Abstract
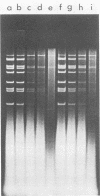
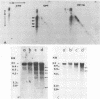
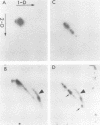
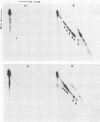
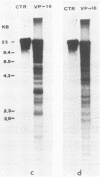
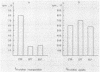
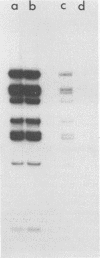
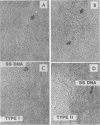
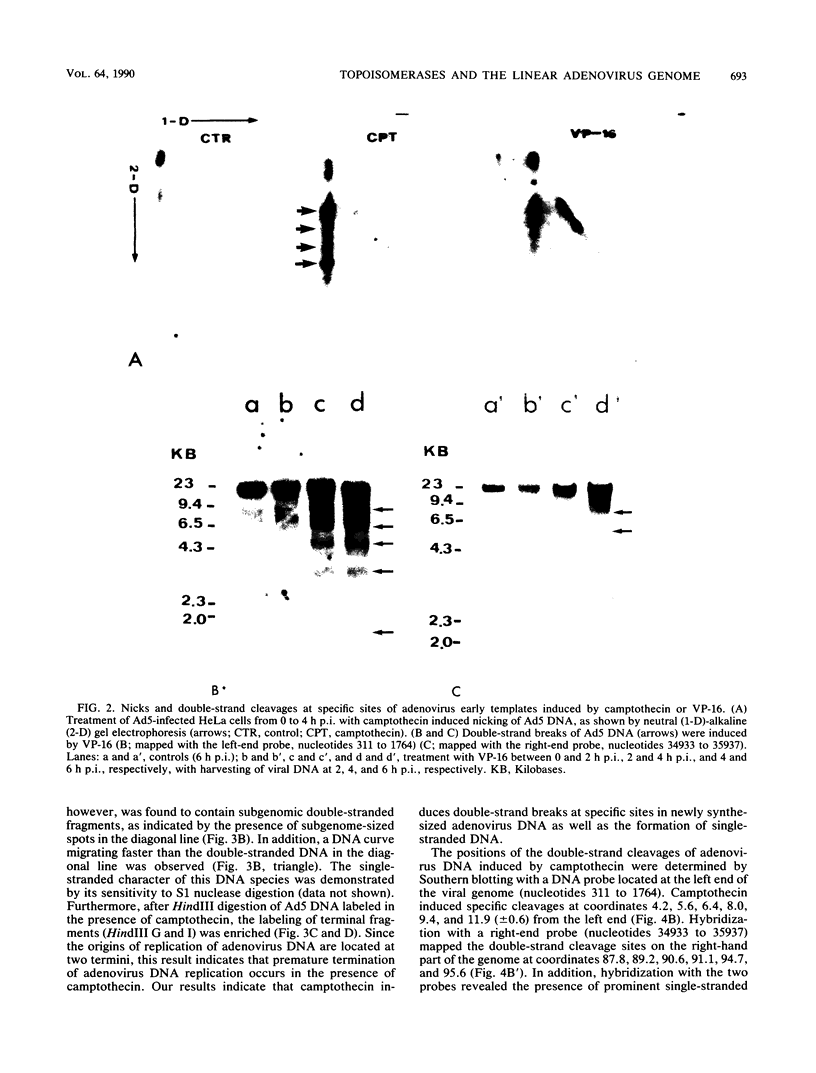
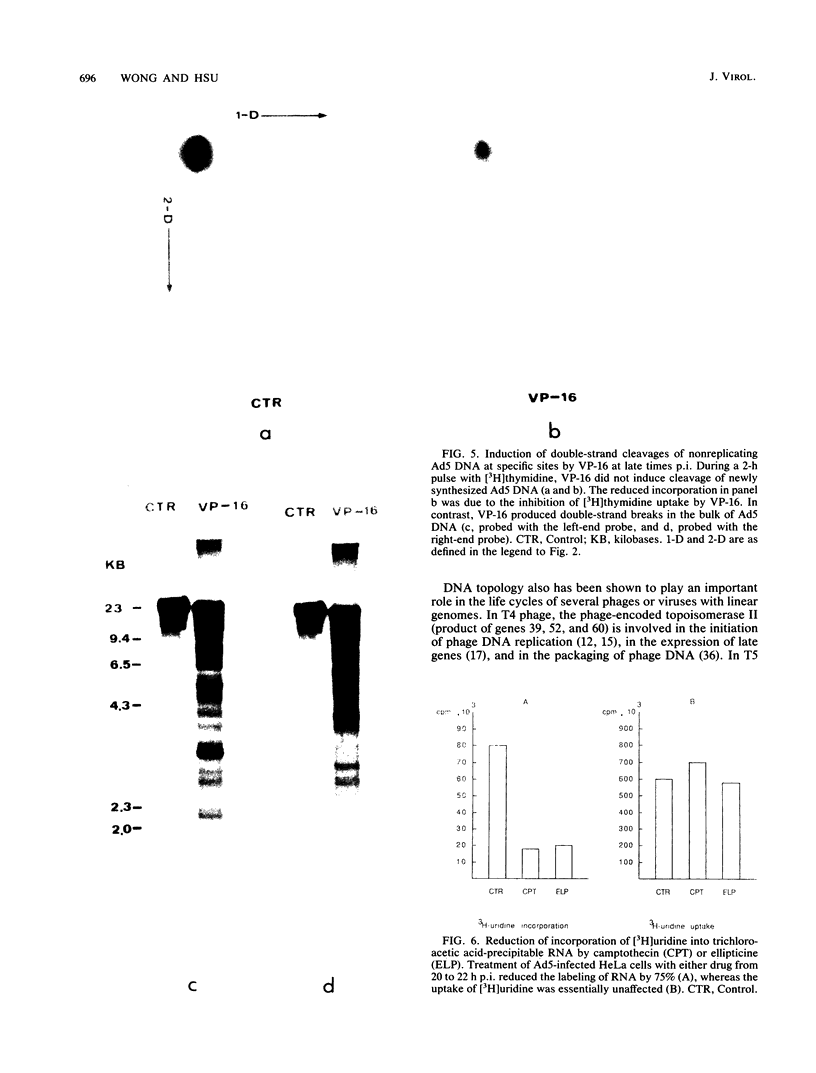
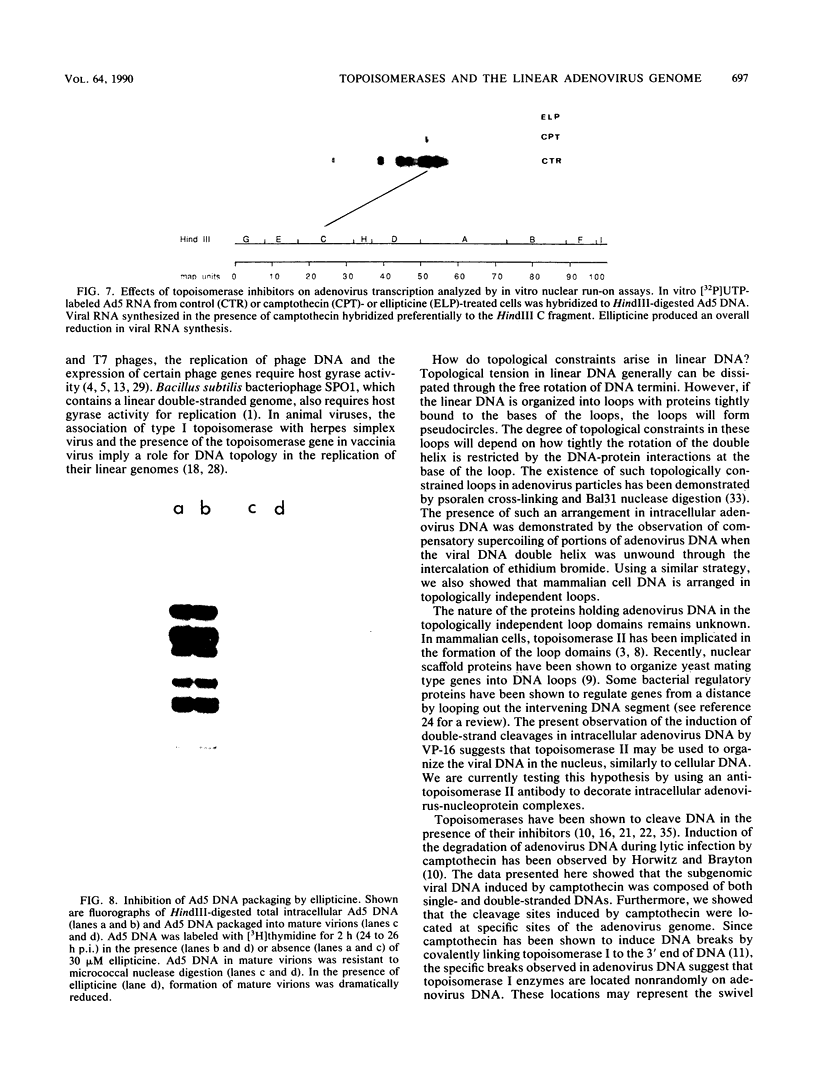
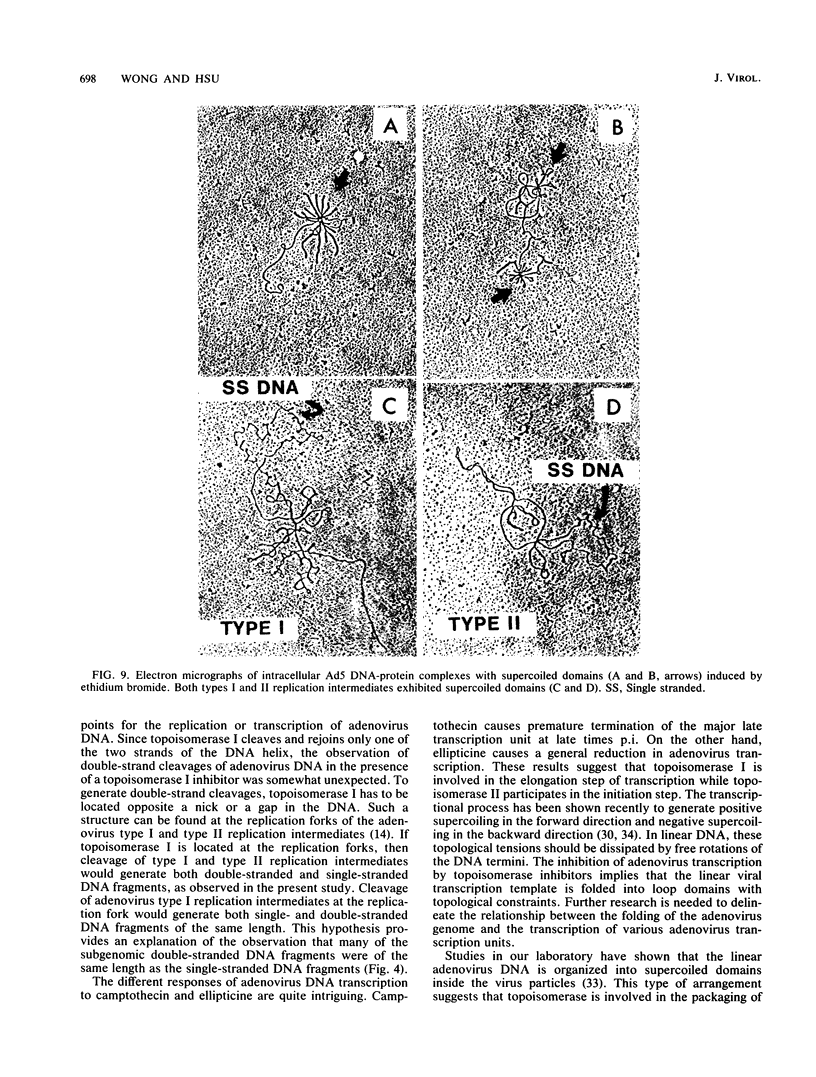
The role of topoisomerases in the replication of human adenovirus type 5 was investigated with topoisomerase inhibitors. Both topoisomerase I and topoisomerase II inhibitors blocked adenovirus replication when added at the time of infection. Both types of inhibitors induced strand cleavages at specific sites in the adenovirus early templates. The cleavage sites were mapped near the 5' and 3' ends of the genes transcribed early during infection. At late times after infection, camptothecin, a topoisomerase I inhibitor, inhibited adenovirus DNA replication and induced the formation of single- and double-stranded fragments with breakpoints located at defined regions of the viral genome. The topoisomerase II inhibitors, VP-16 (etoposide) and ellipticine, did not block adenovirus DNA replication and did not induce an appreciable amount of double-strand cleavages in the newly synthesized DNA. On the other hand, VP-16 promoted double-strand cleavages at specific sites of nonreplicating adenovirus DNA. The packaging of adenovirus DNA into virus particles, which contain supercoiled adenovirus DNA (M.-L. Wong and M.-T. Hsu, Nucleic Acids Res. 17:3535-3550, 1989), was inhibited by the topoisomerase II inhibitors. Transcription of adenovirus major late genes was inhibited by both topoisomerase I and topoisomerase II inhibitors. In addition, camptothecin caused a premature termination of major late transcription. Electron microscopic analysis showed that adenovirus templates late after infection were arranged in topologically constrained loop domains. Together, these data provide evidence for the requirement of topoisomerase activities in the replication, transcription, and packaging of the linear adenovirus genome.