Abstract
Apoptosis is central for control and elimination of viral infections. In chronic hepatitis C virus (HCV) infection, enhanced hepatocyte apoptosis and upregulation of the death-inducing ligands CD95/Fas occur. This study aimed to study the role of serum soluble Fas and hepatic Fas expression as early predictors of advancement of chronic hepatitis C disease. The current study included 50 cases of chronic hepatitis C (CHC) (and negative hepatitis B virus infection), 30 cases of liver cirrhosis (LC) and HCV, and 20 cases of hepatocellular carcinoma (HCC) and HCV admitted to Theodor Bilharz Research Institute, Giza, Egypt. Fifteen wedge liver biopsies, taken during laparoscopic cholecystectomy, were included in the study as normal controls. Assessment of serum soluble Fas level (sFas) and other laboratory investigations, including liver function tests, serologic markers for viral hepatitis, and serum alpha-fetoprotein level (alpha-FP), were determined for all cases. Histopathologic study and immunohistochemistry using monoclonal antibody for CD95 were also done. The sFas was significantly increased in CHC, LC, and HCC cases compared with normal controls (P < .01). The increase of sFas in HCC was also significantly higher than that of CHC (P < .01). However, positive hepatic expression of Fas antigen was higher in CHC than LC with no significant difference; meanwhile, it was significantly lower in HCC (P < .01) compared with CHC. In conclusion, circulating and hepatic Fas expression in chronic hepatitis C infection illustrate the mechanism of liver injury caused by death receptors throughout the multistep process of fibrosis/carcinogenesis. Not only the higher degree of hepatic fibrosis, but also the lower expression of Fas protein, are correlated with the increased incidence of HCC.
Introduction
Hepatitis C virus (HCV) is a major cause of chronic liver disease[1] that leads to cirrhosis in 20%-30% of patients,[2] of whom 20% develop hepatocellular carcinoma (HCC).[3] Liver damage in chronic HCV-infected patients is mostly due to the host's mediated immune response, with cytotoxic T lymphocytes (CTL) playing a crucial role in clearance of viral infection.[4]
Cell surface death receptors are widely expressed in normal and diseased tissues, and their ligands (FasL) are expressed mainly in CTL,[5] immune privileged sites,[6] and in various tumors where specific cytotoxic T cell clones are produced.[7] The expression of FasL on tumor cells is thought to be a mechanism that tumors employ to escape immune surveillance.[8] The activation of CTL and NK cells results in upregulation of FasL, which can transduce the apoptotic death signal to target cells triggering apoptosis.[9]
Soluble Fas (sFas) is generated by alternative mRNA splicing events.[10] Several investigators reported an elevation of sFas level in sera of patients with either inflammatory diseases such as lupus erythematosus or malignancies.[11–14]
In normal cells, the rates of cell proliferation and cell death approximate each other.[15] Malignancy may not only be associated with enhanced cell proliferation but may also be linked to decreased cell death,[8,16] and failure of cellular apoptosis could be attributed to deficiency in Fas expression.[17]
This study was designed to assess the circulating serum level and the hepatic tissue expression of Fas in chronic hepatitis C liver disease, and its sequelae of cirrhosis and/or HCC, to analyze the role of these factors in the multistep process of fibrosis/carcinogenesis.
Subjects and Methods
Study Design and Patients
A total of 125 cases were the subject of the current study. This cross-sectional study involved 110 patients with HCV-related liver pathology, followed up by the clinicians at the Hepato-Gastroenterology Department, Theodor Bilharz Research Institute (TBRI), Giza, Egypt, for evaluation of their chronic liver disease. They were 82 males and 28 females (mean age 45.6 ± 6.4, range 22–60 years) with circulating anti-HCV antibodies, and no serologic evidence of co-infection with hepatitis B virus. All patients gave informed consent prior to participation in the study in conformance with the guidelines of the 1975 Declaration of Helsinki as reflected by approval of the institution's human research ethics committee. All procedures, including liver biopsy, were medically indicated for patient management.
The study included 50 cases of chronic hepatitis C virus infection (CHC) without cirrhosis, 30 cases of CHC with cirrhosis (LC/HCV) and 30 cases of HCC with HCV infection (HCC/HCV). Children, schistosomal patients, and patients with chronic viral diseases other than HCV, nonalcoholic steatohepatitis, autoimmune hepatitis, biliary disorders, and malignancies other than HCC were excluded from the study.
The patients were subjected to thorough clinical examination and were assessed by (1) laboratory investigations; especially liver function tests, (2) ultrasonography; and (3) liver biopsy using ultrasound-guided Menghini needle for histopathologic and immunohistochemical studies.
Fifteen control wedge liver biopsies were obtained from 10 males and 5 females (mean age 42.8 ± 6.6 years) who had no serologic evidence of hepatitis B and/or C viruses,[18] and their consent was obtained to perform laparoscopic cholecystectomy.
