Abstract
(±)-3,4-Methylenedioxymethamphetmine (MDMA, or Ecstasy) is an illicit drug that evokes transporter-mediated release of monoamines, including serotonin (5-HT) and dopamine (DA). Here we monitored the effects of MDMA on neurochemistry and motor activity in rats, as a means to evaluate relationships between 5-HT, DA, and behavior. Male rats undergoing in vivo microdialysis were housed in chambers equipped with photobeams for measurement of ambulation (i.e., forward locomotion) and stereotypy (i.e., head weaving and forepaw treading). Microdialysis probes were placed into the n. accumbens, striatum or prefrontal cortex in separate groups of rats. Dialysate samples were assayed for 5-HT and DA by microbore HPLC-ECD. Rats received two i.v. injections of MDMA, 1 mg/kg followed by 3 mg/kg 60 min later; neurochemical and locomotor parameters were measured concurrently. MDMA produced dose-related elevations in extracellular 5-HT and DA in all regions, with the magnitude of 5-HT release always exceeding that of DA release. MDMA-induced ambulation was positively correlated with dialysate DA levels in all regions (P<0.05-0.0001) and with dialysate 5-HT in striatum and cortex (P<0.001-0.0001). Stereotypy was strongly correlated with dialysate 5-HT in all areas (P<0.001-0.0001) and with dialysate DA in accumbens and striatum (P<0.001-0.0001). These data support previous work and suggest the complex spectrum of behaviors produced by MDMA involves 5-HT and DA in a region- and modality-specific manner.
Keywords: Ambulation, dopamine, MDMA, microdialysis, serotonin, stereotypy
1. Introduction
(±)-3,4-Methylenedioxymethamphetamine (MDMA or Ecstasy) is a popular “club” drug which produces unique psychoactive effects, including mood elevation, altered sensory perception, and feelings of emotional closeness to others (Liechti and Vollenweider, 2001). In recent years, MDMA has received increasing media attention due to its propensity to cause serotonin (5-HT) dysfunction in the brains of laboratory animals (Baumann et al., 2007; Ricaurte et al., 2000) and possibly humans (Gouzoulis-Mayfrank and Daumann, 2006; McCann et al., 2000). From a molecular perspective, MDMA interacts with monoamine transporter proteins to reverse the normal direction of transmitter flux, thereby causing non-exocytotic release of transmitters (Green et al., 2003; Hilber et al., 2005; Rothman and Baumann, 2002). Because MDMA-induced transmitter efflux requires the transporter-dependent internalization of drug molecules (Verrico et al., 2007), transporter blockers (i.e., reuptake inhibitors) can antagonize MDMA’s releasing effects.
Early in vitro experiments suggested that MDMA is a selective 5-HT releaser in nervous tissue (Johnson et al., 1986; Schmidt et al., 1987), but more contemporary investigations reveal the drug releases 5-HT, dopamine (DA) and norepinephrine (NE) with comparable potency (Rothman et al., 2001; Verrico et al., 2007). It is noteworthy that MDMA taken by humans is a mixture of (+) and (−) stereoisomers, and (+)-MDMA is a more potent monoamine releaser when compared to (−)-MDMA (Baumann et al., 2007; Schmidt et al., 1987). In vivo microdialysis findings in rats confirm that MDMA evokes concurrent elevations in extracellular levels of 5-HT and DA in forebrain regions such as n. accumbens, striatum and prefrontal cortex (Baumann et al., 2005; Gough et al., 2002; Gudelsky and Nash, 1996; Kankaanpaa et al., 1998; Shankaran and Gudelsky, 1999; Yamamoto et al., 1995). No published microdialysis studies have reported the effects of MDMA on release of NE, and such studies are warranted.
The administration of MDMA to rats causes hyperactivity characterized by forward locomotion and elements of the 5-HT behavioral syndrome (Gold et al., 1988; Shankaran and Gudelsky, 1999; Spanos and Yamamoto, 1989). When compared to effects of prototypical stimulants like amphetamine, motor effects of MDMA are peculiar in several ways: (1) rats display thigmotaxis, an affinity for the walls of the locomotor chamber, and avoid the center area (Gold et al., 1988); (2) flattened body posture reduces rearing in the vertical plane (Spanos and Yamamoto, 1989); (3) stereotypic movements of the 5-HT syndrome, namely reciprocal forepaw treading and side-to-side head weaving, tend to predominate (Hiramatsu et al., 1989). In accordance with in vitro studies, (+)- MDMA exhibits more potent locomotor stimulant properties than (−)-MDMA (Hiramatsu et al., 1989; Paulus and Geyer, 1992).
Based on the molecular mechanism of MDMA, behaviors produced by the drug likely involve transporter-mediated release of monoamines, followed by activation of multiple 5-HT, DA and NE receptor subtypes (e.g., Bubar et al., 2004; Fletcher et al., 2002; Selken and Nichols, 2007). It is convenient to divide locomotor effects of MDMA into two basic types: ambulation (i.e., forward locomotion) and stereotypy (i.e., forepaw treading and head weaving). In our laboratory, we have quantified both types of behaviors using commercially-available activity monitors and direct observation with behavioral scoring (Baumann et al., 1998; Baumann et al., 2005). Many studies have examined the DA and 5-HT receptor mechanisms underlying MDMA-induced ambulation (reviewed by (Bankson and Cunningham, 2001)), while few have addressed the mechanisms responsible for eliciting 5-HT syndrome. Furthermore, the role of NE receptors has received little attention. A recent study by Selken and Nichols (2007) showed that pretreatment with the α1 receptor antagonist prazosin blocks ambulation produced by i.p. administered MDMA, suggesting the importance of NE mechanisms in MDMA’s locomotor effects.
It is well known that mesolimbic DA neurons are critical mediators of motor activity produced by amphetamine-type stimulants (Gold et al., 1989a; Ikemoto, 2002). Cell bodies of mesolimbic DA neurons reside in the midbrain ventral tegmental area (VTA) and send axonal projections to various forebrain regions, notably the n. accumbens (Moore and Bloom, 1978; Ungerstedt, 1971). The n. accumbens is an important limbic-motor interface receiving afferent inputs from the prefrontal cortex, hippocampus and amygdala, while sending efferent outputs to the ventral pallidum and other areas modulating motor activity (Mogenson et al., 1980; Pennartz et al., 1994). Destruction of DA nerve terminals in the n. accumbens markedly inhibits ambulation produced by systemically injected MDMA (Gold et al., 1989b). Moreover, microinjection of (+)-MDMA into the accumbens stimulates ambulation, and this effect involves DA but not 5-HT (Callaway and Geyer, 1992a). Such findings implicate accumbens DA in the mechanism of MDMA’s locomotor actions. Additionally, pretreatment with D1 or D2 receptor antagonists can reduce ambulation produced by i.p. administered MDMA, suggesting both receptor subtypes are involved (Bubar et al., 2004; Kehne et al., 1996).
Serotonergic mechanisms also play a prominent role in the locomotor effects of MDMA (Bankson and Cunningham, 2001; Geyer, 1996). In rats, pretreatment with selective 5-HT reuptake inhibitors (SSRIs) like fluoxetine can significantly attenuate MDMA-induced 5-HT release (Gudelsky and Nash, 1996; Mechan et al., 2002) and forward locomotion (Callaway et al., 1991; Callaway et al., 1990). Studies in “knockout” mice reveal that deletion of the 5-HT transporter (SERT) gene causes parallel reductions in 5-HT release and ambulation produced by MDMA (Bengel et al., 1998; Trigo et al., 2007). Thus, SERT-mediated release of 5-HT is an important factor in MDMA-induced locomotor activation in both rats and mice.
Determining the role of specific 5-HT receptors in MDMA’s effects in vivo is complicated by the presence of 14 different 5-HT receptor subtypes (Barnes and Sharp, 1999; Hoyer et al., 2002), many of which affect DA function (Alex and Pehek, 2007; Bubar and Cunningham, 2006). Nonetheless, there is general agreement that 5-HT1B, 5-HT2A and 5-HT2C receptor subtypes influence locomotor effects of MDMA. Hyperactivity produced by MDMA is mimicked by administration of the 5-HT1B agonist RU-24969 (Rempel et al., 1993), and 5-HT1B antagonists inhibit MDMA-induced ambulation (Callaway and Geyer, 1992b; Fletcher et al., 2002; McCreary et al., 1999). 5-HT2A antagonists reduce ambulation produced by MDMA (Fletcher et al., 2002; Kehne et al., 1996), whereas 5-HT2C antagonists markedly enhance it (Bankson and Cunningham, 2002; Fletcher et al., 2006). Taken together, these data reveal that 5-HT1B and 5-HT2A receptors facilitate, while 5-HT2C receptors suppress, forward locomotion evoked by MDMA administration. Importantly, the neural circuits underlying serotonergic modulation of MDMA-induced activity are largely unexplored.
The measurement of real-time neurochemistry in awake freely-moving animals provides a powerful approach for assessing the role of monoamines in behavior (Olive et al., 2000; Robinson et al., 1988; Yurek et al., 1998). To this end, a number of investigators have examined the effects of amphetamine-type drugs on locomotor activity in rats undergoing in vivo microdialysis (Kuczenski and Segal, 1989; Kuczenski et al., 1995; Sharp et al., 1987). Sharp et al. (1987) found significant correlations between dialysate DA levels in the n. accumbens and hyperactivity produced by low-dose (+)- amphetamine, such that higher DA levels corresponded to greater ambulation. Kuczenski and Segal (1989) reported an overall positive correlation between dialysate DA levels in the striatum and perseverative stereotypies produced by high-dose (+)-amphetamine, although DA levels alone could not explain the complex nature of such behaviors. These microdialysis findings agree with historical evidence indicating mesolimbic DA neurons mediate amphetamine-induced ambulation, whereas nigrostriatal DA neurons mediate stereotypy (Creese and Iversen, 1974; Kelly et al., 1975; Pijnenburg et al., 1976). The role of 5-HT neurons in modulation of ambulation vs. stereotypy is less clear and warrants further investigation (Bankson and Cunningham, 2001; Geyer, 1996; Kuczenski and Segal, 1989; Kuczenski et al., 1995)
In the present study, we combined in vivo microdialysis with automated analysis of motor activity to examine the effects of MDMA in male rats (Baumann et al., 2005; Rothman et al., 2005). To the best of our knowledge, no study has used this strategy to correlate neurochemical and behavioral effects of MDMA. Microdialysis probes were inserted into guide tubes aimed at the n. accumbens, striatum (i.e., caudate-putamen) or prefrontal cortex in separate groups of rats, and dialysate samples were assayed for 5-HT and DA using high-performance liquid chromatography coupled to electrochemical detection (HPLC-ECD). Rats undergoing microdialysis were housed in chambers equipped with photobeams to allow measurement of ambulation and stereotypies associated with the 5-HT syndrome. Each rat received two i.v. doses of MDMA, 1 mg/kg followed by 3 mg/kg 60 min later; the i.v. injection regimen eliminated handling stress, and afforded rapid bioavailability of MDMA with diminished metabolism. This strategy allowed us to examine interrelationships between 5-HT, DA and behavior in MDMA-treated rats.
2. Materials and methods
2.1. Animals
Male Sprague-Dawley rats weighing 300–350 g were housed under conditions of controlled temperature (22±2°C) and humidity (45±5%) with food and water freely available. Rats were maintained in facilities accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, and procedures were carried out in accordance with the Animal Care and Use Committee of the National Institute on Drug Abuse (NIDA) Intramural Research Program (IRP). Lights were on from 0700–1900 h, and experiments were carried out between 0900 and 1400 h.
2.2. Drugs and reagents
(±)-3,4-Methylenedioxymethamphetamine HCl (MDMA) was provided by NIDA Drug Supply Program, Rockville MD. Racemic MDMA was used for the present experiments because this form of the drug is used by humans, and no evidence indicates that enantiomers of MDMA are available illicitly (Green et al., 2003). MDMA was dissolved in saline immediately before use and administered i.v. in a volume of 1 ml/kg. Sources of reagents required for microdialysis and HPLC-ECD methods have been previously reported (Baumann et al., 2001; Baumann et al., 2005).
