Abstract
Context
Type 2 diabetes is primarily a disease that affects late-middle-aged and elderly individuals. Due to increasing affluence, lifestyle changes, and obesity, however, it is also affecting younger age groups. Therefore, treatment recommendations may need to be revised to reflect these changes. Fortunately, various new treatment options, including insulin analogs with more physiologic time-action profiles and drugs that target the incretin system, are now available.
Evidence acquisition
This review was based on a PubMed literature search done in August 2007, using relevant search terms.
Evidence synthesis
Improving diet and increasing physical activity are important therapeutic interventions in diabetes and associated conditions. However, many people find it difficult to maintain lifestyle changes, which is why the American Diabetes Association recommends lifestyle intervention plus metformin following initial diagnosis. Current guidelines recommend a stepwise approach, with additional oral antidiabetic drugs (OADs) being added as the disease progresses. Insulin therapy should be initiated once OADs fail to control hyperglycemia, as there is good evidence that intensive therapy, with strict glycemic targets, can reduce the long-term microvascular complications that are associated with poorly controlled diabetes. In addition, while the evidence is less conclusive, intensive therapy may also improve long-term macrovascular comorbidities.
Conclusions
It is important that patients with diabetes receive the most effective therapy for maintaining glycemic control and that treatment is modified or augmented in those who are not achieving appropriate glycemic goals. Only by maintaining long-term, effective glycemic control can the microvascular and macrovascular comorbidities associated with diabetes be minimized.
The Changing Demographics of Type 2 Diabetes
Diabetes is becoming a pandemic. In 2000, there were approximately 171 million people with diabetes; estimates for 2030 suggest that the prevalence of this disease will increase to 366 million, most of which will be type 2 disease.[1] The highest percentages of increases in disease prevalence are likely to be in non-Western and developing nations(Figure 1), with major increases in the Middle-East, Sub-Saharan Africa, India, Asia, and Latin America.[1] These increases reflect rapidly changing lifestyles in developing countries. In addition, certain populations in developing countries, such as South Asian Indians, have an increased tendency to develop diabetes compared with Western populations exposed to a similar diet and lifestyle.[2] There is some evidence that certain ethnic populations have genetic predispositions that increase their risk for diabetes. For example, the “thrifty gene” hypothesis proposes that populations from areas that commonly experienced famines and food shortages have more efficient fat storage in order to improve survival chances.[3] While existence of “thrifty genes” remains a point of debate,[4] this adaptation would provide an advantage when food resources are scarce but would predispose individuals to increased obesity and risk for diabetes in response to a Western lifestyle. In addition, when populations who are at increased risk for type 2 diabetes migrate to Western countries, they face increased risk for diabetes due to the change in lifestyle.
Figure 1.
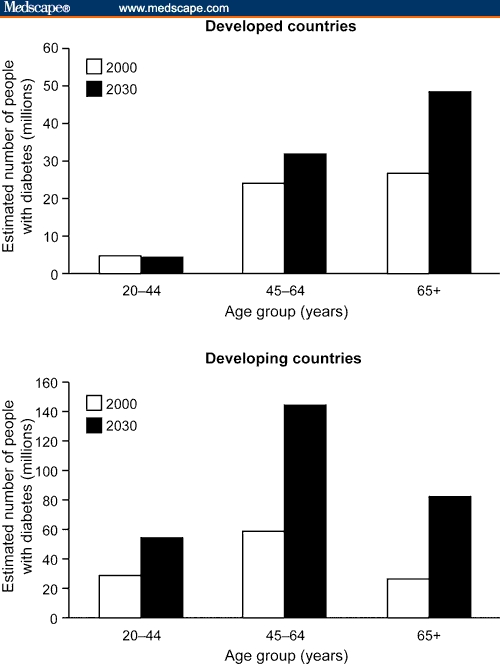
The changing epidemiology of type 2 diabetes. Figure adapted from Wild et al,[1] copyright 2004 American Diabetes Association. Republished with permission.