Laboratory Investigations
Liver function tests were done using commercially available kits. Hepatitis B markers were tested using enzyme immunoassay kits (Abbott Laboratories; North Chicago, Illinois). Chronic hepatitis C (CHC) was confirmed by HCV infection persisting for longer than 6 months (HCV-RNA positive) and increased ALT values. Circulating anti-HCV antibodies were detected using Murex enzyme immunoassay kit (Murex Diagnostics; Dartford, United Kingdom), and the presence of HCV-RNA in patients' sera was detected by real-time polymerase chain using the Amplicor test (Roche Diagnostic Systems; Meylan, France). Meanwhile, serum alpha-FP was tested using the Eurogenetics enzyme immunoassay kit (Tessenderlo, Belgium).[18]
Serum Soluble Fas (sFas) Levels
Blood samples were collected from all subjects; sera were separated by centrifugation and stored at -20° C until used. Serum Fas levels were assayed using an enzyme-linked immunosorbent assay kit (Biosource International, Camarillo, California, USA) according to the manufacturer's instructions. Briefly, 10-fold diluted serum samples or standard Fas proteins (0.23–15.0 ng/mL) were pipetted into the wells of a microtiter plate coated with Fas antibody and another biotinylated Fas antibody was added. The plate was incubated at room temperature for 1 h, followed by incubation with a streptavidin-horseradish peroxidase conjugate for 30 min. A solution including the stabilized chromogen was added to each well and incubated for 30 min at room temperature. The reaction was stopped with sulfuric acid and the optical density was measured at 450 nm wavelength using an ELISA reader (Bio Rad). The concentration of sFas in serum samples was determined from the standard curve. All assays were conducted in duplicates, and the mean concentration of sFas was calculated.
Histopathologic Studies
Core liver biopsies were taken by percutaneous ultrasound-guided Menghini needle. Serial sections (5 micrometers [mcm] thick) from formalin-fixed, paraffin-embedded blocks were processed and stained with hematoxylin and eosin for routine histopathologic diagnosis and Masson trichrome stain for proper evaluation of tissue fibrosis. The activity of chronic hepatitis C (Histologic Activity Index [HAI]) was scored according to the degree of hepatic necroinflammation that depends upon portal inflammation and interface hepatitis (0–4) together with lobular inflammation and/or necrosis (0–4).[19] However, histologic staging is based on the extent of fibrosis and development of cirrhosis (0–4).[19] On the other hand, HCC cases were classified into well-differentiated tumors with acinar or trabecular arrangement of neoplastic hepatocytes, moderately differentiated tumors that revealed moderate anaplasia and hyperchromasia of neoplastic nuclei, and poorly differentiated giant tumor cells with remarkable hyperchromatic large-sized bizzar nuclei.[20]
Immunohistochemical Expression of Hepatic Fas Antigen
Five-mcm-thick sections from formalin-fixed, paraffin-embedded tissue blocks were collected on microscopic slides coated with 3-amino propyl triethoxysilane (Sigma Chemicals; St. Louis, Missouri) for proper fixation of tissue sections on the slides and to minimize staining artifacts. Following de-paraffinization in xylene and rehydration, antigen retrieval was performed by microwaving in 10 mmol/L citrate buffer solution (pH 6.0). Nonspecific antibody binding was prevented by pre-incubation with 100 mcm blocking serum for 30 min at room temperature. Sections were incubated overnight with the primary antibody, mouse anti-human Fas monoclonal antibody (CD95, Clone DX2) (Dako Cytomation Denmark A/S), at an optimal working dilution of 1:40. After thorough washing in buffer solution, the bound antibody was detected with biotinylated anti-rabbit IgG, followed by streptavidine-peroxidase conjugation. Peroxidase activity was developed after incubation with DAB substrate for 5 min and sections were counterstained with Mayer's hematoxylin before mounting. Positive and negative controls were stained appropriately at the same setting. The immunohistochemical expression of hepatic Fas was graded as: negative (-), mild (+ = < 30% positive cells), moderate (++ = 31%-60% positive cells), or intense (+++ = > 60% positive cells).[21,22]
Statistical Analysis
Statistical analysis was performed using SPSS version 11 software. Results, presented as mean ± SD, were compared using one-way ANOVA (analysis of variance), and the least significant difference (LSD) between groups was used. The data were considered significant when P values were below 0.05. Comparison between percent (%) positive cases was done using Chi-square test. Pearson correlation coefficient “r” was used to assess the relationship between 2 variables.
Results
The clinical and laboratory data for HCV-infected patients and control cases are shown in Table 1 .
Table 1.