2.3. Surgical procedures
Twenty four rats received sodium pentobarbital (60 mg/kg, i.p.) for surgical anesthesia. Indwelling catheters made of Silastic Medical Grade tubing (Dow Corning, Midland, MI) were implanted into the r. jugular vein to allow for i.v. drug administration. Intracerebral guide cannulae made of plastic (CMA 12, CMA/Microdialysis, Acton, MA) were implanted above the n. accumbens, striatum or prefrontal cortex in separate groups of rats (N=8 rats/region), according to stereotaxic coordinates (Paxinos and Watson, 2005). Specifically, n. accumbens coordinates were: ML±1.6 mm, AP+1.6 mm from bregma, DV–6.0 mm from dura; striatum coordinates were ML±2.5 mm, AP+1.6 mm from bregma, DV-3.2 mm from dura; prefrontal cortex coordinates were ML±0.8 mm, AP+3.0 mm from bregma, DV-2.5 mm from dura (see Figure 1 for diagram of probe locations). Guide cannulae were secured to the skull using stainless steel screws and dental acrylic. Animals were individually housed post-operatively and allowed 7–10 days for recovery.
Figure 1.
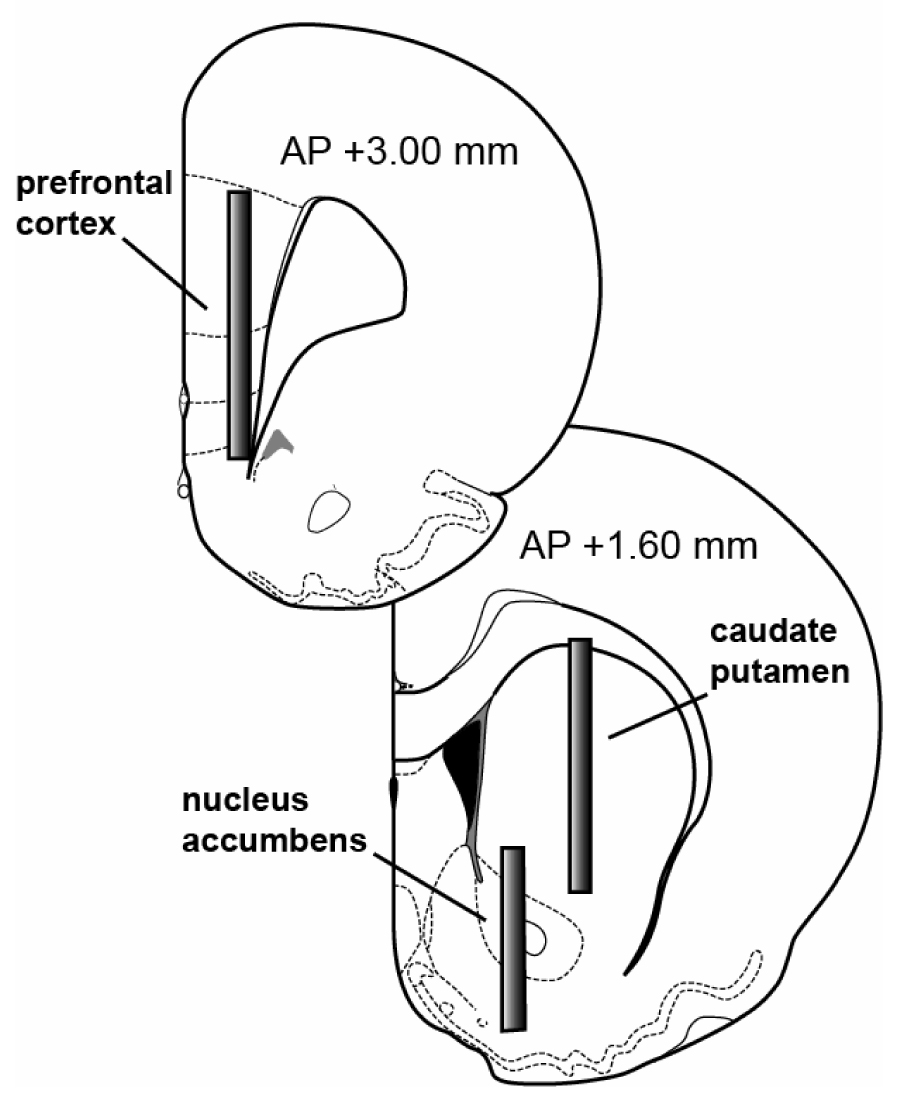
Diagrammatic representation of the placement of microdialysis probes in rat brain for the present study. Anterior-posterior coordinates are noted (Paxinos and Watson, 2005).
2.4. Microdialysis methods
In vivo microdialysis sampling was carried out as described, with minor modifications (Baumann et al., 2001). On the evening before an experiment, rats were moved to the testing room and briefly anesthetized with the short-acting barbiturate, methohexital (10 mg/kg, i.v.). A plastic neck collar was placed on each rat, dialysis probes (CMA/12, CMA Microdialysis) were inserted into guide cannulae, and extension tubes were attached to jugular catheters. The probe exchange surface was 2×0.5 mm for n. accumbens, and 3×0.5 mm for striatum and prefrontal cortex. Each rat was placed into its own activity field arena (Coulbourn Instruments, Allentown, PA) and connected to a tethering system which allowed motor activity within the container. Tubing for microdialysis lines and catheter extensions was connected to a fluid swivel (Instech Laboratories, Inc., Plymouth Meeting, PA). Probes were perfused overnight with artificial cerebrospinal fluid containing 150.0 mM Na, 3.0 mM K, 1.4 mM Ca, 0.8 mM Mg, 1.0 mM P, and 155 mM Cl (Harvard Bioscience, Holliston, MA), pumped at a flowrate of 0.6 µl/min. On the morning of the experiment, dialysate samples were collected at 20-min intervals. Samples were immediately assayed for 5-HT and DA via HPLC-ECD as described below. Once three stable baseline samples were obtained, rats received two sequential i.v. injections of MDMA, 1 mg/kg at time zero followed by 3 mg/kg 60 min later. Microdialysis samples were collected throughout the post-injection period for 120 min. At the end of the experiments, rats were euthanized with CO2, and brains were removed and stored in 10% formalin. Brains were sectioned on a cryostat and mounted on glass slides. The placement of microdialysis probe tips within the specific area of interest was verified by visual inspection, and only rats with correct placements were included in data analyses.
2.5. Analysis of 5-HT and DA
Aliquots of dialysate (5 µL) were injected onto a microbore C18 column that was coupled to an amperometric detector (Model LC-4C, Bioanalytical Systems, Inc., West Lafayette IN). A glassy carbon electrode was set at a potential of +650 mV relative to Ag/AgCl reference. Mobile phase consisting of 150 mM monochloroacetic acid, 150 mM sodium hydroxide, 2.5 mM sodium octanesulfonic acid, 250 µM disodium EDTA, 6% methanol and 6% acetonitrile per liter of water (final pH=5.3) was pumped at 60 µL/min using a syringe pump (Model 260D, ISCO, Lincoln NE, USA). Chromatographic data were acquired on-line and exported to a Millennium software system (Waters Associates, Milford, MA, USA) for peak amplification, integration, and analysis. A monoamine standard mix containing 5-HT, DA and their respective acid metabolites was injected before and after the experiment to insure validity of the constituent retention times. Peak heights of unknowns were compared to peak heights of standards, and the lower limit of assay sensitivity (3×baseline noise) was 50 fg/ 5 µL sample.
2.6. Locomotor Measures
Locomotor measures were obtained as described, with minor modifications (Baumann et al., 2005). During the overnight acclimation period and while undergoing microdialysis, each rat was housed within a square Plexiglass arena (43 cm length × 43 cm width × 43 cm height) that was equipped with an activity monitoring system (Tru Scan, Coulbourn Instruments). A sensor ring lined with photobeams spaced 2.54 cm apart was positioned in the horizontal plane to allow for real-time monitoring of various motor parameters. Activity was monitored in 20-min bins, beginning 60 min before i.v. drug injections and continuing for 120 min thereafter. Ambulation and stereotypy were quantified separately; ambulation is defined as the total distance traveled in the horizontal plane (measured in cm), whereas stereotypy is defined as the number of photobeam breaks less than ± 1.5 beam spaces and back to the original point, that do not exceed 2 sec apart (measured in number of events). Our previous experience shows that i.v. MDMA produces a robust and reproducible pattern of stereotypic movements that can be accounted for by two behaviors: repetitive side-to-side head weaving and forepaw treading (Baumann et al., 2005), both elements of the 5-HT behavioral syndrome (Green, 1984; Jacobs, 1976). Additionally, as i.v. MDMA promotes flattened body posture, minimal activity in the vertical plane is observed under our experimental conditions.
2.7. Data analyses
All group data are mean±SEM. Neurotransmitter data are pg per 5 µL sample. Ambulation data are expressed as cm traveled, while stereotypy data are expressed as number of repetitive events. Raw data were evaluated by one-factor analysis of variance (Dose), followed by Dunnett’s test to compare drug effects to time zero preinjection baseline. Relationships between transmitter levels and motor parameters were assessed by Pearson correlation coefficients (r), using raw data from individual rats. Amounts of 5-HT or DA (pg/5 µL sample) were plotted against corresponding values for ambulation or stereotypy at time points after injection of MDMA. Specifically, values from +20 min (first time-point after 1 mg/kg) through +120 min (last time-point after 3 mg/kg) were used for correlations. By this method, each rat contributed six data points for a given correlation plot. P<0.05 was chosen as the level of statistical significance.
3. Results
3.1. General comments
For the present study, rats had to fulfill a number of criteria in order for their data to be included in the final analyses. Each rat needed: (1) a patent i.v. catheter for drug delivery; (2) detectable basal levels of dialysate 5-HT and DA (> 50 fg/ 5 µL sample); (3) complete sets of transmitter and motor activity data (no missing data points); (4) correct probe placement into the brain region of interest. Based on these requirements, data from 17 of the original 24 rats were used for analyses: N=6 for n. accumbens, N=6 for the striatum, and N=5 for the prefrontal cortex. Two rats from each brain region group were excluded because of undetectable baseline 5-HT levels or incomplete data sets due to technical problems during experiments. One rat from the prefrontal cortex group had an i.v. catheter blockage.
Intravenous MDMA evoked dose-related increases in extracellular levels of 5-HT and DA in the brain regions examined (Fig. 2, Fig. 5 & Fig. 8), with the magnitude of elevation in dialysate 5-HT always exceeding that of dialysate DA. MDMA elicited dose-related stimulation of ambulation and stereotypy (Table 1), and the region of probe placement did not alter the profile of motor activation. As reported previously (Baumann et al., 2005), i.v. MDMA produced a spectrum of motor activity that was characterized by bursts of ambulation interspersed with periods of in-place stereotypic head weaving and forepaw treading. Rats exhibited flattened body posture with splayed hind limbs, and moved in crawling fashion along the perimeter of the arena.
Figure 2.
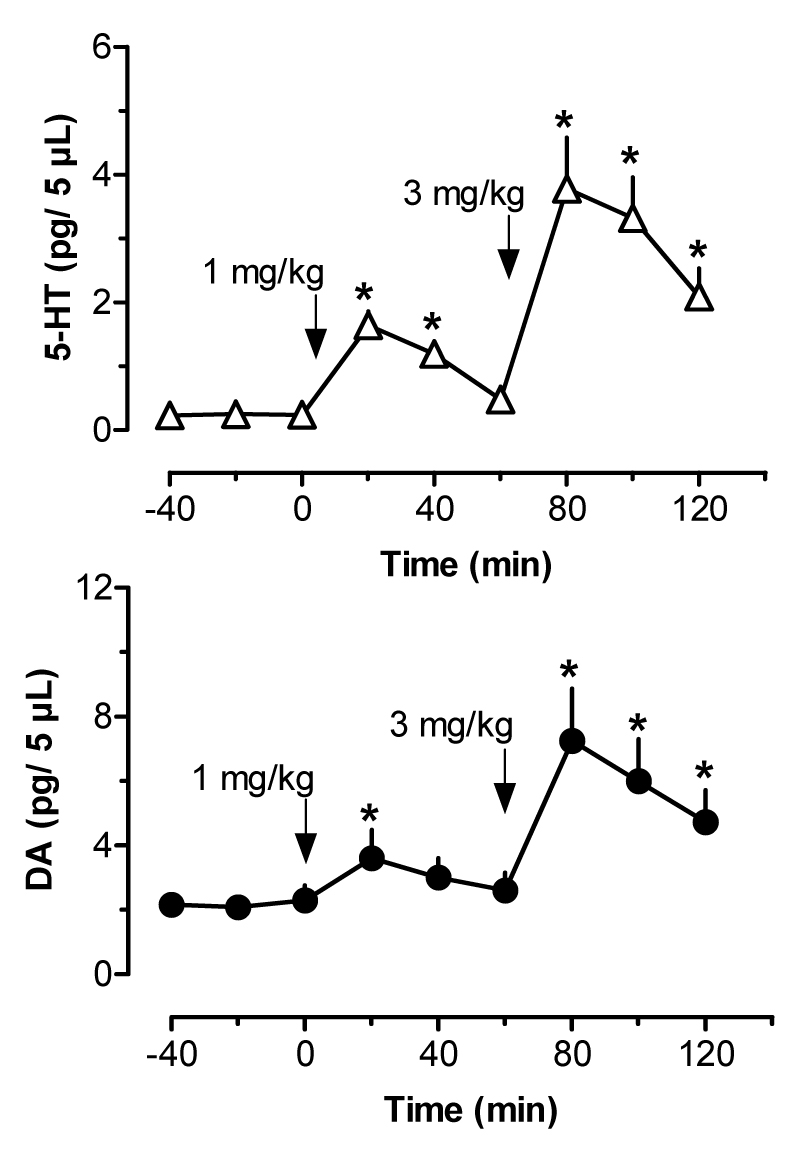
Effects of i.v. MDMA administration on dialysate 5-HT (top panel) and DA (bottom panel) in rat n. accumbens. Rats undergoing microdialysis in the accumbens received two injections of MDMA, 1 mg/kg at 0 min followed by 3 mg/kg at 60 min. Data are mean ±SEM for N=6 rats. Baseline 5-HT and DA levels in accumbens were 0.24±0.04 and 2.18±0.40 pg/5 µL. * = P<0.05 compared to 0 min preinjection control.