Western nations will also be seriously affected. For example, US estimates show that between 2005 and 2050, the prevalence of diabetes will more than double to 48.3 million people.[5] Indeed, it is estimated that at least one third of males and two fifths of females born in the United States in 2000 will be at risk for diabetes.[6] In the United States, minority populations, such as Mexican Americans, other Hispanics, non-Hispanic blacks, American Indians, native Alaskans, native Hawaiians, and Pacific Islanders, have an increased risk for diabetes compared with non-Hispanic whites.[7] Similarly, Aboriginal populations in Australia also appear to be at an increased risk for diabetes compared with the white Australian population.[8] These increased risks in minority and aboriginal populations are likely to reflect a number of factors, including socio economic status, health care provision, educational opportunities, and cultural factors, as well as possible genetic predispositions to diabetes.
Individuals with diabetes who are unable to maintain control of glucose metabolism face a number of debilitating microvascular and macrovascular complications, such as retinopathy, nephropathy, neuropathy, heart disease, and stroke, as well as a greatly increased risk for early death. There are 2 main forms of diabetes: Type 1 diabetes makes up approximately 5% to 10% of cases in the United States[7,9] and results from auto immune destruction of insulin-producing beta-cells in the pancreas. Type 1 diabetes generally starts in childhood and is treated with insulin-replacement therapy. Type 2 diabetes is the predominant form, making up the remaining 90% to 95% of cases in the United States.[7,9] The most important risk factor for type 2 diabetes is obesity: A body mass index (BMI) of 31 kg/m2 confers a 40-fold increased risk, while a BMI of >35 kg/m2 confers a 90-fold increased risk.[10] In addition, obesity is central to other increasingly prevalent disorders, such as prediabetes and the metabolic syndrome, which heighten the risk for diabetes and heart disease.[11, 12] Obesity and type 2 diabetes are states that are characterized by chronic oxidative and inflammatory stress. The central role of chronic inflammation is suggested by its association with the induction of insulin resistance and with atherosclerosis.[13] The effect of diet has also been demonstrated, as macro nutrients, such as glucose, induce pro-inflammatory and pro-oxidative changes, and restricted calorie intake leads to a reversal of oxidative stress and inflammation.[14–16]
In the past, type 2 diabetes was largely a disease of middle-aged and elderly individuals who were often able to control their disease through lifestyle changes and/or use of oral antidiabetic drugs (OADs). However, the increasing numbers of younger individuals who are being affected by type 2 diabetes and current rates of obesity suggest that the mean age of onset will continue to shift downward.
The epidemic rise in type 2 diabetes reflects increasing affluence and changing life styles around the globe. People in both developed and developing countries are leading increasingly sedentary lifestyles and are eating higher-fat and higher-sugar diets than previous generations. Thus, the growing pandemic of type 2 diabetes is following a pandemic rise in obesity. Data from the Centers for Disease Control and Prevention (CDC) highlight the problem in the United States: 32.2% of adults in 2003–2004 were classified as obese.[17] Obesity is also a major problem in minority US populations – for example, in 2003–2004, 45.0% of black American and 36.8% of Mexican American adults were classified as obese compared with 30.6% of white Americans.[17]
The obesity epidemic is not just confined to wealthy, developed nations. For example, although China does not have the same prevalence of obesity as Western countries, obesity rates are increasing rapidly. A 2002 Chinese national survey reported that 14.7% (184 million) of adults were overweight and 2.6% (31 million) of adults were obese.[18] These figures are particularly worrisome because they are based on Western BMI values (≥ 25 kg/m2 overweight, ≥ 30 kg/m2 obese), which may underestimate the true burden of obesity in the Chinese population.[19]
Cultural factors relating to obesity and body image are also important and may underlie high rates of obesity in some minority and ethnic groups. For example, the prevalence of overweight and obesity among Latin Americans has been estimated to be 73%.[20] This may reflect the fact that excess weight is often tolerated, and even celebrated, in the Latino culture and may lessen social pressure to lose weight. Being slightly overweight or plump is equated with being well-cared-for and healthy, whereas being thin may be associated with being under nourished or even neglected, particularly among children. Traditional Latino cuisine is rich in nutrients, high in fiber, and low in fat. However, as Latinos assimilate into American society, the content of their diet becomes lower in nutrients and higher in fat.[20,21] These dietary changes, along with the increasingly sedentary lifestyle that characterizes American life, undoubtedly contribute to the epidemic of overweight and obesity among the Latino population. Cultural factors, including sedentary lifestyles and the association of overweight with well-being, may also contribute to high rates of obesity in Middle-Eastern cultures, especially among women.[22]