Clinical and Laboratory Data of All Studied Cases [18]
| Variables | Controls (n = 15) | CHC Without Cirrhosis (n = 50) | CHC With Cirrhosis (n = 30) | HCC With HCV (n = 30) |
|---|---|---|---|---|
| Age | 42.8 ± 6.6 | 42.4 ± 9.3 | 45.1 ± 5.9 | 48.9 ± 7.2 |
| Gender (M:F) | 2:1 | 4:1 | 17:13 | 5:1 |
| Clinical findings: n (%) | ||||
| Pallor | 0 (0) | 2 (4%) | 5 (16.5%) | 8 (26.6%) |
| Jaundice | 0 (0) | 3 (6%) | 6 (20%) | 13 (43.3%) |
| Palmar erythema | 0 (0) | 0 (0) | 15 (50%) | 17 (56.6%) |
| Spider nevi | 0 (0) | 0 (0) | 14 (46.6%) | 14 (46.6%) |
| Lower-limb edema | 0 (0) | 0 (0) | 16 (53.3%) | 9 (30%) |
| Child-Pugh classification | ||||
| A | 10 (100%) | 50 (100%) | 8 (26.6%) | 1 (3.3%) |
| B | 0 (0) | 9 (30%) | 11 (36.6%) | |
| C | 0 (0) | 13 (43.3%) | 18 (60%) | |
| Ultrasound findings: n (%) | ||||
| Hepatomegaly | 0 (0) | 21 (42%) | 12(40%) | 20(66.6%) |
| Liver echogenicity | ||||
| *Normal | 0 (0) | 26 (52%) | 10(33.3%) | 4 (13.3%) |
| *Hyperechoic | 0 (0) | 24 (48%) | 20(66.6%) | 26 (86.6%) |
| Liver function tests (mean ± SD) | ||||
| AST (U/L) | 8.09 ± 2.11 | 14.91 ± 5.32 | 11.42 ± 4.12 | 18.90 ± 6.47 |
| ALT (U/L) | 7.73 ± 2.04 | 15.09 ± 6.80 | 12.18 ± 5.33 | 16.38 ± 6.32 |
| Alkaline phosphatase (U/L) | 189 ± 60 | 331 ± 77 | 348 ± 69 | 420 ± 72 |
| Albumin (g/dL) | 4.01 ± 0.38 | 3.60 ± 0.85 | 3.80 ± 0.72 | 3.08 ± 0.48 |
| PT concentration | 97.6 ± 3.4 | 89.7 ± 5.8 | 41.4 ± 11.2 | 68.5 ± 3.8 |
| Alpha-fetoprotein (IU/mL) | 3.39 ± 2.9 | 9.36 ± 13.6 | 10.41 ± 15.6 | 25.04 ± 27.9 |
Normal range for ALT and AST: up to 12 U/L; for alkaline phosphatase: up to 250 U/L; for albumin: 3.5–5 g/dL.
Normal range of prothrombin concentration: 85%–100%.
Normal range for alpha-fetoprotein: 0.1–9.6 IU/mL.
Serum sFas
Serum level of soluble Fas (sFas) was measured for all cases of the study (Table 2). Cases of CHC, LC/HCV, and HCC/HCV had higher circulating sFas levels compared with control cases (P < .01).
Table 2.
Serum Level of Circulating Soluble Fas (sFas) in Patients With Chronic Hepatitis C (CHC), Liver Cirrhosis (LC), and Hepatocellular Carcinoma (HCC) as well as Controls
| Histopathologic Diagnosis | No. of Cases | sFas (ng/mL) Mean ± SD |
|---|---|---|
| Controls | 15 | 4.7 ± 1.4 |
| CHC | 50 | 6.4 ± 1.3a |
| LC | 30 | 8.1 ± 3.8a,b |
| HCC | 30 | 12.3 ± 4.7a,b |
Data are expressed as mean ± SD
P < .01 relative to control cases.
P < .01 relative to CHC cases.
Serum sFas was increased, with the HAI being 5.9 ± 1.4 in minimal and mild grades, 6.6 ± 1.3 in moderate, and 7.3 ± 1.2 in severe grades, with no significant differences between the groups. The 50 cases of CHC with variable stages of fibrosis that ranked from 1 to 3 had a mean serum sFas level of 6.4 ± 1.3; meanwhile, stage 4 fibrosis occurred in the LC group (30 cases), in which the level of circulating sFas was significantly increased to 8.1 ± 3.8.
Hepatic Expression of Fas antigen
In the current study, Fas antigen was not detected in the liver tissue of any of the 15 control cases (Table 3).
Table 3.
Expression of CD95 (Fas protein) in Hepatocytes of Studied Groups
| Histopathologic Diagnosis | No. of Cases | Number of Positive Cases n (%) | Intensity of Hepatic Fas (CD95) Expression | ||
|---|---|---|---|---|---|
| +(< 30%) n (%) | ++(31%–60%) n (%) | +++(> 60%) n (%) | |||
| Controls | 15 | 0* | 0 | 0 | 0 |
| CHC | 50 | 44 (88) | 23 (46.0) | 19 (38.0) | 2 (4.0) |
| LC | 30 | 24 (80) | 13 (43.3) | 9 (30) | 2 (6.7) |
| HCC | 30 | 14 (46.7)** | 2 (6.7) | 2 (6.7) | 10 (33.3) |
No Fas expression in normal liver control cases.
Significant difference vs both CHC and LC (P < .01).
Fas expression was found in the diseased groups, mainly in the cytoplasm and membranes of hepatocytes with occasional perinuclear staining.
In CHC, it was detected among infiltrating lymphocytes at the advancing edges of piecemeal necrosis (interface hepatitis). The percentage of cases expressing apoptotic Fas protein in their hepatocytes was higher in the CHC group than in the LC group, with no significant difference (Table 3), but the percentage of positive cases was significantly lower in the HCC group. However, the intensity of expression increased with the development of cirrhosis and was significantly higher in HCC, particularly in poorly differentiated, more aggressive tumors (P < .001) (Table 4).
Table 4.
