Abstract
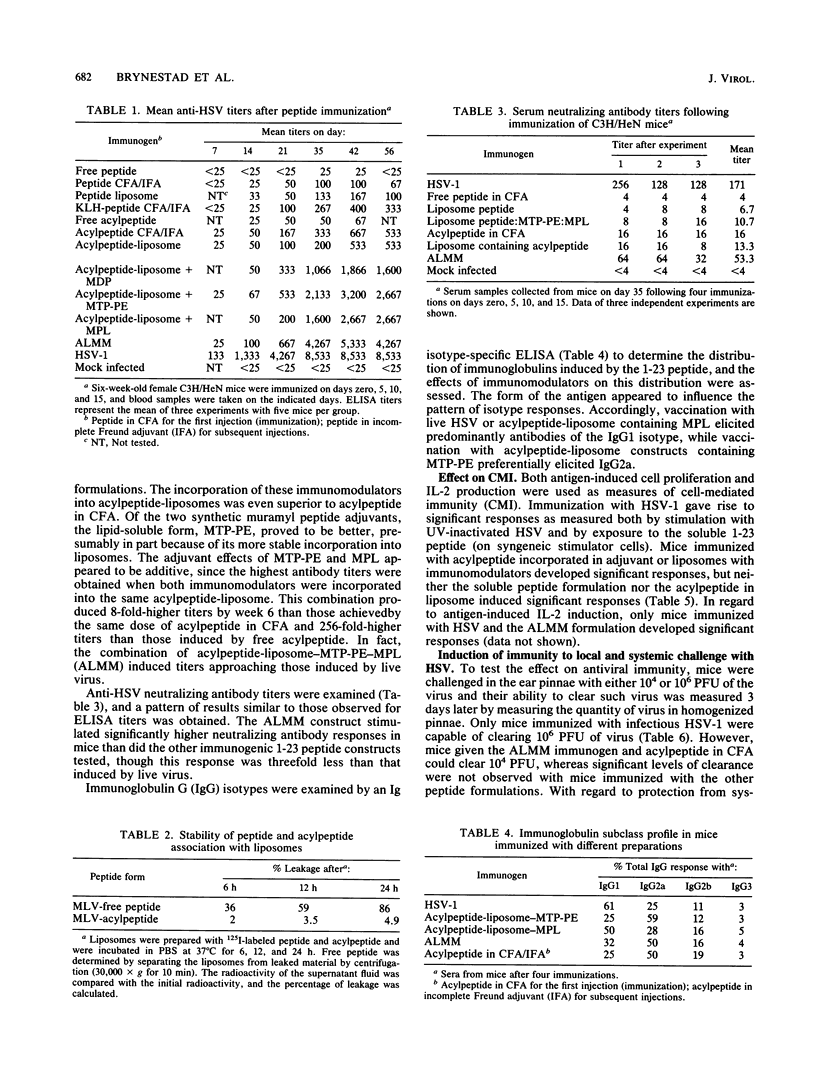
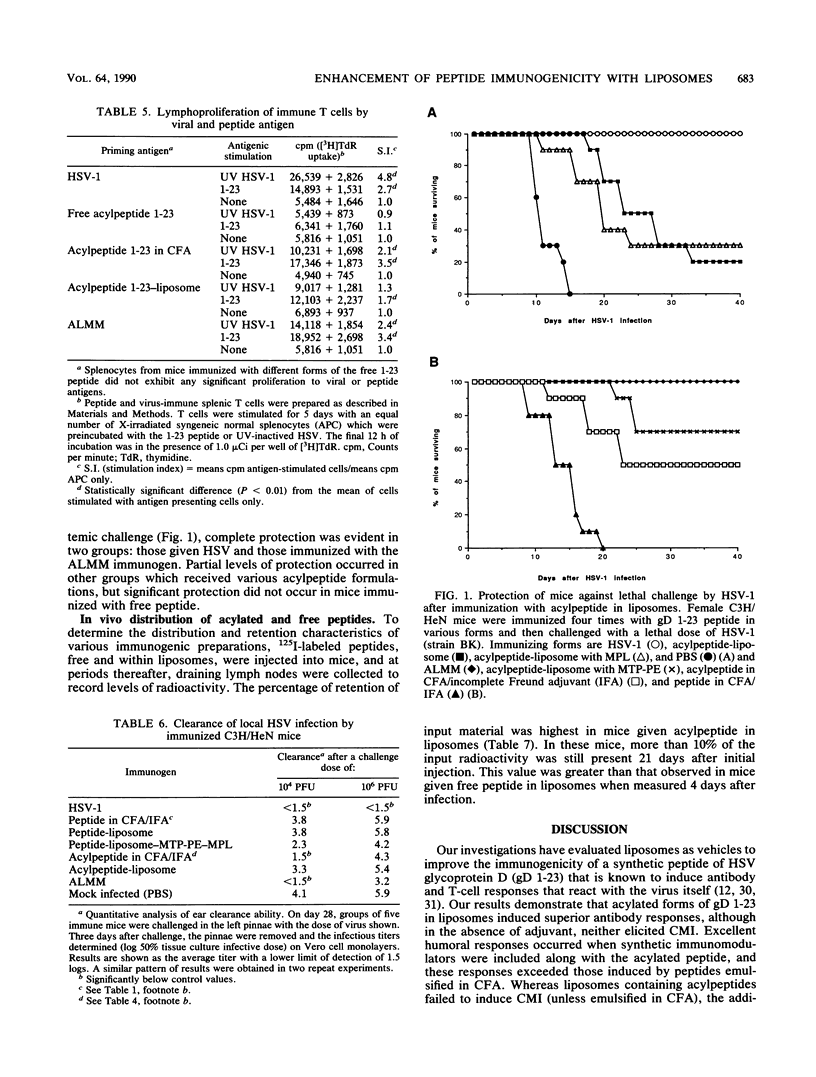
A peptide corresponding to residues 1 to 23 of glycoprotein D of herpes simplex virus type 1 was chemically synthesized and coupled to a fatty acid carrier by standard Merrifield synthesis procedures. The resulting peptide-palmitic acid conjugate (acylpeptide) exhibited enhanced immunogenicity in mice as compared with that exhibited by the free form of the peptide. Incorporation of the acylpeptide into liposomes further increased the immunogenicity of the peptide, while inclusion of the immunomodulators muramyl tripeptide phosphatidylethanolamine and monophosphoryl lipid A into the same liposome stimulated the strongest response. The humoral immune responses induced by the acylpeptide-liposome construct were greater than those induced by peptide in Freund complete adjuvant, and cellular responses were equal. The acylpeptide-immunomodulator-liposome formulation also induced significant levels of protective immunity, although the immunity was less than that induced by herpes simplex virus infection. Acylated peptides, especially in liposomes, were taken up more effectively by draining lymph nodes, which possibly accounts in part for the enhanced immunogenicity of the peptides. Since the acylpeptide-immunoliposome formulation used was nontoxic, it could represent a useful way to enhance immunogenicity of subunit peptides used for vaccine purpose in humans and animals.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Byars N. E. An adjuvant formulation that selectively elicits the formation of antibodies of protective isotypes and of cell-mediated immunity. J Immunol Methods. 1986 Dec 24;95(2):157–168. doi: 10.1016/0022-1759(86)90402-3. [DOI] [PubMed] [Google Scholar]
- Briand J. P., Muller S., Van Regenmortel M. H. Synthetic peptides as antigens: pitfalls of conjugation methods. J Immunol Methods. 1985 Apr 8;78(1):59–69. doi: 10.1016/0022-1759(85)90329-1. [DOI] [PubMed] [Google Scholar]
- Byars N. E., Allison A. C. Adjuvant formulation for use in vaccines to elicit both cell-mediated and humoral immunity. Vaccine. 1987 Sep;5(3):223–228. doi: 10.1016/0264-410x(87)90105-8. [DOI] [PubMed] [Google Scholar]
- Chedid L. A., Parant M. A., Audibert F. M., Riveau G. J., Parant F. J., Lederer E., Choay J. P., Lefrancier P. L. Biological activity of a new synthetic muramyl peptide adjuvant devoid of pyrogenicity. Infect Immun. 1982 Feb;35(2):417–424. doi: 10.1128/iai.35.2.417-424.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chedid L. Adjuvants of immunity. Ann Inst Pasteur Immunol. 1985 Nov-Dec;136D(3):283–291. doi: 10.1016/s0769-2625(85)80113-6. [DOI] [PubMed] [Google Scholar]