A particularly worrisome trend is the increasing problem of obesity in children. Over the past 30 years, the number of children diagnosed as overweight has more than doubled.[23] CDC data from 2003–2004 showed that 17.1% of US children were obese and that there were trends for increasing obesity compared with 1999–2000 data.[17] As with the adult population, rates of childhood obesity are higher in minority US populations. In 2003–2004, 16.3% of white American, 20.0% of black American, and 19.2% of Mexican American children and adolescents were classified as overweight.[17] Childhood and adolescent obesity in countries, such as China, that are under going major economic and social changes is also increasing at an alarming rate. In children aged 7 to 18 years, a 28-fold increase in the prevalence of overweight and a 4-fold increase in the prevalence of obesity was noted between 1985 and 2000.[18]
Again, following the increase in obesity in younger people, type 2 diabetes is being increasingly observed in younger adults.[24,25] This is particularly prevalent in populations that have a high prevalence of diabetes, such as South Asians and Pacific Islanders.[24] However, this changing trend is also being observed more generally: Data from the CDC show that the number of people with diabetes in the United States in the 0- to 44-year age group rose from 951,000 in 1980 to over 2.5 million in 2005.[26] In addition, estimates based on current obesity rates suggest that the population with younger-onset diabetes in the United States will more than double to approximately 5.6 million by 2050.[5] The number of people with younger-onset diabetes (age < 44 y) in developing countries will also increase, from approximately 30 million in 2000 to around 55 million by 2030 (Figure 1).
In addition to increases in diabetes in younger adults, there is an increase in type 2 diabetes in children and adolescents. Fifteen years ago, type 2 diabetes accounted for < 3% of cases of new-onset diabetes in adolescents; however, a recent estimate shows that type 2 diabetes now accounts for 45% of all new cases.[27] Although data for diabetic comorbidities in the younger population are scarce, these individuals are likely to face serious comorbidities in the future. For example, many adolescents with type 2 diabetes present with hypertension and nephropathy at initial diabetes diagnosis.[28] In a Japanese study of 1065 individuals aged < 30 years at diagnosis of type 2 diabetes, 99 (9.3%) had signs of retinopathy, and 135 (12.7%) developed proliferative retinopathy by the age of 35. Thirty-two of the individuals with proliferative retinopathy were blind by the age of 32.[29] There is also evidence of increased cardiovascular risk factors in young people with type 2 diabetes.[28] Therefore, individuals that develop type 2 diabetes in adolescence or early adulthood are likely to face a large burden of comorbidity throughout their lives.
The Changing Medical Perspective of Type 2 Diabetes
Type 2 diabetes was traditionally viewed as a discrete clinical entity involving defects related to insulin resistance and secretion. However, this view is changing. Many clinicians now view type 2 diabetes as part of a continuum of metabolic disorders, including prediabetes and the metabolic syndrome, involving insulin defects as well as other metabolic pathologies.[30] Obesity and sedentary lifestyles are central to disease progression from normal glucose control to increasing metabolic disorder. A dipose tissue is metabolically active and produces such factors as free fatty acids and a dipokines that can influence glucose metabolism.[12] In addition, adipose tissue produces proinflammatory and prothrombotic factors that can create a chronic inflammatory state in the body, and this can increase the risks for cardiovascular and other diseases.[12] The pro-inflammatory and pro-oxidative changes induced by such macro nutrients as glucose promote insulin resistance and a the rosclerosis.[14–16]
Type 2 diabetes is a complex disorder that has 2 primary mechanisms by which glycemic control is lost, but the relative importance of each mechanism is ongoing. The early stages are characterized by “insulin resistance,” in which uptake of glucose by tissue cells is decreased in response to insulin, resulting in post prandial hyper glycemia and defects in pancreatic beta-cell function. Although there are defects in insulin secretion by beta cells in the early stages of type 2 diabetes, beta cells often try to compensate for insulin resistance by secreting more insulin.[31] However, over the longer term, the insulin response to prandial stimuli worsens as beta-cell function deteriorates, resulting in perpetual hyperglycemia.[32]