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso J. C., Sarachu A. N., Grau O. DNA gyrase inhibitors block development of Bacillus subtilis bacteriophage SP01. J Virol. 1981 Sep;39(3):855–860. doi: 10.1128/jvi.39.3.855-860.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson J. D., Huang E. S. Two specific topoisomerase II inhibitors prevent replication of human cytomegalovirus DNA: an implied role in replication of the viral genome. J Virol. 1988 Dec;62(12):4797–4800. doi: 10.1128/jvi.62.12.4797-4800.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockerill P. N., Garrard W. T. Chromosomal loop anchorage of the kappa immunoglobulin gene occurs next to the enhancer in a region containing topoisomerase II sites. Cell. 1986 Jan 31;44(2):273–282. doi: 10.1016/0092-8674(86)90761-0. [DOI] [PubMed] [Google Scholar]
- Constantinou A., Voelkel-Meiman K., Sternglanz R., McCorquodale M. M., McCorquodale D. J. Involvement of host DNA gyrase in growth of bacteriophage T5. J Virol. 1986 Mar;57(3):875–882. doi: 10.1128/jvi.57.3.875-882.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wyngaert M., Hinkle D. C. Involvement of DNA gyrase in replication and transcription of bacteriophage T7 DNA. J Virol. 1979 Feb;29(2):529–535. doi: 10.1128/jvi.29.2.529-535.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drlica K. Biology of bacterial deoxyribonucleic acid topoisomerases. Microbiol Rev. 1984 Dec;48(4):273–289. doi: 10.1128/mr.48.4.273-289.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drlica K., Franco R. J. Inhibitors of DNA topoisomerases. Biochemistry. 1988 Apr 5;27(7):2253–2259. doi: 10.1021/bi00407a001. [DOI] [PubMed] [Google Scholar]