Correlation Analysis of All Cases Studied
| Parameters | r | P |
|---|---|---|
| sFas alpha-fetoprotein | 0.23 | NS |
| sFas grade of inflammation | 0.25 | NS |
| sFas stage of fibrosis | 0.55 | .01 |
| sFas Hepatic Fas | −0.67 | .05 |
| sFas grade of HCC differentiation | 0.43 | .05 |
| Hepatic Fas HAI | 0.53 | .01 |
| Hepatic Fas periportal bridging necrosis | 0.39 | .05 |
| Hepatic Fas intralobular degeneration and focal necrosis | 0.61 | .001 |
| Hepatic Fas portal inflammation | 0.61 | .01 |
| Hepatic Fas fibrosis | 0.59 | .01 |
| Hepatic Fas alpha-fetoprotein | 0.23 | NS |
| Hepatic Fas # grade of HCC differentiation | 0.65 | .001 |
HAI = Histologic Activity Index; HCC = hepatocellular carcinoma
In HCC, Fas immunoreactivity was localized almost exclusively to hepatocytes, whereas the stroma of the tumor was negative (Figures 1 and 2).
Figure 1.
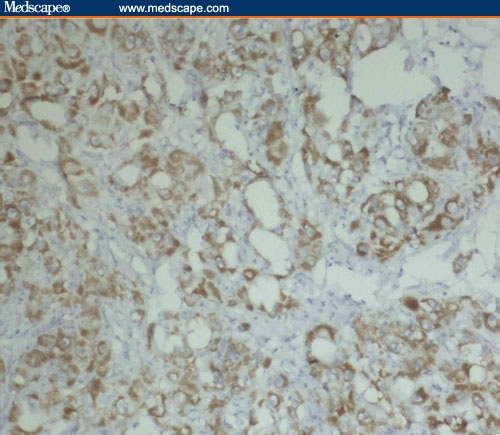
Moderate Fas expression in well-differentiated (acinar pattern) HCC/HCV (immunoperoxidase staining ×20).
Figure 2.
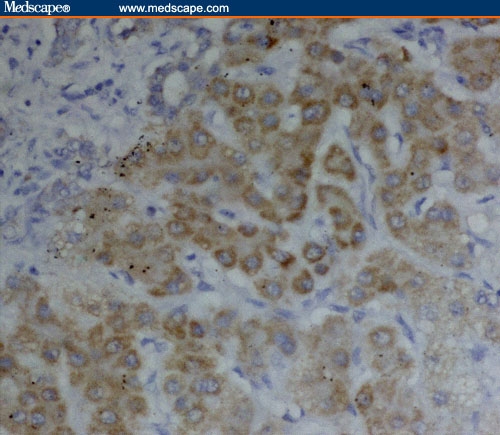
Intense Fas expression in poorly differentiated HCC/HCV (immunoperoxidase staining ×40).
On the other hand, Fas immunoreactivity at sites of inflammation was mainly localized to hepatocytes (Figures 3 and 4) with positive expression in inflammatory cells, fibroblasts, and vascular smooth muscle cells of the portal tracts.
Figure 3.
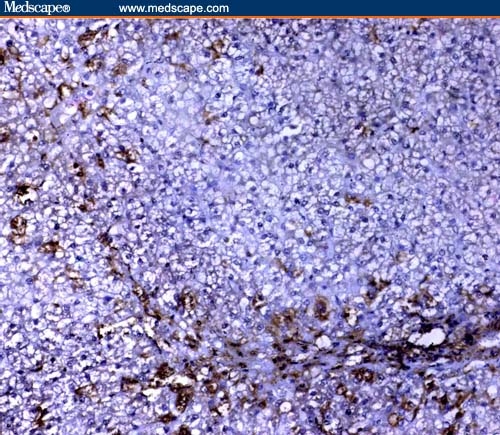
Mild cytoplasmic Fas expression in HCV infection without cirrhosis (immunoperoxidase staining ×20).
Figure 4.
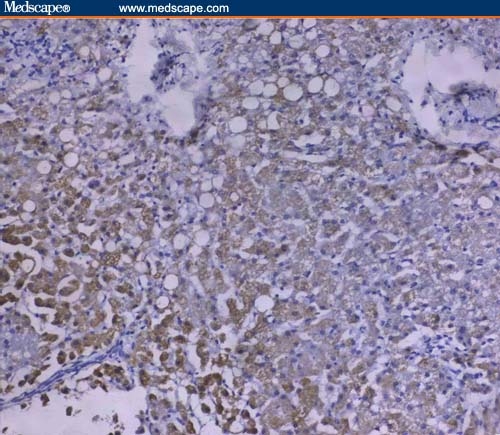
Intense cytoplasmic Fas expression in HCV infection without cirrhosis (immunoperoxidase staining ×20).
In LC, hepatocytes that were positive for Fas were small in size and mainly observed in the regenerative nodules (Figure 5). Fas expression was also detected in the epithelial lining of bile ductules in the adjacent portal tracts.
Figure 5.
