Abstract
We evaluated virus-specific B and T cell responses induced by the attenuated Wa (P1A[8]G1) human rotavirus (AttHRV) oral 2-dose vaccine with or without Lactobacillus acidophilus (LA) colonization in neonatal gnotobiotic (Gn) pigs. The AttHRV vaccinated and LA-fed pigs had a significantly higher magnitude of HRV-specific IFN-γ producing CD8+ T cell responses in ileum and spleen, IgA and IgG antibody-secreting cell responses in ileum, and serum IgM, IgA and IgG antibody and virus neutralizing antibody titers compared to the AttHRV vaccinated pigs without LA colonization. These findings suggest thatL. acidophilus has significant immunopotentiating effects and may be used as a safe oral adjuvant for rotavirus vaccines in neonates.
Keywords: Probiotic Lactobacillus, Gnotobiotic pigs, B and T cell immune responses to rotavirus, vaccine
1. Introduction
Rotaviruses are the most important cause of severe dehydrating diarrhea in children worldwide. About 608,000 deaths in infants and young children are attributed to rotavirus infection per year [1]. The tremendous incidence of RV disease emphasizes the need for vaccines to prevent rotavirus associated morbidity and mortality. Two oral human rotavirus (HRV) vaccines were recently licensed: a three-dose human-bovine reassortant multivalent rotavirus vaccine, RotaTeq™ in the US, Canada and some 30-plus countries; and a two-dose attenuated HRV (AttHRV) monovalent vaccine (G1P1A[8]), Rotarix™ in more than 90 countries, including countries of the European Union, Latin America, Asia and Africa [2]. The efficacy of the Rotarix™ vaccine (the highest dose tested) against any rotavirus diarrhea was 63.5% (20.8–84.4%) [3]. The efficacy of RotaTeq™ vaccine against any G1–G4 rotavirus diarrhea was 48.1–74.9% [4]. Thus, although both of the vaccines are highly effective against severe rotavirus disease and death, the protective efficacy against all cases of rotavirus diarrhea still can be improved to realize the full potential of rotavirus vaccines. No adjuvant is used for either of the vaccines.
Probiotics are live microorganisms which when administered in adequate amounts confer a health benefit on the host. The most commonly used probiotics are lactic acid bacteria (LAB). LAB (e.g. Lactobacillus species) have been widely used in both humans and animals to prevent or treat gastrointestinal disorders [5]. Lactobacilli have also been shown to enhance antigen-specific immune responses induced by viral or bacterial vaccines, such as influenza [6], polio [7], and diphtheria and tetanus vaccines [8]. Accumulating evidence indicates that cytokine profiles induced by different strains of lactobacilli differ significantly and some, but not all Lactobacillus strains have intrinsic adjuvanticity and may be used as vaccine adjuvants [9,10]. Lactobacillus acidophilus NCFM is a scientifically well-documented LAB strain available for commercial use in the US since the mid-1970s in fermented foods and as a probiotic in dietary supplements [11]. Recent in vitro studies showed that L. acidophilus is a strong Th1 cytokine (IL-12, IFN-γ) inducer [12,13]. L. acidophilus significantly up-regulated surface markers on dendritic cells (DCs), including HLA-DR, CD40, CD86 and CD83 [13]. Another commonly used probiotic LAB strain, L. reuteri also activated human DCs and promoted Th1 cytokine IL-12, IL-18 and IFN-γ production [10]. However, other studies showed that L. reuteri inhibited IL-12 and TNF-α production in murine DCs and reduced IL-12 and TNF-α production inhuman PBMC induced by the strong Th1 cytokine inducer L. acidophilus when the two LAB strains were mixed [13].
In our previous studies, gnotobiotic (Gn) pigs were colonized with a 1:1 mixture of L. acidophilus and L. reuteri, and infected 3 days later with virulent HRV (VirHRV) [14]. Although, the Gn pigs were not protected against VirHRV-induced diarrhea, the systemic HRV-specific IFN-γ producing CD4+ T cell responses at post-HRV inoculation day (PID) 28 were significantly elevated in the LAB colonized pigs (Yuan, unpublished data). Therefore, we postulate that LAB may have an adjuvant effect on the immunogenicity of oral HRV vaccines. In this study we chose to test L. acidophilus NCFM strain for its immunostimulating effects on an oral AttHRV vaccine which has been studied previously in Gn pigs [15]. The specific aims of the present study were to determine if oral intake of L. acidophilus NCFM during a period around vaccination with the two-dose live AttHRV vaccine could promote the development of neonatal immune system and enhance the antigen-specific B and T cell immune responses induced by the vaccine, including the intestinal and systemic HRV-specific IFN-γ producing T cell, antibody-secreting cell (ASC), and antibody responses using the neonatal Gn pig model [16]. In addition, in order to confirm the safety and colonization of the L. acidophilus in neonatal Gn pigs and to assess the influence of L. acidophilus colonization on AttHRV replication, we monitored the clinical sign (diarrhea) and measured the fecal and nasal AttHRV shedding after the first dose AttHRV inoculation (PID 0–6) and the fecal L. acidophilus counts throughout.
2. Materials and methods
2.1. Virus
The 34th passage of cell culture adapted Wa strain AttHRV propagated in MA104 cells was used as the AttHRV2x vaccine (5 × 107 fluorescent-forming units [FFU]/dose), and as detector antigens in isotype-specific enzyme-linked-immunosorbent-assay (ELISA), enzyme-linked-immunospot (ELISPOT) assay and as stimulating antigens in the intracellular IFN-γ staining assay as described previously [17].
