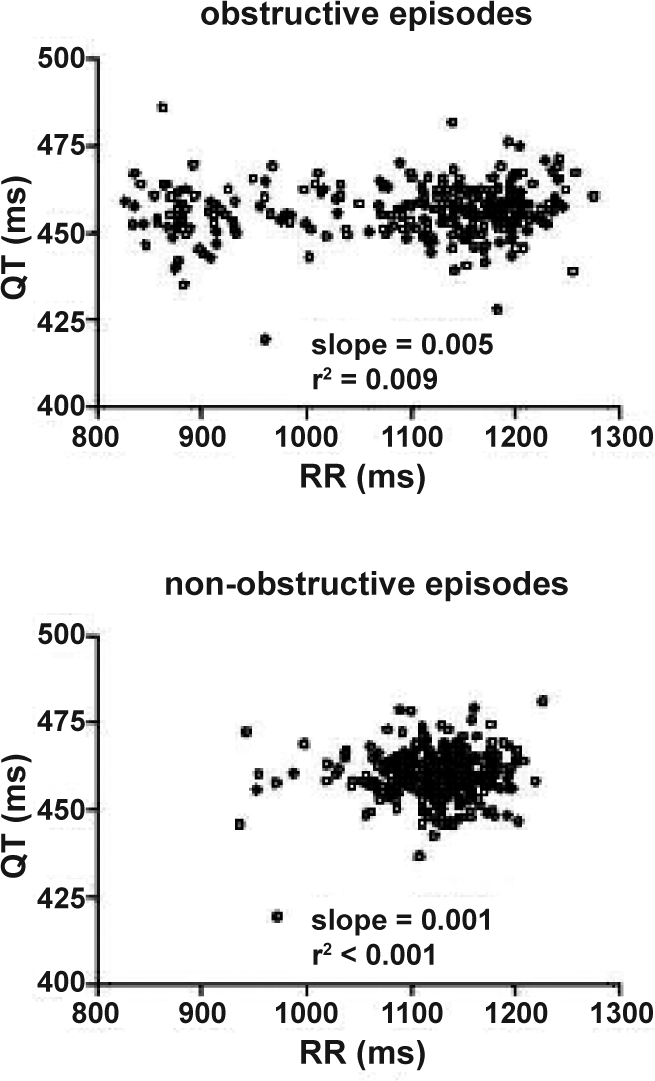
Example of RR/QT dependence computed for the obstructive and an apnea/hypopnea free epochs shown in Figure 1. The correlation between RR and QT interval was low in both conditions, but higher during obstructive events (QT/RRr:2 0.009 vs <0.001).
Correlation Between RDI and ECG Measures
Table 2 describes the relationship between RDI and the ECG measures. Spectral measures of HRV as well as RDI were log-transformed in order to achieve a normal distribution, which is indicated by the prefix log.
The magnitude of RR interval fluctuations (RMSSD), the power of very low frequency heart rate oscillations (logVLF) and the logLF/logHF ratio showed trends for positive correlations with logRDI, although none of them reached statistical significance. Among these HRV measure logVLF showed the strongest correlation with logRDI (r = 0.42; P = 0.06).
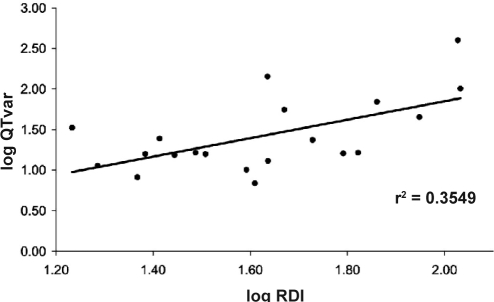
QT variability – without any normalization to heart rate - (logQTvar) was the only ECG-derived measure that was significantly correlated with logRDI (r = 0.6; P = 0.006; see Figure 3). Among the other QT variability indices that are all related to HRV, QTVi showed the highest although nonsignificant correlation (r = 0.38, P = 0.31).
Figure 3.
QT interval variability (QTvar) is significantly correlated with the respiratory disturbance index (RDI). Both measures were log-transformed to achieve normal distributions.
Effects of Oxygen Desaturation on QT Variability
There was a significant correlation between overnight minimum oxygen saturation and logQTvar, with lower minimum oxygen saturation values being associated with increased QT variability (r = −0.55, P = 0.01). The mean overnight minimum oxygen saturation was 83.3% ± 0.2%.
