Abstract
Background
Plasmodium falciparum infection causes cerebral malaria (CM) in a subset of patients with anti-malarial treatment protecting only about 70% to 80% of patients. Why a subset of malaria patients develops CM complications, including neurological sequelae or death, is still not well understood. It is believed that host immune factors may modulate CM outcomes and there is substantial evidence that cellular immune factors, such as cytokines, play an important role in this process. In this study, the potential relationship between the antibody responses to the merozoite surface protein (MSP)-1 complex (which consists of four fragments namely: MSP-183, MSP-130, MSP-138 and MSP-142), MSP-636 and MSP-722 and CM was investigated.
Methods
Peripheral blood antibody responses to recombinant antigens of the two major allelic forms of MSP-1 complex, MSP-636 and MSP-722 were compared between healthy subjects, mild malaria patients (MM) and CM patients residing in a malaria endemic region of central India. Total IgG and IgG subclass antibody responses were determined using ELISA method.
Results
The prevalence and levels of IgG and its subclasses in the plasma varied for each antigen. In general, the prevalence of total IgG, IgG1 and IgG3 was higher in the MM patients and lower in CM patients compared to healthy controls. Significantly lower levels of total IgG antibodies to the MSP-1f38, IgG1 levels to MSP-1d83, MSP-119 and MSP-636 and IgG3 levels to MSP-1f42 and MSP-722 were observed in CM patients as compared to MM patients.
Conclusion
These results suggest that there may be some dysregulation in the generation of antibody responses to some MSP antigens in CM patients and it is worth investigating further whether perturbations of antibody responses in CM patients contribute to pathogenesis.
Background
One life-threatening complication of Plasmodium falciparum infection is cerebral malaria (CM). This complex syndrome affects mainly young children (two to six years old) in sub-Saharan Africa with an estimated incidence of 1.12 cases per 1,000 children per year and an estimated mortality of 18.6% [1]. In addition, a subset of CM survivors have an increased risk of developing persistent neurocognitive sequelae post-recovery [2-4] and reviewed in [5]. In Asia and South America, where the intensity of P. falciparum is much lower than in Africa, all age groups are at risk for CM [1,6-9]. The pathogenesis of CM is complex and it is still poorly understood as to why only a subset of patients develop CM. Various factors, such as sequestration of infected erythrocytes, and inflammatory cytokines and chemokines, have been postulated to play major roles in CM pathogenesis [10-17]. The role of antibodies in CM pathogenesis or protection is not well understood.
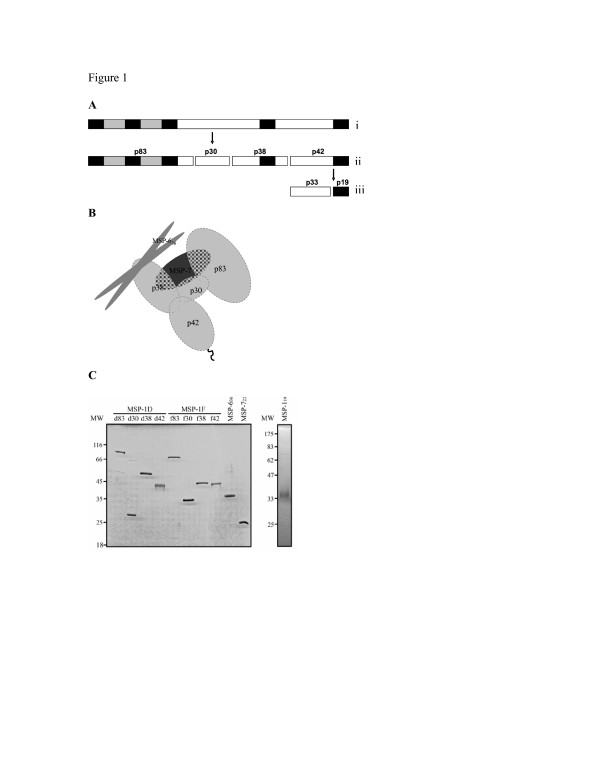
The merozoite surface protein (MSP)-1, a large multiprotein complex exposed on the surface of merozoites, is one of the well characterized antigens of P. falciparum. During late schizogony, MSP-1 is proteolytically processed from its ~190 kDa precursor into four major cleavage products: p83, p30, p38, and p42 [18] designated as MSP-183, MSP-130, MSP-138 and MSP-142 respectively. During erythrocyte invasion, the MSP-142 fragment is further cleaved into MSP-133 and MSP-119 which is essential for invasion (Figure 1A) [19]. The proteolytically processed MSP-1 appears to exist in association with the processed products of MSP-6 and MSP-7 (Figure 1B) [20-22]. Major biochemical and immunological parameters of this multipartite have been described recently [23].
Figure 1.
Schematic representation of the MSP-1 and MSP-1/MSP-6/MSP-7 complex antigens used in the study. The schematic representation of the MSP-1 protein is shown (A). The MSP-1 precursor protein (i) undergoes proteolytic cleavage into four subunits as shown (ii). The MSP-142 molecule is further cleaved to MSP-133 and MSP-119 (iii). A proposed model (adapted from [23]) demonstrating the interactions of MSP-1 protein with the MSP-636 and MSP-722 molecules (B). The two allelic forms of MSP-183, MSP-130, MSP-138 and MSP-142 (D and F), in addition to MSP-636, MSP-722 and MSP-119, were expressed in E. coli and purified [49,50]. The purity of these recombinant proteins was examined by using 12% SDS-PAGE followed by Coomassie staining (C). Molecular weight (MW) is shown in kDa.
Humoral immune responses to MSP-1 protein subunits, especially, MSP-142 and MSP-119 fragments, are known to be protective against P. falciparum infection and clinical malaria [24-33]. Antibodies specific for these antigens have been shown to inhibit both erythrocyte invasion and parasite growth in vitro [26,27]. In some studies, antibody responses to MSP-119 were correlated with clinical immunity to P. falciparum [29,30,34] and with reduced parasitaemia and fever [31]. In addition, presence of several T-cell epitopes within the MSP-142 fragment were identified [35] and these epitopes may provide T- helper function needed for the production of anti-MSP-1 antibodies.
Studies of the msp-1 gene sequence obtained from different P. falciparum isolates demonstrate significant antigenic diversity comprising highly conserved, dimorphic and variable regions. There are two major allelic forms of MSP-1 belonging to either the K1, (as in the FCB-1 strain, here referred to as F allelic form) or the MAD20 (as in the 3D7 strain, here referred to as D allelic form) allelic families [36,37]. Therefore, one would postulate that naturally exposed individuals would mount immune responses to different fragments and allelic forms of MSP-1. However, only a few field studies have investigated humoral responses to both of these allelic forms of the four major subunits of MSP-1 and their associated proteins, MSP-636 and MSP-722[25,38-40] in humans naturally exposed to malaria. A complete characterization of humoral immune responses to this complex protein is therefore required to determine their role in protective immunity and pathogenesis.