2.2. Bacterial strain
The L. acidophilus strain NCFM (LA) (ATCC, Manassas, VA, USA) was used in this study. This strain was propagated in Lactobacilli MRS broth (Weber, Hamilton, NJ, USA) overnight at 37 °C anaerobically (85% nitrogen, 10% hydrogen, 5% carbon dioxide). Cultures were subcultured once and inoculated into 10 ml of MRS broth (Weber). After 24 h, serial dilutions were made in sterile 0.1% peptone water (Becton Dickinson [BD] Biosciences, Sparks, MD, USA) and 0.1 ml of the dilution was spread onto MRS agar (BD) for determining the colony forming units (CFU) per ml. The remaining bacterial suspensions were aliquoted into 1 ml volumes, stored at −80°C. The frozen bacterial suspension was thawed and washed with 0.1% peptone water and titrated 1 day prior to feeding pigs.
2.3. Experimental design
Gnotobiotic pigs were derived near-term and maintained in sterile isolation units as described previously [18]. Pigs were assigned randomly to four groups as follows: AttHRV-inoculated LA-fed (LA+AttHRV+) (n = 7), AttHRV-inoculated non-LA-fed (LA−AttHRV+) (n = 8), non-AttHRV-inoculated LA-fed (LA+AttHRV−) (n = 4), and non-AttHRV-inoculated non-LA-fed (LA−AttHRV−) (n = 4). Pigs were orally dosed with 103, 104, 105, 106 and 106 CFU of LA in 2 ml of 0.1% of peptone water at 3, 5, 7, 9, 11 days of age, respectively. The incremental increase of doses was found to be safe and effective in colonizing neonatal Gn pigs in our previous studies [14]. Non-LA-fed pigs were given an equal volume of peptone water. The LA inoculum was slowly instilled into the mouth at the back of the throat using a needleless syringe. At 5 days of age, pigs were orally inoculated with 5 × 107 FFU AttHRV and reinoculated with the same dose 10 days later (post-inoculation day, PID 10). Non-inoculated pigs were given an equal volume of diluent. Preceding each AttHRV inoculation, pigs received 5 ml of 100mM NaHCO3 to reduce gastric acidity, and then 5 ml of AttHRV inoculum was administered with the same method as described for LA. Pigs were euthanized at PID 28 for isolation of mononuclear cells (MNC) to measure immune responses in intestinal and systemic lymphoid tissues.
2.4. Clinical signs and AttHRV and LA shedding
Pigs were examined daily for diarrhea post first AttHRV inoculation. Fecal consistency was scored as follows: 0, normal; 1, pasty; 2, semi-liquid; 3, liquid. Pigs with daily fecal scores of ≥2 were considered diarrheic. Rectal and nasal swabs (RS and NS) were collected daily for AttHRV shedding (PID 0–6). Rectal swabs were also collected for enumeration of LA shedding on PID 5, 10, 21, and 28. Rotavirus fecal and nasal shedding was determined by antigen capture ELISA and cell culture immunofluorescence (CCIF) assay as described previously [15,19]. Serum samples were collected at PID 0, 10, 21 and 28 for detection of serum antibodies [20].
2.5. Enumeration of LA
Each rectal swab was diluted in 4 ml of 0.1% peptone water (1:10) and a 100 µl aliquot was diluted in 900 µl of 0.1% peptone water and plated onto MRS agar for LA enumeration. Plates were incubated in sealed BBL Gaspak jars (Fisher, Hanover Park, IL, USA) containing Anaerogen paks (BD) for 24 h at 37 °C. The number of CFU on plates with 20–200 colonies were enumerated and recorded. LA shedding was expressed as CFU/ml.
2.6. Isolation of MNC and assessment of B and T cell responses
The MNC from ileum, spleen, and peripheral blood were isolated as previously described [15,19]. The ELISPOT assays were conducted using methods and reagents as described previously to enumerate HRV-specific IgM, IgA and IgG ASC on acetone-fixed, HRV-infected MA104 cell monolayers in 96-well plates [15,21] and total IgSC on plates (Nunc-Immuno, Rochester, NY, USA) coated with antiporcine polyclonal IgM (KPL, Gaithersburg, MD, USA), IgA (Bethyl, Montgomery, TX, USA) and IgG (KPL) antibodies. Intracellular staining and flow cytometry analysis of frequencies of HRV-specific IFN-γ producing CD4+ and CD8+ T cells in ileum, spleen and blood are performed as described in detail elsewhere [17].
2.7. Detection of HRV-specific and total IgM, IgA and IgG and virus neutralizing (VN) antibody responses in serum
Antibody titers to AttHRV in the sera of the Gn pigs were determined by using an indirect isotype-specific antibody ELISA as previously described [22,23]. Total Ig titers were also determined by using an ELISA as previously described [14]. The VN antibody titers in the sera were determined by an infected-cell focus reduction virus neutralization test. Briefly, equal volumes of serially diluted serum samples and virus suspensions containing 360 TCID50/0.09 ml were mixed and incubated for 1 h at 37 °C. The mixtures (50 µl/well) were inoculated onto MA104 cell monolayers in 96-well plates. After 1 h incubation at 37 °C, trypsin (Sigma) (50 µl/well) was added to the final concentration of 0.5 µg/ml. Inoculated MA104 cell monolayers were incubated at 37 °C for 18 to 24 h in a 5% CO2 atmosphere and fixed with 80% acetone. The guinea pig hyperimmune antiserum to 2/6/7 rotavirus-like particles (diluted 1:1000 in PBS containing 0.05% Tween 20 and 2% non-fat-dry-milk) were added and the plates were incubated at 37 °C for 1 h. Horseradish peroxidase (HRP) conjugated goat antiguinea pig IgG (H + L) (KPL) (diluted 1:500) were added and the plates were incubated at 37 °C for 1 h. Aminoethylcarbazole (AEC) substrate kit (Invitrogen, Carlsbad, CA, USA) were used to visualize the HRP-bound infected cells. The VN titer was expressed as the reciprocal of the highest dilution of the sample that reduced the number of infected-cell foci by 100%.
