Abstract
Fatigue is the most common and distressing symptom reported by patients undergoing radiation therapy (RT). However, limited information is available on the trajectories of fatigue, as well as on the predictors of inter-individual variability in fatigue. This study evaluated a sample of patients who underwent RT for prostate cancer to examine how ratings of evening and morning fatigue changed from the time of simulation to four months after the completion of RT and to investigate whether specific patient, disease, and symptom characteristics predicted the initial levels of fatigue and/or characteristics of the trajectories of evening and morning fatigue. Using hierarchical linear modeling (HLM), a large amount of inter-individual variability was demonstrated in the trajectories of evening and morning fatigue. Findings from this study suggest that younger men with a higher level of fatigue at the time of the simulation visit were at increased risk for higher levels of evening and morning fatigue over the course of RT. In addition, the level of morning fatigue over the course of RT appears to depend on the patient’s level of depression at the time of the simulation visit. In future studies, the use of HLM as an analytic tool will assist in the identification of patients who are most at risk for prolonged fatigue trajectories. This type of analysis may lead to the identification of subgroups of patients who are at higher risk for negative outcomes and who require different types of interventions for the fatigue associated with RT.
Keywords: Fatigue, prostate cancer, radiation therapy, hierarchical linear modeling, symptom patterns, symptom trajectories, sleep disturbance, depression
Introduction
Fatigue is one of the most common and distressing symptoms reported by patients receiving radiation therapy (RT) for cancer (1–3). Early cross-sectional studies reported prevalence rates for fatigue that ranged from 31% to 100%, with a mean of 78% (4–8). These studies also demonstrate that fatigue associated with RT has deleterious effects on patients’ functional status and quality of life (QOL) (8–10).
While research on fatigue has become increasingly sophisticated, few longitudinal studies have assessed fatigue in patients before and after RT. In addition, as noted by Bower et al. (11), considerable variability exists in fatigue levels both during and after treatment, which suggests that some patients may experience a slower (or faster) course of recovery. Given this observation, Bower et al. suggested that longitudinal studies that assess patients before, during, and after cancer treatment are required to determine more accurately the prevalence of fatigue and to identify factors that predict inter-individual differences in trajectories of fatigue. These types of studies require the use of valid and reliable assessment tools, as well as more sophisticated statistical methods that take repeated measures over time into account. This approach would enable the identification of patients who are at greatest risk for severe and enduring fatigue based on the dose and duration of therapy. In addition, the determination of predictors of inter-individual differences in fatigue trajectories may provide information on the underlying mechanisms for fatigue associated with RT as well as guide the development of more effective interventions.
The present study was conducted in a sample of patients who underwent RT for prostate cancer. Its aims were to examine how ratings of evening and morning fatigue changed from the time of simulation to four months after the completion of RT and to investigate whether specific patient, disease, and symptom characteristics predicted the initial levels of fatigue and/or characteristics of the trajectories of evening and morning fatigue. These analyses were conducted using one of the more sophisticated statistical methods, namely hierarchical linear modeling (HLM).
Methods
Participants and Settings
This descriptive, longitudinal study recruited 82 men with prostate cancer who met the following inclusion criteria: adults (> 18 years of age); able to read, write, and understand English; Karnofsky Performance Status (KPS) Score of ≥ 60; and scheduled to receive primary or adjuvant RT. Patients were excluded if they had metastatic disease; had more than one cancer diagnosis; or had a diagnosed sleep disorder. They were recruited from RT departments located in a Comprehensive Cancer Center and a community-based oncology program. The study was approved by the Human Subjects Committee at the University of California, San Francisco and at the second study site.
One hundred and eighty-eight patients were approached and 82 consented to participate (43.6% response rate). The major reasons for refusal were being too overwhelmed with their cancer experience or too busy. No differences were found in any of the demographic or disease characteristics between patients who did and did not choose to participate in this study.
Instruments
The study instruments included a demographic questionnaire, the KPS scale (12), the Lee Fatigue Scale (LFS) (13), the General Sleep Disturbance Scale (GSDS) (14), the Center for Epidemiological Studies-Depression Scale (CES-D) (15), the Spielberg State-Trait Anxiety Inventories (STAI-S and STAI-T) (16), and a descriptive numeric rating scale (NRS) for worst pain intensity (17).
The demographic questionnaire provided information on age, marital status, years of education, living arrangements, ethnicity, and employment status. In addition, patients completed a checklist of co-morbidities.
Fatigue severity was measured using the 13-item LFS. Each item is rated using a 0 to 10 numeric rating scale (NRS) and a total score is calculated as the mean of the 13 items that can range from 0 to 10, with higher scores indicating higher levels of fatigue severity. Respondents were asked to rate each item based on how they felt “right now,” within 30 minutes of awakening (i.e., morning fatigue) and prior to bed (i.e., evening fatigue). The LFS has been used with healthy individuals as well as in patients with cancer and HIV (10,18,19). It was chosen for the current study because it is relatively short and easy to administer. The LFS has well established validity and reliability (13,20). In this sample, the Cronbach’s alphas for the LFS for evening and morning ratings were 0.95 and 0.96, respectively.
The GSDS consists of 21 items that evaluate various aspects of sleep disturbance. Each item was rated on a NRS that ranged from 0 (never) to 7 (every day), and the 21 items were summed to yield a total score that could range from 0 (no disturbance) to 147 (extreme sleep disturbance). The GSDS has well established validity and reliability in shift workers, pregnant women, and patients with cancer and HIV (10,14,18,21). In the current study, the Cronbach’s alpha for the GSDS total score was 0.81.
The CES-D consists of 20 items selected to represent the major symptoms in the clinical syndrome of depression. Scores can range from 0 to 60, with scores ≥ 16 indicating the need for individuals to seek clinical evaluation for major depression. The CES-D has well-established concurrent and construct validity (15,22–23). In the current study, the Cronbach’s alpha for the CES-D was 0.83.
The STAI-T and STAI-S inventories consist of 20 items each that are rated from 1 to 4. The scores for each scale are summed and can range from 20 to 80. A higher score indicates greater anxiety. The STAI-T measures an individual’s predisposition to anxiety determined by his/her personality and estimates how a person feels generally. The STAI-S measures an individual’s transitory emotional response to a stressful situation. It evaluates the emotional response of worry, nervousness, tension, and feelings of apprehension related to how people feel “right now” in a stressful situation. The STAI-S and STAI-T inventories have well-established criterion and construct validity, and internal consistency reliability coefficients (16,24–25). In this study, the Cronbach’s alphas for the STAI-T and the STAI-S were 0.86 and 0.91, respectively.
Worst pain intensity was evaluated using a descriptive NRS that ranged from 0 (no pain) to 10 (excruciating pain). A descriptive numeric rating scale is a valid and reliable measure of pain intensity (17). Because the majority of the patients did not have pain (74.4%), for the subsequent longitudinal analyses, pain was recoded as present or absent.
