Abstract
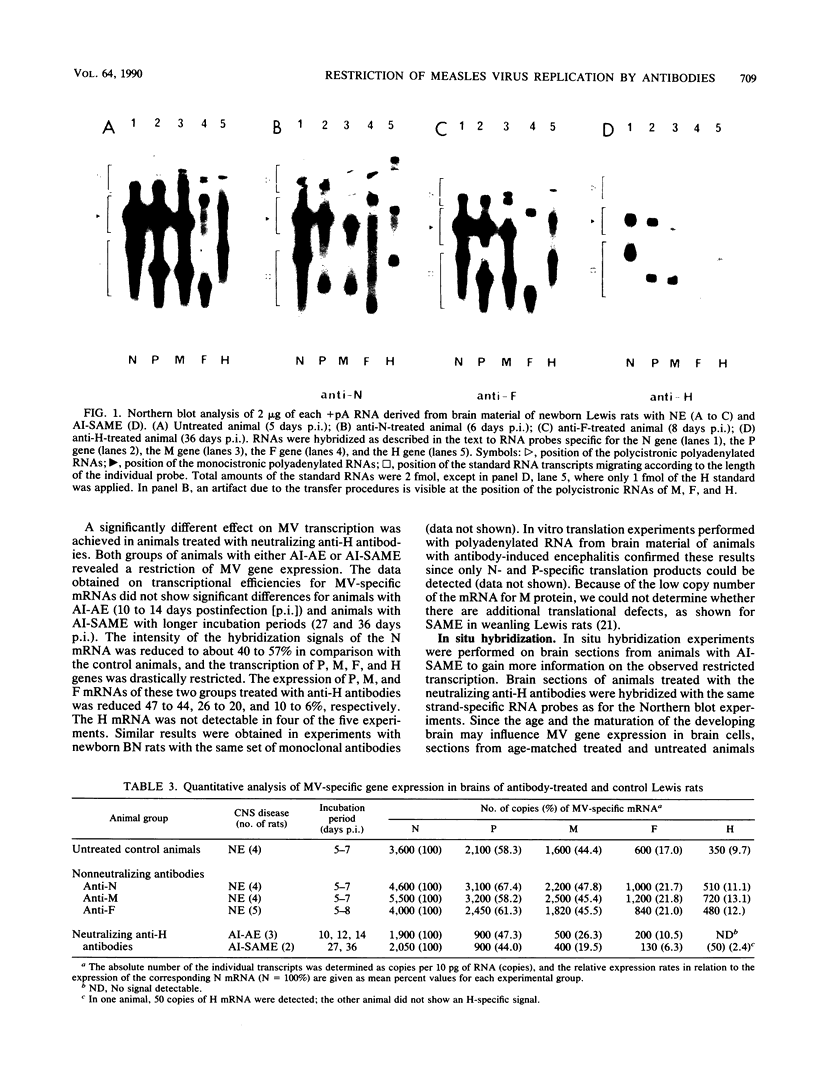
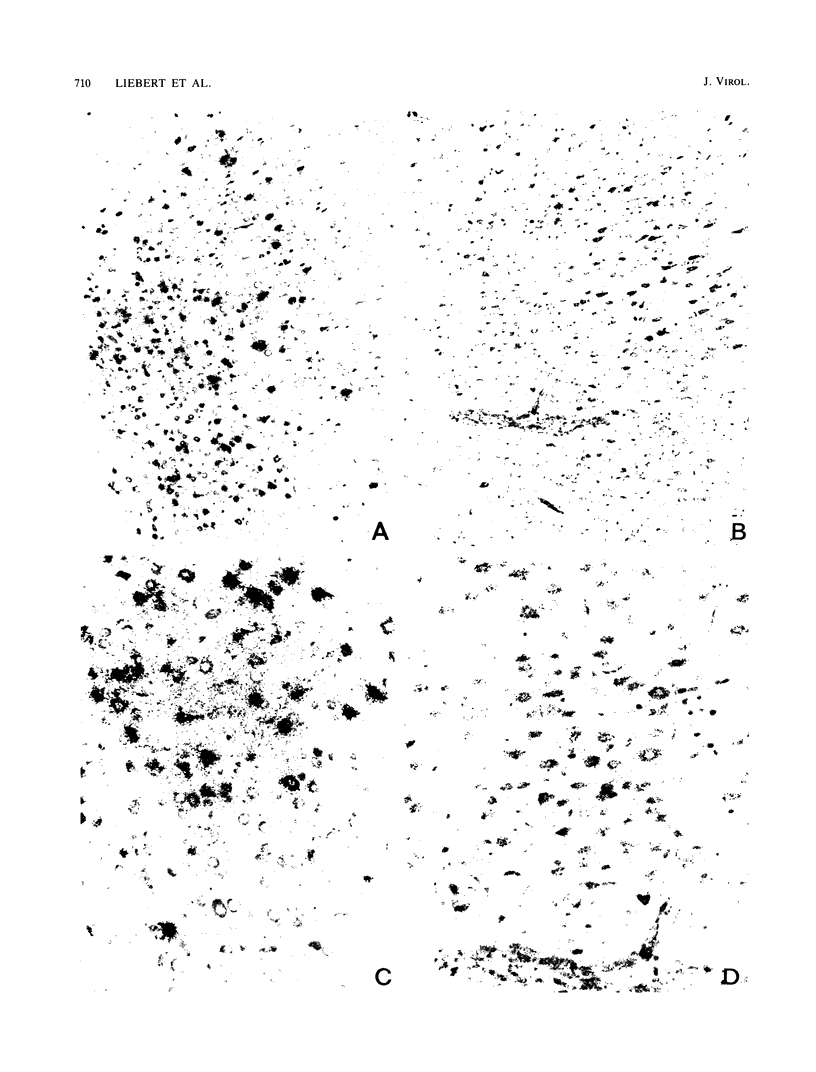
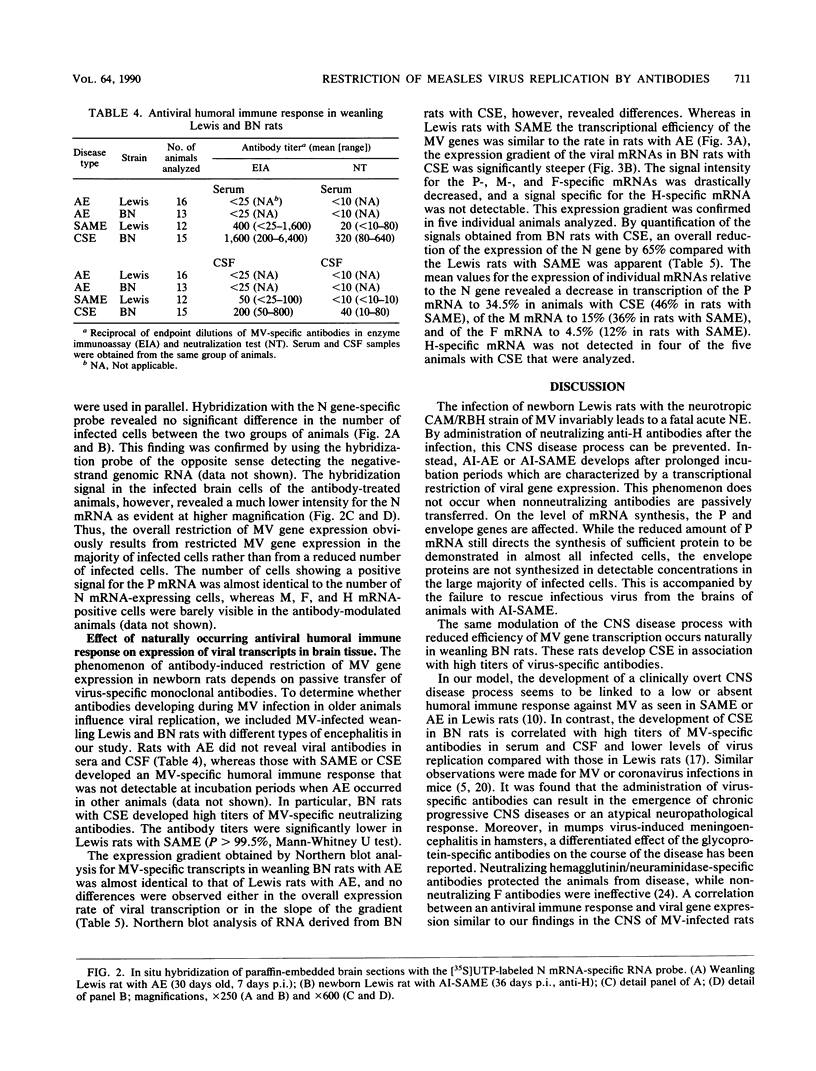
After infection with the neurotropic CAM/RBH measles virus (MV) strain, newborn Lewis rats succumb to an acute necrotizing encephalopathy. Passive transfer of neutralizing monoclonal antibodies directed against MV hemagglutinin prevented this disease process. Instead, either an antibody-induced acute or subacute measles encephalitis developed after a prolonged incubation period with a restricted expression of MV structural proteins. The molecular biological analysis of MV gene expression in brain tissue of rats treated with MV-neutralizing antibodies revealed a transcriptional restriction of viral mRNAs, particularly for the envelope proteins, leading to a steep expression gradient. Based on in situ hybridization, it was concluded that the efficiency of transcription of viral genes at the single-cell level is reduced compared with that of controls. Passive immunization with monoclonal antibodies directed against other MV structural proteins proved to be ineffective. Similar results were obtained in MV-infected weanling Brown Norway rats. These rats developed a clinically silent encephalitis in the presence of high titers of neutralizing antibodies. In such animals, a pronounced attenuation of the viral gene transcription was observed. These findings indicated that neutralizing antibodies directed against a restricted set of specific antigenic sites on the viral hemagglutinin protein expressed on cell membranes exert a modulating effect on the viral gene expression at the level of transcription. This phenomenon contributes to the switch from the acute cytopathic effect to a persistent infection in the central nervous system.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandersen S., Larsen S., Cohn A., Uttenthal A., Race R. E., Aasted B., Hansen M., Bloom M. E. Passive transfer of antiviral antibodies restricts replication of Aleutian mink disease parvovirus in vivo. J Virol. 