The potential adverse effect of malarial antibody responses, including antibodies to the C-terminal region of MSP-1, in the manifestation of CM have been implicated in some studies [41-45]. For example, higher levels of IgM and IgG antibodies to glycosylphosphatidylinositols (GPI) were associated with CM and death in young children in a study conducted in Mali [45]. On the contrary, in another study, reduced anti-GPI antibodies were found in CM patients compared to mild malaria patients [41]. In a Ghanaian study, IgG2 and IgG4 antibody responses to a recombinant P. falciparum RIFIN antigen, RIF-29, were exclusively shown to be associated with CM, suggesting that these antibodies might be involved in CM pathogenesis [44].
In the current study, the antibody response to the two major allelic forms (F and D) of MSP-1 antigens (MSP-183, MSP-130, MSP-138 and MSP-142) and their associated proteins, MSP-636 and MSP-722, was systematically characterized in an Indian cohort and the responses compared between two different group of malaria patients and healthy controls.
Materials and methods
Study area
The blood samples for this study came from a subset of 121 subjects enrolled in a cohort study designed to assess the severe outcomes associated with CM in the Jabalpur Province of Madhya Pradesh state in India. The study sites included Nethaji Subash Chandra Bose (NSCB) Hospital (a referral hospital for the region) in Jabalpur and Civic Hospital (primary hospital) in Maihar, Satna District. This study was approved by the ethical committees of Morehouse School of Medicine, National Institute of Malaria Research in India, Centers for Disease Control and Prevention (CDC) and the National Institute of Health (NIH) in the USA. Jabalpur province is a malaria endemic region with seasonal transmission of malaria accounting for about 23% of all the malaria cases in Madhya Pradesh state [46]. Previous community based studies conducted in this area revealed that malaria affects all age groups with the highest prevalence occurring in children between 8–14 years of age [47].
Study details
Three categories of subjects, namely cerebral malaria (CM) patients, mild malaria (MM) patients and healthy control (HC) subjects were enrolled in the study after the informed consent was obtained. Patients from all ages and both sexes who met the study criteria were enrolled. The presence of P. falciparum parasitaemia was determined by microscopic examination of thin and thick smears. Clinical histories and information were collected for each patient from physician's records and study questionnaires. The following criteria to enroll the subjects into the different clinical groups were used:
Cerebral malaria (CM)
CM was defined as unrousable coma (a non-purposeful response or no response to a painful stimulus) with microscopically diagnosed P. falciparum and have no other clinically evident cause of impaired consciousness such as hypoglycemia, meningitis, and encephalitis following WHO criteria [48]. Patients with mixed infection of malaria parasites were excluded.
Mild malaria (MM)
Patients who had fever with P. falciparum parasitaemia of < 25,000 parasites/μl of blood with no evidence of impaired consciousness or seizures at the time of enrollment with no other past history of mental illness, meningitis or accidental head injury were included.
Healthy control (HC)
HC were recruited from relatives of the patients or persons residing in the same study area who did not have malaria infection (as determined by microscopy) or any other febrile illness at the time of enrollment.
Blood collection
Venous blood samples were collected from children (5 ml) and adults (10 ml) into Becton-Dickinson cell preparation tubes (catalogue #362753, BD Pharmingen, Franklin Lakes, NJ, USA). The blood was centrifuged for 20 minutes at 1500 relative centrifugal force (rcf) to separate plasma. Plasma samples were aliquoted and frozen immediately in liquid nitrogen or at 80°C until use.
Plasmodium falciparum antigens
Relatively pure recombinant antigens of the major processing products of MSP-1 [MSP-183, MSP-130, MSP-138 and MSP-142 belonging to both the K1 allelic form (as in the FCB-1 strain, here referred to as F), and the MAD20 allelic form (as in the 3D7 strain, here referred to as D)] together with MSP-119 (3D7), MSP-636 (3D7) and MSP-722 (3D7) were used. These antigens were prepared as described previously [23,49,50] and the purity of the products that were used in this study are shown in Figure 1C.
Enzyme-linked immunosorbent assay (ELISA)
Microtiter Immulon-2 plates were coated overnight with the MSP complex antigens (500 ng/ml) in phosphate buffered solution at 4°C. Total IgG antibodies were measured using a HRP-conjugated mouse anti-human IgG antibody at 1:8000 dilution (Southern Biotech, Birmingham, AL). IgG subclasses were measured using mouse anti-human IgG subclass antibodies IgG1 (HP6069) at 1:2000, IgG2 (HP6002) at 1:6000, IgG3 (HP6047) at 1:50,000 and IgG4 (HP6023) at 1:20,000 dilution followed by goat anti-mouse IgG-HRP at 1:2000 dilution (Southern Biotech, Birmingham, AL). Samples were tested at a 1:400 dilution and 1:100 dilution for total IgG and subclasses respectively. Ten pooled plasma samples from malaria naïve donors from USA were selected and used as negative controls in each experiment. In addition, each ELISA plate contained a duplicate of blank wells with no plasma sample and the mean background OD value obtained from these wells was subtracted from all other experimental wells. Test samples were considered positive when the OD values were higher than the mean OD plus two standard deviations of pooled negative control sera. The percentage prevalence was calculated as: (total number of positive sera/total number of sera tested) × 100.
Statistical analysis
ELISA absorbance values (measured as optical density, OD) were compared and analysed by a non-parametric test (Kruskal-Wallis test). Differences in the percentage prevalence of the antibodies were calculated using a multiple comparison analysis controlling for age and parasitaemia. Antibody levels for the different subgroups of MSP-1 antigens (D and F) were assessed for correlation by Spearman's rank correlation. All statistical tests were performed using STATA™ 8.2 (College Station, TX, USA) and SAS (9.1, NC, USA).
Results
Study samples
The characteristics of subjects who participated in this study are summarized in Table 1. Among the 121 samples tested for total IgG, 16% were from patients in the HC category, 38% from MM and 46% from CM. Of the 120 samples tested for the subclasses, 15% were from HC, 41% from MM and 44% from CM (Table 1).
Table 1.