Figure 5.
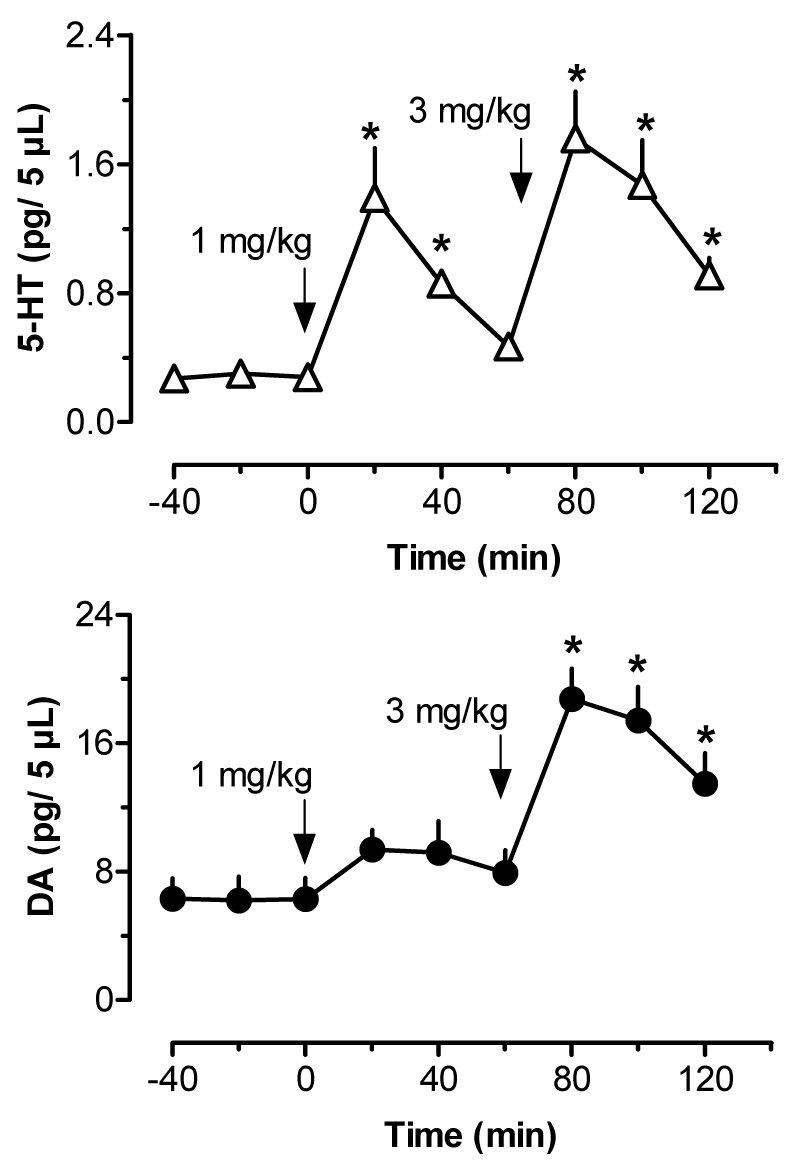
Effects of i.v. MDMA administration on dialysate 5-HT (top panel) and DA (bottom panel) in rat striatum. Rats undergoing microdialysis in the striatum received two injections of MDMA, 1 mg/kg at 0 min followed by 3 mg/kg at 60 min. Data are mean ±SEM for N=6 rats. Baseline 5-HT and DA levels in striatum were 0.28±0.05 and 6.28±1.23 pg/5 µL. * = P<0.05 compared to 0 min preinjection control.
Figure 8.
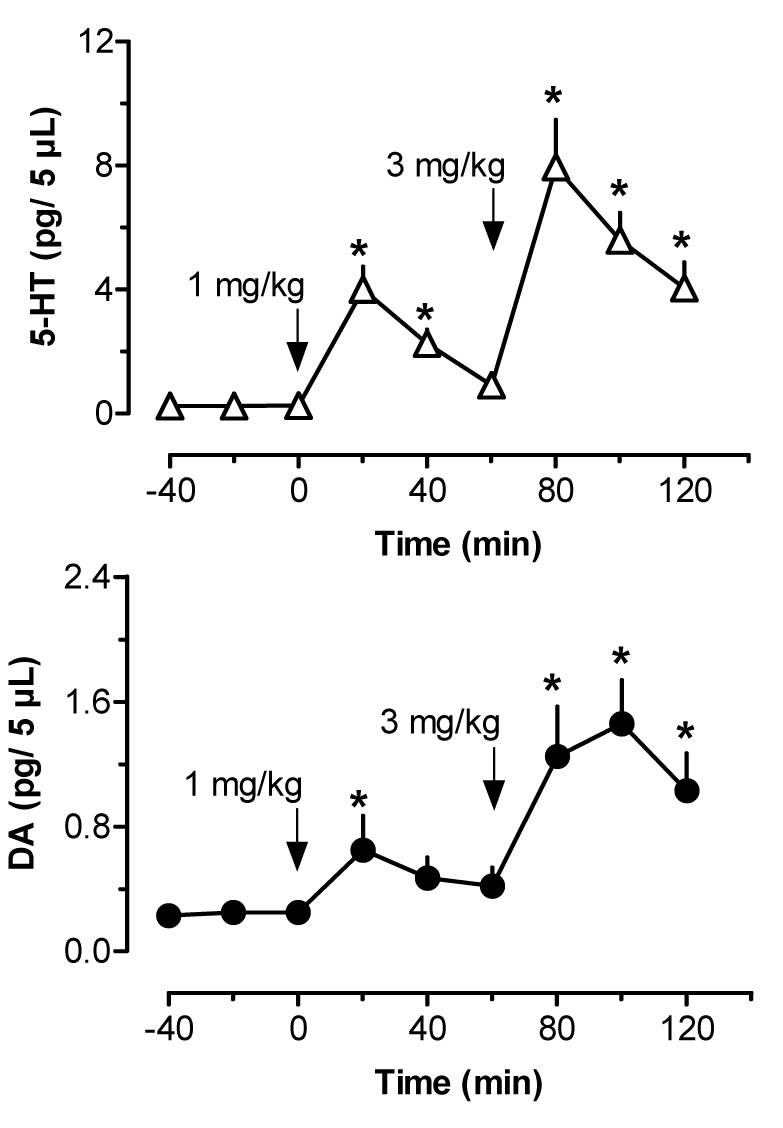
Effects of i.v. MDMA administration on dialysate 5-HT (top panel) and DA (bottom panel) in rat prefrontal cortex. Rats undergoing microdialysis in prefrontal cortex received two injections of MDMA, 1 mg/kg at 0 min followed by 3 mg/kg at 60 min. Data are mean ±SEM for N=5 rats. Baseline 5-HT and DA levels in cortex were 0.24±0.05 and 0.25±0.03 pg/5 µL. * = P<0.05 compared to 0 min preinjection control.
Table 1.
Effects of i.v. MDMA on locomotor activation in rats undergoing in vivo microdialysis
| Dialysis Probe Site | Ambulation (cm) | Stereotypy (events) | ||||
|---|---|---|---|---|---|---|
| Baseline (0 min) | 1 mg/kg (+20 min) | 3 mg/kg (+80 min) | Baseline (0 min) | 1 mg/kg (+20 min) | 3 mg/kg (+80 min) | |
| Nucleus Accumbens | 87±40 | 838±162* | 1343±249* | 89±21 | 421±57* | 790±82* |
| Caudate Nucleus | 157±50 | 749±98* | 1429±277* | 174±47 | 431±37* | 896±63* |
| Prefrontal Cortex | 116±56 | 624±196* | 1893±338* | 123±34 | 353±42* | 799±66* |
Rats received 1 mg/kg MDMA at time zero, followed by 3 mg/kg 60 min later. Values are mean±SEM for N=5–6 rats/group expressed as peak effects obtained in the first sampling period after drug administration
P<0.05 compared to baseline control value for each brain region.
3.2. Effects of MDMA in n. accumbens
Figure 2 depicts the effects of i.v. MDMA administration on levels of 5-HT (upper panel) and DA (lower panel) in dialysate samples from n. accumbens. MDMA significantly increased dialysate 5-HT (F[8,45]=12.31, P<0.0001), with levels of 5-HT peaking at 8-and 18-fold above baseline after 1 and 3 mg/kg injections, respectively. Dialysate DA increased significantly over the same time course (F[8,45]=4.26, P<0.001), and DA levels reached a maximum of 1.5- and 3-fold above baseline in response to 1 and 3 mg/kg. As shown in Table 1, rats undergoing microdialysis in n. accumbens displayed dose-related stimulation of ambulation (F[8,45]=9.89, P<0.0001) and stereotypy (F[8,45]=14.61, P<0001) in response to MDMA. Figure 3 shows correlation plots of accumbens 5-HT versus ambulation (top panel) and stereotypy (lower panel). Dialysate 5-HT levels were positively correlated with stereotypy (r=0.5276, P<0.0001) but not with ambulation (r=0.3214, NS). The data in Figure 4 demonstrate that accumbens DA levels were positively correlated with ambulation (r=0.6409, P<0.0001) and somewhat less so with stereotypy (r=0.4490, P<0.01).
Figure 3.
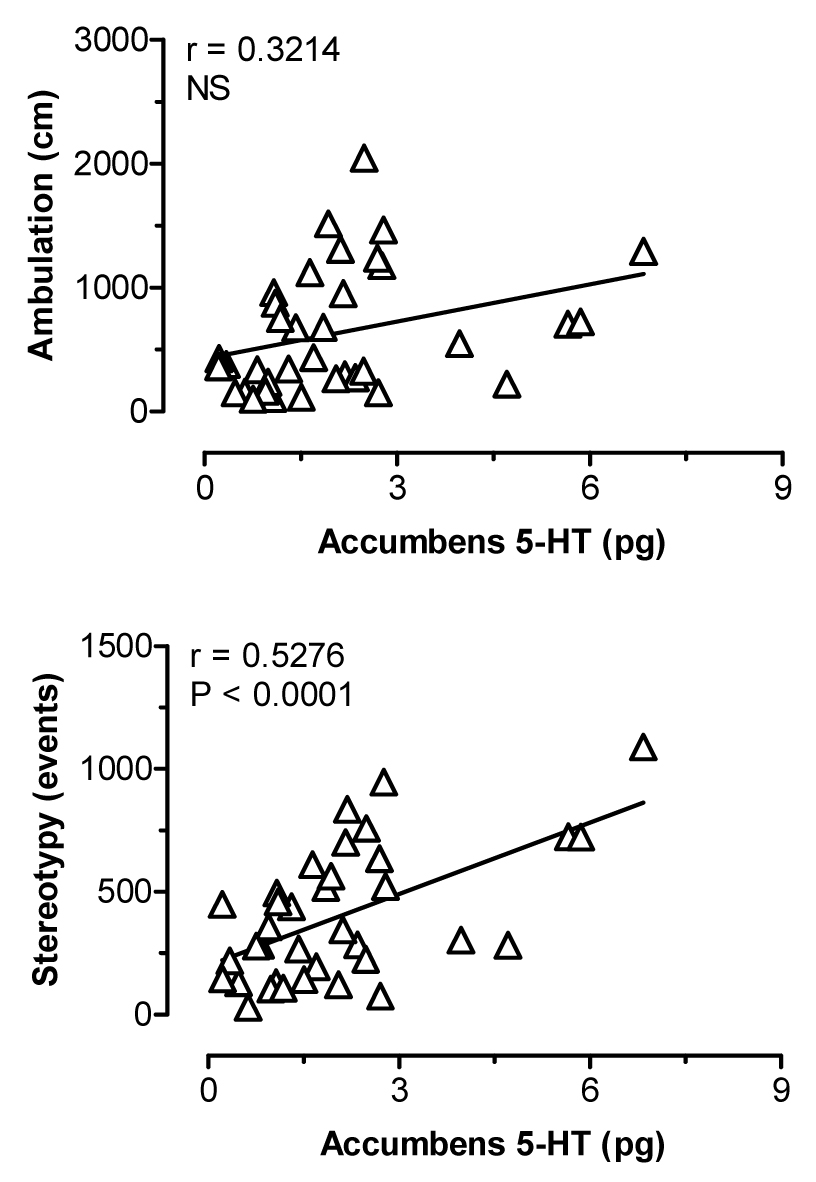
Correlations between dialysate 5-HT in n. accumbens versus ambulation (top panel) and stereotypy (bottom panel) produced by MDMA. Data points represent raw values from individual rats, obtained from +20 min (first sample after 1 mg/kg) through +120 min (last sample after 3 mg/kg). Pearson correlation coefficients (r) are shown with corresponding P values.