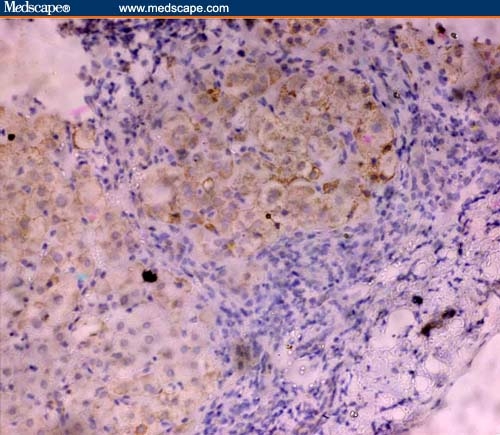
Intensity of Fas tissue expression in the liver cirrhotic nodule with absent immunoreactivity in the internodular fibrotic matrix (immunoperoxidase staining ×20).
A negative correlation was detected between sFas and hepatic Fas expression (r = 0.67, P < .05); however, a positive correlation was observed between the latter and grade of HCC differentiation (r = 0.65, P < .001) where Fas was expressed more intensely in poorly differentiated tumors. Also, correlation studies showed no significant correlation between serum alpha-FP level and the degree of tissue immunoreactivity to Fas protein in CHC, LC, and HCC cases.
Discussion
Death of hepatocytes and other hepatic cell types is a characteristic feature of liver diseases as diverse as cholestasis, viral hepatitis, ischemia reperfusion, and liver preservation for transplantation and drug/toxicant-induced injury.[23] Cell death typically follows one of 2 patterns: oncotic necrosis or apoptosis. Necrosis is typically the consequence of acute metabolic perturbation with ATP depletions, and occurs in ischemia/reperfusion and acute drug-induced hepatotoxicity. By contrast, apoptosis represents the execution of ATP-dependent death, often initiated by death ligand/death receptor interactions such as Fas ligand (on cytotoxic T lymphocytes) to the Fas receptor on hepatocytes, which leads to caspase activation cascade.[24] A common event leading to both apoptosis and necrosis is mitochondrial permeabilization and dysfunction, although the mechanistic basis of mitochondrial injury may vary in different settings.[25]
In the present study, the level of serum sFas in chronic active hepatitis was found to be significantly higher than that of healthy subjects (P < .01). Also, the percentage of cases with elevated sFas level was significantly higher among HCC than among CHC cases. Cancer cells upregulate or stimulate sFas production to protect themselves from Fas-mediated apoptosis,[26] whether through antagonizing the FasL-mediated killing or by alteration of Fas receptors.[11] Kakiuchi and colleagues[27] reported that sFas was elevated in parallel with severity of liver injury and suggested that upregulation of hepatic Fas expression may induce activation of type 1 interferon (IFN)-induced liver cell injury; however, the essential function of sFas to protect hepatocytes against Fas-mediated liver injury was not evident in their clinical setting.
In our study, serum sFas concentration in patients with cirrhosis due to HCV was found to be higher than in control cases, as previously recorded by Yoneyana and colleagues,[28] and this elevation might be due to decreased clearance from the liver.[29] Increased serum sFas level was also found in HCC patients.[30] Taken together, these findings indicate that the level of serum sFas increases as the disease progresses, suggesting that elevated sFas levels may reflect the degree of liver cell damage.[31]
No Fas expression could be detected in the liver tissue of healthy subjects. This result was consistent with those of Hiramatsu and colleagues[21] and Mochizulki and colleagues,[32] who also did not detect Fas antigen in their cases; whereas Leithauser and colleagues[33] detected it in normal hepatocytes and bile duct epithelium. In this study, the Fas antigen expression increased concurrently with histopathologic activity and was correlated moderately with the periportal bridging necrosis. This was in accordance with results reported by Hiramatsu and colleagues,[21] who found the same relationship. Our observation of a strong correlation between intralobular degeneration plus focal necrosis and Fas antigen expression was also emphasized by Mochizulki and colleagues.[32] It is possible that homeostatic mechanisms may prevent massive tissue damage by limiting apoptosis, and although Fas antigen expression increased with inflammation in CHC, it cannot be definitively said to be synthesized de novo; only its high or low expression may be noted. Tsugeno and colleagues[34] supported this by demonstrating Fas antigen expression in normal tissue as well as in chronic viral hepatitis, autoimmune hepatitis, fatty liver, and patients with portal fibrosis. It is important to emphasize here the pivotal role of CTLs which express Fas ligand on their surfaces. The CTLs that are associated with MHC class I antigens on target cells recognize the cells infected with viruses and give the death signal by interaction of Fas antigen-Fas ligand binding.[35] This explains the high frequency of apoptotic cells in piecemeal necrosis, and the positive correlation between portal inflammation and Fas antigen expression supports this.[15]
Investigations of the epidemiology and pathogenesis of HCC have defined HCV infection to be an important driving force in the multistep process of hepatocarcinogenesis.[19] Although molecular events contributing to hepatocarcinogenesis have been explored, the mechanism through which patients with chronic hepatitis develop HCC remains unknown.[36] HCV infection induces DNA damage that leads to a modulator phenotype. It has been suggested that the core protein may be essentially involved in HCV infection.[37] Hepatitis C virus core protein has been found to be involved in apoptosis, either in a positive or negative way.[36,38] Several studies have demonstrated a link between apoptosis and hepatic fibrosis, which is the most serious consequence of chronic liver injury.[39] Zhang and colleagues[40] demonstrated that the expression of Fas and FasL increased in the course of liver fibrosis, and would be furthered by interleukin-10. Experimentally, the upregulation of Fas expression occurs on stimulation with interferon-gamma released from T cells. Hepatic fibrogenesis accompanied by hepatocellular necrosis and inflammation was also suggested to be caused by cytokines and transforming growth factor-beta1 released by Kupfer cells and infiltrating T lymphocytes. These data indicate that both Fas expression and hepatic fibrosis can be affected by cytokines. Because hepatic fibrosis is associated with a high incidence of HCC, it was speculated that Fas expression may be involved in the incidence of HCC,[36,41] which was not emphasized in this work as the accelerated tumor phenotype is attributable to suppression of apoptosis rather than enhanced proliferation. Therefore, therapeutic strategies that inhibit selectively anti-apoptotic signals in tumor cells provide powerful tools for treating liver cancer.[42]
Alpha-FP gene expression promotes the expression of Fas ligand in hepatoma cells and enhances the expression of Fas in lymphocytes.[8] Alpha-FP is observed during massive hepatic necrosis and HCC; however, the use of alpha-FP as a diagnostic marker for HCC is of limited value due to its low sensitivity and specificity.[43] In this study, 26% of cases of chronic liver disease (CHC and LC) and 52.3% of HCC cases showed a remarkable increase in alpha-FP levels above normal values. Similarly, Mohamedein and colleagues[44] detected that estimation of alpha-FP alone can diagnose only 51% of HCC cases, and Garcia and colleagues[45] reported that 20% of HCC cases showed normal serum levels of alpha-FP.
