Abstract
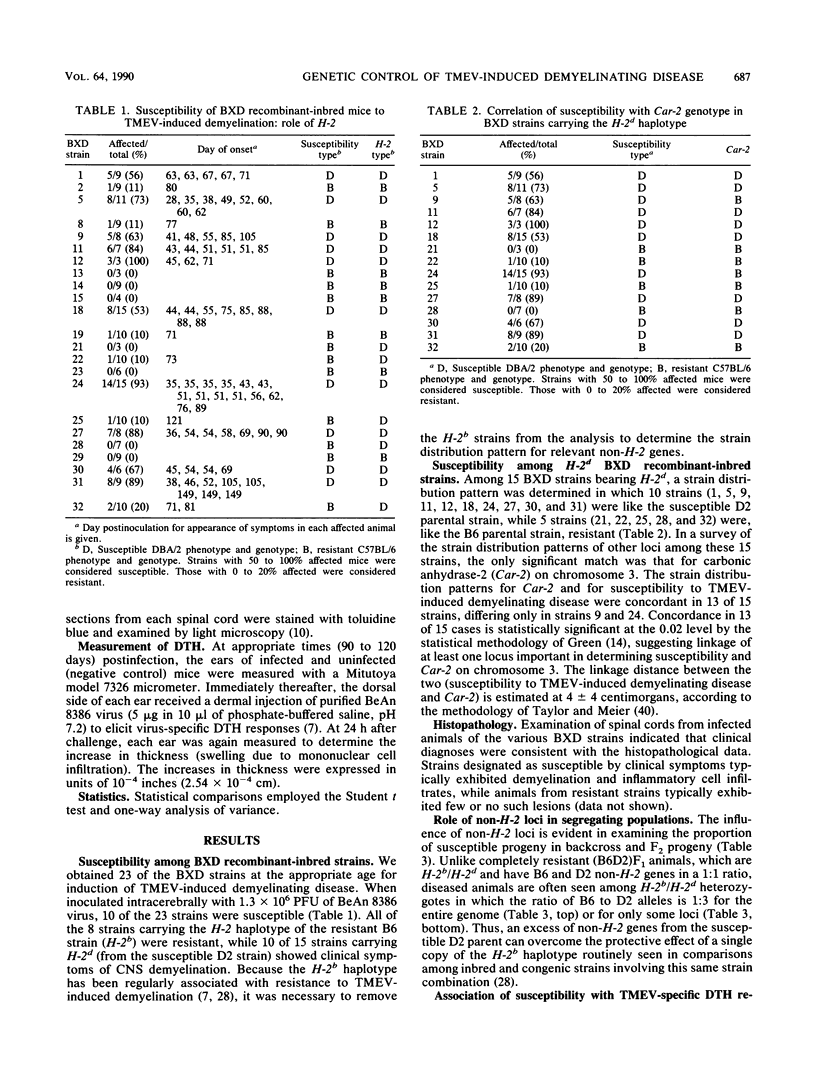
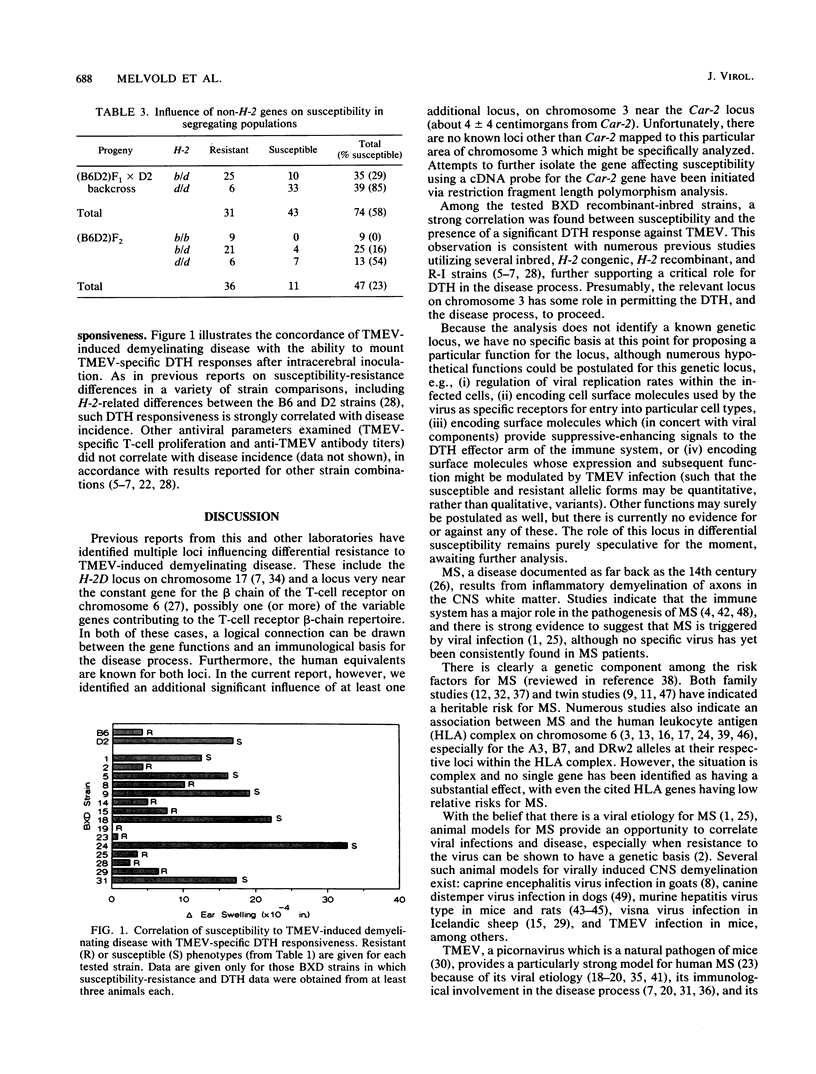
Theiler's virus-induced demyelinating disease results from a chronic infection in the white matter of the central nervous system and provides an excellent model for human multiple sclerosis. Like multiple sclerosis, there are genetic risk factors in disease development, including genes associated with the major histocompatibility complex and with those encoding the beta chain of the T-cell receptor. Comparisons of the susceptible DBA/2 and resistant C57BL/6 strains have indicated an important role for the H-2D locus and for a non-H-2 gene (not involving the beta chain of the T-cell receptor) in differential susceptibility. In the present report, analysis of recombinant-inbred strains (BXD) between the DBA/2 and C57BL/6 strains indicated that this non-H-2 locus is located at the centromeric end of chromosome 3 near (4 +/- 4 centimorgans) the carbonic anhydrase-2 (Car-2) enzyme locus.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnason B. G., Fuller T. C., Lehrich J. R., Wray S. H. Histocompatibility types and measles antibodies in multiple sclerosis and optic neuritis. J Neurol Sci. 1974 Aug;22(4):419–428. doi: 10.1016/0022-510x(74)90078-1. [DOI] [PubMed] [Google Scholar]
- Bang F. B. Genetics of resistance of animals to viruses: I. Introduction and studies in mice. Adv Virus Res. 1978;23:269–348. doi: 10.1016/S0065-3527(08)60102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor J. R., Compston A., McDonald W. I. The significance of the association between HLA and multiple sclerosis. Br Med Bull. 1978 Sep;34(3):279–284. doi: 10.1093/oxfordjournals.bmb.a071512. [DOI] [PubMed] [Google Scholar]
- Bloom B. R. Immunological changes in multiple sclerosis. Nature. 1980 Sep 25;287(5780):275–276. doi: 10.1038/287275a0. [DOI] [PubMed] [Google Scholar]
- Clatch R. J., Lipton H. L., Miller S. D. Characterization of Theiler's murine encephalomyelitis virus (TMEV)-specific delayed-type hypersensitivity responses in TMEV-induced demyelinating disease: correlation with clinical signs. J Immunol. 1986 Feb 1;136(3):920–927. [PubMed] [Google Scholar]
- Clatch R. J., Lipton H. L., Miller S. D. Class II-restricted T cell responses in Theiler's murine encephalomyelitis virus (TMEV)-induced demyelinating disease. II. Survey of host immune responses and central nervous system virus titers in inbred mouse strains. Microb Pathog. 1987 Nov;3(5):327–337. doi: 10.1016/0882-4010(87)90003-9. [DOI] [PubMed] [Google Scholar]