Figure 4.
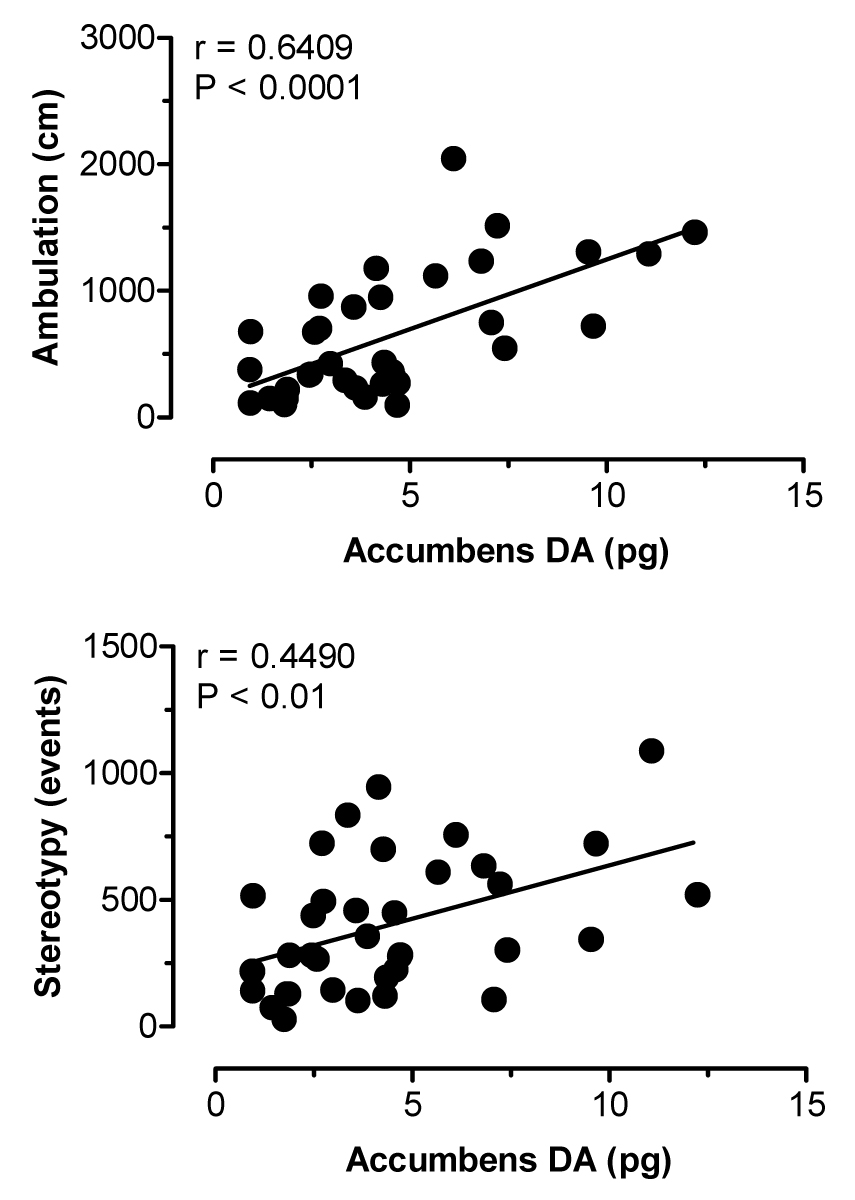
Correlations between dialysate DA in n. accumbens versus ambulation (top panel) and stereotypy (bottom panel) produced by MDMA. Data points represent raw values from individual rats, obtained from +20 min (first sample after 1 mg/kg) through +120 min (last sample after 3 mg/kg). Pearson correlation coefficients (r) are shown with corresponding P values.
3.3. Effects of MDMA in striatum
Figure 5 illustrates the effects of i.v. MDMA on levels of 5-HT (upper panel) and DA (lower panel) in striatal dialysate samples. MDMA significantly elevated dialysate 5-HT (F[8,45]=12.22, P<0.0001), with peak levels reaching 5- and 7-fold above baseline after 1 and 3 mg/kg doses, respectively. Dialysate DA increased significantly as well (F[8,45]=10.56, P<0.0001), and DA levels rose to 1.5- and 3.5-fold above baseline in response to 1 and 3 mg/kg. Rats undergoing microdialysis in striatum exhibited dose-related stimulation of ambulation (F[8,45]=11.22, P<0.0001) and stereotypy (F[8,45]=15.97, P<0001) after MDMA injections. Figure 6 shows correlation plots of striatal 5-HT versus ambulation (top panel) and stereotypy (lower panel). Dialysate 5-HT levels displayed strong positive correlations with ambulation (r=0.7141, P<0.0001) and stereotypy (r=0.6211, P<0.0001). The data in Figure 7 demonstrate that striatal DA levels correlated significantly with stereotypy (r=0.6634, P<0.001), and somewhat less so with ambulation (r=0.4273, P<0.01).
Figure 6.
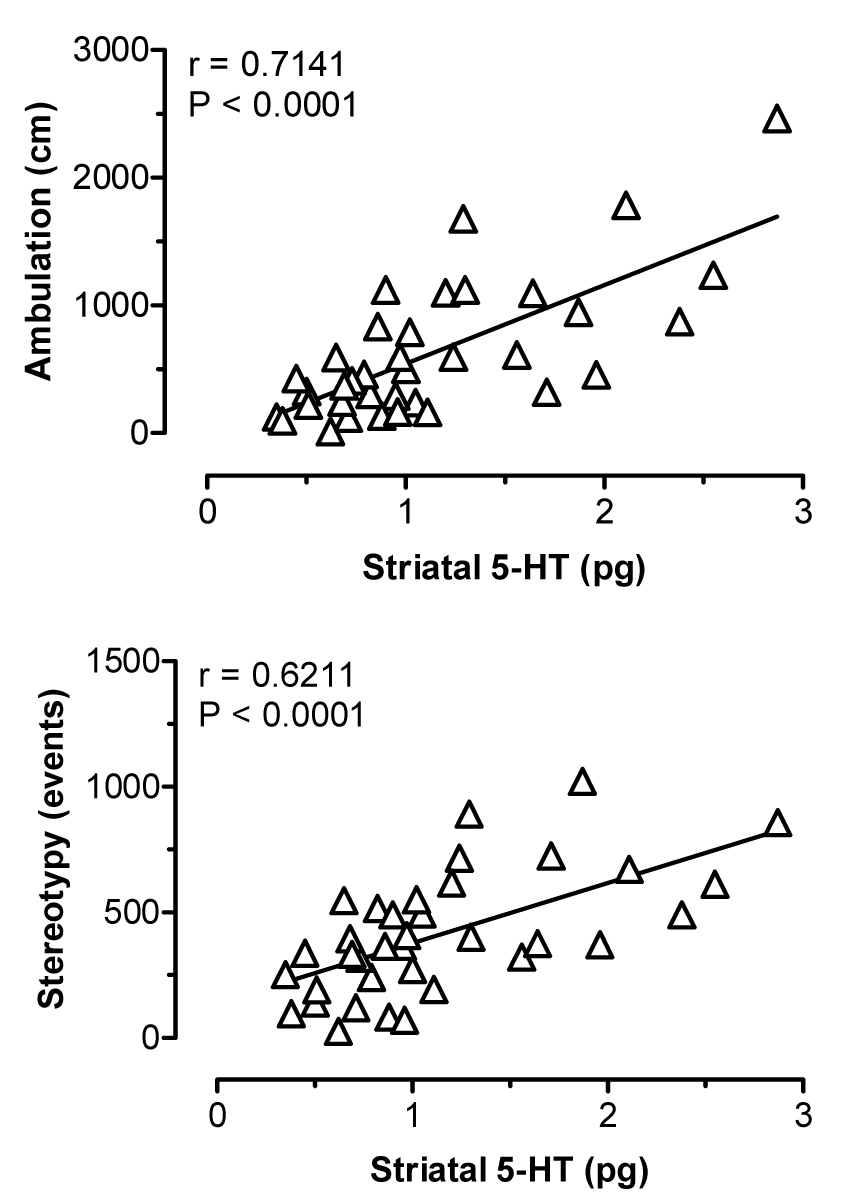
Correlations between dialysate 5-HT in striatum versus ambulation (top panel) and stereotypy (bottom panel) produced by MDMA. Data points represent raw values from individual rats, obtained from +20 min (first sample after 1 mg/kg) through +120 min (last sample after 3 mg/kg). Pearson correlation coefficients (r) are shown with corresponding P values.
Figure 7.
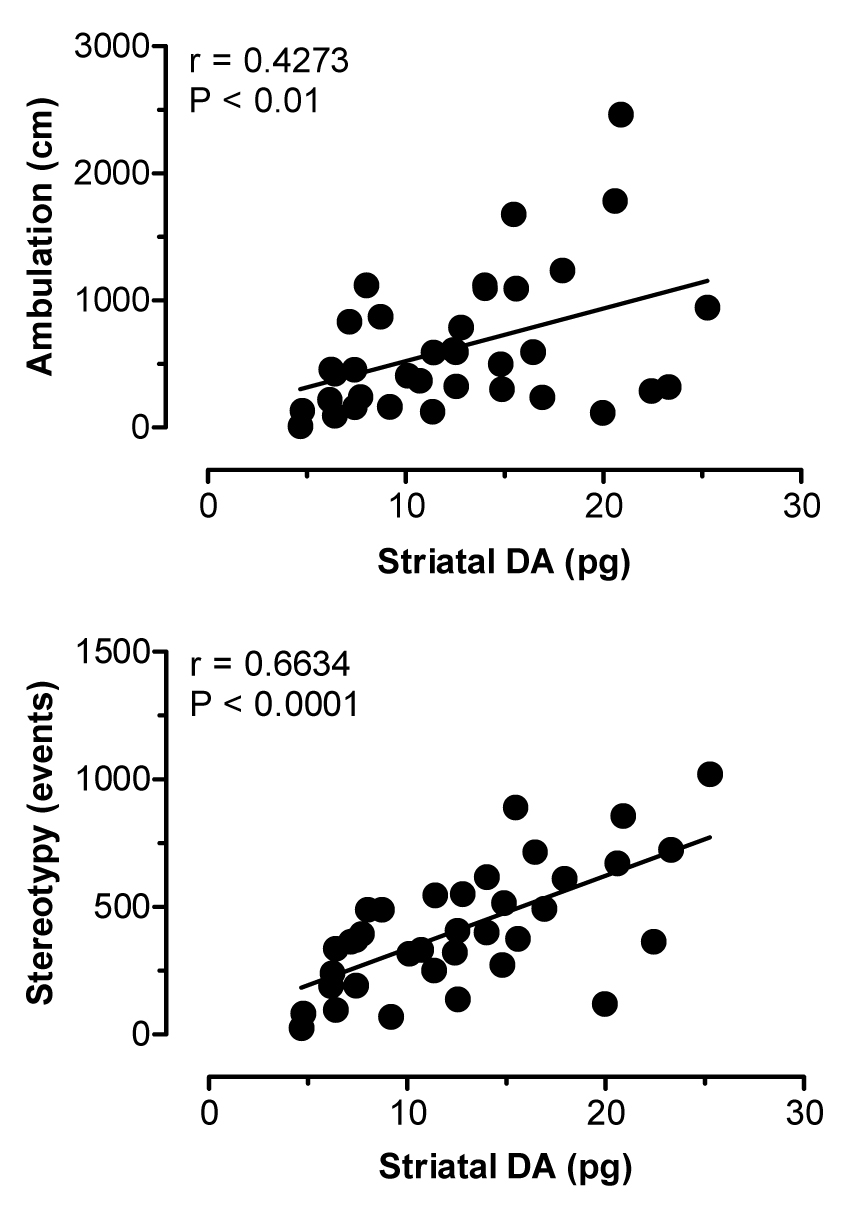
Correlations between dialysate DA in striatum versus ambulation (top panel) and stereotypy (bottom panel) produced by MDMA. Data points represent raw values from individual rats, obtained from +20 min (first sample after 1 mg/kg) through +120 min (last sample after 3 mg/kg). Pearson correlation coefficients (r) are shown with corresponding P values.