Effects of Sleep Stage on Heart Rate and QT Interval Variability
All HRV measures changed significantly with the stage of sleep, except for logHF (Table 3). Post hoc analysis revealed an increase in the mean RR interval in stage 2 sleep compared to wake. Overall HRV, as expressed by SDNN, was significantly different between S2 and REM sleep, and between SWS and REM sleep, respectively. The magnitude of beat-to-beat fluctuations, as expressed by RMSSD, differed only between REM sleep and SWS. Spectral analysis of HRV showed significant differences in the very low frequency (VLF) band between all sleep stages, except between REM sleep and wake. The low frequency (LF) power of HRV was reduced in SWS compared with REM and S2 sleep.
Table 3.
Sleep Stage Effects on HRV and QT Variability Measures in OSA Patients
| REM | S2 | SWS | Wake | ANOVA | Sign Post hoc | |
|---|---|---|---|---|---|---|
| Heart rate variability measures | ||||||
| Heart rate | 70 ± 9 | 69 ± 8 | 71 ± 9 | 73 ± 10 | 0.014 | S2vsWake |
| meanNN | 873 ± 111 | 890 ± 109 | 870 ± 120 | 837 ± 123 | 0.014 | S2vsWake |
| sdNN | 64 ± 28 | 48 ± 19 | 55 ± 24 | 55 ± 24 | 0.0014 | S2vsREM, SWSvsREM |
| Rmssd | 52 ± 31 | 44 ± 22 | 40 ± 25 | 47 ± 24 | 0.02 | SWSvsREM |
| logVLF | 3.26 ± 0.32 | 2.81 ± 0.36 | 2.58 ± 0.41 | 3.05 ± 0.39 | <0.0001 | REMvsS2, REMvsSWS, SWSvsWake, S2vsWake |
| logLF | 2.98 ± 0.33 | 2.84 ± 0.34 | 2.60 ± 0.47 | 3.05 ± 0.39 | <0.0001 | REMvsSWS, S2vsSWS, SWSvsWake |
| logHF | 2.52 ± 0.54 | 2.58 ± 0.53 | 2.48 ± 0.64 | 2.49 ± 0.47 | 0.7 | – |
| logLF/logHF | 1.22 ± 0.21 | 1.13 ± 0.15 | 1.10 ± 0.17 | 1.15 ± 0.16 | 0.007 | REMvsSWS |
| QT variability measures | ||||||
| logQTvar | 1.39 ± 0.55 | 1.26 ± 0.53 | 1.38 ± 0.59 | 1.34 ± 0.433 | 0.3 | – |
| QTVi | −1.43 ± 0.51 | −1.34 ± 0.50 | −1.03 ± 0.62 | −1.33 ± 0.43 | <0.0001 | SWSvsREM, SWSvsS2, SWSvsWake |
| QT/RRslope | 0.035 ± 0.023 | 0.024 ± 0.039 | 0.027 ± 0.034 | 0.042 ± 0.017 | 0.03 | S2vsWake |
| QT/RRr2 | 0.21 ± 0.12 | 0.17 ± 0.13 | 0.12 ± 0.12 | 0.18 ± 0.11 | 0.01 | REMvsSWS |
| QT/RRcoherence | 0.43 ± 0.12 | 0.41 ± 0.13 | 0.35 ± 0.14 | 0.37 ± 0.12 | 0.007 | REMvsSWS |
Data presented as mean ± standard deviation; n = 20. The last column shows the sleep stages that were significantly different from each other, using the Tukey multiple comparison post hoc test.
Among the QT variability measures, logQTvar showed no significant sleep stage effect. The other QT variability measures, however, which are all related to heart rate, displayed a significant sleep stage effect. QTVi values during wake were similar to those of healthy controls that have been reported by Berger (−1.29 ± 0.51). During SWS QTVi values were increased compared to REM, S2, and wake. RR/QT coherence as well as QT/RRr2 were both increased in REM sleep compared to SWS. RR/QT slope was increased in wake compared to S2 sleep.