- Gasser S. M., Laemmli U. K. The organisation of chromatin loops: characterization of a scaffold attachment site. EMBO J. 1986 Mar;5(3):511–518. doi: 10.1002/j.1460-2075.1986.tb04240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann J. F., Laroche T., Brand A. H., Gasser S. M. RAP-1 factor is necessary for DNA loop formation in vitro at the silent mating type locus HML. Cell. 1989 Jun 2;57(5):725–737. doi: 10.1016/0092-8674(89)90788-5. [DOI] [PubMed] [Google Scholar]
- Horwitz M. S., Brayton C. Camptothecin: mechanism of inhibition of adenovirus formation. Virology. 1972 Jun;48(3):690–698. doi: 10.1016/0042-6822(72)90153-5. [DOI] [PubMed] [Google Scholar]
- Hsiang Y. H., Hertzberg R., Hecht S., Liu L. F. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem. 1985 Nov 25;260(27):14873–14878. [PubMed] [Google Scholar]
- Huang W. M. Nucleotide sequence of a type II DNA topoisomerase gene. Bacteriophage T4 gene 39. Nucleic Acids Res. 1986 Oct 10;14(19):7751–7765. doi: 10.1093/nar/14.19.7751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Tomizawa J. I. Involvement of DNA gyrase in bacteriophage T7 DNA replication. Nature. 1977 Nov 3;270(5632):78–80. doi: 10.1038/270078a0. [DOI] [PubMed] [Google Scholar]
- Lechner R. L., Kelly T. J., Jr The structure of replicating adenovirus 2 DNA molecules. Cell. 1977 Dec;12(4):1007–1020. doi: 10.1016/0092-8674(77)90165-9. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Liu C. C., Alberts B. M. T4 DNA topoisomerase: a new ATP-dependent enzyme essential for initiation of T4 bacteriophage DNA replication. Nature. 1979 Oct 11;281(5731):456–461. doi: 10.1038/281456a0. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Rowe T. C., Yang L., Tewey K. M., Chen G. L. Cleavage of DNA by mammalian DNA topoisomerase II. J Biol Chem. 1983 Dec 25;258(24):15365–15370. [PubMed] [Google Scholar]
- Muller M. T., Bolles C. S., Parris D. S. Association of type I DNA topoisomerase with herpes simplex virus. J Gen Virol. 1985 Jul;66(Pt 7):1565–1574. doi: 10.1099/0022-1317-66-7-1565. [DOI] [PubMed] [Google Scholar]