Characteristics of the study participants
| HC | MM | CMa | |
| Total No. of samples for IgG | 19 | 46 | 56 |
| Total No. of samples for subclasses | 18 | 49 | 53 |
| No. of males used for total IgG | 5 | 20 | 18 |
| No. of males used for subclasses | 4 | 28 | 36 |
| No. of females used for total IgG | 14 | 26 | 38 |
| No. of females used for subclasses | 14 | 21 | 17 |
| Median age for samples used for total IgG (yr) | 30.0 | 18.5 | 22.0 |
| Median age for samples used for subclasses (Yr) | 27.0 | 19.0 | 22.0 |
| Median parasitaemia (parasite/μl) of samples used for total IgG | N/A | 1,507 | 960 |
| Median parasitaemia (parasite/μl) of samples used for subclasses | N/A | 1,413 | 987 |
The demographic and parasitological characteristics of the study participants are shown. A total of 121 samples were used for the total IgG assay and 120 for the IgG subclasses assay. HC = healthy controls, MM = mild malaria and CM = cerebral malaria. Seven patients in the CM group died of CM. Both age and parasitaemia data are represented as median. a CM patients include seven patients who died of CM
Prevalence and levels of total IgG antibodies to various antigens of MSP-1 complex
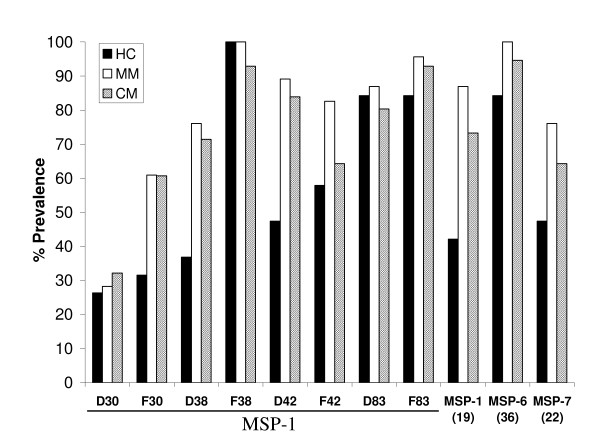
As shown in Figure 2, total IgG antibody prevalence to the different antigens varied greatly. Overall, the highest prevalence of IgG response was seen in the MM group followed by the CM group and the HC group, the latter showing the lowest prevalence with a few exceptions. However, these differences were significantly different between HC and MM groups only for MSP-1d38 (P < 0.006), MSP-1d42 (P < 0.003) and MSP-119 (P < 0.01) antigens. MSP-1d30 and MSP-1f30 showed the lowest responses while the MSP-1f38, MSP-1d83, MSP-1f83 and MSP-636 antigens showed the highest responses. The F allelic forms of MSP-130 and MSP-138 demonstrated higher responses compared to the D allelic forms.
Figure 2.
Prevalence of anti-MSP total IgG for the different subunits. The total IgG antibody response to the various MSP-1 subunits, MSP-636 and MSP-722 was measured by ELISA. The percentage prevalence was calculated as follows; (total number of positive sera/total number of sera tested) × 100. The prevalence of total IgG antibodies was statistically different between HC and MM for the indicated antigens (* = P < 0.05).
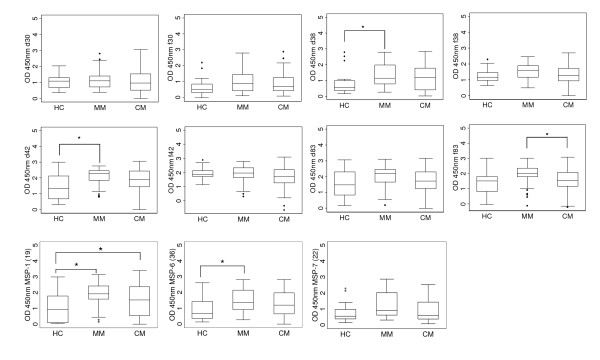
The mean antibody levels reported in Figure 3 illustrate important differences in the IgG antibody levels between the three groups. Significantly higher levels of antibody responses in the MM group compared to the HC group were observed for MSP-1d38, MSP-1d42, MSP-119 and MSP-636 antigens (P < 0.05). The CM and the HC group had similar levels of antibodies except in the case of MSP-119, which showed higher levels of antibody responses in the CM group compared to HC group (P < 0.05). Although there was a trend towards lower levels of antibody responses in CM as compared to the MM group for several antigens, the difference was significant only for MSP-1f83 (P < 0.05).
Figure 3.
Levels of total IgG antibody responses to MSP-1 subunits, MSP-636 and MSP-722 antigens. Total IgG antibody responses were measured. Box plots depict median values with 25th- and 75th-percentile values represented by the bottom and top edges of boxes. Small diamonds indicate values that fall outside of the error bars. Only a few antigens demonstrated statistically significant differences (* = p < 0.05) when the antibody levels were compared among the different disease categories. The different subunits and allelic forms of MSP-1 are represented as d30, f30, d38, f38, d42, f42, d83 and f83.
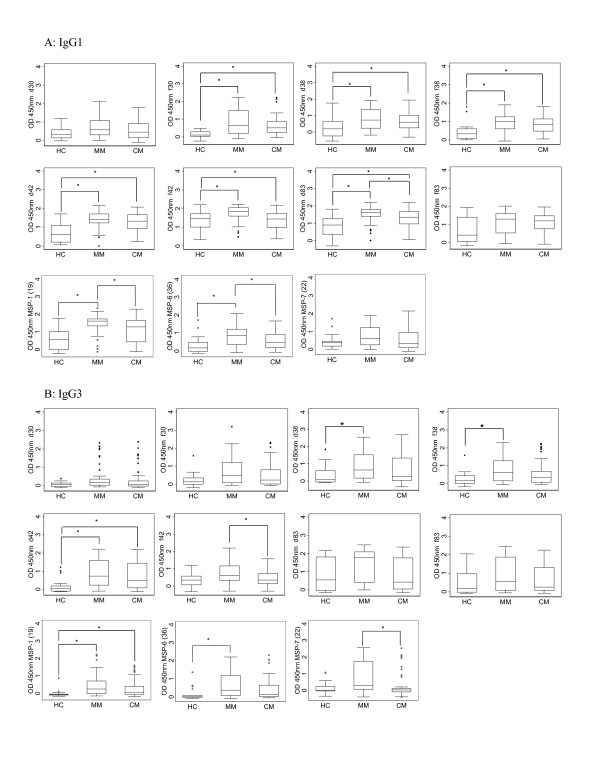
IgG subclass responses to MSP complex antigens
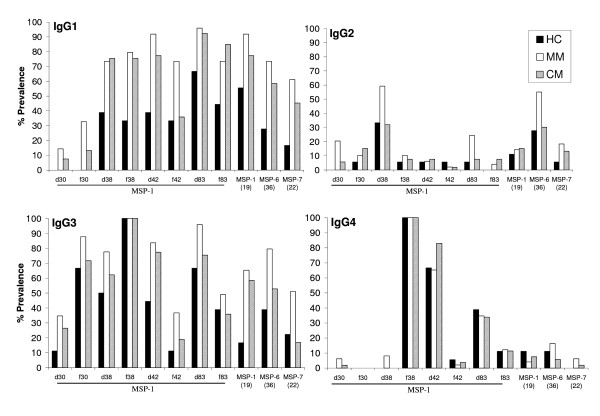
As previously reported [51], a skewing towards IgG1 and IgG3 subclasses in the prevalence and levels of antibody responses to most of the antigens was observed. IgG2 and IgG4 responses were generally very low with a few exceptions (Figures 4). Interestingly, all the plasmas were positive for anti-MSP-1f38 IgG3 and IgG4 (100% prevalence) regardless of disease category (Figure 4). As observed with total IgG responses, the MM group showed higher prevalence of IgG1 responses to eight antigens and IgG3 responses to for five antigens compared to HC. The CM group showed higher prevalence of IgG1 responses to three antigens and higher IgG3 responses to two antigens as compared to the HC group. Significantly lower prevalence of anti- MSP-1f30 and MSP-1f42 IgG1 (p < 0.05), anti- MSP-1d83, MSP-636 and MSP-722 IgG3 (p < 0.05) and anti- MSP-1d38 and MSP-636 IgG2 (p < 0.05) were observed in CM patients compared to MM patients. The prevalence of IgG2 antibody responses was below 20% for all antigens except for MSP-1d38, MSP-1d83 (in MM cases) and MSP-636. Only MSP-1f38, MSP-1d42 and MSP-1d83 antigens showed IgG4 antibody in more than 20% of the patients and there were little differences in the prevalence of IgG4 responses among the three patient categories.