Study Procedures
At the time of the simulation visit (i.e., approximately one week prior to the start of RT), patients were approached by a research nurse to discuss participation in the study. After obtaining written informed consent, they were asked to complete the baseline study questionnaires. Patients were taught to complete the LFS before going to bed each night (i.e., evening fatigue) and upon arising each morning (i.e., morning fatigue) for two consecutive days. Assessments were done at the time of the simulation visit (i.e., baseline), weekly during the course of RT, every two weeks for two months, and once a month for two months following the completion of RT. The majority of the patients completed 16 assessments.
Data Analysis
Descriptive statistics and frequency distributions were generated on the sample characteristics and baseline symptom severity scores using SPSS™ Version 14.0. For each of the 16 assessments, a mean score for each of the LFSs (i.e., evening and morning) was calculated for use in the subsequent statistical analyses.
HLM, based on full maximum likelihood estimation, was done using the software developed by Raudenbush and colleagues (26). The repeated measures of fatigue were conceptualized as being nested within individuals. Compared with other methods of analyzing change, HLM has two major advantages. First, HLM can accommodate unbalanced designs which allows for the analysis of data when the number and the spacing of the assessments vary across respondents. Although every patient was to be assessed on a pre-specified schedule, the actual number of assessments was not the same for all of the patients because some patients had longer periods of RT and some had scheduling conflicts. Second, HLM has the ability to model individual change, which helps to identify more complex patterns of change that are often overlooked by other methods (26,27).
With HLM, the repeated measures of the outcome variables (i.e., evening and morning fatigue) are nested within individuals and the analysis of change in fatigue scores has two levels: within persons (Level 1) and between persons (Level 2). At Level 1, the outcome is conceptualized as varying within individuals and is a function of person-specific change parameters plus error. At Level 2, these person-specific change parameters are multivariate outcomes that vary across individuals. These Level 2 outcomes can be modeled as a function of demographic or clinical characteristics that vary between individuals, plus an error associated with the individual. Combining Level 1 with Level 2 results in a mixed model with fixed and random effects (26,28,29).
Separate HLM analyses were done to evaluate changes over time in ratings of evening and morning fatigue. Each HLM analysis proceeded in two stages. First, intra-individual variability in fatigue over time was examined. In this study, time in weeks, refers to the length of time from the simulation visit to four months after the completion of RT (i.e., six months with a total of 16 assessments). Three Level 1 models, which represented that the patients’ fatigue levels (a) did not change over time (i.e., no time effect), (b) changed at a constant rate (i.e., linear time effect), and (c) changed at a rate that accelerates or decelerates over time (i.e., quadratic effect) were compared. At this point, the Level 2 model was constrained to be unconditional (i.e., no predictors) and likelihood ratio tests were used to determine the best model. These analyses answered the first research question and identified the change parameters that best described individual changes in evening and morning fatigue over time.
The second stage of the HLM analysis, which answered the second research question, examined inter-individual differences in the trajectories of evening and morning fatigue by modeling the individual change parameters (i.e., intercept, linear and quadratic slopes) as a function of proposed predictors at Level 2. Table 1 presents a list of the proposed predictors that was developed based on a review of the literature of fatigue in men with prostate cancer who underwent RT. To improve estimation efficiency and construct a model that was parsimonious, an exploratory Level 2 analysis was done in which each potential predictor was assessed to see if it would result in a better fitting model if it alone was added as a Level 2 predictor. Predictors with a t-value of < 2.0, which indicates a lack of a significant effect, were dropped from subsequent model testing. All of the potentially significant predictors from the exploratory analyses were entered into the model to predict each individual change parameter. Only predictors that maintained a significant contribution in conjunction with other variables were retained in the final model. A P-value of <0.05 indicates statistical significance.
Table 1.
Potential Predictors of Intercept, Linear Coefficient, and Quadratic Coefficient for Evening and Morning Fatigue
| Potential Predictors | Evening Fatigue | Morning Fatigue | ||||
|---|---|---|---|---|---|---|
| Intercept | Linear Coefficient | Quadratic Coefficient | Intercept | Linear Coefficient | Quadratic Coefficient | |
| Person | ||||||
| Age | ■ | ■ | ■ | ■ | ■ | |
| Lives alone | ||||||
| Marital status | ||||||
| Education | ■ | ■ | ||||
| Employment status | ■ | ■ | ■ | ■ | ■ | ■ |
| Disease and Treatment | ||||||
| KPS Score | ■ | ■ | ■ | ■ | ||
| Number of comorbidities | ||||||
| Pretreatment PSA | ||||||
| Gleason score | ||||||
| Total dose of RT received | ||||||
| Hormonal therapy prior to RT | ||||||
| Symptoms | ||||||
| Baseline GSDS score | ■ | ■ | ■ | ■ | ■ | ■ |
| Baseline CES-D | ■ | ■ | ■ | ■ | ■ | ■ |
| Presence of pain at baseline | ||||||
| Baseline trait anxiety score | ■ | ■ | ■ | ■ | ■ | ■ |
| Baseline state anxiety score | ■ | ■ | ■ | ■ | ■ | |
■ = From exploratory analyses had a t-value > 2.00
Abbreviations: CES-D = Center for Epidemiologic Studies- Depression; GSDS -= General Sleep Disturbance Score; KPS = Karnofsky Performance Status, RT = Radiation Therapy
Results
Patient Characteristics and Symptom Severity Scores
The demographic, disease, and treatment characteristics of the 82 patients are presented in Table 2. These men with prostate cancer were approximately 67 years of age, were well educated, and had a KPS score of 95.7. Most of the patients were married or partnered (69.5%), White (76.8%), and not employed (53.7%). The distribution of clinical stage was 48.8% with T1, 42.5% with T2, and 8.8% with T3. Over 50% of the patients received hormonal therapy prior to the initiation of RT. The mean symptom severity scores for the 82 patients, at the time of the simulation visit, are listed in Table 2.
Table 2.