- Nagata K., Guggenheimer R. A., Hurwitz J. Adenovirus DNA replication in vitro: synthesis of full-length DNA with purified proteins. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4266–4270. doi: 10.1073/pnas.80.14.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poddar S. K., Bauer W. R. Type I topoisomerase activity after infection of enucleated, synchronized mouse L cells by vaccinia virus. J Virol. 1986 Feb;57(2):433–437. doi: 10.1128/jvi.57.2.433-437.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier Y., Schwartz R. E., Zwelling L. A., Kohn K. W. Effects of DNA intercalating agents on topoisomerase II induced DNA strand cleavage in isolated mammalian cell nuclei. Biochemistry. 1985 Nov 5;24(23):6406–6410. doi: 10.1021/bi00344a014. [DOI] [PubMed] [Google Scholar]
- Porter S. E., Champoux J. J. Mapping in vivo topoisomerase I sites on simian virus 40 DNA: asymmetric distribution of sites on replicating molecules. Mol Cell Biol. 1989 Feb;9(2):541–550. doi: 10.1128/mcb.9.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss G. J., Drlica K. DNA supercoiling and prokaryotic transcription. Cell. 1989 Feb 24;56(4):521–523. doi: 10.1016/0092-8674(89)90574-6. [DOI] [PubMed] [Google Scholar]
- Ptashne M. Gene regulation by proteins acting nearby and at a distance. Nature. 1986 Aug 21;322(6081):697–701. doi: 10.1038/322697a0. [DOI] [PubMed] [Google Scholar]
- Riou J. F., Gabillot M., Philippe M., Schrevel J., Riou G. Purification and characterization of Plasmodium berghei DNA topoisomerases I and II: drug action, inhibition of decatenation and relaxation, and stimulation of DNA cleavage. Biochemistry. 1986 Apr 8;25(7):1471–1479. doi: 10.1021/bi00355a001. [DOI] [PubMed] [Google Scholar]
- Ross W. E., Glaubiger D., Kohn K. W. Qualitative and quantitative aspects of intercalator-induced DNA strand breaks. Biochim Biophys Acta. 1979 Mar 28;562(1):41–50. doi: 10.1016/0005-2787(79)90124-2. [DOI] [PubMed] [Google Scholar]