Linear Regression Model
The first step of regression analysis, inserting the variables age, gender, and BMI in the model, showed that none significantly explained QT variability (ANOVA: P = 0.19; R2 = 0.25, see Table 4). Subsequently, when RDI and all HRV variables were inserted into the model in a stepwise fashion, RDI and the HRV frequency domain measures logHF and logLF were significantly predictive of logQTvar (ANOVA: P < 0.001; increase in R2 adjusted = 0.757). RDI explained 38% of the variance and logLF and logHF each another 10%.
Table 4.
Linear Regressions Between logQTvar as Outcome Variable and logRDI as Well as Measures of Heart Rate Variability as Input Variables
| Model variables | β | P |
|---|---|---|
| Gender | −0.256 | 0.79 |
| BMI | −0.604 | 0.08 |
| Age | −0.511 | 0.002 |
| logRDI | 1.153 | <0.001 |
| logHF | −1.047 | 0.002 |
| logLF | 0.733 | 0.015 |
The first iteration (gender, BMI, and age are entered) resulted in R2 (adjusted) = 0.108. The second iteration (inclusion of logRDI) resulted in R2 (adjusted) = 0.532. The second and third iteration (inclusion of logHF and logLF, respectively) increased R2 (adjusted) to 0.639 and 0.757, respectively. β, standardized regression coefficients; P, 2-tailed significance level.
DISCUSSION
Our major finding is that QT interval duration variability (QTvar) was significantly correlated with RDI in patients with obstructive sleep apnea. Those patients with greater severity of OSA, as determined by a higher RDI, had greater fluctuations in QT interval duration. As vagal ventricular effects are minor and still debated, it is likely that increased QT variability reflects alterations in the activity of cardiac sympathetic nerves.
Sudden Cardiac Death in Sleep Apnea
OSA patients may be at higher risk of sudden cardiac death.14 In contrast to the general population, death occurs more often at night in OSA patients,3 suggesting the involvement of a sleep related mechanism/event. Several previous studies have reported an association between OSA and cardiac arrhythmias.3,4,15 Assessing cardiac sympathetic tone in OSA patients during sleep is thus a crucial step in advancing the hypothesis that OSA-related sudden death is casually related to an elevated cardiac sympathetic nerve activity.
Noninvasive Evaluation of Cardiac Autonomic Activity
A common noninvasive method of assessing cardiac sympathetic activity is based on frequency domain analysis of the HRV. The power spectrum of HRV typically shows 2 distinct periodic components: 1) a high frequency (HF) oscillation at the respiratory frequency—respiratory sinus arrhythmia—and, 2) a low frequency oscillation (LF), around 0.1 Hz. The former is exclusively mediated by fast acting vagal efferents, whereas the latter is a composite of both sympathetic and vagal effects. Thus, sympathetic effects cannot be clearly distinguished from vagal effects. In a recent animal study with direct recording of cardiac sympathetic nerve activity, the authors found that an increase in sympathetic nerve activity was not accompanied by any increase in the low frequency power of HRV.17 As a further major limitation HRV measures reflect neural effects only at the level of the sinoatrial node. With accumulating evidence that different regions of the heart could receive quite different and sometimes oppositely directed neural influences (see16 for review), HRV is clearly not an ideal way to asses sympathetic outflow to the ventricular myocardium. These considerations emphasize the necessity to use a different approach to assess potential proarrhythmic neural influences in OSA patients, focusing on changes in the ventricular myocardium. The QT interval reflects the duration of ventricular repolarization and depends on neurotransmitter release in the ventricular myocardium and on heart rate. It is important to point out that the relationship between beat-to-beat dynamics of heart rate and QT interval is complex with some long-lasting adjustments.18 Repetitive obstructive episodes are accompanied by a predominant cyclical heart rate alteration, whereas the parallel fluctuations in QT intervals appear to be more subtle (Figure 1). The differences in response might be partly caused by different neural pathways, where the fast cyclical heart rate changes might be predominately caused by alternating vagal withdrawal/activation, whereas the subtle QT changes might be predominantly caused by changes in the sympathetic outflow. A major technical limitation of QT interval analysis is the difficulty in determination of the T-wave end. For this reason we used an algorithm that it is independent from T-wave end (see Methods for details).