Figure 4.
Prevalence of anti-MSP IgG subclasses for the different subunits. The prevalences of the four IgG subclasses are shown. Test samples were considered positive if their OD values were higher than the mean plus two standard deviations of 10 pooled plasmas from malaria naïve donors from North America. The percentage prevalence was then calculated as described in figure 2.
The differences in the median of antibody levels (IgG1 and IgG3) are reported in Figure 5. IgG2 and IgG4 responses were not included as there were only negligible amounts of antibodies present. Some antigens, such as MSP-1f42, MSP-1f83, MSP-119 and MSP-722, evoked more IgG1 antibodies than IgG3 while MSP-1d30, MSP-1f30 and MSP-1f38 elicited more IgG3 antibodies than other subclasses. Significantly higher levels of IgG1 for 8 antigens and IgG3 for five antigens antibody levels were found in MM patients compared to HC. The CM group showed higher levels of IgG1 for six antigens (Figure 5A) and IgG3 for two antigens as compared to HC (Figure 5B). Significantly lower antibody levels in CM patients compared to MM patients for anti- MSP-1d83, MSP-119 and MSP-636 IgG1 antibodies (P < 0.05) (Figure 5A) and anti- MSP-1f42 and MSP-722 IgG3 antibodies (P < 0.05) (Figure 5B) were observed.
Figure 5.
Levels of IgG subclasses antibody responses to MSP-1 subunits, MSP-636 and MSP-722 antigens. The IgG1 (A) and IgG3 (B) antibody responses to the two allelic forms of each of the MSP-1 subunits, MSP-636 and MSP-722 were measured. The box plots depict median values with 25th- and 75th-percentile values represented by the bottom and top edges of boxes. Small diamonds indicate values that fall outside of the error bars. Statistically significant differences are depicted with the * (p < 0.05).
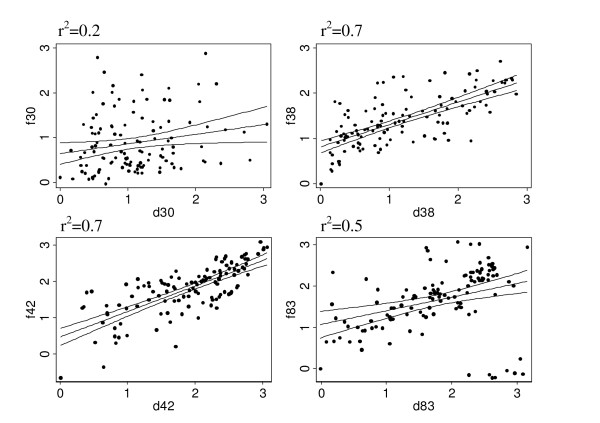
Correlation of the two dimorphic alleles of MSP-1 antigens for total IgG
In order to examine to what extent the two alleles are related to each other in terms of eliciting antibody responses, correlations in antibody responses between the two allelic forms were determined. In general, there was a strong correlation in the IgG antibody responses between MSP-138,, MSP-142 and MSP-183 allelic forms. The correlation was lowest for the MSP-130 antigen (Figure 6). A similar result was observed for IgG1 (p30; r2 = 0.4, p38; r2 = 0.7, p42; r2 = 0.6, p83; r2= 0.4) and IgG3 (p30; r2 = 0.5, p38; r2 = 0.7, p42; r2 = 0.6, p83; r2 = 0.7) antibody responses.
Figure 6.
Correlation of antibody responses between the two dimorphic alleles, MSP-1D and MSP-1F. To determine if there was a correlation in antibody responses between the two allelic forms of MSP-1, a scatter plot of anti-MSP-1D versus MSP-1F total IgG antibody responses was generated. The lines represent the fitted values and the 95% confident interval lines. The Spearman correlation coefficients are shown in the upper left corner.
Discussion
In this study the differences in antibody responses to MSP complex proteins among CM patients, MM patients and HC subjects in a malaria-endemic part of India was investigated. This is one of the first studies to address systematically the antibody responses to the two major allelic forms of all the four major subunits of MSP-1 antigens (MSP-130 MSP-138 MSP-183, MSP-142) together with their associated proteins, MSP-636 and MSP-722 in malaria patients. The HC group tended to have lower antibody levels than MM and CM patients consistent with previous studies, which demonstrated that antibodies to merozoite antigens were higher in parasitaemic compared to aparasitaemic subjects [38,52-54]. An important finding is the observation that CM patients showed significantly lower antibody responses to some of the MSP family of antigens as compared to MM patients. These differences included both low prevalence and low mean IgG levels and IgG subclasses in the CM group. On the contrary, MM patients showed significantly elevated IgG responses to many of the antigens compared to the HC group.
The association of lower antibody titers to certain P. falciparum antigens with malaria severity (such as CM) has been demonstrated in previous studies. For example, significantly lower levels of P. falciparum anti-GPI IgGs were observed in CM patients as compared to MM patients in a study in Senegal [41]. In the Senegal study, the differences in the responses to MSP-119 antigen were slightly lower in the CM patients compared to the MM patients, although significantly lower levels were only observed in the CM non-survivors sub-group. In the current study, antibody responses to the MSP-119 antigen and some other MSP complex antigens were lower in CM patients as compared to MM patients. On the contrary, other studies have reported higher levels of anti- GPI antibodies in CM patients compared to non-severe malaria patients or HC subjects [45]. In another study, higher levels of IgG2 and IgG4 antibody responses to the variant surface glycoprotein RIF-29 were found exclusively in CM children but not in the non-cerebral malaria controls [44]. Overall, findings from the current study are consistent with the hypothesis that CM patients may have some deficiency in mounting optimal antibody responses to some antigens essential for clinical protection. However, additional evidence to confirm this hypothesis is required and validation will depend on further studies.
The seropositivity of antibodies varied considerably depending on the MSP antigen as previously demonstrated [39]. Antibody responses were relatively high for most of the antigens, which was not surprising given that this study was conducted in a malaria endemic region where the majority of people are exposed to malaria. Both MSP-142 and MSP-183 demonstrated high antibody prevalence compared to the other fragments, consistent with a previous study [39]. Anti-MSP-636 antibodies were shown to be generated in individuals naturally infected with P. falciparum [55]. Additionally, these antibodies were thought to play a role in the inhibition of erythrocyte invasion [55,56] and parasite multiplication [23,57]. This study demonstrates that naturally exposed residents in central India also generate both anti-MSP-636 and anti-MSP-722 antibodies. In contrast, the antibody prevalence to the MSP-1d30 antigen was low as had been observed in previous studies [39], suggesting that this may be a poorly immunogenic antigen.