- Clatch R. J., Melvold R. W., Miller S. D., Lipton H. L. Theiler's murine encephalomyelitis virus (TMEV)-induced demyelinating disease in mice is influenced by the H-2D region: correlation with TEMV-specific delayed-type hypersensitivity. J Immunol. 1985 Aug;135(2):1408–1414. [PubMed] [Google Scholar]
- Cork L. C., Davis W. C. Ultrastructural features of viral leukoencephalomyelitis of goats. Lab Invest. 1975 Mar;32(3):359–365. [PubMed] [Google Scholar]
- Currier R. D., Eldridge R. Possible risk factors in multiple sclerosis as found in a national twin study. Arch Neurol. 1982 Mar;39(3):140–144. doi: 10.1001/archneur.1982.00510150010003. [DOI] [PubMed] [Google Scholar]
- Dal Canto M. C., Lipton H. L. Primary demyelination in Theiler's virus infection. An ultrastructural study. Lab Invest. 1975 Dec;33(6):626–637. [PubMed] [Google Scholar]
- Eldridge R., McFarland H., Sever J., Sadowsky D., Krebs H. Familial multiple sclerosis: clinical, histocompatibility, and viral serological studies. Ann Neurol. 1978 Jan;3(1):72–80. doi: 10.1002/ana.410030111. [DOI] [PubMed] [Google Scholar]
- Francis D. A., Batchelor J. R., McDonald W. I., Hern J. E., Downie A. W. Multiple sclerosis and HLA DQw1. Lancet. 1986 Jan 25;1(8474):211–211. doi: 10.1016/s0140-6736(86)90684-7. [DOI] [PubMed] [Google Scholar]
- Haase A. T. Pathogenesis of lentivirus infections. Nature. 1986 Jul 10;322(6075):130–136. doi: 10.1038/322130a0. [DOI] [PubMed] [Google Scholar]
- Jersild C., Fog T., Hansen G. S., Thomsen M., Svejgaard A., Dupont B. Histocompatibility determinants in multiple sclerosis, with special reference to clinical course. Lancet. 1973 Dec 1;2(7840):1221–1225. doi: 10.1016/s0140-6736(73)90970-7. [DOI] [PubMed] [Google Scholar]
- Lamoureux G., Duquette P., Lapierre Y., Cosgrove B., Bourret G., Labrie L. HLA antigens-linked genetic control in multiple sclerosis patients resistant and susceptible to infection. J Neurol. 1983;230(2):91–104. doi: 10.1007/BF00313636. [DOI] [PubMed] [Google Scholar]
- Lipton H. L., Dal Canto M. C. Chronic neurologic disease in Theiler's virus infection of SJL/J mice. J Neurol Sci. 1976 Nov;30(1):201–207. doi: 10.1016/0022-510x(76)90267-7. [DOI] [PubMed] [Google Scholar]
- Lipton H. L., Dal Canto M. C. Susceptibility of inbred mice to chronic central nervous system infection by Theiler's murine encephalomyelitis virus. Infect Immun. 1979 Oct;26(1):369–374. doi: 10.1128/iai.26.1.369-374.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton H. L., Dal Canto M. C. Theiler's virus-induced demyelination: prevention by immunosuppression. Science. 1976 Apr 2;192(4234):62–64. doi: 10.1126/science.176726. [DOI] [PubMed] [Google Scholar]
- Lipton H. L., Melvold R. Genetic analysis of susceptibility to Theiler's virus-induced demyelinating disease in mice. J Immunol. 1984 Apr;132(4):1821–1825. [PubMed] [Google Scholar]