2.8. Statistical analyses
Mean duration of virus nasal and fecal shedding and diarrhea, mean cumulative scores, mean peak titers of virus nasal and fecal shedding from PID 1 to 6 and isotype-specific ELISA and VN antibody titers among the groups were compared using one-way analysis of variance (ANOVA-general linear model), followed by Duncan’s multiple range test. Fisher’s exact test was used to compare proportions of pigs with diarrhea, virus nasal and fecal shedding among groups. The numbers of HRV-specific ASC and total IgSC and the frequencies of IFN-γ producing T cells were compared among groups using the Kruskal–Wallis rank sum test. When differences among the groups were detected, the same test was used in a pairwise fashion to clarify the nature of the differences. Statistical significance was assessed at p < 0.05 for all comparisons. All statistical analyses were performed using SAS program (SAS Institute, NC, USA).
3. Results
3.1. LA colonization
On PID 5, 10, 21, and 28, the average daily fecal LA counts in the LA+AttHRV+ pigs ranged between 1.2 × 106 and 5.8 × 106 CFU/ml, and were similar to LA+AttHRV− pigs (5.2 × 105 and 4.7 × 106 CFU/ml). On PID 28, 3 weeks after the last LA feeding, the LA count in the LA+AttHRV+ pigs was 1.2 × 106 CFU/ml and in LA+AttHRV− pigs 5.2 × 105 CFU/ml, confirming that LA effectively colonized the intestine of Gn pigs (data not shown). The bacterial colonies on MRS agar plates from the intestinal content samples had identical morphology as the colonies from the original LA inoculum. The serially diluted intestinal contents and rectal swab samples were also plated on regular blood agar plates and cultured aerobically at 37 °C over night. No bacterial growth was detected on the blood agar plates from any of the pigs, confirming that no extraneous bacterial contamination occurred.
3.2. Diarrhea and virus shedding after first dose AttHRV inoculation
The clinical signs (diarrhea) and virus shedding in pigs from all groups are summarized in Table 1. LA feeding alone did not cause diarrhea or any other side effects in neonatal Gn pigs. One pig each in the LA+AttHRV+ and LA−AttHRV+ groups, but not in the two AttHRV− groups had transient diarrhea, therefore the diarrhea was due to AttHRV inoculation, not LA. The fecal consistency scores were significantly lower in the LA+AttHRV+ group than the LA−AttHRV+ group; hence LA improved the fecal consistency of the pigs after the first dose AttHRV. LA also reduced the duration of diarrhea of the one diarrheic pig from 2 days to 1 (Table 1). After the first dose of AttHRV inoculation, none of the LA+AttHRV+ and 50% of LA−AttHRV+ pigs shed virus nasally; and 29% of LA+AttHRV+ and 25% of LA−AttHRV+ pigs shed virus fecally as detected by ELISA and CCIF. The LA+AttHRV+ pigs had significantly delayed (6 versus 2) days-to-onset of virus fecal shedding than the LA−AttHRV+ pigs. No significant differences between LA+AttHRV+ and LA−AttHRV+ pigs in proportions and mean durations of fecal virus shedding and mean peak titers of fecal virus shedding were observed, indicating that intestinal replication of the AttHRV vaccine was delayed but not reduced by LA colonization.
Table 1.
Summary of AttHRV shedding and diarrhea in Gn pigs
| Treatment |
n |
After first dose of AttHRV inoculation |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Virus shedding (nasal)a |
Virus shedding (fecal)a |
Diarrhea |
|||||||||||
| % Shed* | Mean days to onset** | Mean duration days** | Mean peak titer shed (FFU/ml)b,** | % Shed* | Mean days to onset** | Mean duration days** | Mean peak titer shed (FFU/ml)b,** | % with diarrhea* | Mean days to onset** | Mean duration daysc,** | Mean cumulative scored,** | ||
| LA+AttHRV+ | 7 | 0A | nae | 0 | 0 | 29A | 6A (0) | 1A (0) | 3.9 × 103A (1.8 × 103) | 14.3A | 6.0 | 1.0 | 4.2B (0.5) |
| LA−AttHRV+ | 8 | 50A | 1.3 (0)f | 2.3 (1) | 3 × 104 (4.5 × 104) | 25A | 2B (0) | 2.5A (1) | 1 × 103A (3.3 × 102) | 12.5A | 2.0 | 2.0 | 6.1A (0.3) |
| LA+AttHRV− | 4 | 0A | na | 0 | 0 | 0A | na | 0 | 0 | 0A | na | 0 | 3.8B (0.5) |
| LA−AttHRV− | 4 | 0A | na | 0 | 0 | 0A | na | 0 | 0 | 0A | na | 0 | 4.1B |
Determined by ELISA and cell culture immunofluorescence infectivity assays.
FFU, fluorescent focus forming units.
Duration of diarrhea determined by number of days with fecal scores greater or equal to 2: feces were scored as follows: 0, normal; 1, pasty; 2, semi-liquid; 3, liquid.
Mean cumulative score calculation included all the pigs in each group.
na, not applicable.
Standard error of the mean.
Proportions in the same column, with different superscript letters (A and B) differ significantly (Fisher’s exact test).
Means in the same column, with different superscript letters (A and B) differ significantly (one-way ANOVA followed by Duncan’s multiple range test).