Demographic, Disease, and Treatment Characteristics of the Patients (n=82)
| Characteristic | Mean (Standard Deviation) |
|---|---|
| Age (years) | 67.1 (7.8) |
| Education (years) | 16.0 (3.2) |
| Karnofsky Performance Status Score | 95.7 (6.9) |
| Number of comorbidities | 4.6 (2.5) |
| Lives alone | 23.2% |
| Marital status | |
| Married/partnered | 69.5% |
| Divorced/separated | 13.4% |
| Other | 17.1% |
| Ethnicity | |
| Black | 18.3% |
| White | 76.8% |
| Other | 4.9% |
| Employed | |
| Yes | 46.3% |
| No | 53.7% |
| Pre-treatment PSA level (nanograms/milliliter) | 10.9 (7.9) |
| Gleason score | |
| 5 or 6 | 39.0% |
| 7 | 47.7% |
| ≥ 8 | 13.4% |
| Gleason score | 6.8 (0.9) |
| Clinical stage | |
| T1 | 48.8% |
| T2 | 42.5% |
| T3 | 8.8% |
| Prostatectomy prior to RT | 9.8% |
| Hormonal therapy prior to RT | 51.2% |
| RT treatment plan | |
| Whole pelvis + conformal boost after surgery | 9.8% |
| Whole pelvis + conformal boost | 75.6% |
| Whole pelvis + high dose RT | 4.9% |
| Whole pelvis + seed implant | 9.8% |
| Total dose of RT (cGys) | 6902 (958.2) |
| Mean symptom severity scores at baseline | |
| LFS score for evening fatigue | 3.5 (2.1) |
| LFS score for morning fatigue | 1.8 (1.8) |
| GSDS score | 33.4 (16.3) |
| CES-D score | 5.9 (5.7) |
| Trait Anxiety Inventory score | 31.3 (7.9) |
| State Anxiety Inventory score | 27.8 (7.8) |
Abbreviations: CES-D = Center for Epidemiologic Studies Depression Scale, GSDS = General Sleep Disturbance Scale, LFS = Lee Fatigue Scale, PSA = Prostate Specific Antigen, RT = radiation therapy
Individual and Mean Change in Evening and Morning Fatigue
The first HLM analyses examined how evening and morning levels of fatigue changed from the time of the simulation visit to four months after the completion of RT. Two models were estimated in which the function of time was linear and quadratic. For both evening fatigue (Deviance χ2,4 = 69.55) and morning fatigue (Deviance χ2,4 = 70.41), the goodness-of-fit tests of the deviance between the linear and quadratic models indicated that a quadratic model fit the data significantly better than a linear model (both P < 0.0001).
Evening Fatigue
The estimates of the quadratic change model are presented in Table 3 (unconditional model). Because the model had no covariates (i.e., unconditional), the intercept represents the estimated amount of evening fatigue (i.e., 3.6 on a 0 to 10 scale) at the time of the simulation visit. The estimated linear rate of change in evening fatigue, for each additional week, was 0.078 (P<0.0001) and the estimated quadratic rate of change per week was −0.003 (P<0.0001). It is important to remember that it is the weighted combination of the linear and quadratic terms that define each curve. Figure 1 displays the trajectory for evening fatigue from the time of the simulation visit to four months after the completion of RT. Evening fatigue increased over the course of RT (i.e., weeks 1 to 9) and then declined after the completion of RT. It should be noted that the mean fatigue scores for the various groups depicted in all of the figures are estimated or predicted means based on the HLM analyses.
Table 3.
Hierarchical Linear Models of Evening and Morning Fatigue
| Evening Fatigue | Coefficient (SE) | ||
|---|---|---|---|
| Variable | Unconditional Model | Final Model | |
| Fixed Effects | |||
| Intercept | 3.623 (0.235)* | 3.623 (0.196)* | |
| Timea (linear rate of change) | 0.078 (0.021)* | 0.079 (0.022)** | |
| Time2 (quadratic rate of change) | −0.003 (0.0001)* | −0.003 (0.001)* | |
| Time invariant covariates | |||
| Intercept: | Age | −0.052 (0.020)+ | |
| Baseline GSDS | 0.064 (0.012)* | ||
| Linear: | Baseline evening fatigue × time | −0.050 (0.011)* | |
| Baseline GSDS × time | 0.004 (0.002)++ | ||
| Quadratic: | Baseline evening fatigue × time2 | 0.003 (0.001)* | |
| Baseline GSDS × time2 | −0.0002 (0.001)** | ||
| Variance components | |||
| In intercept | 4.247* | 2.864* | |
| In linear rate | 0.021* | 0.025* | |
| In quadratic fit | 0.00003* | 0.00004* | |
| Goodness-of-fit deviance(parameters estimated) | 3613.973 (10) | 3555.958 (16) | |
| Model comparison (χ2 [df]) | 58.015 (6) | ||
| Morning Fatigue | Coefficient (SE) | ||
| Variable | Unconditional Model | Final Model | |
| Fixed Effects | |||
| Intercept | 2.044 (0.179)* | 2.047 (0.123)* | |
| Timea (linear rate of change) | 0.035 (0.019) | 0.035 (0.018) | |
| Time2 (quadratic rate of change) | −0.003 (0.001)** | −0.003 (0.001)* | |
| Time invariant covariates | |||
| Intercept: | Age | −0.042 (0.015)+ | |
| Baseline GSDS | 0.039 (0.009)* | ||
| Baseline CES-D | 0.094 (0.027)** | ||
| Linear: | Baseline morning fatigue × time | −0.036 (0.012)+ | |
| Baseline CES-D × time | 0.009 (0.004)++ | ||
| Quadratic: | Baseline morning fatigue × time2 | 0.001 (0.0005)+ | |
| Baseline CES-D × time2 | −0.0004 (0.0002)+ | ||
| Variance components | |||
| In intercept | 2.380* | 0.996* | |
| In linear rate | 0.017* | 0.016* | |
| In quadratic fit | 0.00002* | 0.0002* | |
| Goodness-of-fit deviance(parameters estimated) | 3398.231 (10) | 3331.428 (17) | |
| Model comparison (χ2 [df]) | 66.803 (7) | ||
p < 0.0001
p = 0.001
p = 0.01
p = 0.02
Time was coded 0 at the time of the simulation visit
Figure 1.
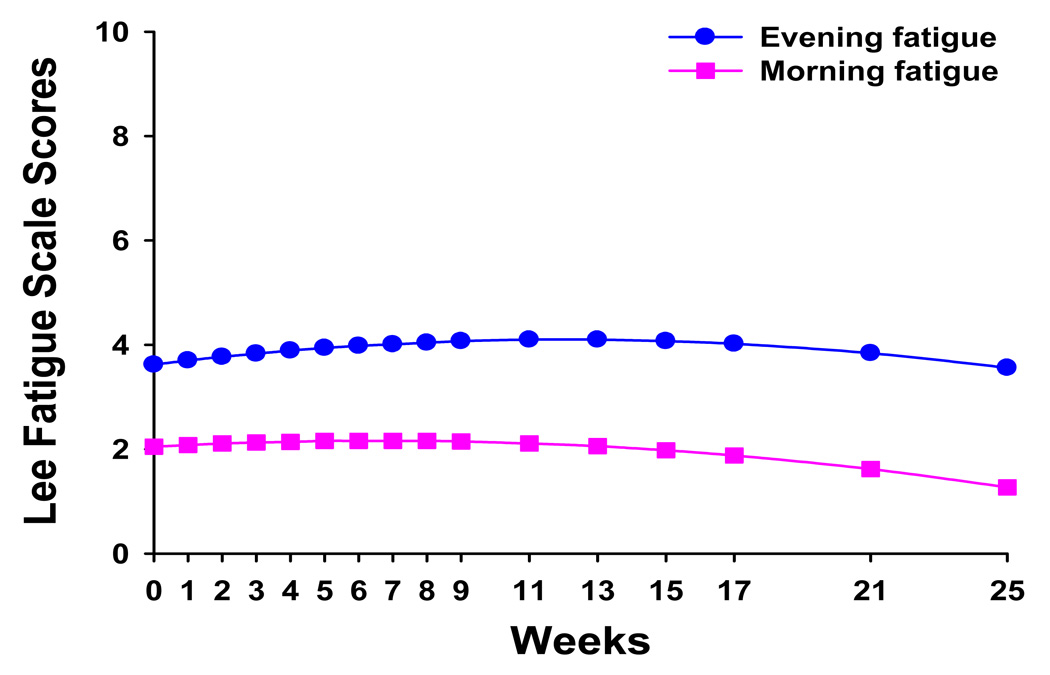
Trajectories of evening and morning fatigue over the 25 weeks of the study.