A number of previous studies have analyzed HRV in patients with OSA, aiming to investigate autonomic dysfunction in heart rate control during sleep. Depending on the applied methodology and the investigated patient cohort, the results differ substantially,8,19,20 possibly due to confounders such as hypertension. In our study we therefore examined associations between the severity of OSA and HRV and QT variability in a group of OSA patients without relevant comorbidities or medications. In agreement with a previous study,8 we found that the very low frequency (VLF) power was the most sensitive HRV index of respiratory-related cardiac changes. Shiomi et al.21 have clearly demonstrated that this increase in the VLF power is a reflection of cyclical oscillations in heart rate induced by repetitive obstructive episodes (similar to those shown in Figure 1). Our results fully support this explanation of VLF power increase. Importantly, we found that QT variability was also elevated in 5-min epochs that contained at least one apneic episode.
It is possible that rate-dependency is the major mechanism linking QT variability to RDI in our patients. Furthermore, obesity is associated with hyperinsulinemia which may affect cardiac repolarization measures.22 To gain more insight into the causes of QT variability we developed a linear regression model. After controlling for BMI, age, and gender, we found the most significant contributor to QTvar was RDI, explaining 38% of the total variance. The high frequency (HF) power of HRV was the second significant contributor, explaining another 10% of the variance. An increase in QTvar was associated with a decrease in HF power. Finally, QTvar was associated with an increase in low frequency (LF) power of HRV that explained another 10% of the variance. The association between QTvar and LF power might be partly caused by the alternating tachycardia/bradycardia sequences due to obstructive episodes. We also found that minimum oxygen saturation significantly correlated with changes in QTvar. However, after adjusting for RDI, min O2 sat was not a significant independent predictor of QTvar. The correlation between very low frequency (VLF) power of HRV and QTvar is rather low (r = 0.27, n.s.) and thus it does not significantly contribute to QTvar. Thus we posit that in OSA patients, QTvar is elevated due to increased variability in the autonomic neural outflow to the ventricular myocardium that is independent from and not reflected by heart rate.
Effect of Sleep Stage
Heart rate in our patients showed only marginal sleep stage dependence. Overall heart rate variability (SDNN) was increased in REM compared to NREM sleep, similar to results reported in healthy controls.23,24 This was associated with an increased beat-to-beat variability (RMSSD) that was significantly different between REM and SWS. In healthy subjects SWS is characterized by a predominant respiratory sinus arrhythmia,25 and the relative breathing stability in SWS compared to REM sleep may largely explain these findings.
Frequency domain analysis of HRV showed increased VLF power values in REM sleep compared to S2 sleep and SWS, where in the latter one VLF values were even lower. During wake the VLF power values were similar to those during REM sleep. A similar, but less pronounced behavior was found for the LF power values of HRV. On the contrary, HF power did not show any sleep stage effect. The effect of sleep stage on the LF/HF ratio is in line with the general observation that the differences in HRV are greatest between SWS and REM sleep.21
QT variability per se does not appear to be influenced by sleep stage. However, if QT variability relating to HRV is quantified, as by the measures QTVi, RR/QT slope, RR/QTr2 and RR/QT coherence, significant sleep stage effects are observed that are likely an indirect reflection of the sleep stage dependence of HRV.
Clinical Implications
Providing evidence of sleep apnea in patients undergoing Holter monitoring and identifying patients with OSA at risk of SCD are of clinical importance. Our study suggests that beat-to-beat QT interval analysis may be more sensitive than standard HRV parameters, and maybe a useful prognostic measure. Increased beat-to-beat changes in repolarization duration may predispose to electrical instability in the myocardium. Increased QT interval variability has been associated with ventricular arrhythmias and sudden cardiac death, although underlying mechanisms are not fully understood.12,26 The apparent independence of QTvar from the actual sleep architecture might have implications for a simple ECG-based screening tool for OSA. The applied QT estimation algorithm by Berger et al. has proven to be reliable and would allow an automated analysis in a clinical environment requiring only a minimum of operator intervention.
Limitations
Our study was performed in a small number of patients without overt comorbidities. Extrapolation of the findings of this study to use QTvar routinely as a diagnostic measure would require confirmation of the findings in a broader patient sample, with a range of OSA severities and comorbidities. In addition, QT analysis requires ECG recordings of reasonable quality. Although the applied implementation of the Berger algorithm provides robust functions to automatically exclude ectopic beats and artefacts, the effect of noise on the QT estimation accuracy has yet to be investigated.
CONCLUSION
Obstructive sleep apnea is associated with increased QT interval variability during sleep. The variance of beat-to-beat QT intervals correlates more strongly to the severity of OSA (as determined by RDI) than standard measures of heart rate variability, and depends on blood oxygenation, but not on sleep stage. Beat-to-beat QT interval analysis provides a useful marker of autonomic effects on the heart in patients with OSA.