1989 Jan;63(1):9–17. doi: 10.1128/jvi.63.1.9-17.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baczko K., Liebert U. G., Billeter M., Cattaneo R., Budka H., ter Meulen V. Expression of defective measles virus genes in brain tissues of patients with subacute sclerosing panencephalitis. J Virol. 1986 Aug;59(2):472–478. doi: 10.1128/jvi.59.2.472-478.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baczko K., Liebert U. G., Cattaneo R., Billeter M. A., Roos R. P., ter Meulen V. Restriction of measles virus gene expression in measles inclusion body encephalitis. J Infect Dis. 1988 Jul;158(1):144–150. doi: 10.1093/infdis/158.1.144. [DOI] [PubMed] [Google Scholar]
- Barrett P. N., Koschel K., Carter M., ter Meulen V. Effect of measles virus antibodies on a measles SSPE virus persistently infected C6 rat glioma cell line. J Gen Virol. 1985 Jul;66(Pt 7):1411–1421. doi: 10.1099/0022-1317-66-7-1411. [DOI] [PubMed] [Google Scholar]
- Buchmeier M. J., Lewicki H. A., Talbot P. J., Knobler R. L. Murine hepatitis virus-4 (strain JHM)-induced neurologic disease is modulated in vivo by monoclonal antibody. Virology. 1984 Jan 30;132(2):261–270. doi: 10.1016/0042-6822(84)90033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter M. J., Willcocks M. M., Löffler S., Ter Meulen V. Comparison of lytic and persistent measles virus matrix proteins by competition radioimmunoassay. J Gen Virol. 1983 Aug;64(Pt 8):1801–1805. doi: 10.1099/0022-1317-64-8-1801. [DOI] [PubMed] [Google Scholar]
- Carter M. J., Willcocks M. M., Löffler S., ter Meulen V. Relationships between monoclonal antibody-binding sites on the measles virus haemagglutinin. J Gen Virol. 1982 Nov;63(Pt 1):113–120. doi: 10.1099/0022-1317-63-1-113. [DOI] [PubMed] [Google Scholar]