3.3. Intestinal and systemic HRV-specific IFN-γ producing T cell responses were significantly enhanced in LA-fed vaccinated pigs
Fig. 1 depicts mean frequencies of HRV-specific IFN-γ producing T cells in ileum, spleen and blood of the AttHRV-vaccinated pigs with or without LA. The frequencies of IFN-γ producing CD4+ T cells in the LA+AttHRV+ pigs were higher (2–22 fold, but not significantly) than the LA−AttHRV+ pigs in all lymphoid tissues. The frequencies of IFN-γ producing CD8+ T cells in the LA+AttHRV+ pigs were significantly higher in ileum (12 fold) and spleen (45 fold) and higher in blood (7 fold) than the LA−AttHRV+ pigs, thus LA feeding significantly increased the intestinal and systemic HRV-specific IFN-γ producing CD8+ T cell responses induced by AttHRV. HRV-specific IFN-γ producing T cells were not detected in the LA+AttHRV− or LA−AttHRV− pigs (data not shown).
Fig. 1.
HRV-specific IFN-γ producing T cell responses in the Gn pigs vaccinated with AttHRV with or without LA. Mononuclear cells from the ileum, spleen and peripheral blood of pigs were extracted and assayed on PID 28. Frequencies of IFN-γ+CD4+ and IFN-γ+CD8+ T cells among CD3+ MNC were determined by an intracellular staining and flow cytometry assay after the MNC were stimulated with purified AttHRV antigen for 17 h. Frequencies from the mock-stimulated MNC were subtracted from the frequencies of the AttHRV-stimulated MNC. Data represent the adjusted mean frequency (n = 4 for LA+AttHRV+; n = 7 for LA−AttHRV+) of HRV-specific IFN-γ producing T cells. Bars with different letters (A and B) on top differ significantly for the same tissue (Kruskal–Wallis Test, p < 0.05).
3.4. Intestinal HRV-specific IgA and IgG ASC responses were significantly enhanced in LA-fed vaccinated pigs
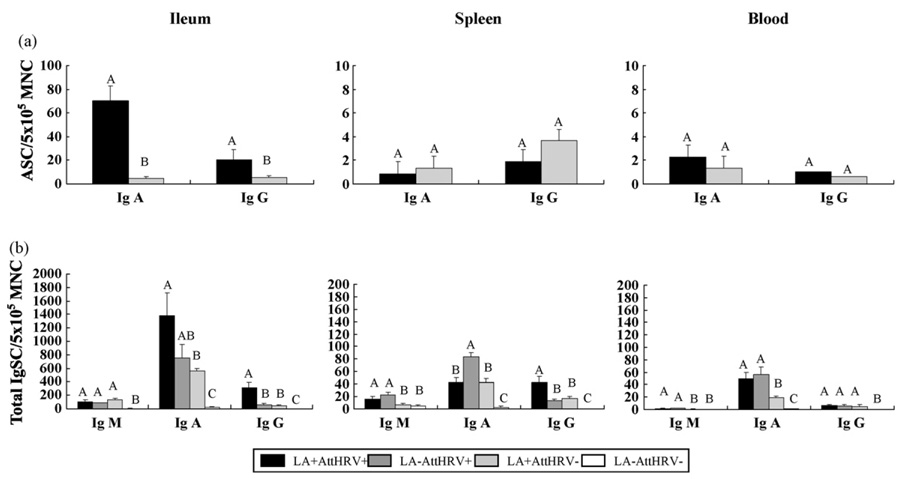
The HRV-specific ASC responses in ileum, spleen and blood at PID 28 are depicted in Fig. 2a. Zero or very low numbers of IgM ASC were detected in the AttHRV+ groups (LA+AttHRV+ and LA−AttHRV+); no any HRV-specific ASC were detected in the AttHRV− groups (LA+AttHRV− and LA−AttHRV−) in any lymphoid tissues (data not shown). In ileum, the numbers of IgA ASC in the LA+AttHRV+ pigs were significantly higher (70 versus 4.6/5 × 105 MNC) than the LA−AttHRV+ pigs. Significantly higher numbers of IgG ASC were also detected in the ileum of LA+AttHRV+ pigs (21 versus 5.4/5 × 105 MNC) compared to the LA−AttHRV+ pigs. In spleen, numbers of IgA and IgG ASC in the LA+AttHRV+ pigs were lower but not significantly lower (1.6 and 2 fold, respectively) than the LA−AttHRV+ pigs. The numbers of IgA and IgG ASC in the blood of LA+AttHRV+ were statistically similar to the LA−AttHRV+ pigs. Thus, LA feeding significantly increased the intestinal HRV-specific IgA and IgG ASC responses induced by the AttHRV vaccine at PID 28.
Fig. 2.
(a and b) HRV-specific ASC and total Ig SC responses in Gn pigs vaccinated with AttHRV with or without LA. Mononuclear cells from the ileum, spleen and peripheral blood of pigs were extracted and assayed on PID 28. Enzyme-linked-immunospot assays for determining HRV-specific ASC and total IgSC numbers were performed on the day of MNC extraction. Data represent the mean numbers of HRV-specific ASC (a) and total IgSC (b) per 5 × 105 MNC, respectively (n = 4–7). Bars with different letters (A–C) on top differ significantly among groups for the same tissue and the same antibody isotype (Kruskal–Wallis Test, p < 0.05).