In conclusion, Fas antigen, the triggering molecule of apoptosis, plays an important role in the pathogenesis of chronic viral hepatitis. Circulating soluble Fas (sFas) is increased in the advanced stages of HCV. This increase seems to be caused by the host-related immunologic factors and could be considered as a predictive marker for tumorigenesis in HCC. However, serum alpha-FP is not a satisfactory marker for HCC.
Hepatic Fas antigen is upregulated in accordance with the severity of liver inflammation in HCV. Not only the higher degree of fibrosis, but also the lower hepatic expression of Fas protein, are correlated with the increased incidence of HCC.
Acknowledgements
This work was supported by Theodor Bilharz Research Institute internal project No. 74 D, Immunology Department (Prof. Azza El Bassiouny).
Footnotes
Reader Comments on: Circulating and Hepatic Fas Expression in HCV-Induced Chronic Liver Disease and Hepatocellular Carcinoma See reader comments on this article and provide your own.
Readers are encouraged to respond to the author at drmonanosseir@yahoo.com or to George Lundberg, MD, Editor in Chief of The Medscape Journal of Medicine, for the editor's eyes only or for possible publication as an actual Letter in the Medscape Journal via email: glundberg@medscape.net
Contributor Information
Azza E. I. El Bassiouny, Theodor Bilharz Research Institute, Giza, Egypt Author's email: azzaelbassiouny@yahoo.com.
Nora E. I. El-Bassiouni, Theodor Bilharz Research Institute, Giza, Egypt.
Mona M. F. Nosseir, Theodor Bilharz Research Institute, Giza, Egypt Author's email: drmonanosseir@yahoo.com.
Mona M.K. Zoheiry, Theodor Bilharz Research Institute, Giza, Egypt.
Eman G. El-Ahwany, Theodor Bilharz Research Institute, Giza, Egypt.
Faten Salah, Theodor Bilharz Research Institute, Giza, Egypt.
Zeinab S.O. Omran, Theodor Bilharz Research Institute, Giza, Egypt.
Raafat A. Ibrahim, Theodor Bilharz Research Institute, Giza, Egypt.
References
- 1.Barth H, Ulsenheimer A, Pape GR. Presentation of hepatitis C virus-like particles by human dendritic cells. Blood. 2005;105:3605–3614. doi: 10.1182/blood-2004-05-1952. [DOI] [PubMed] [Google Scholar]
- 2.Shin JY, Hur W, Wang JS, et al. HCV core protein promotes liver fibrogenesis via up-regulation of CTGF with TGF-beta1. Exp Mol Med. 2005;37:138–145. doi: 10.1038/emm.2005.19. [DOI] [PubMed] [Google Scholar]
- 3.Schinoni MI, Parana R. Apoptosis and progression of hepatic fibrosis in liver diseases. Acta Gastroenterol Latinoam. 2006;36:211–217. [PubMed] [Google Scholar]
- 4.Fischer R, Baumert T, Blum HE. Hepatitis C virus infection and apoptosis. World J Gastroenterol. 2007;13:4865–4872. doi: 10.3748/wjg.v13.i36.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suda T, Takashi T, Goldstein R, Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993;75:1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- 6.Griffith T, Brunter T, Fletcher S, Green D, Ferguson T. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 7.Lee SH, Kim SY, Lee YH, et al. Detection of soluble Fas mRNA using in situ reverse transcriptase-polymerase chain reaction. Lab Investig. 1998;78:453–459. [PubMed] [Google Scholar]
- 8.Lee WC, YU MC, Chen MF. Prognostic impact of Fas ligand on hepatocellular carcinoma after hepatectomy. World J Surg. 2004;28:792–796. doi: 10.1007/s00268-004-7254-2. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi N, Mita E. Fas system and apoptosis in viral hepatitis. J Gastroenterol Hepatol. 1997;12:S223–S226. doi: 10.1111/j.1440-1746.1997.tb00504.x. [DOI] [PubMed] [Google Scholar]
- 10.Casino I, Fiucci G, Papoff G, Ruberti G. Three functional soluble forms of the human apoptosis-inducing Fas molecule are produced by alternative splicing. J Immunol. 1995;154:2706–2713. [PubMed] [Google Scholar]
- 11.Ching J, Zhou T, Liu C, et al. Protection from Fas mediated apoptosis by a soluble form of Fas molecule. Science. 1994;263:1759–1762. doi: 10.1126/science.7510905. [DOI] [PubMed] [Google Scholar]