- Lipton H. L. Theiler's virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect Immun. 1975 May;11(5):1147–1155. doi: 10.1128/iai.11.5.1147-1155.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlin D. E., McFarland H. F. Histocompatibility studies and multiple sclerosis. Arch Neurol. 1976 Jun;33(6):395–398. doi: 10.1001/archneur.1976.00500060001001. [DOI] [PubMed] [Google Scholar]
- McFarlin D. E., McFarland H. F. Multiple sclerosis (first of two parts). N Engl J Med. 1982 Nov 4;307(19):1183–1188. doi: 10.1056/NEJM198211043071905. [DOI] [PubMed] [Google Scholar]
- Medaer R. Does the history of multiple sclerosis go back as far as the 14th century? Acta Neurol Scand. 1979 Sep;60(3):189–192. doi: 10.1111/j.1600-0404.1979.tb02968.x. [DOI] [PubMed] [Google Scholar]
- Melvold R. W., Jokinen D. M., Knobler R. L., Lipton H. L. Variations in genetic control of susceptibility to Theiler's murine encephalomyelitis virus (TMEV)-induced demyelinating disease. I. Differences between susceptible SJL/J and resistant BALB/c strains map near the T cell beta-chain constant gene on chromosome 6. J Immunol. 1987 Mar 1;138(5):1429–1433. [PubMed] [Google Scholar]
- Multiple sclerosis in twins. N Engl J Med. 1987 Jul 2;317(1):50–52. doi: 10.1056/NEJM198707023170111. [DOI] [PubMed] [Google Scholar]
- Pevear D. C., Calenoff M., Rozhon E., Lipton H. L. Analysis of the complete nucleotide sequence of the picornavirus Theiler's murine encephalomyelitis virus indicates that it is closely related to cardioviruses. J Virol. 1987 May;61(5):1507–1516. doi: 10.1128/jvi.61.5.1507-1516.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pétursson G., Nathanson N., Georgsson G., Panitch H., Pálsson P. A. Pathogenesis of visna. I. Sequential virologic, serologic, and pathologic studies. Lab Invest. 1976 Oct;35(4):402–412. [PubMed] [Google Scholar]
- Rabinowitz S. G., Lipton H. L. Cellular immunity in chronic Theiler's virus central nervous system infection. J Immunol. 1976 Aug;117(2):357–363. [PubMed] [Google Scholar]
- Roberts D. F., Bates D. The genetic contribution to multiple sclerosis. Evidence from North-East England. J Neurol Sci. 1982 May;54(2):287–293. doi: 10.1016/0022-510x(82)90189-7. [DOI] [PubMed] [Google Scholar]
- Rodriguez M., David C. S. Demyelination induced by Theiler's virus: influence of the H-2 haplotype. J Immunol. 1985 Sep;135(3):2145–2148. [PubMed] [Google Scholar]
- Rodriguez M., Leibowitz J. L., Lampert P. W. Persistent infection of oligodendrocytes in Theiler's virus-induced encephalomyelitis. Ann Neurol. 1983 Apr;13(4):426–433. doi: 10.1002/ana.410130409. [DOI] [PubMed] [Google Scholar]