3.5. Total intestinal IgA and IgG SC responses were enhanced in LA-fed vaccinated pigs
The mean numbers of total IgM, IgA and IgG SC at PID 28 are depicted in Fig. 2b. The total IgSC responses are theoretically consisted of B cell responses to AttHRV, LA and undefined background antigens (likely food antigens) in the Gn pigs. The numbers of all 3 isotypes of IgSC were low (0–21/5 × 105 MNC) in all lymphoid tissues of LA−AttHRV− pigs and were significantly lower than other groups (except for the similar numbers of IgM SC in spleen and blood to the LA+AttHRV− pigs), indicating that the total IgSC responses were induced mainly by LA and/or AttHRV. In spleen and blood, the numbers of IgM SC were significantly higher in the two AttHRV+ pigs compared to the AttHRV− groups. The highest numbers of IgA SC in ileum were detected in the LA+AttHRV+ pigs, which were higher than the LA−AttHRV+ pigs and significantly higher than the LA+AttHRV− pigs. In spleen, the mean numbers of IgA SC were highest in the LA−AttHRV+ group and were significantly higher than the two LA+ groups. In blood, the numbers of IgA SC were significantly higher in the two AttHRV+ groups compared to the LA+AttHRV− group. The numbers of IgG SC in ileum and spleen of the LA+AttHRV+ pigs were significantly higher than the LA−AttHRV+ and LA+AttHRV− pigs. Thus, both LA alone and AttHRV alone stimulated development of total IgSC responses; however, AttHRV was more effective than LA in stimulating IgA SC in ileum and significantly more effective in spleen and blood. LA plus AttHRV further enhanced development of the total IgA and IgG SC responses in ileum.
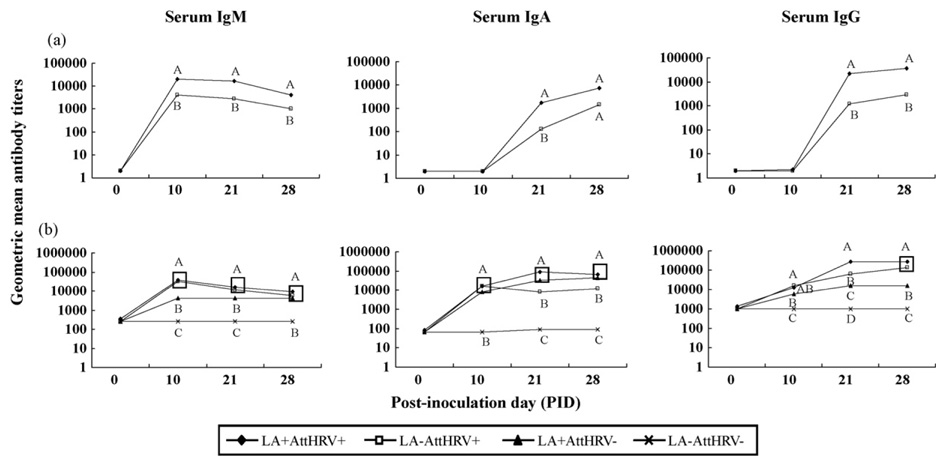
3.6. Isotype-specific antibody and VN antibody responses to HRV were significantly enhanced in LA-fed vaccinated pigs
Fig. 3a depicts HRV-specific serum IgM, IgA and IgG antibody responses at post-AttHRV inoculation day 0, 10, 21 and 28. No HRV-specific serum IgM, IgA, IgG or VN antibodies were detected in LA+AttHRV− and LA−AttHRV− pigs throughout the study (data not shown). The serum IgM antibody titers in the LA+AttHRV+ pigs were significantly higher (4–6 fold) than the LA−AttHRV+ pigs at PID 10, 21 and 28. The serum IgA antibody titers in the LA+AttHRV+ pigs were significantly higher (14 fold) than the LA−AttHRV+ pigs at PID 21. Although the differences were not significant at PID 28, the serum IgA antibody titers were higher (5 fold) in the LA+AttHRV+ pigs than in the LA−AttHRV+ pigs. The serum IgG antibody titers were significantly higher (12–18 fold) in the LA+AttHRV+ pigs than the LA−AttHRV+ pigs at PID 21 and 28. The VN titers in the LA+AttHRV+ pigs (GMT= 832± 192) were also significantly higher than the LA−AttHRV+ pigs (GMT= 138±55). Thus, LA feeding significantly increased the titers of HRV-specific IgM, IgA, IgG and VN antibodies induced by the two-dose AttHRV vaccine.
Fig. 3.
(a and b) Geometric mean titers (GMT) of isotype-specific antibodies to HRV and total Ig GMT in Gn pigs vaccinated with AttHRV with or without LA. Data represent the GMT of HRV-specific antibody (a) and total Ig (b) determined by ELISA at each time point (n = 7–9). Data points on lines marked with different letters (A–D) differ significantly (one-way ANOVA followed by Duncan’s multiple range test on log10 transferred titers, p = 0.05).
3.7. Serum total Ig responses stimulated by LA and AttHRV
Fig. 3b depicts serum total Ig GMT at post-AttHRV inoculation day 0, 10, 21 and 28. Similar to the IgSC responses, all 3 isotypes of total Ig titers were low in the LA−AttHRV− pigs and were significantly lower than the other 3 treatment groups. The total IgM titers in the two AttHRV+ groups were significantly higher than the LA+AttHRV− group at PID 10 and 21. The total IgA GMTs of the 3 treatment groups were comparable at PID 10. At PID 21 and 28, the total IgA titers were significantly higher in the two LA+ groups than the LA−AttHRV+ group. The total IgG GMT were highest in the two AttHRV+ groups, followed by the LA+AttHRV− group at PID 10 and 28. At PID 21, the total IgG titers in the LA+AttHRV+ pigs were significantly higher than the LA−AttHRV+ and the LA+AttHRV− pigs. Thus, both LA and AttHRV promoted development of serum total Ig responses. AttHRV was more effective in stimulating serum total IgM and IgG responses than LA, whereas LA was more effective in stimulating serum total IgA responses than AttHRV. LA plus AttHRV induced the highest serum total IgA and IgG titers at PID 21.