Results from this study confirm that the entire MSP-1/MSP-6/MSP-7 complex contains B-cell epitopes capable of generating specific antibodies in naturally exposed individuals.
The F allelic form of MSP-130 and MSP-138 antigens showed higher seroprevalence than the corresponding D allelic forms, suggesting that individuals generate specific antibodies to the different allelic forms of these antigens. The higher prevalence of antibodies to the F allelic forms of MSP-130 and MSP-138 antigens may be due to a predominant presence of P. falciparum parasites with this allele in this population in India. However, there is no published information to verify the relative proportions of these alleles within this population. A few studies that have investigated the prevalence of the different MSP-1 alleles in India have either demonstrated the predominance of the MAD20 allele (D), as defined by sequence analysis of the 16th and 17th block [58], or no bias to any allele [59,60].
However, the MSP-142 and MSP-183 antigens showed similar antibody prevalence to the two allelic forms of the antigens, suggesting the development of antibodies cross reactive to both allelic forms. Significant correlations in the antibody levels elicited by the two allelic forms of MSP-1 were observed except for the p30 fragment. The MSP-130 is located within the dimorphic region of the MSP-1 protein [36]. Therefore, different epitopes in these dimorphic regions may be presented to B-cells generating heterogeneity in antibody responses between the two alleles. These results demonstrate that, at least for p38, p42 and p83, responses to one allelic form predict positive responses to the other allelic form. It is possible that conserved epitopes between the two allelic forms are presented to the immune system leading to the generation of cross-reactive antibodies. In fact, a previous study demonstrated a high degree of cross-inhibition between antibodies generated against the D and the F allelic forms of MSP-1 [39].
IgG subclass analysis showed that IgG1 and IgG3 were the predominant subclasses for most of the antigens studied, consistent with previous studies [34,51,61]. While mixed IgG1/IgG3 responses to all MSP antigens used was detected, the relative proportions of these two subclasses were different for the different MSP antigens, implying that IgG class switching may be greatly influenced by the characteristics of the antigen as previously suggested [40,51,62]. It has been suggested that conserved antigens, such as MSP-119, typically induce IgG1, while highly polymorphic antigens induce IgG3 [63]. Interestingly in this study, such a correlation was not observed except for the MSP-119 antigen which elicited more IgG1 than IgG3 as previously observed [40,51,61,64]. In contrast, a mixed response to the conserved MSP-636 was observed while a previous study in southern-central Vietnam demonstrated a skewing towards IgG1 [63]. Similarly, predominant IgG3 responses were observed for the highly polymorphic block 2 region (found within the p83 subunit) of MSP-1 in an African population [40] and for polymorphic MSP-722 in Vietnam [63]. However, in the current study, MSP-722 induced more IgG1 antibodies than IgG3 and MSP-1d83 showed a mixed response, while MSP-1f83 elicited more IgG1 antibodies than IgG3. These differences may be due to differences in innate characteristics of the host population or epidemiologic differences.
Conclusion
Observations from the current study provide insight into the intricate pattern of acquired humoral responses to the MSP-1/MSP-6/MSP-7 complex. The results demonstrate that MSP-1/MSP-6/MSP-7 complex antigens are immunogenic in humans naturally exposed to P. falciparum infection in India, supporting the view that full size MSP-1 is a particularly interesting vaccine candidate. In addition, this study suggests that there may be a dysregulation in the generation of optimal antibody responses to some of the MSP-1 protein fragments in CM patients and it remains to be determined if such differences contribute to susceptibility of individuals to CM.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
NWL: Participated in the study design, performed the experiments and data analysis and wrote the manuscript. JET: Participated in the study design, data analysis and editing of the manuscript. VJ: Participated in the cohort study design, enrolled patients, collected clinical and epidemiologic data and biological samples and helped in the editing of the manuscript. ACN: Participated in designing the cohort, enrollment of subjects, clinical evaluation and editing of the manuscript. CWK: Participated in the expression and purification of the recombinant proteins, study design and assisted in editing the manuscript. UW: Participated in the expression and purification of the recombinant proteins, study design and in the editing of the manuscript. HB: Facilitated the expression and purification of the recombinant proteins, planning of the study and contributed to the editing of the manuscript. APD: Participated in planning of the study, supervision of the study in India and editing of the manuscript. NS: Participated in the cohort study design, enrollment of study subjects, planning of the experiments and editing of the manuscript. JKS: Participated in the cohort study design, planning of experiments and assisted in the editing of the manuscript. VU: Participated in the study design, planning of the experiments, data analysis, and contributed to manuscript writing and editing.
Acknowledgments
Acknowledgements
This study was supported by National Institutes of Health grant numbers NIH-FIC (R21-TW006804-02S1), NIH-RCMI (RR03034), and NIH-NIGM-MBRS (SO6GM08248) to Jonathan Stiles, Morehouse School of Medicine. We also acknowledge the American Society of Microbiology/Coordinating Center for Infectious Diseases (ASM/CCID) fellowship granted to NWL and JET.
We would like to acknowledge and thank all the study participants for their willingness to participate in the study and Ira Goldman and Amanda Poe for the critical review of the manuscript.
We also acknowledge the Atlanta Research and Education Foundation for the financial support.
Contributor Information
Naomi W Lucchi, Email: frd9@cdc.gov.
Jon Eric Tongren, Email: Tongren@maine.gov.
Vidhan Jain, Email: vidhanjain78@yahoo.com.
Avinash C Nagpal, Email: nagpal_avinash@yahoo.com.
Christian W Kauth, Email: C.Kauth@gdels.de.
Ute Woehlbier, Email: uwoehlbier@yahoo.de.
Hermann Bujard, Email: h.bujard@zmbh.uni-heidelberg.de.
Aditya P Dash, Email: apdash2@rediffmail.com.
Neeru Singh, Email: neeru.singh@gmail.com.
Jonathan K Stiles, Email: jstiles@msm.edu.
Venkatachalam Udhayakumar, Email: vxu0@cdc.gov.