ABBREVIATIONS
- ECG
electrocardiogram
- HF
high frequency power
- HRV
heart rate variability
- LF
low frequency power
- LF/HF
ratio of low-to-high frequency power
- meanNN
mean normal-to-normal RR interval
- OSA
obstructive sleep apnea
- QT/RR r2
residuals of the regression line fitted to QT vs. RR plots
- QTvar
variance of beat-to-beat QT intervals
- QTVi
QT variability index
- RDI
respiratory disturbance index
- RMSSD
root-mean-square of the beat-to-beat differences
- SCD
sudden cardiac death
- SDNN
standard deviation of normal-to-normal RR interval
- VLF
very low frequency power
ACKNOWLEDGMENTS
The authors are grateful to Prof Ronald Berger for generously providing the QT analysis software, to Mr. Barry Fetics for his kind support in using this software, and to Dr Richard Woodman for the assistance with statistical analysis. This study was supported by grants from the Australian Research Council, National Heart Foundation of Australia and National Health and Medical Research Council of Australia. Dr Sanders is supported by the National Heart Foundation of Australia.
The work was performed at the Adelaide Institute for Sleep Health, Repatriation General Hospital, Adelaide, Australia
Funding: M.B. holds a Australian Postdoctoral Fellowship (ARC grant #DP0663345); J.S. and P.C. are funded by the National Health and Medical Research Council of Australia (NHMRC; grant #324732); R.D.M. is supported by the NHMRC (fellowship #324745); P.S. holds the Knapman Chair of Cardiology Research from the National Heart Foundation of Australia (NHF); E.N. is a holder of the NHF fellowship (#CR06A2710).
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. New Eng J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.McNicholas WT, Bonsigore MR Management Committee of EU COST ACTION B26. Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J. 2007;29:156–78. doi: 10.1183/09031936.00027406. [DOI] [PubMed] [Google Scholar]
- 3.Gami A, Howard D, Olson E, Somers V. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352:1206–14. doi: 10.1056/NEJMoa041832. [DOI] [PubMed] [Google Scholar]
- 4.Guilleminault C, Connolly SJ, Winkle RA. Cardiac arrhythmia and conduction disturbances during sleep in 400 patients with sleep apnea syndrome. Am J Cardiol. 1983;52:490–4. doi: 10.1016/0002-9149(83)90013-9. [DOI] [PubMed] [Google Scholar]
- 5.Tsuji H, Larson MG, Venditti FJ, Jr, et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996;94:2850–5. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- 6.Sandercock GR, Brodie DA. The role of heart rate variability in prognosis for different modes of death in chronic heart failure. Pacing Clin Electrophysiol. 2006;29:892–904. doi: 10.1111/j.1540-8159.2006.00457.x. [DOI] [PubMed] [Google Scholar]
- 7.Wiklund U, Olofsson BO, Franklin K, Blom H, Bjerle P, Niklasson U. Autonomic cardiovascular regulation in patients with obstructive sleep apnoea: a study based on spectral analysis of heart rate variability. Clin Physiol. 2000;20:234–41. doi: 10.1046/j.1365-2281.2000.00251.x. [DOI] [PubMed] [Google Scholar]
- 8.Roche F, Duverney D, Court-Fortune I, et al. Cardiac interbeat interval increment for the identification of obstructive sleep apnea. Pacing Clin Electrophysiol. 2002;25:1192–9. doi: 10.1046/j.1460-9592.2002.01192.x. [DOI] [PubMed] [Google Scholar]
- 9.Malik M, Acar B, Gang Y, Yap Y, Hnatkova K, Camm A. QT dispersion does not represent electrocardiographic interlead heterogeneity of ventricular repolarization. J Cardiovasc Electrophysiol. 2000;11:835–43. doi: 10.1111/j.1540-8167.2000.tb00061.x. [DOI] [PubMed] [Google Scholar]
- 10.Berger RD, Kasper EK, Baughman KL, Marban E, Calkins H, Tomaselli GF. Beat-to-beat QT interval variability: novel evidence for repolarization lability in ischemic and nonischemic dilated cardiomyopathy. Circulation. 1997;96:1557–65. doi: 10.1161/01.cir.96.5.1557. [DOI] [PubMed] [Google Scholar]