- Cattaneo R., Rebmann G., Schmid A., Baczko K., ter Meulen V., Billeter M. A. Altered transcription of a defective measles virus genome derived from a diseased human brain. EMBO J. 1987 Mar;6(3):681–688. doi: 10.1002/j.1460-2075.1987.tb04808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R., Schmid A., Eschle D., Baczko K., ter Meulen V., Billeter M. A. Biased hypermutation and other genetic changes in defective measles viruses in human brain infections. Cell. 1988 Oct 21;55(2):255–265. doi: 10.1016/0092-8674(88)90048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörries R., Liebert U. G., ter Meulen V. Comparative analysis of virus-specific antibodies and immunoglobulins in serum and cerebrospinal fluid of subacute measles virus-induced encephalomyelitis (SAME) in rats and subacute sclerosing panencephalitis (SSPE). J Neuroimmunol. 1988 Oct;19(4):339–352. doi: 10.1016/0165-5728(88)90014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinami R. S., Oldstone M. B. Alterations in expression of measles virus polypeptides by antibody: molecular events in antibody-induced antigenic modulation. J Immunol. 1980 Jul;125(1):78–85. [PubMed] [Google Scholar]
- Fujinami R. S., Oldstone M. B. Antiviral antibody reacting on the plasma membrane alters measles virus expression inside the cell. Nature. 1979 Jun 7;279(5713):529–530. doi: 10.1038/279529a0. [DOI] [PubMed] [Google Scholar]
- Kobune K., Kobune F., Yamanouchi K., Nagashima K., Yoshikawa Y., Hayami M. Neurovirulence of rat brain-adapted measles virus. Jpn J Exp Med. 1983 Jun;53(3):177–180. [PubMed] [Google Scholar]
- Liebert U. G., Baczko K., Budka H., ter Meulen V. Restricted expression of measles virus proteins in brains from cases of subacute sclerosing panencephalitis. J Gen Virol. 1986 Nov;67(Pt 11):2435–2444. doi: 10.1099/0022-1317-67-11-2435. [DOI] [PubMed] [Google Scholar]
- Liebert U. G., ter Meulen V. Virological aspects of measles virus-induced encephalomyelitis in Lewis and BN rats. J Gen Virol. 1987 Jun;68(Pt 6):1715–1722. doi: 10.1099/0022-1317-68-6-1715. [DOI] [PubMed] [Google Scholar]