3.4. Effects of MDMA in prefrontal cortex
Figure 8 depicts the effects of i.v. MDMA administration on levels of 5-HT (upper panel) and DA (lower panel) in dialysate samples from prefrontal cortex. MDMA evoked a marked increase in dialysate 5-HT (F[8,36]=14.82, P<0.0001), where levels of cortical 5-HT peaked at 17- and 33-fold above baseline after 1 and 3 mg/kg doses, respectively. Dialysate DA increased significantly over the same time course (F[8,36]=5.99, P<0.001), and DA levels achieved a maximum of 2.5- and 6-fold above baseline in response to 1 and 3 mg/kg. MDMA stimulated dose-related ambulation (F[8,36]=9.68, P<0.0001) and stereotypy (F[8,36]=16.91, P<0001) in rats undergoing dialysis in prefrontal cortex. Figure 9 shows correlation plots of cortical 5-HT versus ambulation (top panel) and stereotypy (lower panel). Dialysate 5-HT levels were positively correlated with ambulation (r=0.5792, P<0.001) and stereotypy (r=0.6259, P<0.001). The data in Figure 10 demonstrate that cortical DA levels were correlated with ambulation (r=0.3774, P<0.05), while there was no significant correlation between DA and stereotypy in this region (r=0.2901, NS).
Figure 9.
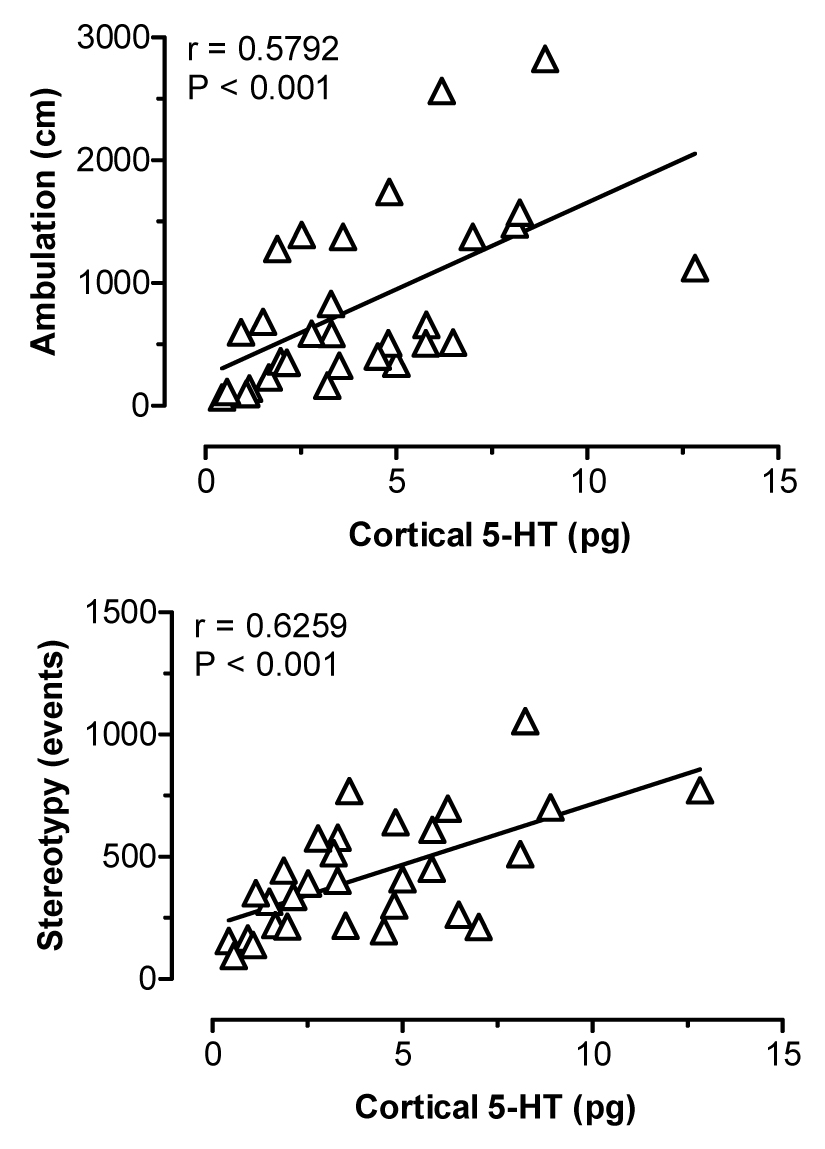
Correlations between dialysate 5-HT in prefrontal cortex versus ambulation (top panel) and stereotypy (bottom panel) produced by MDMA. Data points represent raw values from individual rats, obtained from +20 min (first sample after 1 mg/kg) through +120 min (last sample after 3 mg/kg). Pearson correlation coefficients (r) are shown with corresponding P values.
Figure 10.
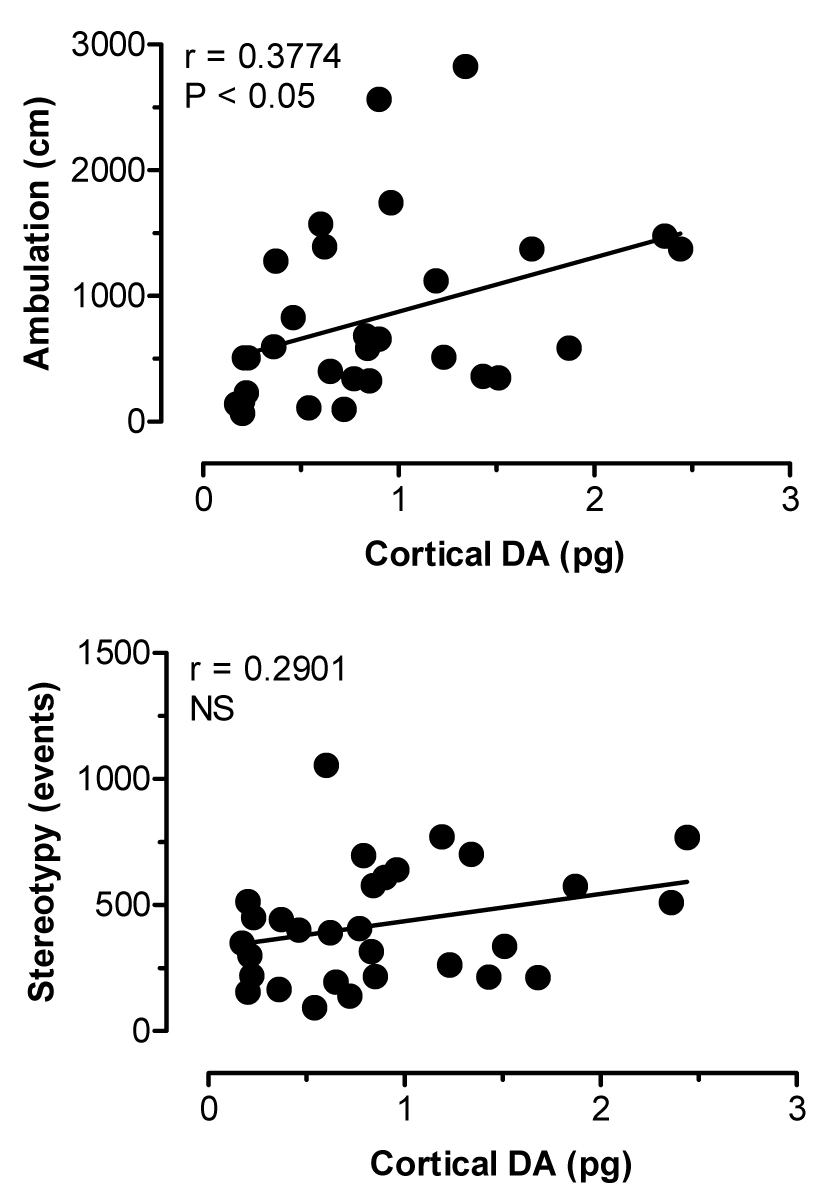
Correlations between dialysate DA in prefrontal cortex versus ambulation (top panel) and stereotypy (bottom panel) produced by MDMA. Data points represent raw values from individual rats, obtained from +20 min (first sample after 1 mg/kg) through +120 min (last sample after 3 mg/kg). Pearson correlation coefficients (r) are shown with corresponding P values.
4. Discussion
The aim of this study was to use in vivo microdialysis to examine the involvement of brain monoamines in locomotor activity produced by MDMA. Dialysis probes were implanted into brain areas implicated in motor behaviors, while dialysate 5-HT and DA were measured by a sensitive HPLC-ECD assay. Consistent with previous findings, MDMA caused concurrent increases in dialysate 5-HT and DA in all brain regions examined (Baumann et al., 2005; Gough et al., 2002; Gudelsky and Nash, 1996; Kankaanpaa et al., 1998). Dialysate monoamine levels were positively correlated with ambulation and stereotypy in a region-specific manner, and stratification of the correlation data based on the degree of statistical significance [i.e., marginal (P<0.05), moderate (P<0.01) and high (P<0.001-0.0001)] reveals important information. Dialysate 5-HT levels in all brain areas were highly correlated with MDMA-induced stereotypy, whereas 5-HT in striatum and cortex correlated with ambulation. Dialysate DA levels in the accumbens were highly correlated with ambulation, as predicted by previous microdialysis studies evaluating locomotor effects of amphetamine (Sharp et al., 1987). Additionally, DA in striatum was moderately correlated with ambulation, while DA in striatum and accumbens was correlated with stereotypy. Although such correlations can not establish cause-and-effect relationships, our data support the hypothesis that central 5-HT and DA systems are involved with the complex spectrum of locomotor activity produced by MDMA (Bankson and Cunningham, 2001; Geyer, 1996).
The elevation of extracellular 5-HT and DA levels evoked by MDMA is consistent with the known molecular mechanism of the drug, which involves transporter-mediated release of monoamines (Green et al., 2003; Hilber et al., 2005; Rothman and Baumann, 2002; Verrico et al., 2007). It seems logical to assume that extracellular NE levels also increase after MDMA administration, but as mentioned previously, the effects of MDMA on dialysate NE have not been reported. MDMA exhibits somewhat greater affinity for SERT versus DA transporters (DAT) (Crespi et al., 1997; Rothman et al., 2001), and recent in vitro data show MDMA is a more efficacious releaser of 5-HT than DA (Verrico et al., 2007). Accordingly, we found that MDMA produced greater effects on 5-HT release, when compared to DA release, in all brain areas examined. The propensity for MDMA to increase dialysate 5-HT more than DA has been reported by some investigators (Gudelsky and Nash, 1996; Shankaran and Gudelsky, 1999) but not others (Gough et al., 2002; Kankaanpaa et al., 1998).
Discrepancies regarding the relative effect of MDMA on 5-HT vs. DA release could be related to a variety of differences across microdialysis studies. In particular, technical difficulty with measuring baseline 5-HT is an important factor that is often overlooked (see (Baumann and Rutter, 2003)). Many investigators rely on HPLC methods which use reciprocating pump heads to deliver mobile phase; the resulting oscillations in detector output increase baseline noise and can artificially elevate baseline 5-HT measurements, especially if noise pulsations are mistaken for “genuine” 5-HT peaks. In our lab, we developed a microbore HPLC method which uses pulse-free delivery of mobile phase, thereby increasing sensitivity for the detection of 5-HT nearly 10-fold (Baumann et al., 2001; Baumann et al., 2005). In the present study, for example, baseline levels of 5-HT in rat striatum were exquisitely low ~0.05 pg/µL, whereas those reported by Gough et al. (2002) were 8-fold higher. MDMA administration increased extracellular 5-HT to about the same absolute amount in both studies, yet data expressed as % control values differ markedly. In general, elevated baseline 5-HT measurements will tend to diminish the apparent magnitude of evoked 5-HT release.