- 11.Desai N, Raghunandan DS, Mallavarapu M, Berger RD, Yeragani VK. Beat-to-beat heart rate and QT variability in patients with congestive cardiac failure: blunted response to orthostatic challenge. Ann Noninvasive Electrocardiol. 2004;9:323–9. doi: 10.1111/j.1542-474X.2004.94559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atiga WL, Calkins H, Lawrence JH, Tomaselli GF, Smith JM, Berger RD. Beat-to-beat repolarization lability identifies patients at risk for sudden cardiac death. J Cardiovasc Electrophysiol. 1998;9:899–908. doi: 10.1111/j.1540-8167.1998.tb00130.x. [DOI] [PubMed] [Google Scholar]
- 13.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996. Heart rate variability - standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 14.Grimm W, Becker HF. Obesity, sleep apnea syndrome, and rhythmogenic risk. Herz. 2006;31:213–8. doi: 10.1007/s00059-006-2800-3. quiz 219. [DOI] [PubMed] [Google Scholar]
- 15.Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107:2589–94. doi: 10.1161/01.CIR.0000068337.25994.21. [DOI] [PubMed] [Google Scholar]
- 16.Paton JF, Boscan P, Pickering AE, Nalivaiko E. The yin and yang of cardiac autonomic control: vago-sympathetic interactions revisited. Brain Res Rev. 2005;49:555–65. doi: 10.1016/j.brainresrev.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Watson AM, Hood SG, Ramchandra R, McAllen RM, May CN. Increased cardiac sympathetic nerve activity in heart failure is not due to desensitization of the arterial baroreflex. Am J Physiol. 2007;293:H798–804. doi: 10.1152/ajpheart.00147.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franz MR, Swerdlow CD, Liem LB, et al. Cycle length dependence of human action potential duration in vivo. Effects of single extrastimuli, sudden sustained rate acceleration and deceleration, and different steady-state frequencies. J Clin Invest. 1988;82:972–9. doi: 10.1172/JCI113706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aytemir K, Deniz A, Yavuz B, et al. Increased myocardial vulnerability and autonomic nervous system imbalance in obstructive sleep apnea syndrome. Resp Med. 2007;101:1277–82. doi: 10.1016/j.rmed.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 20.Yang A, Schafer H, Manka R, et al. Influence of obstructive sleep apnea on heart rate turbulence. Basic Res Cardiol. 2005;100:439–45. doi: 10.1007/s00395-005-0536-5. [DOI] [PubMed] [Google Scholar]
- 21.Shiomi T, Guilleminault C, Sasanabe R, Hirota I, Maekawa M, Kobayashi T. Augmented very low frequency component of heart rate variability during obstructive sleep apnea. Sleep. 1996;19:370–7. doi: 10.1093/sleep/19.5.370. [DOI] [PubMed] [Google Scholar]
- 22.Gastaldelli A, Emdin M, Conforti F, Camastra S, Ferrannini E. Insulin prolongs the QTc interval in humans. Am J Physiol. 2000;279:R2022–5. doi: 10.1152/ajpregu.2000.279.6.R2022. [DOI] [PubMed] [Google Scholar]
- 23.Bonnet MH, Arand DL. Heart rate variability: sleep stage, time of night, and arousal influences. Electroencephalogr Clin Neurophysiol. 1997;102:390–6. doi: 10.1016/s0921-884x(96)96070-1. [DOI] [PubMed] [Google Scholar]
- 24.Busek P, Vankova J, Opavsky J, Salinger J, Nevsimalova S. Spectral analysis of the heart rate variability in sleep. Physiol Res. 2005;54:369–76. [PubMed] [Google Scholar]
- 25.Brandenberger G, Buchheit M, Ehrhart J, Simon C, Piquard F. Is slow wave sleep an appropriate recording condition for heart rate variability analysis? Autonom Neurosci. 2005;121:81–6. doi: 10.1016/j.autneu.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Piccirillo G, Magrì D, Matera S, et al. QT variability strongly predicts sudden cardiac death in asymptomatic subjects with mild or moderate left ventricular systolic dysfunction: a prospective study. Eur Heart J. 2007 Jun;28:1344–50. doi: 10.1093/eurheartj/ehl367. [DOI] [PubMed] [Google Scholar]