Morning Fatigue
As shown in Table 3, in the unconditional model, the intercept represents the estimated amount of morning fatigue (i.e., 2.0) at the time of the simulation visit. The estimated linear rate of change in morning fatigue, for each additional week, was 0.035 (P<0.0001) and the estimated quadratic rate of change per week was −0.003 (P<0.001). Figure 1 displays the trajectory for morning fatigue from the time of the simulation visit to four months after the completion of RT. Morning fatigue increased over the course of RT and then declined after the completion of RT.
Although the results indicate a sample-wide increase followed by a decrease in evening and morning fatigue, they do not imply that all patients exhibited the same trajectory. The variance in individual change parameters estimated by the models (i.e., variance components, Table 3), suggested that substantial inter-individual differences existed in the trajectories of evening and morning fatigue; which are illustrated in Figure 2. These results suggested that further examinations of inter-individual differences in the individual change parameters were warranted.
Figure 2.
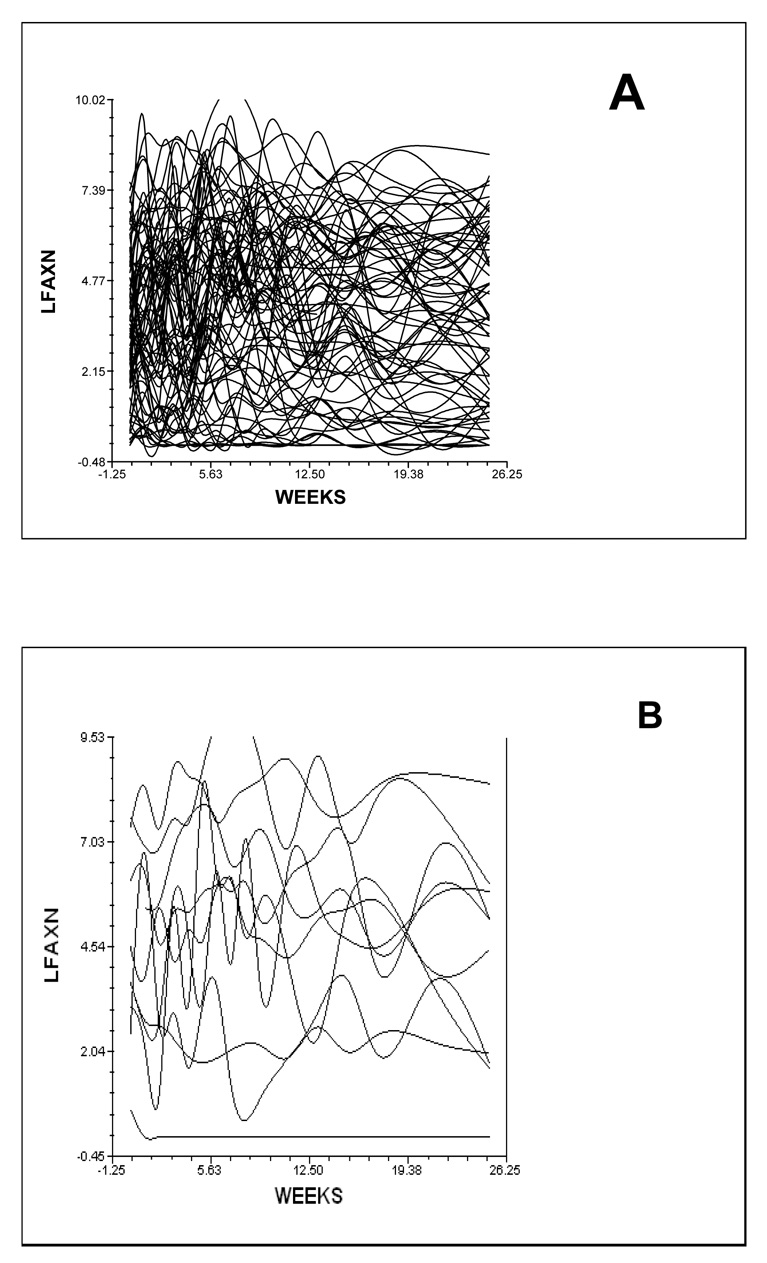
Spaghetti plot of the 82 patients’ individual trajectories of evening fatigue (A) and a plot of ten individual evening fatigue trajectories (B) over the 25 weeks of the study.
Inter-Individual Differences in the Trajectories of Evening and Morning Fatigue
The second stage of the HLM analyses tested the hypothesis that the pattern of change over time in evening and morning fatigue varied based on specific person, disease, treatment, and symptom variables that were found to influence fatigue levels of patients who underwent RT for prostate cancer. Exploratory analyses were done with each of the potential predictors listed in Table 1. To improve estimation efficiency and construct models that were parsimonious, exploratory Level 2 analyses were done in which each potential predictor was assessed to see if it would result in a better fitting model if it alone was added as a Level 2 predictor. Predictors with a t-value of < 2.0, indicating lack of a significant effect, were dropped from subsequent model testing. All of the significant predictors from the exploratory analyses were entered into the models to predict each individual change parameter. Only predictors that maintained a significant contribution in conjunction with other variables were retained in the final models.
Evening Fatigue
As shown in the final model in Table 3, the two variables that predicted inter-individual differences in the intercept for evening fatigue were age and baseline level of sleep disturbance (i.e., baseline GSDS score). Baseline evening fatigue was entered in Level 2 as a predictor of the slope parameters to control for intra-individual differences in evening fatigue at baseline. The two variables that predicted inter-individual differences in the slope parameters for evening fatigue were baseline level of evening fatigue and baseline GSDS score.