References
- Newton CR, Krishna S. Severe falciparum malaria in children: current understanding of pathophysiology and supportive treatment. Pharmacol Ther. 1998;79:1–53. doi: 10.1016/S0163-7258(98)00008-4. [DOI] [PubMed] [Google Scholar]
- Carter JA, Mung'ala-Odera V, Neville BG, Murira G, Mturi N, Musumba C, Newton CR. Persistent neurocognitive impairments associated with severe falciparum malaria in Kenyan children. J Neurol Neurosurg Psychiatry. 2005;76:476–481. doi: 10.1136/jnnp.2004.043893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JA, Neville BG, White S, Ross AJ, Otieno G, Mturi N, Musumba C, Newton CR. Increased prevalence of epilepsy associated with severe falciparum malaria in children. Epilepsia. 2004;45:978–981. doi: 10.1111/j.0013-9580.2004.65103.x. [DOI] [PubMed] [Google Scholar]
- Idro R, Ndiritu M, Ogutu B, Mithwani S, Maitland K, Berkley J, Crawley J, Fegan G, Bauni E, Peshu N, Marsh K, Neville B, Newton C. Burden, features, and outcome of neurological involvement in acute falciparum malaria in Kenyan children. Jama. 2007;297:2232–2240. doi: 10.1001/jama.297.20.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mung'Ala-Odera V, Snow RW, Newton CR. The burden of the neurocognitive impairment associated with Plasmodium falciparum malaria in sub-saharan Africa. Am J Trop Med Hyg. 2004;71:64–70. [PubMed] [Google Scholar]
- Kamble MB, Raut PP, Hussain ZF. Cerebral malaria in rural India. Indian J Pediatr. 2002;69:659–661. doi: 10.1007/BF02722697. [DOI] [PubMed] [Google Scholar]
- Marsh K, Forster D, Waruiru C, Mwangi I, Winstanley M, Marsh V, Newton C, Winstanley P, Warn P, Peshu N, Pasvol G, Snow R. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–1404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- Molyneux ME, Taylor TE, Wirima JJ, Borgstein A. Clinical features and prognostic indicators in paediatric cerebral malaria: a study of 131 comatose Malawian children. Q J Med. 1989;71:441–459. [PubMed] [Google Scholar]
- Singh N, Chand SK, Mishra AK, Bharti PK, Singh MP, Ahluwalia TP, Dash AP. Epidemiology of malaria in an area of low transmission in central India. Am J Trop Med Hyg. 2006;75:812–816. [PubMed] [Google Scholar]
- Montgomery J, Milner DA, Jr, Tse MT, Njobvu A, Kayira K, Dzamalala CP, Taylor TE, Rogerson SJ, Craig AG, Molyneux ME. Genetic analysis of circulating and sequestered populations of Plasmodium falciparum in fatal pediatric malaria. J Infect Dis. 2006;194:115–122. doi: 10.1086/504689. [DOI] [PubMed] [Google Scholar]
- Taylor TE, Fu WJ, Carr RA, Whitten RO, Mueller JS, Fosiko NG, Lewallen S, Liomba NG, Molyneux ME. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med. 2004;10:143–145. doi: 10.1038/nm986. [DOI] [PubMed] [Google Scholar]
- Pongponratn E, Riganti M, Punpoowong B, Aikawa M. Microvascular sequestration of parasitized erythrocytes in human falciparum malaria: a pathological study. Am J Trop Med Hyg. 1991;44:168–175. doi: 10.4269/ajtmh.1991.44.168. [DOI] [PubMed] [Google Scholar]
- Clark IA, Rockett KA, Cowden WB. Role of TNF in cerebral malaria. Lancet. 1991;337:302–303. doi: 10.1016/0140-6736(91)90915-C. [DOI] [PubMed] [Google Scholar]
- Armah H, Dodoo AK, Wiredu EK, Stiles JK, Adjei AA, Gyasi RK, Tettey Y. High-level cerebellar expression of cytokines and adhesion molecules in fatal, paediatric, cerebral malaria. Ann Trop Med Parasitol. 2005;99:629–647. doi: 10.1179/136485905X51508. [DOI] [PubMed] [Google Scholar]
- Armah HB, Wilson NO, Sarfo BY, Powell MD, Bond VC, Anderson W, Adjei AA, Gyasi RK, Tettey Y, Wiredu EK, Tongren JE, Udhayakumar V, Stiles JK. Cerebrospinal fluid and serum biomarkers of cerebral malaria mortality in Ghanaian children. Malar J. 2007;6:147. doi: 10.1186/1475-2875-6-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain V, Armah HB, Tongren JE, Ned RM, Wilson NO, Crawford S, Joel PK, Singh MP, Nagpal AC, Dash AP, Udhayakumar V, Singh N, Stiles JK. Plasma IP-10, apoptotic and angiogenic factors associated with fatal cerebral malaria in India. Malar J. 2008;7:83. doi: 10.1186/1475-2875-7-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarfo BY, Singh S, Lillard JW, Quarshie A, Gyasi RK, Armah H, Adjei AA, Jolly P, Stiles JK. The cerebral-malaria-associated expression of RANTES, CCR3 and CCR5 in post-mortem tissue samples. Ann Trop Med Parasitol. 2004;98:297–303. doi: 10.1179/000349804225003271. [DOI] [PubMed] [Google Scholar]
- Blackman MJ, Ling IT, Nicholls SC, Holder AA. Proteolytic processing of the Plasmodium falciparum merozoite surface protein-1 produces a membrane-bound fragment containing two epidermal growth factor-like domains. Mol Biochem Parasitol. 1991;49:29–33. doi: 10.1016/0166-6851(91)90127-R. [DOI] [PubMed] [Google Scholar]
- Blackman MJ, Scott-Finnigan TJ, Shai S, Holder AA. Antibodies inhibit the protease-mediated processing of a malaria merozoite surface protein. J Exp Med. 1994;180:389–393. doi: 10.1084/jem.180.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford WH, Gunder B, Harris A, Heidrich HG, Holder AA, Blackman MJ. A 22 kDa protein associated with the Plasmodium falciparum merozoite surface protein-1 complex. Mol Biochem Parasitol. 1996;80:159–169. doi: 10.1016/0166-6851(96)02696-5. [DOI] [PubMed] [Google Scholar]
- Pachebat JA, Ling IT, Grainger M, Trucco C, Howell S, Fernandez-Reyes D, Gunaratne R, Holder AA. The 22 kDa component of the protein complex on the surface of Plasmodium falciparum merozoites is derived from a larger precursor, merozoite surface protein 7. Mol Biochem Parasitol. 2001;117:83–89. doi: 10.1016/S0166-6851(01)00336-X. [DOI] [PubMed] [Google Scholar]
- Trucco C, Fernandez-Reyes D, Howell S, Stafford WH, Scott-Finnigan TJ, Grainger M, Ogun SA, Taylor WR, Holder AA. The merozoite surface protein 6 gene codes for a 36 kDa protein associated with the Plasmodium falciparum merozoite surface protein-1 complex. Mol Biochem Parasitol. 2001;112:91–101. doi: 10.1016/S0166-6851(00)00350-9. [DOI] [PubMed] [Google Scholar]
- Kauth CW, Woehlbier U, Kern M, Mekonnen Z, Lutz R, Mucke N, Langowski J, Bujard H. Interactions between merozoite surface proteins 1, 6, and 7 of the malaria parasite Plasmodium falciparum. J Biol Chem. 2006;281:31517–31527. doi: 10.1074/jbc.M604641200. [DOI] [PubMed] [Google Scholar]