To the best of our knowledge, no studies have used microdialysis to evaluate correlations between monoamines and behavior in MDMA-treated rats. Early investigations examined concurrent neurochemical and locomotor effects of amphetamine in rats under going microdialysis (Kuczenski and Segal, 1989; Kuczenski et al., 1995; Sharp et al., 1987). Sharp et al (1987) reported that dialysate DA in the n. accumbens correlated with amphetamine-induced ambulation, whereas dialysate DA in striatum correlated with stereotypy. By contrast, Kuczenski et al. (1995) measured neurochemical and locomotor effects of amphetamine stereoisomers, and found no significant relationships between dialysate monoamines and behavior across a broad range of doses.
In our experiments, conditions were optimized to increase the probability of establishing correlations between monoamines and behavior. First, we used a within-subjects design where each rat provided data after two drug doses, thereby reducing inter-individual variability. Second, MDMA was administered via i.v. catheters which offered advantages over i.p. or s.c. routes. Rats were not disturbed during i.v. injections, eliminating handling stress that might influence subsequent motor activity. The i.v. route afforded rapid drug bioavailability with minimal metabolism; MDMA is extensively metabolized in rats, and certain metabolites display distinct pharmacological actions which could complicate data interpretation (de la Torre and Farre, 2004; Schmidt et al., 1987). Finally, we used low doses of MDMA that produce a consistent repertoire of behaviors. It is noteworthy that 1 mg/kg i.v. MDMA has been used for self-administration training in rats (Ratzenboeck et al., 2001; Schenk et al., 2003), and this dose is similar to those used recreationally by humans (Cole et al., 2002; Green et al., 2003; Harris et al., 2002).
As mentioned in the Introduction, DA nerve terminals in the n. accumbens are important mediators of amphetamine-induced locomotor activity, especially ambulation (i.e. forward locomotion) (Kelly et al., 1975; Pijnenburg et al., 1976). 6-Hydroxydopamine lesions of the n. accumbens reduce hyperactivity produced by systemic administration of MDMA (Gold et al., 1989a; Gold et al., 1989b), while local injection of (+)-MDMA into the accumbens elicits ambulation (Callaway and Geyer, 1992a). Our correlation data from the n. accumbens are fully consistent with the lesion and microinjection findings. We found that MDMA-induced ambulation was highly correlated with dialysate DA (P<0.0001), but not with dialysate 5-HT, in the n. accumbens. On the other hand, 5-HT in this region was highly correlated with stereotypy (P<0.0001), implicating accumbens 5-HT in the repetitive movements produced by MDMA. One recent investigation showed that neurotoxic lesions of 5-HT terminals in the ventral striatum can significantly diminish locomotor effects of MDMA (Ludwig and Schwarting, 2007), but the effects of such lesions on ambulation vs. stereotypy were not described. In any case, our data support the involvement of accumbens DA, and perhaps 5-HT, in locomotor activation produced by MDMA. The precise manner in which monoamine release in the accumbens might stimulate motor activity is not known, but GABA output neurons from the n. accumbens project to the ventral pallidum (Pennartz et al., 1994; Swerdlow et al., 1986), a region involved in the control of motor centers in the brainstem. Accordingly, it is tempting to speculate that MDMA-induced monoamine release in the n. accumbens stimulates locomotion via modulation of GABA efferents projecting to the ventral pallidum (Amalric and Koob, 1993). Future studies should examine the validity of this hypothesis.
DA nerve terminals in the striatum are implicated in stereotypy produced by amphetamines (Creese and Iversen, 1974; Kelly et al., 1975). Historically, the term “stereotypy” has been used to describe repetitive behaviors elicited by high-dose amphetamine administration, such as head-bobbing, patterned sniffing and gnawing (Kuczenski and Segal, 1989; Kuczenski et al., 1995). In our study, stereotypy refers to specific repetitive elements of the 5-HT behavioral syndrome (Green, 1984; Jacobs, 1976), namely side-to-side head weaving and forepaw treading (Baumann et al., 2005; Shankaran and Gudelsky, 1999; Spanos and Yamamoto, 1989). We found that MDMA-induced stereotypy was highly correlated with dialysate 5-HT and DA in rat striatum (P<0.0001), suggesting that both transmitters are involved in repetitive movements. Our data agree with findings showing 5-HT-mediated head weaving and forepaw treading are diminished by lesions of nigrostriatal DA neurons (Andrews et al., 1982). Likewise, the selective 5-HT1A agonist 8-OH-DPAT elicits robust 5-HT syndrome in rats, but this effect requires intact catecholamine systems (Hillegaart et al., 2000; Tricklebank et al., 1984). Thus, it appears that 5-HT and DA are required for full expression of the 5-HT syndrome. Morley et al. (2005) recently provided evidence that 5-HT1A sites mediate some aspects of 5-HT syndrome produced by MDMA, but the brain areas involved are not known. Further investigation is needed to determine the role of specific brain regions and receptor subtypes in mediating MDMA-induced 5-HT syndrome.
Ambulation produced by MDMA was strongly correlated with striatal levels of dialysate 5-HT (P<0.0001) and somewhat less so with DA (P<0.01). A number of investigators have shown that pretreatment with antagonists selective for 5-HT2A receptors can diminish hyperactivity produced by MDMA (Fletcher et al., 2002; Kehne et al., 1996; McCreary et al., 1999). As the striatum is enriched in 5-HT2A sites (Bubser et al., 2001; Lopez-Gimenez et al., 1997), this region may be involved in the serotonergic stimulation of ambulation. In particular, 5-HT2A receptors exert a stimulatory influence over DA neurons in the nigrostriatal pathway (Lucas and Spampinato, 2000). MDMA-induced DA release in the striatum is reduced by pretreatment with 5-HT2A antagonists (Schmidt et al., 1994; Yamamoto et al., 1995), indicating 5-HT release can facilitate DA release via stimulation of 5-HT2A receptors. In studies pertinent to the present work, Ball et al. (2003; 2005) examined the effects of MDMA on single unit activity in striatum of conscious freely-moving rats. These investigators demonstrated that systemic MDMA injections excite most striatal output cells, and the degree of cell excitation is positively correlated with ambulation (Ball et al., 2003). Prior treatment with antagonists selective for 5-HT2A or D2 receptors reduces MDMA-induced striatal excitations and ambulation in a parallel fashion (Ball and Rebec, 2005). The single unit data, coupled with our correlation data, provide compelling evidence that striatal 5-HT and DA are involved in motor effects of MDMA. Exactly how monoamine release in the striatum can stimulate locomotion is unclear, but this process likely involves excitation of striatal output neurons projecting to the globus pallidus or substantia nigra pars reticulata (Rebec, 2006), as these regions are implicated in the control of motor pathways (Grillner et al., 2005).
In the prefrontal cortex, dialysate 5-HT was highly correlated with ambulation and stereotypy (P<0.001) while dialysate DA was marginally correlated with ambulation, but not stereotypy. Our data suggest the relative importance of cortical 5-HT rather than DA in mediating MDMA’s motor effects. It is noteworthy that 5-HT2A antagonists can diminish evoked DA release in the prefrontal cortex (Gobert and Millan, 1999; Pehek et al., 2001), indicating 5-HT facilitates mesocortical DA function analogous to effects in nigrostriatal DA neurons. NE release in the prefrontal cortex also appears to play a key role in locomotor-activating effects of MDMA (Selken and Nichols, 2007) and other amphetamine-type stimulants (Blanc et al., 1994; Darracq et al., 1998). Specifically, cortical infusion of the α1 antagonist prazosin reduces ambulation produced by systemic MDMA injection (Selken and Nichols, 2007), suggesting the importance of α1 adrenergic receptors. Electrophysiological studies reveal that systemic MDMA administration inhibits cell firing in most cells of the prefrontal cortex, and this effect is mediated via activation of 5-HT receptors by released 5-HT (Pan and Wang, 1991a, Pan and Wang, 1991b). It seems possible that MDMA-induced suppression of cell firing in the prefrontal cortex influences descending outputs to subcortical areas controlling motor activity (Tzschentke and Schmidt, 2000), and this hypothesis warrants further inquiry.
Our previous microdialysis findings indicate that central 5-HT release is capable of antagonizing locomotor stimulant effects mediated by DA release (Rothman and Baumann, 2006; Rothman et al., 2005), and this view seems at odds with MDMA data presented herein. For instance, amphetamine analogs which display high potency at SERT relative to DAT elicit less ambulation than DAT-selective analogs (Rothman and Baumann, 2006). In fact, SERT-selective 5-HT releasers such as fenfluramine and chlorphentermine do not stimulate ambulation, and prior treatment with fenfluramine attenuates locomotor effects of the DA releaser phentermine (Baumann et al., 2000; Rothman et al., 1998). Based on the work of others (Di Matteo et al., 2001; Di Matteo et al., 2000), we have speculated that 5-HT can counteract the effects of DA via activation of 5-HT2C receptor mechanisms in the brain. 5-HT2C receptors are characterized by high levels of constitutive activity which affords potent inhibition of tonic and evoked DA release in the mesolimbic, nigrostriatal and mesocortical systems (Alex and Pehek, 2007; Bubar and Cunningham, 2006). Indeed, ambulation produced by MDMA and other psychomotor stimulants is markedly enhanced by pretreatment with 5-HT2C antagonists (Bankson and Cunningham, 2002; Fletcher et al., 2002; Fletcher et al., 2006; McCreary and Cunningham, 1999). These findings suggest that one overarching role of 5-HT is to dampen the magnitude of locomotor activation, via agonist actions at 5-HT2C receptors. Nonetheless, the evidence from a variety of experiments, including the correlation data presented here, demonstrates that MDMA co-activates specific 5-HT pathways that are coupled to stimulation of ambulation and stereotypy (Bankson and Cunningham, 2001; Geyer, 1996).
In summary, our microdialysis data suggest that motor behaviors elicited by MDMA depend upon central 5-HT and DA systems in a region- and modality-specific manner. As with most amphetamine analogs, DA release in the n. accumbens is involved with MDMA-induced ambulation (Gold et al., 1989a; Gold et al., 1989b; Ikemoto, 2002), whereas DA release in the striatum seems to be involved with both stereotypy and ambulation. In contrast to other stimulants, MDMA displays predominant serotonergic effects which modulate locomotor activity in a complex manner: 5-HT1B and 5-HT2A receptors facilitate, while 5-HT2C receptors inhibit, ambulatory effects of the drug. We found that stereotypy was highly correlated with dialysate 5-HT in the accumbens, striatum and prefrontal cortex, implicating these regions as substrates for repetitive aspects of the 5-HT syndrome. Future experiments should combine in vivo microdialysis with anatomical (e.g., discrete lesions) and pharmacological (e.g., selective antagonists) methods to characterize the circuitry and receptor mechanisms responsible for the effects of MDMA.