4. Discussion
We evaluated virus-specific B and T cell immune responses induced by the two-dose oral AttHRV vaccine with or without LA feeding in Gn pigs. We demonstrated that LA significantly enhanced the immunogenicity of the AttHRV vaccine as indicated by the significantly higher magnitude of HRV-specific IFN-γ producing CD8+ T cell responses in ileum and spleen, IgA and IgG ASC responses in ileum, and serum IgM, IgA, IgG and VN antibody responses in the AttHRV-vaccinated and LA-fed pigs compared to the AttHRV vaccinated pigs without LA. These findings indicate that L. acidophilus NCFM has a strong potentiating effect on both cellular and humoral immunity induced by the oral rotavirus vaccine. We also evaluated the effect of LA, AttHRV and LA plus AttHRV on the development of total B cell compartment by comparing the total IgSC responses in intestinal and systemic lymphoid tissues and the Ig titers in serum among the 4 groups. Total Ig responses were induced by either LA or AttHRV, but LA induced significantly lower IgA and IgG SC in ileum, IgM and IgG SC in spleen and IgM and IgA SC in blood compared to AttHRV, indicating that AttHRV was more effective in promoting development of the neonatal immune system than LA colonization. However, LA enhanced development of the intestinal IgA and IgG SC responses in the LA+AttHRV+ pigs, which were in parallel with the enhanced virus-specific intestinal IgA and IgG ASC responses. Challenge studies are needed to assess the effect of LA on improving the protective efficacy of the AttHRV vaccine in Gn pigs. The significantly elevated virus-specific immune responses after AttHRV vaccination in LA-fed pigs are likely to improve the protective efficacy of the vaccine, because previous studies of rotavirus protective immunity in neonatal Gn pigs have shown that the magnitude of virus-specific serum IgA antibody, intestinal IgA ASC and IFN-γ producing T cell responses are positively correlated with protection rates against rotavirus diarrhea upon challenge the Gn pigs with the virulent Wa HRV [15–17,20,23]. Serum antibody titers were also correlated with protection against reinfection in humans [24–26].
A recent study by Olivares et al. showed that oral intake of L. fermentum CECT5716 significantly enhanced serum Th1 type cytokine and influenza-specific IgA antibody responses to an intramuscular influenza vaccine in adults [6]. Hori et al. reported that mice infected with influenza virus and fed L. casei had significantly higher IFN-γ production by nasal lymphocytes than non-fed mice [27]. These and our results collectively demonstrated that probiotic lactobacilli can increase both mucosal and systemic T and B cell responses to viral infection or vaccines. Therefore, these particular Lactobacillus strains may be used as oral adjuvants to improve the immunogenicity and protective efficacy of oral or parental vaccines, such as rotavirus and influenza vaccines.
Oral intake of lactobacilli during a period around vaccination is a safe and easy way for enhancing the effectiveness of vaccines. Because neonatal Gn pigs are totally devoid of maternal antibodies, they are likely more susceptible than human infants to potential adverse effects of the lactobacilli or vaccines. We confirmed that the LA feeding regimen did not induce any adverse effect in neonatal Gn pigs, yet was highly effective in enhancing the immunogenicity of the AttHRV vaccine. The AttHRV vaccine caused transient diarrhea in 13% (2/15) Gn pigs after the first dose. Although LA feeding did not prevent the diarrhea, there was a delay in the onset and a reduction in the diarrheic days of the pig, and the mean cumulative fecal consistency scores in the LA+AttHRV+ group were significantly lower than the LA−AttHRV group. LA mixed with L. reuteri and Bifidobacterium infantis BBI significantly decreased the duration and incidence of diarrhea in children of 12–32 mo of age [11]. The effect of LA alone in reducing rotavirus diarrhea has not been reported [11]. Further studies with a larger number of pigs in each group may be needed to confirm if LA alone has an effect on reducing the severity of rotavirus diarrhea after rotavirus infection, and in this case, reducing the side effects of AttHRV vaccine.
After the first dose of AttHRV inoculation, none of the LA+AttHRV+ pigs shed virus nasally compared to 50% of LA−AttHRV+ pigs, whereas both groups shed virus fecally (29% versus 25%). These results are consistent with a previous study that AttHRV induced a higher rate of nasal shedding than of fecal shedding (79% versus 17%) in neonatal Gn pigs [28]. The potential mechanism by which LA inhibited the AttHRV nasal shedding might be that LA inhibited the virus adhesion to the upper respiratory tract epithelial surfaces. It has been suggested that Lactobacillus may bind to receptors on the cell surface so that the receptors were not accessible or not recognized by the pathogen [29–31]. We found that after LA feeding, LA colonized effectively not only in the gut, but also in the oral/nasal cavity and on the tonsils (Zhang and Yuan, unpublished data). However, LA did not affect the AttHRV fecal shedding, except delayed the onset. It is likely that the differences in the biological environment (temperature, density of virus receptors, density of colonizing LA, proteolytic enzymes, etc.) between intestinal and upper respiratory tract have led to the different replication capability of the AttHRV and the observed differential effects of LA. Further studies are needed to identify the mechanism. Nonetheless, our results showed that LA intestinal colonization did not reduce vaccine replication rate in the gut.