- Siddiqui WA, Tam LQ, Kramer KJ, Hui GS, Case SE, Yamaga KM, Chang SP, Chan EB, Kan SC. Merozoite surface coat precursor protein completely protects Aotus monkeys against Plasmodium falciparum malaria. Proc Natl Acad Sci USA. 1987;84:3014–3018. doi: 10.1073/pnas.84.9.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolle R, Fruh K, Doumbo O, Koita O, N'Diaye M, Fischer A, Dietz K, Bujard H. A prospective study of the association between the human humoral immune response to Plasmodium falciparum blood stage antigen gp190 and control of malarial infections. Infect Immun. 1993;61:40–47. doi: 10.1128/iai.61.1.40-47.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman MJ, Heidrich HG, Donachie S, McBride JS, Holder AA. A single fragment of a malaria merozoite surface protein remains on the parasite during red cell invasion and is the target of invasion-inhibiting antibodies. J Exp Med. 1990;172:379–382. doi: 10.1084/jem.172.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SP, Case SE, Gosnell WL, Hashimoto A, Kramer KJ, Tam LQ, Hashiro CQ, Nikaido CM, Gibson HL, Lee-Ng CT, Barr PJ, Yokota BT, Hut GS. A recombinant baculovirus 42-kilodalton C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 protects Aotus monkeys against malaria. Infect Immun. 1996;64:253–261. doi: 10.1128/iai.64.1.253-261.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Yaman F, Genton B, Kramer KJ, Chang SP, Hui GS, Baisor M, Alpers MP. Assessment of the role of naturally acquired antibody levels to Plasmodium falciparum merozoite surface protein-1 in protecting Papua New Guinean children from malaria morbidity. Am J Trop Med Hyg. 1996;54:443–448. doi: 10.4269/ajtmh.1996.54.443. [DOI] [PubMed] [Google Scholar]
- Egan AF, Burghaus P, Druilhe P, Holder AA, Riley EM. Human antibodies to the 19 kDa C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 inhibit parasite growth in vitro. Parasite Immunol. 1999;21:133–139. doi: 10.1046/j.1365-3024.1999.00209.x. [DOI] [PubMed] [Google Scholar]
- O'Donnell RA, de Koning-Ward TF, Burt RA, Bockarie M, Reeder JC, Cowman AF, Crabb BS. Antibodies against merozoite surface protein (MSP)-1(19) are a major component of the invasion-inhibitory response in individuals immune to malaria. J Exp Med. 2001;193:1403–1412. doi: 10.1084/jem.193.12.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch OH, Udhayakumar V, Hightower AW, Oloo AJ, Hawley WA, Nahlen BL, Bloland PB, Kaslow DC, Lal AA. A longitudinal investigation of IgG and IgM antibody responses to the merozoite surface protein-1 19-kiloDalton domain of Plasmodium falciparum in pregnant women and infants: associations with febrile illness, parasitemia, and anemia. Am J Trop Med Hyg. 1998;58:211–219. doi: 10.4269/ajtmh.1998.58.211. [DOI] [PubMed] [Google Scholar]
- Riley EM, Morris-Jones S, Blackman MJ, Greenwood BM, Holder AA. A longitudinal study of naturally acquired cellular and humoral immune responses to a merozoite surface protein (MSP1) of Plasmodium falciparum in an area of seasonal malaria transmission. Parasite Immunol. 1993;15:513–524. doi: 10.1111/j.1365-3024.1993.tb00639.x. [DOI] [PubMed] [Google Scholar]
- Shi Q, Lynch MM, Romero M, Burns JM., Jr Enhanced protection against malaria by a chimeric merozoite surface protein vaccine. Infect Immun. 2007;75:1349–1358. doi: 10.1128/IAI.01467-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi YP, Sayed U, Qari SH, Roberts JM, Udhayakumar V, Oloo AJ, Hawley WA, Kaslow DC, Nahlen BL, Lal AA. Natural immune response to the C-terminal 19-kilodalton domain of Plasmodium falciparum merozoite surface protein 1. Infect Immun. 1996;64:2716–2723. doi: 10.1128/iai.64.7.2716-2723.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udhayakumar V, Anyona D, Kariuki S, Shi YP, Bloland PB, Branch OH, Weiss W, Nahlen BL, Kaslow DC, Lal AA. Identification of T and B cell epitopes recognized by humans in the C-terminal 42-kDa domain of the Plasmodium falciparum merozoite surface protein (MSP)-1. J Immunol. 1995;154:6022–6030. [PubMed] [Google Scholar]
- Tanabe K, Mackay M, Goman M, Scaife JG. Allelic dimorphism in a surface antigen gene of the malaria parasite Plasmodium falciparum. J Mol Biol. 1987;195:273–287. doi: 10.1016/0022-2836(87)90649-8. [DOI] [PubMed] [Google Scholar]
- Miller LH, Roberts T, Shahabuddin M, McCutchan TF. Analysis of sequence diversity in the Plasmodium falciparum merozoite surface protein-1 (MSP-1) Mol Biochem Parasitol. 1993;59:1–14. doi: 10.1016/0166-6851(93)90002-F. [DOI] [PubMed] [Google Scholar]
- Fruh K, Doumbo O, Muller HM, Koita O, McBride J, Crisanti A, Toure Y, Bujard H. Human antibody response to the major merozoite surface antigen of Plasmodium falciparum is strain specific and short-lived. Infect Immun. 1991;59:1319–1324. doi: 10.1128/iai.59.4.1319-1324.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woehlbier U, Epp C, Kauth CW, Lutz R, Long CA, Coulibaly B, Kouyate B, Arevalo-Herrera M, Herrera S, Bujard H. Analysis of antibodies directed against merozoite surface protein 1 of the human malaria parasite Plasmodium falciparum. Infect Immun. 2006;74:1313–1322. doi: 10.1128/IAI.74.2.1313-1322.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh DR, Dobano C, Elhassan IM, Marsh K, Elhassan A, Hviid L, Khalil EA, Theander TG, Arnot DE, McBride JS. Differential patterns of human immunoglobulin G subclass responses to distinct regions of a single protein, the merozoite surface protein 1 of Plasmodium falciparum. Infect Immun. 2001;69:1207–1211. doi: 10.1128/IAI.69.2.1207-1211.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perraut R, Diatta B, Marrama L, Garraud O, Jambou R, Longacre S, Krishnegowda G, Dieye A, Gowda DC. Differential antibody responses to Plasmodium falciparum glycosylphosphatidylinositol anchors in patients with cerebral and mild malaria. Microbes Infect. 2005;7:682–687. doi: 10.1016/j.micinf.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Perraut R, Marrama L, Diouf B, Sokhna C, Tall A, Nabeth P, Trape JF, Longacre S, Mercereau-Puijalon O. Antibodies to the conserved C-terminal domain of the Plasmodium falciparum merozoite surface protein 1 and to the merozoite extract and their relationship with in vitro inhibitory antibodies and protection against clinical malaria in a Senegalese village. J Infect Dis. 2005;191:264–271. doi: 10.1086/426398. [DOI] [PubMed] [Google Scholar]
- Al-Yaman F, Genton B, Reeder JC, Mokela D, Anders RF, Alpers MP. Humoral response to defined Plasmodium falciparum antigens in cerebral and uncomplicated malaria and their relationship to parasite genotype. Am J Trop Med Hyg. 1997;56:430–435. doi: 10.4269/ajtmh.1997.56.430. [DOI] [PubMed] [Google Scholar]