Acknowledgements
This research was generously supported by the Intramural Research Program of NIDA, NIH, DHHS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alex KD, Pehek EA. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol Ther. 2007;113:296–320. doi: 10.1016/j.pharmthera.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amalric M, Koob GF. Functionally selective neurochemical afferents and efferents of the mesocorticolimbic and nigrostriatal dopamine system. Prog Brain Res. 1993;99:209–226. doi: 10.1016/s0079-6123(08)61348-5. [DOI] [PubMed] [Google Scholar]
- Andrews CD, Fernando JC, Curzon G. Differential involvement of dopamine-containing tracts in 5-hydroxytryptamine-dependent behaviours caused by amphetamine in large doses. Neuropharmacology. 1982;21:63–68. doi: 10.1016/0028-3908(82)90212-x. [DOI] [PubMed] [Google Scholar]
- Ball KT, Budreau D, Rebec GV. Acute effects of 3,4- methylenedioxymethamphetamine on striatal single-unit activity and behavior in freely moving rats: differential involvement of dopamine D(1) and D(2) receptors. Brain Res. 2003;994:203–215. doi: 10.1016/j.brainres.2003.09.037. [DOI] [PubMed] [Google Scholar]
- Ball KT, Rebec GV. Role of 5-HT2A and 5-HT2C/B receptors in the acute effects of 3,4-methylenedioxymethamphetamine (MDMA) on striatal single-unit activity and locomotion in freely moving rats. Psychopharmacology (Berl) 2005;181:676–687. doi: 10.1007/s00213-005-0038-z. [DOI] [PubMed] [Google Scholar]
- Bankson MG, Cunningham KA. 3,4-Methylenedioxymethamphetamine (MDMA) as a unique model of serotonin receptor function and serotonin-dopamine interactions. J Pharmacol Exp Ther. 2001;297:846–852. [PubMed] [Google Scholar]
- Bankson MG, Cunningham KA. Pharmacological studies of the acute effects of (+)−3,4- methylenedioxymethamphetamine on locomotor activity: role of 5-HT(1B/1D) and 5-HT(2) receptors. Neuropsychopharmacology. 2002;26:40–52. doi: 10.1016/S0893-133X(01)00345-1. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Dersch CM, Brockington A, Rice KC, Rothman RB. Effects of phentermine and fenfluramine on extracellular dopamine and serotonin in rat nucleus accumbens: therapeutic implications. Synapse. 2000;36:102–113. doi: 10.1002/(SICI)1098-2396(200005)36:2<102::AID-SYN3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Dersch CM, Rothman RB. 1-(m-chlorophenyl)piperazine (mCPP) dissociates in vivo serotonin release from long-term serotonin depletion in rat brain. Neuropsychopharmacology. 2001;24:492–501. doi: 10.1016/S0893-133X(00)00221-9. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Rothman RB. Functional consequences of central serotonin depletion produced by repeated fenfluramine administration in rats. J Neurosci. 1998;18:9069–9077. doi: 10.1523/JNEUROSCI.18-21-09069.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Budzynski AG, Partilla JS, Blough BE, Rothman RB. N-substituted piperazines abused by humans mimic the molecular mechanism of 3,4-methylenedioxymethamphetamine (MDMA, or 'Ecstasy') Neuropsychopharmacology. 2005;30:550–560. doi: 10.1038/sj.npp.1300585. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Rutter JJ. Application of in vivo microdialysis methods to the study of psychomotor stimulant drugs. In: Waterhouse BD, editor. Methods in Drug Abuse Research: Cellular and Circuit Level Analyses. Boca Raton: CRC Press; 2003. pp. 51–86. [Google Scholar]
- Baumann MH, Wang X, Rothman RB. 3,4-Methylenedioxymethamphetamine (MDMA) neurotoxicity in rats: a reappraisal of past and present findings. Psychopharmacology (Berl) 2007;189:407–424. doi: 10.1007/s00213-006-0322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Mossner R, Westphal H, Lesch KP. Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine ("Ecstasy") in serotonin transporter-deficient mice. Mol Pharmacol. 1998;53:649–655. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- Blanc G, Trovero F, Vezina P, Herve D, Godeheu AM, Glowinski J, Tassin JP. Blockade of prefronto-cortical alpha 1-adrenergic receptors prevents locomotor hyperactivity induced by subcortical D-amphetamine injection. Eur J Neurosci. 1994;6:293–298. doi: 10.1111/j.1460-9568.1994.tb00272.x. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. Serotonin 5-HT2A and 5-HT2C receptors as potential targets for modulation of psychostimulant use and dependence. Curr Top Med Chem. 2006;6:1971–1985. doi: 10.2174/156802606778522131. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Pack KM, Frankel PS, Cunningham KA. Effects of dopamine D1- or D2- like receptor antagonists on the hypermotive and discriminative stimulus effects of (+)-MDMA. Psychopharmacology (Berl) 2004;173:326–336. doi: 10.1007/s00213-004-1790-1. [DOI] [PubMed] [Google Scholar]
- Bubser M, Backstrom JR, Sanders-Bush E, Roth BL, Deutch AY. Distribution of serotonin 5-HT(2A) receptors in afferents of the rat striatum. Synapse. 2001;39:297–304. doi: 10.1002/1098-2396(20010315)39:4<297::AID-SYN1012>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Callaway CW, Geyer MA. Stimulant effects of 3,4-methylenedioxymethamphetamine in the nucleus accumbens of rat. Eur J Pharmacol. 1992a;214:45–51. doi: 10.1016/0014-2999(92)90094-k. [DOI] [PubMed] [Google Scholar]
- Callaway CW, Geyer MA. Tolerance and cross-tolerance to the activating effects of 3,4- methylenedioxymethamphetamine and a 5-hydroxytryptamine1B agonist. J Pharmacol Exp Ther. 1992b;263:318–326. [PubMed] [Google Scholar]
- Callaway CW, Johnson MP, Gold LH, Nichols DE, Geyer MA. Amphetamine derivatives induce locomotor hyperactivity by acting as indirect serotonin agonists. Psychopharmacology (Berl) 1991;104:293–301. doi: 10.1007/BF02246026. [DOI] [PubMed] [Google Scholar]
- Callaway CW, Wing LL, Geyer MA. Serotonin release contributes to the locomotor stimulant effects of 3,4-methylenedioxymethamphetamine in rats. J Pharmacol Exp Ther. 1990;254:456–464. [PubMed] [Google Scholar]
- Cole JC, Bailey M, Sumnall HR, Wagstaff GF, King LA. The content of ecstasy tablets: implications for the study of their long-term effects. Addiction. 2002;97:1531–1536. doi: 10.1046/j.1360-0443.2002.00222.x. [DOI] [PubMed] [Google Scholar]
- Creese I, Iversen SD. The role of forebrain dopamine systems in amphetamine induced stereotyped behavior in the rat. Psychopharmacologia. 1974;39:345–357. doi: 10.1007/BF00422974. [DOI] [PubMed] [Google Scholar]
- Crespi D, Mennini T, Gobbi M. Carrier-dependent and Ca(2+)-dependent 5-HT and dopamine release induced by (+)-amphetamine, 3,4- methylendioxymethamphetamine, p-chloroamphetamine and (+)-fenfluramine. Br J Pharmacol. 1997;121:1735–1743. doi: 10.1038/sj.bjp.0701325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darracq L, Blanc G, Glowinski J, Tassin JP. Importance of the noradrenaline-dopamine coupling in the locomotor activating effects of D-amphetamine. J Neurosci. 1998;18:2729–2739. doi: 10.1523/JNEUROSCI.18-07-02729.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre R, Farre M. Neurotoxicity of MDMA (ecstasy): the limitations of scaling from animals to humans. Trends Pharmacol Sci. 2004;25:505–508. doi: 10.1016/j.tips.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Di Matteo V, De Blasi A, Di Giulio C, Esposito E. Role of 5-HT(2C) receptors in the control of central dopamine function. Trends Pharmacol Sci. 2001;22:229–232. doi: 10.1016/s0165-6147(00)01688-6. [DOI] [PubMed] [Google Scholar]
- Di Matteo V, Di Giovanni G, Di Mascio M, Esposito E. Biochemical and electrophysiological evidence that RO 60-0175 inhibits mesolimbic dopaminergic function through serotonin(2C) receptors. Brain Res. 2000;865:85–90. doi: 10.1016/s0006-8993(00)02246-0. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Korth KM, Robinson SR, Baker GB. Multiple 5-HT receptors are involved in the effects of acute MDMA treatment: studies on locomotor activity and responding for conditioned reinforcement. Psychopharmacology (Berl) 2002;162:282–291. doi: 10.1007/s00213-002-1104-4. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Sinyard J, Higgins GA. The effects of the 5-HT(2C) receptor antagonist SB242084 on locomotor activity induced by selective, or mixed, indirect serotonergic and dopaminergic agonists. Psychopharmacology (Berl) 2006;187:515–525. doi: 10.1007/s00213-006-0453-9. [DOI] [PubMed] [Google Scholar]
- Geyer MA. Serotonergic functions in arousal and motor activity. Behav Brain Res. 1996;73:31–35. doi: 10.1016/0166-4328(96)00065-4. [DOI] [PubMed] [Google Scholar]
- Gobert A, Millan MJ. Serotonin (5-HT)2A receptor activation enhances dialysate levels of dopamine and noradrenaline, but not 5-HT, in the frontal cortex of freely-moving rats. Neuropharmacology. 1999;38:315–317. doi: 10.1016/s0028-3908(98)00188-9. [DOI] [PubMed] [Google Scholar]
- Gold LH, Geyer MA, Koob GF. Neurochemical mechanisms involved in behavioral effects of amphetamines and related designer drugs. NIDA Res Monogr. 1989a;94:101–126. [PubMed] [Google Scholar]
- Gold LH, Hubner CB, Koob GF. A role for the mesolimbic dopamine system in the psychostimulant actions of MDMA. Psychopharmacology (Berl) 1989b;99:40–47. doi: 10.1007/BF00634450. [DOI] [PubMed] [Google Scholar]
- Gold LH, Koob GF, Geyer MA. Stimulant and hallucinogenic behavioral profiles of 3,4-methylenedioxymethamphetamine and N-ethyl-3,4- methylenedioxyamphetamine in rats. J Pharmacol Exp Ther. 1988;247:547–555. [PubMed] [Google Scholar]
- Gough B, Imam SZ, Blough B, Slikker W, Jr, Ali SF. Comparative effects of substituted amphetamines (PMA, MDMA, and METH) on monoamines in rat caudate: a microdialysis study. Ann N Y Acad Sci. 2002;965:410–420. doi: 10.1111/j.1749-6632.2002.tb04182.x. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Daumann J. Neurotoxicity of methylenedioxyamphetamines (MDMA; ecstasy) in humans: how strong is the evidence for persistent brain damage? Addiction. 2006;101:348–361. doi: 10.1111/j.1360-0443.2006.01314.x. [DOI] [PubMed] [Google Scholar]
- Green AR. 5-HT-mediated behavior. Animal studies. Neuropharmacology. 1984;23:1521–1528. doi: 10.1016/0028-3908(84)90096-0. [DOI] [PubMed] [Google Scholar]
- Green AR, Mechan AO, Elliott JM, O'Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, "ecstasy") Pharmacol Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- Grillner S, Hellgren J, Menard A, Saitoh K, Wikstrom MA. Mechanisms for selection of basic motor programs--roles for the striatum and pallidum. Trends Neurosci. 2005;28:364–370. doi: 10.1016/j.tins.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Gudelsky GA, Nash JF. Carrier-mediated release of serotonin by 3,4- methylenedioxymethamphetamine: implications for serotonin-dopamine interactions. J Neurochem. 1996;66:243–249. doi: 10.1046/j.1471-4159.1996.66010243.x. [DOI] [PubMed] [Google Scholar]
- Harris DS, Baggott M, Mendelson JH, Mendelson JE, Jones RT. Subjective and hormonal effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology (Berl) 2002;162:396–405. doi: 10.1007/s00213-002-1131-1. [DOI] [PubMed] [Google Scholar]
- Hilber B, Scholze P, Dorostkar MM, Sandtner W, Holy M, Boehm S, Singer EA, Sitte HH. Serotonin-transporter mediated efflux: a pharmacological analysis of amphetamines and non-amphetamines. Neuropharmacology. 2005;49:811–819. doi: 10.1016/j.neuropharm.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Hillegaart V, Magnusson O, Ahlenius S. A9 and A10 dopamine nuclei as a site of action for effects of 8-OH-DPAT on locomotion in the rat. Pharmacol Biochem Behav. 2000;67:55–63. doi: 10.1016/s0091-3057(00)00292-6. [DOI] [PubMed] [Google Scholar]
- Hiramatsu M, Nabeshima T, Kameyama T, Maeda Y, Cho AK. The effect of optical isomers of 3,4-methylenedioxymethamphetamine (MDMA) on stereotyped behavior in rats. Pharmacol Biochem Behav. 1989;33:343–347. doi: 10.1016/0091-3057(89)90511-x. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Ventral striatal anatomy of locomotor activity induced by cocaine, D-amphetamine, dopamine and D1/D2 agonists. Neuroscience. 2002;113:939–955. doi: 10.1016/s0306-4522(02)00247-6. [DOI] [PubMed] [Google Scholar]