To illustrate the effects of the three different predictors on patients’ trajectories of evening fatigue, Figure 3 displays the adjusted change curves of evening fatigue that were estimated based on differences in age (i.e., younger/older calculated based on one standard deviation above and below the mean age of the patients), baseline level of evening fatigue (i.e., low fatigue/high fatigue calculated based on one standard deviation above and below the mean baseline evening LFS score), and baseline level of sleep disturbance (i.e. low sleep/high sleep calculated based on one standard deviation above and below the mean baseline GSDS score).
Figure 3.
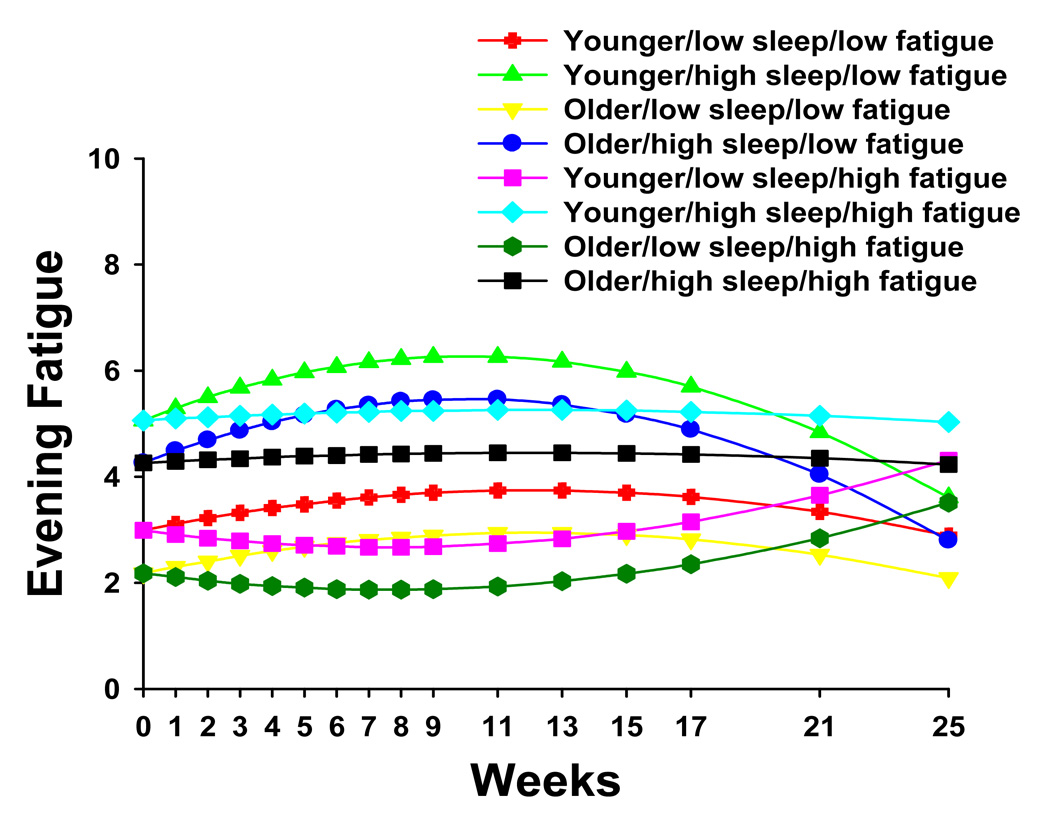
Trajectories of evening fatigue by age (i.e., younger/older), level of sleep disturbance at baseline (i.e., low sleep/high sleep), and level of evening fatigue at baseline (i.e., low fatigue/high fatigue).
Morning Fatigue
As shown in the final model in Table 3, the three variables that predicted inter-individual differences in the intercept for morning fatigue were age, baseline GSDS score, and baseline CES-D score. Baseline morning fatigue was entered in Level 2 as a predictor of the slope parameters to control for intra-individual differences in morning fatigue at baseline. The two variables that predicted inter-individual differences in the slope parameters for morning fatigue were baseline level of morning fatigue and baseline CES-D score.
To illustrate the effects of the different predictors on patients’ trajectories of morning fatigue, Figure 4 and Figure 5 display the adjusted change curves of morning fatigue that were estimated based on differences in baseline levels of depression (i.e., low CES-D and high CES-D respectively, calculated based on one standard deviation above and below the mean baseline CES-D score) as well as age (younger/older calculated based on one standard deviation above and below the mean age of the patients), baseline level of morning fatigue (i.e., low fatigue/high fatigue calculated based on one standard deviation above and below the mean baseline morning LFS score), and baseline level of sleep disturbance (i.e. low sleep/high sleep calculated based on one standard deviation above and below the mean GSDS score). Rather than place sixteen trajectories on a single plot, two figures were made to illustrate differences in morning fatigue trajectories based on low (Figure 4) and high (Figure 5) CES-D scores.
Figure 4.
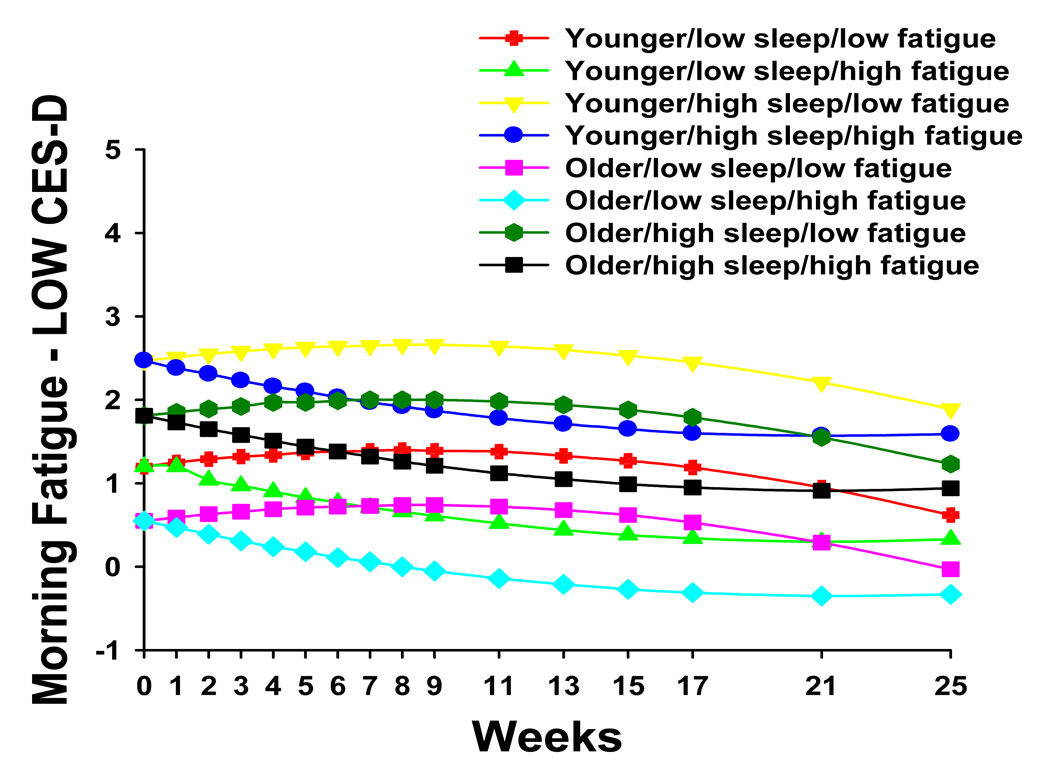
Trajectories of morning fatigue by lower level of depression at baseline (i.e., LOW CES-D) and by age (i.e., younger/older), level of sleep disturbance at baseline (i.e., low sleep/high sleep), and level of morning fatigue at baseline (i.e., low fatigue/high fatigue)
Figure 5.