In our previous studies, the protective efficacy of the Wa two-dose AttHRV vaccine (G1P1A[8]) against rotavirus diarrhea in Gn pigs was substantially lower (33%) than that of the two-dose RIX4414 AttHRV vaccine (Rotarix™, G1P1A[8]) in human infants (63.5% efficacy against all case diarrhea) [3,15]. This difference may be partially explained by the lack of immunostimulation of the neonatal immune system by commensal microflora in Gn pigs. However, the Wa three-dose AttHRV vaccine conferred a protection rate against rotavirus diarrhea of 67% in Gn pigs [32], similar to the efficacy of RIX4414 vaccine in humans. Because human infants are colonized with lactobacilli hours after birth, the effect of LA observed in Gn pigs may not be extrapolated directly to humans. Further challenge studies in Gn pigs and “conventionalized” Gn pigs (Gn pigs seeded with the gut microflora from a suckling conventional pig to mimic formula-fed human infants) are needed to confirm LA’s effect on improving the protective efficacy of oral live HRV vaccines before clinical trials in humans.
In conclusion, we demonstrated that L. acidophilus NCFM has a significant immunostimulating effect on the intestinal and systemic HRV-specific T and B cell immune responses induced by the AttHRV vaccine and is safe in neonates; therefore it may have the potential to be used as an adjuvant for rotavirus vaccines.
Acknowledgements
This work was supported by grants from the National Institutes of Health (1R21AT002524 to LY and R01AI033561 to LJS) and Ohio Agricultural Research and Development Center, The Ohio State University (OHOA1208 to LY). We thank Dr. Juliette Hanson, Ms. Peggy Lewis and Mr. Rich McCormick for animal care and technical assistance. Salaries and research support were provided by state and federal funds appropriated to the Ohio Agricultural Research and Development Center (OARDC) of The Ohio State University.
References
- 1.Parashar UD, Gibson CJ, Bresse JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerg Infect Dis. 2006;12(2):304–306. doi: 10.3201/eid1202.050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernstein DI. A live attenuated human rotavirus vaccine. Drugs Today (Barc) 2007;43(5):281–291. doi: 10.1358/dot.2007.43.5.1101728. [DOI] [PubMed] [Google Scholar]
- 3.Araujo EC, Clemens SA, Oliveira CS, Justino MC, Rubio P, Gabbay YB, et al. Safety, immunogenicity, and protective efficacy of two doses of RIX4414 live attenuated human rotavirus vaccine in healthy infants. J Pediatr (Rio J) 2007;83(3):217–224. doi: 10.2223/JPED.1600. [DOI] [PubMed] [Google Scholar]
- 4.Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354(1):23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 5.Ouwehand A, Isolauri E, Salminen S. The role of the intestinal microflora for the development of the immune system in early childhood. Eur J Nutr. 2004;41 Suppl. 1:I32–I37. doi: 10.1007/s00394-002-1105-4. [DOI] [PubMed] [Google Scholar]
- 6.Olivares M, Diaz-Ropero MP, Sierra S, Lara-Villoslada F, Fonolla J, Navas M, et al. Oral intake of Lactobacillus fermentum CECT5716 enhances the effects of influenza vaccination. Nutrition (Burbank, Los Angeles County, Calif) 2007;23(3):254–260. doi: 10.1016/j.nut.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 7.de Vrese M, Rautenberg P, Laue C, Koopmans M, Herremans T, Schrezenmeir J. Probiotic bacteria stimulate virus-specific neutralizing antibodies following a booster polio vaccination. Eur J Nutr. 2005;44(7):406–413. doi: 10.1007/s00394-004-0541-8. [DOI] [PubMed] [Google Scholar]
- 8.West CE, Gothefors L, Granstrom M, Kayhty H, Hammarstrom ML, Hernell O. Effects of feeding probiotics during weaning on infections and antibody responses to diphtheria, tetanus and Hib vaccines. Pediatr Allergy Immunol. 2008;19:53–60. doi: 10.1111/j.1399-3038.2007.00583.x. [DOI] [PubMed] [Google Scholar]
- 9.Maassen CB, van Holten-Neelen C, Balk F, den Bak-Glashouwer MJ, Leer RJ, Laman JD, et al. Strain-dependent induction of cytokine profiles in the gut by orally administered Lactobacillus strains. Vaccine. 2000;18(23):2613–2623. doi: 10.1016/s0264-410x(99)00378-3. [DOI] [PubMed] [Google Scholar]
- 10.Mohamadzadeh M, Olson S, Kalina WV, Ruthel G, Demmin GL, Warfield KL, et al. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc Natl Acad Sci USA. 2005;102(8):2880–2885. doi: 10.1073/pnas.0500098102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanders ME, Klaenhammer TR. Invited review: the scientific basis of Lactobacillus acidophilus NCFM functionality as a probiotic. J Dairy Sci. 2001;84(2):319–331. doi: 10.3168/jds.S0022-0302(01)74481-5. [DOI] [PubMed] [Google Scholar]
- 12.Gackowska L, Michalkiewicz J, Krotkiewski M, Helmin-Basa A, Kubiszewska I, Dzierzanowska D. Combined effect of different lactic acid bacteria strains on the mode of cytokines pattern expression in human peripheral blood mononuclear cells. J Physiol Pharmacol. 2006;57 Suppl. 9:13–21. [PubMed] [Google Scholar]