- Jacobs BL. An animal behavior model for studying central serotonergic synapses. Life Sci. 1976;19:777–785. doi: 10.1016/0024-3205(76)90303-9. [DOI] [PubMed] [Google Scholar]
- Johnson MP, Hoffman AJ, Nichols DE. Effects of the enantiomers of MDA, MDMA and related analogues on [3H]serotonin and [3H]dopamine release from superfused rat brain slices. Eur J Pharmacol. 1986;132:269–276. doi: 10.1016/0014-2999(86)90615-1. [DOI] [PubMed] [Google Scholar]
- Kankaanpaa A, Meririnne E, Lillsunde P, Seppala T. The acute effects of amphetamine derivatives on extracellular serotonin and dopamine levels in rat nucleus accumbens. Pharmacol Biochem Behav. 1998;59:1003–1009. doi: 10.1016/s0091-3057(97)00527-3. [DOI] [PubMed] [Google Scholar]
- Kehne JH, Ketteler HJ, McCloskey TC, Sullivan CK, Dudley MW, Schmidt CJ. Effects of the selective 5-HT2A receptor antagonist MDL 100,907 on MDMA-induced locomotor stimulation in rats. Neuropsychopharmacology. 1996;15:116–124. doi: 10.1016/0893-133X(95)00160-F. [DOI] [PubMed] [Google Scholar]
- Kelly PH, Seviour PW, Iversen SD. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975;94:507–522. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal D. Concomitant characterization of behavioral and striatal neurotransmitter response to amphetamine using in vivo microdialysis. J Neurosci. 1989;9:2051–2065. doi: 10.1523/JNEUROSCI.09-06-02051.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS, Cho AK, Melega W. Hippocampus norepinephrine, caudate dopamine and serotonin, and behavioral responses to the stereoisomers of amphetamine and methamphetamine. J Neurosci. 1995;15:1308–1317. doi: 10.1523/JNEUROSCI.15-02-01308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME, Vollenweider FX. Which neuroreceptors mediate the subjective effects of MDMA in humans? A summary of mechanistic studies. Hum Psychopharmacol. 2001;16:589–598. doi: 10.1002/hup.348. [DOI] [PubMed] [Google Scholar]
- Lopez-Gimenez JF, Mengod G, Palacios JM, Vilaro MT. Selective visualization of rat brain 5-HT2A receptors by autoradiography with [3H]MDL 100,907. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:446–454. doi: 10.1007/pl00005075. [DOI] [PubMed] [Google Scholar]
- Lucas G, Spampinato U. Role of striatal serotonin2A and serotonin2C receptor subtypes in the control of in vivo dopamine outflow in the rat striatum. J Neurochem. 2000;74:693–701. doi: 10.1046/j.1471-4159.2000.740693.x. [DOI] [PubMed] [Google Scholar]
- Ludwig V, Schwarting RK. Neurochemical and behavioral consequences of striatal injection of 5,7-dihydroxytryptamine. J Neurosci Methods. 2007;162:108–118. doi: 10.1016/j.jneumeth.2006.12.011. [DOI] [PubMed] [Google Scholar]
- McCann UD, Eligulashvili V, Ricaurte GA. (+/−)3,4-Methylenedioxymethamphetamine ('Ecstasy')-induced serotonin neurotoxicity: clinical studies. Neuropsychobiology. 2000;42:11–16. doi: 10.1159/000026665. [DOI] [PubMed] [Google Scholar]
- McCreary AC, Bankson MG, Cunningham KA. Pharmacological studies of the acute and chronic effects of (+)-3, 4-methylenedioxymethamphetamine on locomotor activity: role of 5-hydroxytryptamine(1A) and 5-hydroxytryptamine(1B/1D) receptors. J Pharmacol Exp Ther. 1999;290:965–973. [PubMed] [Google Scholar]
- McCreary AC, Cunningham KA. Effects of the 5-HT2C/2B antagonist SB 206553 on hyperactivity induced by cocaine. Neuropsychopharmacology. 1999;20:556–564. doi: 10.1016/S0893-133X(98)00087-6. [DOI] [PubMed] [Google Scholar]
- Mechan AO, Esteban B, O'Shea E, Elliott JM, Colado MI, Green AR. The pharmacology of the acute hyperthermic response that follows administration of 3,4-methylenedioxymethamphetamine (MDMA, 'ecstasy') to rats. Br J Pharmacol. 2002;135:170–180. doi: 10.1038/sj.bjp.0704442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Moore RY, Bloom FE. Central catecholamine neuron systems: anatomy and physiology of the dopamine systems. Annu Rev Neurosci. 1978;1:129–169. doi: 10.1146/annurev.ne.01.030178.001021. [DOI] [PubMed] [Google Scholar]
- Olive MF, Mehmert KK, Hodge CW. Microdialysis in the mouse nucleus accumbens: a method for detection of monoamine and amino acid neurotransmitters with simultaneous assessment of locomotor activity. Brain Res Brain Res Protoc. 2000;5:16–24. doi: 10.1016/s1385-299x(99)00054-9. [DOI] [PubMed] [Google Scholar]
- Pan HS, Wang RY. The action of (+/−)-MDMA on medial prefrontal cortical neurons is mediated through the serotonergic system. Brain Res. 1991a;543:56–60. doi: 10.1016/0006-8993(91)91047-5. [DOI] [PubMed] [Google Scholar]
- Pan HS, Wang RY. MDMA: further evidence that its action in the medial prefrontal cortex is mediated by the serotonergic system. Brain Res. 1991b;539:332–336. doi: 10.1016/0006-8993(91)91640-m. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Geyer MA. The effects of MDMA and other methylenedioxy-substituted phenylalkylamines on the structure of rat locomotor activity. Neuropsychopharmacology. 1992;7:15–31. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Elsevier; 2005. [DOI] [PubMed] [Google Scholar]
- Pehek EA, McFarlane HG, Maguschak K, Price B, Pluto CP. M100,907, a selective 5-HT(2A) antagonist, attenuates dopamine release in the rat medial prefrontal cortex. Brain Res. 2001;888:51–59. doi: 10.1016/s0006-8993(00)03004-3. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, Groenewegen HJ, Lopes da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog Neurobiol. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Pijnenburg AJ, Honig WM, Van der Heyden JA, Van Rossum JM. Effects of chemical stimulation of the mesolimbic dopamine system upon locomotor activity. Eur J Pharmacol. 1976;35:45–58. doi: 10.1016/0014-2999(76)90299-5. [DOI] [PubMed] [Google Scholar]
- Ratzenboeck E, Saria A, Kriechbaum N, Zernig G. Reinforcing effects of MDMA ("ecstasy") in drug-naive and cocaine-trained rats. Pharmacology. 2001;62:138–144. doi: 10.1159/000056086. [DOI] [PubMed] [Google Scholar]
- Rebec GV. Behavioral electrophysiology of psychostimulants. Neuropsychopharmacology. 2006;31:2341–2348. doi: 10.1038/sj.npp.1301160. [DOI] [PubMed] [Google Scholar]
- Rempel NL, Callaway CW, Geyer MA. Serotonin1B receptor activation mimics behavioral effects of presynaptic serotonin release. Neuropsychopharmacology. 1993;8:201–211. doi: 10.1038/npp.1993.22. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Yuan J, McCann UD. (+/−)3,4-Methylenedioxymethamphetamine ('Ecstasy')-induced serotonin neurotoxicity: studies in animals. Neuropsychobiology. 2000;42:5–10. doi: 10.1159/000026664. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Jurson PA, Bennett JA, Bentgen KM. Persistent sensitization of dopamine neurotransmission in ventral striatum (nucleus accumbens) produced by prior experience with (+)-amphetamine: a microdialysis study in freely moving rats. Brain Res. 1988;462:211–222. doi: 10.1016/0006-8993(88)90549-5. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Balance between dopamine and serotonin release modulates behavioral effects of amphetamine-type drugs. Ann N Y Acad Sci. 2006;1074:245–260. doi: 10.1196/annals.1369.064. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Therapeutic and adverse actions of serotonin transporter substrates. Pharmacol Ther. 2002;95:73–88. doi: 10.1016/s0163-7258(02)00234-6. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Woolverton WL, Anderson KG, Negus SS, Mello NK, Roth BL, Baumann MH. Development of a rationally designed, low abuse potential, biogenic amine releaser that suppresses cocaine self-administration. J Pharmacol Exp Ther. 2005;313:1361–1369. doi: 10.1124/jpet.104.082503. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Elmer GI, Shippenberg TS, Rea W, Baumann MH. Phentermine and fenfluramine. Preclinical studies in animal models of cocaine addiction. Ann N Y Acad Sci. 1998;844:59–74. [PubMed] [Google Scholar]
- Schenk S, Gittings D, Johnstone M, Daniela E. Development, maintenance and temporal pattern of self-administration maintained by ecstasy (MDMA) in rats. Psychopharmacology (Berl) 2003;169:21–27. doi: 10.1007/s00213-003-1407-0. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Levin JA, Lovenberg W. In vitro and in vivo neurochemical effects of methylenedioxymethamphetamine on striatal monoaminergic systems in the rat brain. Biochem Pharmacol. 1987;36:747–755. doi: 10.1016/0006-2952(87)90729-5. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Sullivan CK, Fadayel GM. Blockade of striatal 5-hydroxytryptamine2 receptors reduces the increase in extracellular concentrations of dopamine produced by the amphetamine analogue 3,4-methylenedioxymethamphetamine. J Neurochem. 1994;62:1382–1389. doi: 10.1046/j.1471-4159.1994.62041382.x. [DOI] [PubMed] [Google Scholar]
- Selken J, Nichols DE. Alpha1-adrenergic receptors mediate the locomotor response to systemic administration of (+/−)-3,4-methylenedioxymethamphetamine (MDMA) in rats. Pharmacol Biochem Behav. 2007;86:622–630. doi: 10.1016/j.pbb.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankaran M, Gudelsky GA. A neurotoxic regimen of MDMA suppresses behavioral, thermal and neurochemical responses to subsequent MDMA administration. Psychopharmacology (Berl) 1999;147:66–72. doi: 10.1007/s002130051143. [DOI] [PubMed] [Google Scholar]
- Sharp T, Zetterstrom T, Ljungberg T, Ungerstedt U. A direct comparison of amphetamine-induced behaviours and regional brain dopamine release in the rat using intracerebral dialysis. Brain Res. 1987;401:322–330. doi: 10.1016/0006-8993(87)91416-8. [DOI] [PubMed] [Google Scholar]
- Spanos LJ, Yamamoto BK. Acute and subchronic effects of methylenedioxymethamphetamine [(+/−)MDMA] on locomotion and serotonin syndrome behavior in the rat. Pharmacol Biochem Behav. 1989;32:835–840. doi: 10.1016/0091-3057(89)90044-0. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Vaccarino FJ, Amalric M, Koob GF. The neural substrates for the motor-activating properties of psychostimulants: a review of recent findings. Pharmacol Biochem Behav. 1986;25:233–248. doi: 10.1016/0091-3057(86)90261-3. [DOI] [PubMed] [Google Scholar]
- Tricklebank MD, Forler C, Fozard JR. The involvement of subtypes of the 5-HT1 receptor and of catecholaminergic systems in the behavioural response to 8- hydroxy-2-(di-n-propylamino)tetralin in the rat. Eur J Pharmacol. 1984;106:271–282. doi: 10.1016/0014-2999(84)90714-3. [DOI] [PubMed] [Google Scholar]
- Trigo JM, Renoir T, Lanfumey L, Hamon M, Lesch KP, Robledo P, Maldonado R. 3,4-methylenedioxymethamphetamine self-administration is abolished in serotonin transporter knockout mice. Biol Psychiatry. 2007;62:669–679. doi: 10.1016/j.biopsych.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. Functional relationship among medial prefrontal cortex, nucleus accumbens, and ventral tegmental area in locomotion and reward. Crit Rev Neurobiol. 2000;14:131–142. [PubMed] [Google Scholar]
- Ungerstedt U. Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiol Scand Suppl. 1971;367:1–48. doi: 10.1111/j.1365-201x.1971.tb10998.x. [DOI] [PubMed] [Google Scholar]
- Verrico CD, Miller GM, Madras BK. MDMA (Ecstasy) and human dopamine, norepinephrine, and serotonin transporters: implications for MDMA-induced neurotoxicity and treatment. Psychopharmacology (Berl) 2007;189:489–503. doi: 10.1007/s00213-005-0174-5. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Nash JF, Gudelsky GA. Modulation of methylenedioxymethamphetamine-induced striatal dopamine release by the interaction between serotonin and gamma-aminobutyric acid in the substantia nigra. J Pharmacol Exp Ther. 1995;273:1063–1070. [PubMed] [Google Scholar]
- Yurek DM, Hipkens SB, Hebert MA, Gash DM, Gerhardt GA. Age-related decline in striatal dopamine release and motoric function in brown Norway/Fischer 344 hybrid rats. Brain Res. 1998;791:246–256. doi: 10.1016/s0006-8993(98)00110-3. [DOI] [PubMed] [Google Scholar]