- Schaack J., Schedl P., Shenk T. Topoisomerase I and II cleavage of adenovirus DNA in vivo: both topoisomerase activities appear to be required for adenovirus DNA replication. J Virol. 1990 Jan;64(1):78–85. doi: 10.1128/jvi.64.1.78-85.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman S., Moss B. Identification of a vaccinia virus gene encoding a type I DNA topoisomerase. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7478–7482. doi: 10.1073/pnas.84.21.7478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck T. R., Drlica K. Involvement of DNA gyrase in bacteriophage T7 growth. J Virol. 1985 Jan;53(1):296–298. doi: 10.1128/jvi.53.1.296-298.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao Y. P., Wu H. Y., Liu L. F. Transcription-driven supercoiling of DNA: direct biochemical evidence from in vitro studies. Cell. 1989 Jan 13;56(1):111–118. doi: 10.1016/0092-8674(89)90989-6. [DOI] [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]
- Wong M. L., Hsu M. T. Linear adenovirus DNA is organized into supercoiled domains in virus particles. Nucleic Acids Res. 1989 May 11;17(9):3535–3550. doi: 10.1093/nar/17.9.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M. L., Hsu M. T. Psoralen-cross-linking study of the organization of intracellular adenovirus nucleoprotein complexes. J Virol. 1988 Apr;62(4):1227–1234. doi: 10.1128/jvi.62.4.1227-1234.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. Y., Shyy S. H., Wang J. C., Liu L. F. Transcription generates positively and negatively supercoiled domains in the template. Cell. 1988 May 6;53(3):433–440. doi: 10.1016/0092-8674(88)90163-8. [DOI] [PubMed] [Google Scholar]
- Yang L., Rowe T. C., Nelson E. M., Liu L. F. In vivo mapping of DNA topoisomerase II-specific cleavage sites on SV40 chromatin. Cell. 1985 May;41(1):127–132. doi: 10.1016/0092-8674(85)90067-4. [DOI] [PubMed] [Google Scholar]
- Zachary A., Black L. W. Topoisomerase II and other DNA-delay and DNA-arrest mutations impair bacteriophage T4 DNA packaging in vivo and in vitro. J Virol. 1986 Oct;60(1):97–104. doi: 10.1128/jvi.60.1.97-104.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]