- Miller C. A., Carrigan D. R. Reversible repression and activation of measles virus infection in neural cells. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1629–1633. doi: 10.1073/pnas.79.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby E., Chen S. N., Togashi T., Shesberadaran H., Johnson K. P. Five measles virus antigens demonstrated by use of mouse hybridoma antibodies in productively infected tissue culture cells. Arch Virol. 1982;71(1):1–11. doi: 10.1007/BF01315171. [DOI] [PubMed] [Google Scholar]
- Rammohan K. W., McFarland H. F., McFarlin D. E. Induction of subacute murine measles encephalitis by monoclonal antibody to virus haemagglutinin. Nature. 1981 Apr 16;290(5807):588–589. doi: 10.1038/290588a0. [DOI] [PubMed] [Google Scholar]
- Schneider-Schaulies S., Liebert U. G., Baczko K., Cattaneo R., Billeter M., ter Meulen V. Restriction of measles virus gene expression in acute and subacute encephalitis of Lewis rats. Virology. 1989 Aug;171(2):525–534. doi: 10.1016/0042-6822(89)90622-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmann-Dorsch C., Koschel K. Coupling of viral membrane proteins to phosphatidylinositide signalling system. FEBS Lett. 1989 Apr 24;247(2):185–188. doi: 10.1016/0014-5793(89)81330-4. [DOI] [PubMed] [Google Scholar]
- Wolinsky J. S., Waxham M. N., Server A. C. Protective effects of glycoprotein-specific monoclonal antibodies on the course of experimental mumps virus meningoencephalitis. J Virol. 1985 Mar;53(3):727–734. doi: 10.1128/jvi.53.3.727-734.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa Y., Yamanouchi K. Effect of papaverine treatment on replication of measles virus in human neural and nonneural cells. J Virol. 1984 May;50(2):489–496. doi: 10.1128/jvi.50.2.489-496.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Meulen V., Carter M. J. Measles virus persistency and disease. Prog Med Virol. 1984;30:44–61. [PubMed] [Google Scholar]