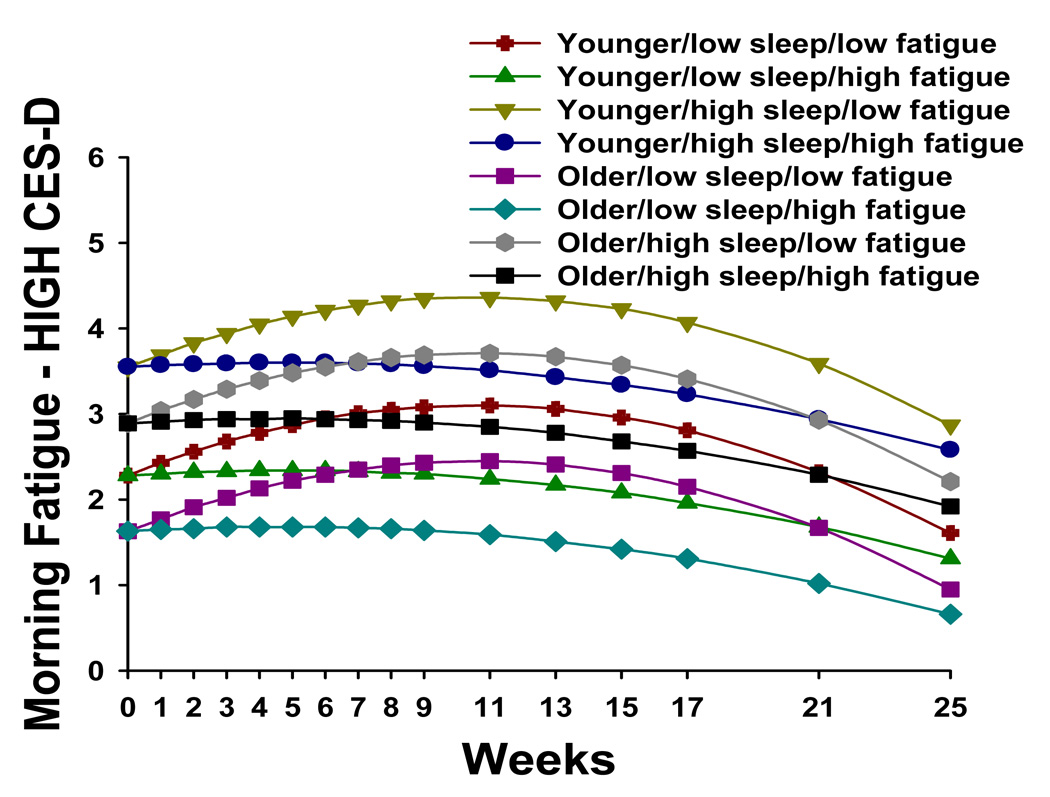
Trajectories of morning fatigue by higher level of depression at baseline (i.e., HIGH CES-D) and by age (i.e., younger/older), level of sleep disturbance at baseline (i.e., low sleep/high sleep), and level of morning fatigue at baseline (i.e., low fatigue/high fatigue)
Discussion
This study is the first to evaluate for inter-individual differences in evening and morning fatigue trajectories before, during, and after RT in men with prostate cancer using more sophisticated statistical methods (i.e., HLM). In addition, this study is the first to determine the predictors for these inter-individual differences in trajectories of evening and morning fatigue. Consistent with other longitudinal studies of men with prostate cancer (3,30–32), based on the unconditional model, the severity of both evening and morning fatigue increased during RT and then decreased following the completion of RT. Of note, for both evening and morning fatigue, the incremental weekly increases from the beginning of to the completion of RT (i.e., nine weeks) were relatively small (i.e., 0.078 and 0.035 points per week, respectively) for the sample overall. For evening fatigue, this value equates with approximately a one point increase in fatigue severity from the beginning of to the end of RT, which is higher than that reported by Choo et al. (31), but lower than that reported by Monga et al. (30). The differences in fatigue among these three studies may relate to the instruments that were used to measure fatigue, the timing of the measurements, or to differences in sample characteristics.
While the incremental increases in evening and morning fatigue for the sample as a whole were modest, the use of HLM, compared to the more traditional statistical approaches that are used to evaluate for changes over time in some dependent variable (e.g., repeated measures analysis of variance), provided evidence of a large amount of inter-individual variability in the trajectories of evening and morning fatigue in these men with prostate cancer. In addition, the HLM analyses provide some insights into which of these patients were at increased risk for more severe and prolonged fatigue trajectories.
In terms of evening fatigue, estimated mean evening fatigue scores at the time of the simulation visit ranged from 2.2 to 5.1 (actual scores ranged from 0 to 7.6), which are in the mild to moderate range (11). Based on the HLM analysis, younger men and men with higher levels of sleep disturbance reported higher levels of evening fatigue at the time of the simulation visit. In addition, because HLM has the ability to determine predictors, not only of the intercept but of the slopes of the trajectories of evening fatigue, eight different evening fatigue trajectories were generated based on meaningful values (i.e., ±1 standard deviation) for the various predictors of the intercept and slopes. As shown in Figure 3, four of these evening fatigue trajectories increased over the course of RT and then decreased following the completion of RT (i.e., week 9). These four trajectories were characterized by lower levels of evening fatigue at baseline. Two trajectories remained relatively constant across the six months of the study and were characterized by higher levels of both evening fatigue and sleep disturbance at baseline. Finally, two trajectories decreased over the course of RT and then increased following the completion of RT. These trajectories were characterized by lower levels of sleep disturbance and higher levels of fatigue at the time of the simulation visit. While the findings from this study warrant replication, they suggest that the risk factors for higher levels of evening fatigue in men who undergo RT for prostate cancer include younger age, as well as higher levels of baseline fatigue and sleep disturbance. In addition, these findings suggest that HLM may be a useful statistical approach to determine patient characteristics that can be used to identify patients who are most at risk for prolonged and severe fatigue trajectories.