- Cohen G. H., Dietzschold B., Ponce de Leon M., Long D., Golub E., Varrichio A., Pereira L., Eisenberg R. J. Localization and synthesis of an antigenic determinant of herpes simplex virus glycoprotein D that stimulates the production of neutralizing antibody. J Virol. 1984 Jan;49(1):102–108. doi: 10.1128/jvi.49.1.102-108.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutelier J. P., van der Logt J. T., Heessen F. W., Warnier G., Van Snick J. IgG2a restriction of murine antibodies elicited by viral infections. J Exp Med. 1987 Jan 1;165(1):64–69. doi: 10.1084/jem.165.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFreitas E. C., Dietzschold B., Koprowski H. Human T-lymphocyte response in vitro to synthetic peptides of herpes simplex virus glycoprotein D. Proc Natl Acad Sci U S A. 1985 May;82(10):3425–3429. doi: 10.1073/pnas.82.10.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. J., Cerini C. P., Heilman C. J., Joseph A. D., Dietzschold B., Golub E., Long D., Ponce de Leon M., Cohen G. H. Synthetic glycoprotein D-related peptides protect mice against herpes simplex virus challenge. J Virol. 1985 Dec;56(3):1014–1017. doi: 10.1128/jvi.56.3.1014-1017.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. J., Long D., Pereira L., Hampar B., Zweig M., Cohen G. H. Effect of monoclonal antibodies on limited proteolysis of native glycoprotein gD of herpes simplex virus type 1. J Virol. 1982 Feb;41(2):478–488. doi: 10.1128/jvi.41.2.478-488.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogler W. E., Wade R., Brundish D. E., Fidler I. J. Distribution and fate of free and liposome-encapsulated [3H]nor-muramyl dipeptide and [3H]muramyl tripeptide phosphatidylethanolamine in mice. J Immunol. 1985 Aug;135(2):1372–1377. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Heber-Katz E., Dietzschold B. Immune response to synthetic herpes simplex virus peptides: the feasibility of a synthetic vaccine. Curr Top Microbiol Immunol. 1986;130:51–64. doi: 10.1007/978-3-642-71440-5_5. [DOI] [PubMed] [Google Scholar]
- Heber-Katz E., Hollosi M., Dietzschold B., Hudecz F., Fasman G. D. The T cell response to the glycoprotein D of the herpes simplex virus: the significance of antigen conformation. J Immunol. 1985 Aug;135(2):1385–1390. [PubMed] [Google Scholar]
- Hopp T. P. Immunogenicity of a synthetic HBsAg peptide: enhancement by conjugation to a fatty acid carrier. Mol Immunol. 1984 Jan;21(1):13–16. doi: 10.1016/0161-5890(84)90084-1. [DOI] [PubMed] [Google Scholar]
- Klaus G. G., Pepys M. B., Kitajima K., Askonas B. A. Activation of mouse complement by different classes of mouse antibody. Immunology. 1979 Dec;38(4):687–695. [PMC free article] [PubMed] [Google Scholar]