- 12.Knipping E, Depatin KM, Stricker K, Hellig B, Eder A, Krammer PH. Identification of soluble APO-1 in supernatants of human B- and T-cell lines and increased serum levels in B- and T-cell leukaemias. Blood. 1995;85:1562–1569. [PubMed] [Google Scholar]
- 13.Midis GP, Shen Y, Owen LB. Elevated soluble Fas (sFas) levels in non haematopoietic human malignancy. Cancer Res. 1996;56(3870–3874) [PubMed] [Google Scholar]
- 14.Osario LM, Angular M, De Santiago A, Hachiya T, Mellstedt H, Jondal M. Increased serum levels of soluble Fas in progressive B-CLL. Eur J Haematol. 2001;66:342–346. doi: 10.1034/j.1600-0609.2001.066005342.x. [DOI] [PubMed] [Google Scholar]
- 15.Kiyici M, Gurel S, Budak F, et al. Fas antigen (CD95) expression and apoptosis in hepatocytes of patients with chronic viral hepatitis. Euro J Gastroenterol Hepatol. 2003;15:1079–1084. doi: 10.1097/00042737-200310000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Wu JX. Apoptosis and angiogenesis: tow promising tumor markers in breast cancer. Anticancer Res. 1996;16:2233–2240. [PubMed] [Google Scholar]
- 17.Pan G, Vickers SM, Pickens A, et al. Apoptosis and tumorigenesis in human cholangiocarcinoma Cells: Involvement of Fas/APO-1 (CD95) and Calmoulin. Am J Pathol. 1999;155:193–203. doi: 10.1016/S0002-9440(10)65113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El Bassiouny AEI, Zoheiry MMK, Nosseir MMF, El-Ahwany EG, El-Bassiouni NEI. Expression of cyclooxygenase-2 and transforming growth factor-beta1 in HCV-induced chronic liver disease and hepatocellular carcinoma. MedGenMed. 2007;9(3):45. Published online 2007 August 28. [PMC free article] [PubMed] [Google Scholar]
- 19.Scheuer PJ. Chronic hepatitis. In: Scheuer PJ, Lefkowitch JH, editors. Liver Biopsy Interpretation. 6th ed. London: Saunders, Elsevier Science, Inc.; 2003. pp. 151–172. [Google Scholar]
- 20.Ishak KG, Anthony PP, Sobin LH. World Health Organization. International Histological Classification of Tumors. 2nd ed. Berlin: Springer-Verlag; 1994. Histological typing of tumors of the liver; pp. 91–118. [Google Scholar]
- 21.Hiramatsu N, Hayashi N, Katayama K, et al. Immunohistochemical detection of Fas antigen in liver tissue of patients with chronic hepatitis C. Hepatology. 1993;46:1354–1359. [PubMed] [Google Scholar]
- 22.Chen NL, Bai L, Deng T. Study on pathway of apoptosis in chronic liver disease. Zhonghua Chuanranbing Zazhi. 2003;21:122–124. [Google Scholar]
- 23.Rosai J. source>Rosai and Ackerman's Surgical Pathology. 9th ed. Vol. 2. London: Saunders, Elsevier Science; 2004. Gastrointestinal tract, liver; pp. 776–856. Chapter 13. [Google Scholar]
- 24.Nagai H, Miyaki D, Matsui T, et al. Changes of cytokines in cirrhosis patients with advanced hepatocellular carcinoma treated by intra-arterial chemotherapy. Cancer Chemother Pharmacol. 2007 doi: 10.1007/s00280-007-0602-9. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 25.Malhi H, Gores GJ, Lemasters JJ. Apoptosis and necrosis in the liver: a tale of two deaths. Hepatology. 2006;43:S31–S44. doi: 10.1002/hep.21062. [DOI] [PubMed] [Google Scholar]
- 26.Owen-Schaub LB, Angelo LS, Radinesky R, Ware CF, Gesner DP. Should Fas/APO-1 in tumor cells a potential regulator of apoptosis? Cancer Lett. 1995;94:1–8. doi: 10.1016/0304-3835(95)03834-j. [DOI] [PubMed] [Google Scholar]
- 27.Kakiuchi Y, Yuki N, Igoda K, Sugiyasu Y, Kaneko A, Kato M. Circulating soluble Fas levels in patients with hepatitis C virus infection and interferon therapy. J Gastroenterol. 2004;39:1189–1195. doi: 10.1007/s00535-004-1470-2. [DOI] [PubMed] [Google Scholar]
- 28.Yoneyama K, Goto T, Miura K, et al. The expression of Fas and Fas ligand, and the effects of interferon in chronic liver diseases with hepatitis C virus. Hepatol Res. 2002;24:327–337. doi: 10.1016/s1386-6346(02)00137-7. [DOI] [PubMed] [Google Scholar]