No cross-sectional or longitudinal studies of fatigue associated with RT have distinguished between ratings of the severity of evening and morning fatigue. However, consistent with work by Lee et al. (13,19), patients’ ratings of evening fatigue were higher than their ratings of morning fatigue. As illustrated in Figure 4 and Figure 5, estimated mean morning fatigue scores at the time of the simulation visit ranged from 0.55 to 3.55 (actual scores ranged from 0 to 7.38). These morning fatigue scores are in the mild to moderate range (11).
As with evening fatigue, patients’ age and their baseline level of sleep disturbance were predictors of their baseline levels of morning fatigue. In contrast, depression scores at the time of the simulation visit predicted variability in baseline levels, as well as in the trajectories, of morning fatigue. That baseline levels of depression predicted variability in the trajectories of morning but not evening fatigue is consistent with the fact that a complaint of morning fatigue is one of the major diagnostic criteria for clinical depression (33,34). In addition, these findings of differences in the predictors and trajectories of evening and morning fatigue suggest that both of these fatigue measures should be assessed in future studies of cancer-related fatigue. This approach may provide insights into the underlying mechanisms of cancer-related fatigue, as well as provide direction for the development of intervention studies for this significant clinical problem.
As shown in Figure 4, for patients with lower CES-D scores at baseline, estimated mean morning fatigue scores at the time of the simulation visit (i.e., intercept) ranged from 0.55 to 2.47. In contrast, as shown in Figure 5, for patients with higher CES-D scores at baseline, estimated mean morning fatigue scores at the time of the simulation visit ranged from 1.63 to 3.55. This finding suggests that patients with higher levels of depression are at greater risk for higher levels of morning fatigue at the start of RT.
In addition, when the patients with higher levels of depression at baseline (i.e., eight groups in Figure 5) were compared with the patients with lower levels of depression at baseline (i.e., eight groups in Figure 4), all of the morning fatigue trajectories were consistently higher in these groups. As shown in Figure 4, in patients with lower levels of depression at baseline, four of the morning fatigue trajectories increased and then decreased over the course of RT. These four trajectories were characterized by lower levels of morning fatigue at baseline. The other four morning fatigue trajectories decreased slightly over the course of RT and were characterized by higher levels of morning fatigue at baseline.
In a similar fashion, as shown in Figure 5, in those patients with higher levels of depression at baseline, the four morning fatigue trajectories with the largest increases followed by decreases in fatigue were characterized by lower levels of morning fatigue at baseline. It should be noted that in this sample of men with prostate cancer, mean CES-D scores were low (i.e., 5.9 ± 5.7) with only 7.3% of the sample having CES-D scores of ≥ 16. However, the association between depression and morning fatigue suggests that this symptom warrants clinical evaluation in these patients.
One needs to remember throughout this discussion that the mean fatigue scores for the various groups depicted in all of the figures are estimated or predicted means based on the HLM analyses. That said, taken together, these analyses of the trajectories of evening and morning fatigue suggest that younger men with a higher level of fatigue at the time of the simulation visit are at increased risk for higher levels of evening and morning fatigue over the course of RT. In addition, since sleep disturbance was a predictor of both evening and morning fatigue, this symptom needs to be evaluated routinely in patients who will initiate RT. Finally, the level of morning fatigue over the course of RT appears to depend on the patient’s level of depression at the time of the simulation visit and warrants clinical evaluation. These findings warrant replication before definitive conclusions can be made about the impact of these predictors on evening and morning fatigue trajectories of men undergoing RT for prostate cancer.
It should be noted that, consistent with previous reports (3,32), none of the disease or treatment characteristics (i.e., KPS score, number of comorbidities, pretreatment PSA, Gleason score, total dose of RT received, hormonal therapy prior to RT) were predictors of initial levels of evening or morning fatigue, or of the various fatigue trajectories. This finding may be due to the relatively homogeneous characteristics of this sample.
Limitations of this study include the relatively small sample size, the single cancer diagnosis, and the single gender. Therefore, the findings from this study cannot be generalized to cancer patients with different cancer diagnoses who undergo RT, or to female patients. While the refusal rate for this study was relatively high (43.6%), it should be noted that the major reasons for refusal were being overwhelmed or too busy. One can speculate that the patients who refused may have been more fatigued and that the data presented in this paper are conservative estimates of the trajectories of evening and morning fatigue.
In summary, findings from this study suggest that HLM is a useful statistical approach to analyze longitudinal data on symptoms. This statistical approach provides evidence of a large amount of inter-individual variability in the trajectories of evening and morning fatigue before, during, and following RT for prostate cancer. The ability of HLM to identify predictors of inter-individual variability will assist in the identification of patients who are most at risk for prolonged fatigue trajectories and who may require different types of interventions for the fatigue associated with RT. In addition, these types of statistical analyses of longitudinal data may identify specific patient characteristics that will need to be considered as potential covariates or stratification criteria in the design of intervention studies for cancer-related fatigue.
Acknowledgments
The assistance of the research nurses on the project, Carol Maroten and Mary Cullen, and the support from the physicians and nurses at the study sites were greatly appreciated.