- 13.Zeuthen LH, Christensen HR, Frokiaer H. Lactic acid bacteria inducing a weak interleukin-12 and tumor necrosis factor alpha response in human dendritic cells inhibit strongly stimulating lactic acid bacteria but act synergistically with gram-negative bacteria. Clin Vaccine Immunol. 2006;13(3):365–375. doi: 10.1128/CVI.13.3.365-375.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang W, Azevedo MS, Gonzalez AM, Saif LJ, Van Nguyen T, Wen K, et al. Influence of probiotic Lactobacilli colonization on neonatal B cell responses in a gnotobiotic pig model of human rotavirus infection and disease. Vet Immunol Immunopathol. 2008;122(1–2):175–181. doi: 10.1016/j.vetimm.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan L, Ward LA, Rosen BI, To TL, Saif LJ. Systematic and intestinal antibody-secreting cell responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J Virol. 1996;70(5):3075–3083. doi: 10.1128/jvi.70.5.3075-3083.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan L, Saif LJ. Induction of mucosal immune responses and protection against enteric viruses: rotavirus infection of gnotobiotic pigs as a model. Vet Immunol Immunopathol. 2002;87(3–4):147–160. doi: 10.1016/S0165-2427(02)00046-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan L, Wen K, Azevedo M, Gonzalez A, Zhang W, Saif L. Virus-specific intestinal IFN-γ producing T cell responses induced by human rotavirus infection and vaccines are correlated with protection against rotavirus diarrhea in gnotobiotic pigs. Vaccine. 2008;26:3322–3331. doi: 10.1016/j.vaccine.2008.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer RC, Bohl EH, Kohler EM. Procurement and maintenance of germ-free seine for microbiological investigations. Appl Environ Microbiol. 1964;12:295–300. doi: 10.1128/am.12.4.295-300.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ward LA, Rosen BI, Yuan L, Saif LJ. Pathogenesis of an attenuated and a virulent strain of group A human rotavirus in neonatal gnotobiotic pigs. J Gen Virol. 1996;77(Pt 7):1431–1441. doi: 10.1099/0022-1317-77-7-1431. [DOI] [PubMed] [Google Scholar]
- 20.Azevedo MS, Yuan L, Iosef C, Chang KO, Kim Y, Nguyen TV, et al. Magnitude of serum and intestinal antibody responses induced by sequential replicating and nonreplicating rotavirus vaccines in gnotobiotic pigs and correlation with protection. Clin Diagn Lab Immunol. 2004;11(1):12–20. doi: 10.1128/CDLI.11.1.12-20.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan L, Kang SY, Ward LA, To TL, Saif LJ. Antibody-secreting cell responses and protective immunity assessed in gnotobiotic pigs inoculated orally or intra-muscularly with inactivated human rotavirus. J Virol. 1998;72(1):330–338. doi: 10.1128/jvi.72.1.330-338.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parreno V, Hodgins DC, de Arriba L, Kang SY, Yuan L, Ward LA, et al. Serum and intestinal isotype antibody responses to Wa human rotavirus in gnotobiotic pigs are modulated by maternal antibodies. J Gen Virol. 1999;80(Pt 6):1417–1428. doi: 10.1099/0022-1317-80-6-1417. [DOI] [PubMed] [Google Scholar]
- 23.To TL, Ward LA, Yuan L, Saif LJ. Serum and intestinal isotype antibody responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J Gen Virol. 1998;79(Pt 11):2661–2672. doi: 10.1099/0022-1317-79-11-2661. [DOI] [PubMed] [Google Scholar]
- 24.Velazquez FR, Matson DO, Guerrero ML, Shults J, Calva JJ, Morrow AL, et al. Serum antibody as a marker of protection against natural rotavirus infection and disease. J Infect Dis. 2000;182(6):1602–1609. doi: 10.1086/317619. [DOI] [PubMed] [Google Scholar]
- 25.Jiang B, Gentsch JR, Glass RI. The role of serum antibodies in the protection against rotavirus disease: an overview. Clin Infect Dis. 2002;34(10):1351–1361. doi: 10.1086/340103. [DOI] [PubMed] [Google Scholar]
- 26.O’Ryan ML, Matson DO, Estes MK, Pickering LK. Acquisition of serum isotype-specific and G type-specific antirotavirus antibodies among children in day care centers. Pediatr Infect Dis J. 1994;13(10):890–895. doi: 10.1097/00006454-199410000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Hori T, Kiyoshima J, Shida K, Yasui H. Augmentation of cellular immunity and reduction of influenza virus titer in aged mice fed Lactobacillus casei strain Shirota. Clin Diagn Lab Immunol. 2002;9(1):105–108. doi: 10.1128/CDLI.9.1.105-108.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Azevedo MS, Yuan L, Jeong KI, Gonzalez A, Nguyen TV, Pouly S, et al. Viremia and nasal and rectal shedding of rotavirus in gnotobiotic pigs inoculated with Wa human rotavirus. J Virol. 2005;79(9):5428–5436. doi: 10.1128/JVI.79.9.5428-5436.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernet MF, Brassart D, Neeser JR, Servin AL. Lactobacillus acidophilus LA 1 binds to cultured human intestinal cell lines and inhibits cell attachment and cell invasion by enterovirulent bacteria. Gut. 1994;35(4):483–489. doi: 10.1136/gut.35.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blomberg L, Henriksson A, Conway PL. Inhibition of adhesion of Escherichia coli K88 to piglet ileal mucus by Lactobacillus spp. Appl Environ Microbiol. 1993;59(1):34–39. doi: 10.1128/aem.59.1.34-39.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michail S, Abernathy F. Lactobacillus plantarum reduces the in vitro secretory response of intestinal epithelial cells to enteropathogenic Escherichia coli infection. J Pediatr Gastroenterol Nutr. 2002;35(3):350–355. doi: 10.1097/00005176-200209000-00021. [DOI] [PubMed] [Google Scholar]
- 32.Yuan L, Geyer A, Saif LJ. Short-term immunoglobulin A B-cell memory resides in intestinal lymphoid tissues but not in bone marrow of gnotobiotic pigs inoculated with Wa human rotavirus. Immunology. 2001;103(2):188–198. doi: 10.1046/j.1365-2567.2001.01229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]