- Koff W. C., Showalter S. D., Hampar B., Fidler I. J. Protection of mice against fatal herpes simplex type 2 infection by liposomes containing muramyl tripeptide. Science. 1985 Apr 26;228(4698):495–497. doi: 10.1126/science.2984772. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lawman M. J., Courtney R. J., Eberle R., Schaffer P. A., O'Hara M. K., Rouse B. T. Cell-mediated immunity to herpes simplex virus: specificity of cytotoxic T cells. Infect Immun. 1980 Nov;30(2):451–461. doi: 10.1128/iai.30.2.451-461.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew F. Y. New aspects of vaccine development. Clin Exp Immunol. 1985 Nov;62(2):225–241. [PMC free article] [PubMed] [Google Scholar]
- Martin S., Rouse B. T. The mechanisms of antiviral immunity induced by a vaccinia virus recombinant expressing herpes simplex virus type 1 glycoprotein D: clearance of local infection. J Immunol. 1987 May 15;138(10):3431–3437. [PubMed] [Google Scholar]
- Nash A. A., Phelan J., Wildy P. Cell-mediated immunity in herpes simplex virus-infected mice: H-2 mapping of the delayed-type hypersensitivity response and the antiviral T cell response. J Immunol. 1981 Apr;126(4):1260–1262. [PubMed] [Google Scholar]
- Naylor P. T., Larsen H. S., Huang L., Rouse B. T. In vivo induction of anti-herpes simplex virus immune response by type 1 antigens and lipid A incorporated into liposomes. Infect Immun. 1982 Jun;36(3):1209–1216. doi: 10.1128/iai.36.3.1209-1216.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K., Qureshi N., Ribi E., Cantrell J. L. Separation and characterization of toxic and nontoxic forms of lipid A. Rev Infect Dis. 1984 Jul-Aug;6(4):439–443. doi: 10.1093/clinids/6.4.439. [DOI] [PubMed] [Google Scholar]
- Watari E., Dietzschold B., Szokan G., Heber-Katz E. A synthetic peptide induces long-term protection from lethal infection with herpes simplex virus 2. J Exp Med. 1987 Feb 1;165(2):459–470. doi: 10.1084/jem.165.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijer W. J., Drijfhout J. W., Geerligs H. J., Bloemhoff W., Feijlbrief M., Bos C. A., Hoogerhout P., Kerling K. E., Popken-Boer T., Slopsema K. Antibodies against synthetic peptides of herpes simplex virus type 1 glycoprotein D and their capability to neutralize viral infectivity in vitro. J Virol. 1988 Feb;62(2):501–510. doi: 10.1128/jvi.62.2.501-510.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyckoff J. H., 3rd, Osmand A. P., Eisenberg R. J., Cohen G. H., Rouse B. T. Functional T cell recognition of synthetic peptides corresponding to continuous antibody epitopes of herpes simplex virus type 1 glycoprotein D. Immunobiology. 1988 May;177(2):134–148. doi: 10.1016/S0171-2985(88)80034-2. [DOI] [PubMed] [Google Scholar]
- van Rooijen N., van Nieuwmegen R. Use of liposomes as biodegradable and harmless adjuvants. Methods Enzymol. 1983;93:83–95. doi: 10.1016/s0076-6879(83)93036-7. [DOI] [PubMed] [Google Scholar]