This research was supported by a grant from the National Institute of Nursing Research (NR04835). Additional support for the corresponding author’s program of research was provided through unrestricted grants from Endo Pharmaceuticals, PriCara, Unit of Ortho-McNeil, Inc., and Purdue Pharma LP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lawrence DP, Kupelnick B, Miller K, Devine D, Lau J. Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients. J Natl Cancer Inst Monogr. 2004;32:40–50. doi: 10.1093/jncimonographs/lgh027. [DOI] [PubMed] [Google Scholar]
- 2.Jereczek-Fossa BA, Marsiglia HR, Orecchia R. Radiotherapy-related fatigue. Crit Rev Oncol Hematol. 2002;41(3):317–325. doi: 10.1016/s1040-8428(01)00143-3. [DOI] [PubMed] [Google Scholar]
- 3.Hickok JT, Roscoe JA, Morrow GR, et al. Frequency, severity, clinical course, and correlates of fatigue in 372 patients during 5 weeks of radiotherapy for cancer. Cancer. 2005;104(8):1772–1778. doi: 10.1002/cncr.21364. [DOI] [PubMed] [Google Scholar]
- 4.Greenberg DB, Sawicka J, Eisenthal S, Ross D. Fatigue syndrome due to localized radiation. J Pain Symptom Manage. 1992;7(1):38–45. doi: 10.1016/0885-3924(92)90106-r. [DOI] [PubMed] [Google Scholar]
- 5.Haylock PJ, Hart LK. Fatigue in patients receiving localized radiation. Cancer Nurs. 1979;2(6):461–467. [PubMed] [Google Scholar]
- 6.Irvine D, Vincent L, Graydon JE, Bubela N, Thompson L. The prevalence and correlates of fatigue in patients receiving treatment with chemotherapy and radiotherapy. A comparison with the fatigue experienced by healthy individuals. Cancer Nurs. 1994;17(5):367–378. [PubMed] [Google Scholar]
- 7.King KB, Nail LM, Kreamer K, Strohl RA, Johnson JE. Patients' descriptions of the experience of receiving radiation therapy. Oncol Nurs Forum. 1985;12(4):55–61. [PubMed] [Google Scholar]
- 8.Smets EM, Visser MR, Willems-Groot AF, et al. Fatigue and radiotherapy: (A) experience in patients undergoing treatment. Br J Cancer. 1998;78(7):899–906. doi: 10.1038/bjc.1998.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curt GA, Breitbart W, Cella D, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologist. 2000;5(5):353–360. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- 10.Miaskowski C, Lee KA. Pain, fatigue, and sleep disturbances in oncology outpatients receiving radiation therapy for bone metastasis: a pilot study. J Pain Symptom Manage. 1999;17(5):320–332. doi: 10.1016/s0885-3924(99)00008-1. [DOI] [PubMed] [Google Scholar]
- 11.Bower JE, Ganz PA, Desmond KA, et al. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18(4):743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 12.Karnofsky D. Performance scale. New York: Plenum Press; 1977. [Google Scholar]
- 13.Lee KA, Hicks G, Nino-Murcia G. Validity and reliability of a scale to assess fatigue. Psychiatry Res. 1991;36(3):291–298. doi: 10.1016/0165-1781(91)90027-m. [DOI] [PubMed] [Google Scholar]
- 14.Lee KA. Self-reported sleep disturbances in employed women. Sleep. 1992;15(6):493–498. doi: 10.1093/sleep/15.6.493. [DOI] [PubMed] [Google Scholar]
- 15.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Measure. 1977;1(3):385–401. [Google Scholar]
- 16.Spielberger CG, Gorsuch RL, Suchene R, Vagg PR, Jacobs GA. Manual for the State-Anxiety (Form Y): Self Evaluation Questionnaire. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 17.Jensen MP. The validity and reliability of pain measures in adults with cancer. J Pain. 2003;4(1):2–21. doi: 10.1054/jpai.2003.1. [DOI] [PubMed] [Google Scholar]
- 18.Lee KA, DeJoseph JF. Sleep disturbances, vitality, and fatigue among a select group of employed childbearing women. Birth. 1992;19(4):208–213. doi: 10.1111/j.1523-536x.1992.tb00404.x. [DOI] [PubMed] [Google Scholar]
- 19.Lee KA, Portillo CJ, Miramontes H. The fatigue experience for women with human immunodeficiency virus. J Obstet Gynecol Neonatal Nurs. 1999;28(2):193–200. doi: 10.1111/j.1552-6909.1999.tb01984.x. [DOI] [PubMed] [Google Scholar]
- 20.Lee KA, Lentz MJ, Taylor DL, Mitchell ES, Woods NF. Fatigue as a response to environmental demands in women's lives. Image J Nurs Sch. 1994;26(2):149–154. doi: 10.1111/j.1547-5069.1994.tb00935.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee KA, Portillo CJ, Miramontes H. The influence of sleep and activity patterns on fatigue in women with HIV/AIDS. J Assoc Nurses AIDS Care. 2001;12 Suppl:19–27. doi: 10.1177/105532901773742257. [DOI] [PubMed] [Google Scholar]
- 22.Sheehan TJ, Fifield J, Reisine S, Tennen H. The measurement structure of the Center for Epidemiologic Studies Depression Scale. J Pers Assess. 1995;64(3):507–521. doi: 10.1207/s15327752jpa6403_9. [DOI] [PubMed] [Google Scholar]
- 23.Carpenter JS, Andrykowski MA, Wilson J, et al. Psychometrics for two short forms of the Center for Epidemiologic Studies-Depression Scale. Issues Ment Health Nurs. 1998;19(5):481–494. doi: 10.1080/016128498248917. [DOI] [PubMed] [Google Scholar]
- 24.Bieling PJ, Antony MM, Swinson RP. The State-Trait Anxiety Inventory, Trait version: structure and content re-examined. Behav Res Ther. 1998;36(7–8):777–788. doi: 10.1016/s0005-7967(98)00023-0. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy BL, Schwab JJ, Morris RL, Beldia G. Assessment of state and trait anxiety in subjects with anxiety and depressive disorders. Psychiatr Q. 2001;72(3):263–276. doi: 10.1023/a:1010305200087. [DOI] [PubMed] [Google Scholar]
- 26.Raudenbush SW, Bryk AS. Hierarchical linear models: Application and data analysis methods. 2nd ed. Thousand Oaks: Sage Publications; 2002. [Google Scholar]
- 27.Raudenbush SW. Comparing personal trajectories and drawing causal inferences from longitudinal data. Annu Rev Psychol. 2001;52:501–525. doi: 10.1146/annurev.psych.52.1.501. [DOI] [PubMed] [Google Scholar]
- 28.Li LW. Longitudinal changes in the amount of informal care among publicly paid home care recipients. Gerontologist. 2005;45(4):465–473. doi: 10.1093/geront/45.4.465. [DOI] [PubMed] [Google Scholar]
- 29.Li LW. From caregiving to bereavement: trajectories of depressive symptoms among wife and daughter caregivers. J Gerontol B Psychol Sci Soc Sci. 2005;60(4):P190–P198. doi: 10.1093/geronb/60.4.p190. [DOI] [PubMed] [Google Scholar]
- 30.Monga U, Kerrigan AJ, Thornby J, Monga TN, Zimmermann KP. Longitudinal study of quality of life in patients with localized prostate cancer undergoing radiotherapy. J Rehabil Res Dev. 2005;42(3):391–399. doi: 10.1682/jrrd.2004.06.0071. [DOI] [PubMed] [Google Scholar]
- 31.Choo R, Pearce A, Danjoux C, et al. Prospective evaluation of quality of life in prostate cancer patients receiving combined treatment of postoperative radiotherapy plus androgen suppression for pt3 or positive resection margin after radical prostatectomy. Eur Urol. 2007;52(6):1645–1650. doi: 10.1016/j.eururo.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 32.Truong PT, Berthelet E, Lee JC, et al. Prospective evaluation of the prevalence and severity of fatigue in patients with prostate cancer undergoing radical external beam radiotherapy and neoadjuvant hormone therapy. Can J Urol. 2006;13(3):3139–3146. [PubMed] [Google Scholar]
- 33.Gelenberg AJ, Hopkins HS. Assessing and treating depression in primary care medicine. Am J Med. 2007;120(2):105–108. doi: 10.1016/j.amjmed.2006.05.059. [DOI] [PubMed] [Google Scholar]
- 34.Maurer D, Colt R. An evidence-based approach to the management of depression. Prim Care. 2006;33(4):923–941. doi: 10.1016/j.pop.2006.09.003. [DOI] [PubMed] [Google Scholar]