- Schreiber N, Brattig N, Evans J, Tsiri A, Horstmann RD, May J, Klinkert MQ. Cerebral malaria is associated with IgG2 and IgG4 antibody responses to recombinant Plasmodium falciparum RIFIN antigen. Microbes Infect. 2006;8:1269–1276. doi: 10.1016/j.micinf.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Cissoko Y, Daou M, Lyke KE, Dicko A, Diarra I, Kone A, Guindo A, Traore K, Krishnegowda G, Diallo DA, Doumbo OK, Plowe CV, Gowda DC, Sztein MB. Serum antibody levels to glycosylphosphatidylinositols in specimens derived from matched Malian children with severe or uncomplicated Plasmodium falciparum malaria and healthy controls. Am J Trop Med Hyg. 2006;75:199–204. [PMC free article] [PubMed] [Google Scholar]
- Sharma VP. Re-emergence of malaria in India. Indian J Med Res. 1996;103:26–45. [PubMed] [Google Scholar]
- Singh N, Valecha N. Evaluation of a rapid diagnostic test, 'Determine malaria pf', in epidemic-prone, forest villages of central India (Madhya Pradesh) Ann Trop Med Parasitol. 2000;94:421–427. doi: 10.1080/00034980057446. [DOI] [PubMed] [Google Scholar]
- Severe falciparum malaria. World Health Organization, Communicable Diseases Cluster. Trans R Soc Trop Med Hyg. 2000;94:S1–90. [PubMed] [Google Scholar]
- Epp C, Kauth CW, Bujard H, Lutz R. Expression and purification of Plasmodium falciparum MSP-1(42): A malaria vaccine candidate. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;786:61–72. doi: 10.1016/S1570-0232(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Kauth CW, Epp C, Bujard H, Lutz R. The merozoite surface protein 1 complex of human malaria parasite Plasmodium falciparum: interactions and arrangements of subunits. J Biol Chem. 2003;278:22257–22264. doi: 10.1074/jbc.M302299200. [DOI] [PubMed] [Google Scholar]
- Tongren JE, Drakeley CJ, McDonald SL, Reyburn HG, Manjurano A, Nkya WM, Lemnge MM, Gowda CD, Todd JE, Corran PH, Riley EM. Target antigen, age, and duration of antigen exposure independently regulate immunoglobulin G subclass switching in malaria. Infect Immun. 2006;74:257–264. doi: 10.1128/IAI.74.1.257-264.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Yaman F, Genton B, Anders RF, Falk M, Triglia T, Lewis D, Hii J, Beck HP, Alpers MP. Relationship between humoral response to Plasmodium falciparum merozoite surface antigen-2 and malaria morbidity in a highly endemic area of Papua New Guinea. Am J Trop Med Hyg. 1994;51:593–602. doi: 10.4269/ajtmh.1994.51.593. [DOI] [PubMed] [Google Scholar]
- al-Yaman F, Genton B, Falk M, Anders RF, Lewis D, Hii J, Beck HP, Alpers MP. Humoral response to Plasmodium falciparum ring-infected erythrocyte surface antigen in a highly endemic area of Papua New Guinea. Am J Trop Med Hyg. 1995;52:66–71. doi: 10.4269/ajtmh.1995.52.66. [DOI] [PubMed] [Google Scholar]
- Cavanagh DR, Elhassan IM, Roper C, Robinson VJ, Giha H, Holder AA, Hviid L, Theander TG, Arnot DE, McBride JS. A longitudinal study of type-specific antibody responses to Plasmodium falciparum merozoite surface protein-1 in an area of unstable malaria in Sudan. J Immunol. 1998;161:347–359. [PubMed] [Google Scholar]
- Pearce JA, Triglia T, Hodder AN, Jackson DC, Cowman AF, Anders RF. Plasmodium falciparum merozoite surface protein 6 is a dimorphic antigen. Infect Immun. 2004;72:2321–2328. doi: 10.1128/IAI.72.4.2321-2328.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez R, Valbuena J, Rodriguez LE, Ocampo M, Vera R, Curtidor H, Puentes A, Garcia J, Ramirez LE, Patarroyo ME. Plasmodium falciparum merozoite surface protein 6 (MSP-6) derived peptides bind erythrocytes and partially inhibit parasite invasion. Peptides. 2006;27:1685–1692. doi: 10.1016/j.peptides.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Singh S, Soe S, Roussilhon C, Corradin G, Druilhe P. Plasmodium falciparum merozoite surface protein 6 displays multiple targets for naturally occurring antibodies that mediate monocyte-dependent parasite killing. Infect Immun. 2005;73:1235–1238. doi: 10.1128/IAI.73.2.1235-1238.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamillapalli A, Sunil S, Diwan SS, Sharma SK, Tyagi PK, Adak T, Joshi H, Malhotra P. Polymorphism and epitope sharing between the alleles of merozoite surface protein-1 of Plasmodium falciparum among Indian isolates. Malar J. 2007;6:95. doi: 10.1186/1475-2875-6-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi H, Valecha N, Verma A, Kaul A, Mallick PK, Shalini S, Prajapati SK, Sharma SK, Dev V, Biswas S, Nanda N, Malhotra MS, Subbarao SK, Dash AP. Genetic structure of Plasmodium falciparum field isolates in eastern and north-eastern India. Malar J. 2007;6:60. doi: 10.1186/1475-2875-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj DK, Das BR, Dash AP, Supakar PC. Genetic diversity in the merozoite surface protein 1 gene of Plasmodium falciparum in different malaria-endemic localities. Am J Trop Med Hyg. 2004;71:285–289. [PubMed] [Google Scholar]
- Branch OH, Oloo AJ, Nahlen BL, Kaslow D, Lal AA. Anti-merozoite surface protein-1 19-kDa IgG in mother-infant pairs naturally exposed to Plasmodium falciparum: subclass analysis with age, exposure to asexual parasitemia, and protection against malaria. V. The Asembo Bay Cohort Project. J Infect Dis. 2000;181:1746–1752. doi: 10.1086/315424. [DOI] [PubMed] [Google Scholar]
- Da Silveira LA, Dorta ML, Kimura EA, Katzin AM, Kawamoto F, Tanabe K, Ferreira MU. Allelic diversity and antibody recognition of Plasmodium falciparum merozoite surface protein 1 during hypoendemic malaria transmission in the Brazilian amazon region. Infect Immun. 1999;67:5906–5916. doi: 10.1128/iai.67.11.5906-5916.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Crouch L, Richie TL, Nhan DH, Coppel RL. Naturally acquired antibody responses to the components of the Plasmodium falciparum merozoite surface protein 1 complex. Parasite Immunol. 2003;25:403–412. doi: 10.1111/j.1365-3024.2003.00647.x. [DOI] [PubMed] [Google Scholar]
- Egan AF, Chappel JA, Burghaus PA, Morris JS, McBride JS, Holder AA, Kaslow DC, Riley EM. Serum antibodies from malaria-exposed people recognize conserved epitopes formed by the two epidermal growth factor motifs of MSP1(19), the carboxy-terminal fragment of the major merozoite surface protein of Plasmodium falciparum. Infect Immun. 1995;63:456–466. doi: 10.1128/iai.63.2.456-466.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]