- Rodriguez M., Leibowitz J., David C. S. Susceptibility to Theiler's virus-induced demyelination. Mapping of the gene within the H-2D region. J Exp Med. 1986 Mar 1;163(3):620–631. doi: 10.1084/jem.163.3.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadovnick A. D., Macleod P. M. The familial nature of multiple sclerosis: empiric recurrence risks for first, second-, and third-degree relatives of patients. Neurology. 1981 Aug;31(8):1039–1041. doi: 10.1212/wnl.31.8.1039. [DOI] [PubMed] [Google Scholar]
- Spielman R. S., Nathanson N. The genetics of susceptibility to multiple sclerosis. Epidemiol Rev. 1982;4:45–65. doi: 10.1093/oxfordjournals.epirev.a036251. [DOI] [PubMed] [Google Scholar]
- Svejgaard A., Platz P., Ryder L. P. HLA and disease 1982--a survey. Immunol Rev. 1983;70:193–218. doi: 10.1111/j.1600-065x.1983.tb00715.x. [DOI] [PubMed] [Google Scholar]
- Taylor B. A., Meier H. Mapping the adrenal lipid depletion gene of the AKR/J mouse strain. Genet Res. 1975 Dec;26(3):307–312. doi: 10.1017/s0016672300016104. [DOI] [PubMed] [Google Scholar]
- Waksman B. Mechanisms in multiple sclerosis. Nature. 1985 Nov 14;318(6042):104–105. doi: 10.1038/318104a0. [DOI] [PubMed] [Google Scholar]
- Watanabe R., Wege H., ter Meulen V. Adoptive transfer of EAE-like lesions from rats with coronavirus-induced demyelinating encephalomyelitis. Nature. 1983 Sep 8;305(5930):150–153. doi: 10.1038/305150a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wege H., Watanabe R., ter Meulen V. Relapsing subacute demyelinating encephalomyelitis in rats during the course of coronavirus JHM infection. J Neuroimmunol. 1984 Aug;6(5):325–336. doi: 10.1016/0165-5728(84)90022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner L. P. Pathogenesis of demyelination induced by a mouse hepatitis. Arch Neurol. 1973 May;28(5):298–303. doi: 10.1001/archneur.1973.00490230034003. [DOI] [PubMed] [Google Scholar]
- Weitkamp L. R. Multiple sclerosis susceptibility. Interaction between sex and HLA. Arch Neurol. 1983 Jul;40(7):399–401. doi: 10.1001/archneur.1983.04050070029004. [DOI] [PubMed] [Google Scholar]
- Williams A., Eldridge R., McFarland H., Houff S., Krebs H., McFarlin D. Multiple sclerosis in twins. Neurology. 1980 Nov;30(11):1139–1147. doi: 10.1212/wnl.30.11.1139. [DOI] [PubMed] [Google Scholar]
- Wisniewski H. M., Bloom B. R. Primary demyelination as a nonspecific consequence of a cell-mediated immune reaction. J Exp Med. 1975 Feb 1;141(2):346–359. doi: 10.1084/jem.141.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiśniewski H., Raine C. S., Kay W. J. Observations on viral demyelinating encephalomyelitis. Canine distemper. Lab Invest. 1972 May;26(5):589–599. [PubMed] [Google Scholar]