- 29.Seichima M, Takemura M, Saito K, Ando K, Noma A. Increased serum soluble Fas (sFas) concentrations in HCV-positive patients with liver cirrhosis. J Hepatol. 1997;27:424–427. doi: 10.1016/s0168-8278(97)80191-2. [DOI] [PubMed] [Google Scholar]
- 30.Jodo S, Kobayashi S, Nakajima Y, et al. Elevated serum levels of soluble Fas/ Apo-1 (Cdas) in patients with hepatocellular carcinoma. Clin Exp Immunol. 1998;112:166–171. doi: 10.1046/j.1365-2249.1998.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ozaslan E, Kilioaeslan A, Simsek H, Fater G, Kirazli S. Elevated serum soluble Fas levels in the various stages of hepatitis C virus-induced liver disease. J Inter Med Res. 2003;31:384–391. doi: 10.1177/147323000303100505. [DOI] [PubMed] [Google Scholar]
- 32.Mochizulki K, Hayashi N, Hiramatsu N, et al. Fas antigen expression in liver tissue of patients with chronic hepatitis B. J Hepatol. 1996;24:1–7. doi: 10.1016/s0168-8278(96)80178-4. [DOI] [PubMed] [Google Scholar]
- 33.Leithauser F, Dhein J, Mechtersheimer G, et al. Constitutive and induced expression of Apo-1: a new member of the nerve growth factor/tumor necrosis factor receptor superfamily in normal and neoplastic cells. Lab Invest. 1993;69:415–429. [PubMed] [Google Scholar]
- 34.Tsugeno H, Yamada G, Takatani M, et al. Immunohistochemical study of Fas antigen in human liver tissues. Hepatology. 1994;202:145A. [Google Scholar]
- 35.Seino KI, Kayagaki N, Fukao K, Okumura K, Yagita H. Contribution of Fas ligand to T-cell mediated hepatic injury in mice. Gastroenterology. 1997;113:1315–1322. doi: 10.1053/gast.1997.v113.pm9322527. [DOI] [PubMed] [Google Scholar]
- 36.Hirashima N, Matsumoto Y, Ohono T, Kimura Y, Hasegawa I, Ueda R. Hepatic Fas protein expression might be a predictive factor for hepatocellular carcinoma development in patient with chronic hepatitis C undergoing interferon therapy. J Clin Gastroenterol. 2002;34:263–267. doi: 10.1097/00004836-200203000-00014. [DOI] [PubMed] [Google Scholar]
- 37.Moriya K, Fujii H, Shintani Y, et al. Hepatitis C core protein induces hepatocellular carcinoma in transgenic mice. Nat Med. 1998;4:1065–1071. doi: 10.1038/2053. [DOI] [PubMed] [Google Scholar]
- 38.Ray RB, Meyer K, Ray R. Suppression of apoptotic cell death by hepatic C virus core protein. Virology. 1996;226:176–182. doi: 10.1006/viro.1996.0644. [DOI] [PubMed] [Google Scholar]
- 39.Akazawa Y, Gores GJ. Death receptor mediated liver injury. Semin Liver Dis. 2007;27:327–338. doi: 10.1055/s-2007-991510. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L, Wang X, Zheng W, Shi M. The effects of interleukin-10 on the expression of Fas and FasL in rat hepatic stellate cells. Med Chem. 2006;2:611–616. doi: 10.2174/1573406410602060611. [DOI] [PubMed] [Google Scholar]
- 41.Kamegaya Y, Hiasa Y, Zukerberg L, et al. Hepatitis C virus acts as a tumor accelerator by blocking apoptosis in a mouse model of hepatocarcinogenesis. Hepatology. 2005;41:660–667. doi: 10.1002/hep.20621. [DOI] [PubMed] [Google Scholar]
- 42.Fabregat I, Roncero C, Fernandz M. Survival and apoptosis: a deregulated balance in liver cancer. Liver Int. 2007;27:155–162. doi: 10.1111/j.1478-3231.2006.01409.x. [DOI] [PubMed] [Google Scholar]
- 43.El Bassiouny A, Nosseir M, Zoheiry M, El-Ahwany E, Ghali A, El-Bassiouni N. Immunohistochemical expression of CD95 (Fas), c-myc and epidermal growth factor receptor in hepatitis C virus infection, cirrhotic liver disease and hepatocellular carcinoma. APMIS. 2006;114:420–427. doi: 10.1111/j.1600-0463.2006.apm_323.x. [DOI] [PubMed] [Google Scholar]
- 44.Mohammedein A, Yousif-Kadaru AG, Ahmed SA. A carboxy prothrombin (PIVKA II) as a tumor marker for hepatocellular carcinoma and other liver diseases. East Afr Med J. 1995;72:584–587. [PubMed] [Google Scholar]
- 45.Garcia CD, Guzman de la FJ, Munoz ELE. Primary liver cancer Its epidemiological, clinical and biochemical characteristics. Rev Gastroenterol Mex. 1994;59:17–22. [PubMed] [Google Scholar]