The term “prediabetes” defines an earlier stage in the disease process, characterized by impaired fasting plasma glucose (5.6–6.9 mmol/L) and/or impaired glucose tolerance, as measured by the oral glucose tolerance test (postchallenge plasma glucose, 7.8–11.1 mmol/L).[33,34] Estimates suggest that at least 200 million people worldwide – 54 million people in the United States alone – have impaired glucose tolerance or prediabetes, a key stage in the progression toward type 2 diabetes.[7,24] Prediabetic individuals are often undiagnosed and a symptomatic, but approximately 40% will progress to diabetes in 5–10 years.[24] Overall, prediabetes is associated with an approximately 6-fold increased risk for diabetes compared with individuals who have normal glucose tolerance.[35] There is also evidence that impaired glucose tolerance is a major risk factor for cardiovascular disease.[36,37]
“The metabolic syndrome” is a term that describes a group of linked risk factors that predispose an individual to cardiovascular disease and diabetes. Various clinical organizations have published varied definitions for the metabolic syndrome; however, the main features are insulin resistance, a high waist circumference, elevated triglyceride levels, low high-density lipoprotein cholesterol levels, and elevated blood pressure.[11] Individuals with 3 or more of these risk factors are classified as having the metabolic syndrome, which is associated with a 1.5- to 3-fold increased risk for new-onset coronary heart disease and a 3- to 5-fold increased risk for diabetes.[12] The high prevalence of the metabolic syndrome is a major cause for concern. It is estimated that approximately 34% of Americans, 24% of Greeks, 20% to 25% of urban South Asians, and 15% of Australians are affected.[2, 11] It is likely that health systems around the world will face an increasing burden of diabetes and cardiovascular disease in the years to come because of the prevalence of this syndrome.
Type 2 Diabetes and Associated Metabolic Disorders: Current Management Strategies
As obesity is a major factor in a number of chronic diseases, including diabetes, treatments that reduce the burden of obesity would be beneficial. While lifestyle changes, such as improved diet and increased physical activity, are the most effective means by which to lose weight, many individuals do not maintain weight loss by lifestyle changes alone. Obviously, there is great interest in pharmacologic antiobesity treatments; however, current medications have a number of limitations including lack of long-term efficacy and unacceptable side effects.[38] For example, both of the 2 drugs currently approved in the United States, orlistat (Xenical, Roche Laboratories Inc) and sibutramine (Meridia, Abbott Laboratories), produce a relatively low amount (3% to 4%) of additional weight loss compared with diet alone and have adverse side effects, such as fecal urgency/incontinence with orlistat and possible tachycardia and hypertension with sibutramine.[38] In addition, the novel antiobesity drug rimonabant (Accomplia, sanofi-aventis), which is a cannabinoid-receptor (CB1) antagonist, was not approved by the Food and Drug Administration (FDA) because of concerns about increased rates of psychiatric adverse events.[39]. This raises doubts about the safety profile of the CB1 antagonist class of antiobesity drugs.
Given the large number of people affected by prediabetes and the metabolic syndrome who are at increased risk for diabetes, there have been a number of trials examining the ability of lifestyle changes and/or drug therapy to prevent the onset of diabetes in these at-risk populations. In one such study, 3234 individuals with impaired glucose tolerance or impaired fasting glucose received an intensive lifestyle intervention (consisting of a 16-lesson program to help participants achieve a 7% reduction in initial body weight through diet and to increase their participation in moderate-intensity exercise to ≥ 150 minutes/week) achieved a 58% reduction in the incidence of diabetes compared with a control group.[40] The reduction in diabetes incidence observed with an intensive lifestyle intervention was greater than the 31% reduction seen in the group given metformin and standard lifestyle advice.[40] Similar lifestyle intervention studies in Finland and China produced similar results with a 43% (P = .0001) reduction in the relative risk for diabetes in the Finnish study and a 42% (P < .005) reduction in the Chinese study.[41,42] These studies make it clear that positive lifestyle modification should be a goal for all patients with type 2 diabetes. However, it should also be remembered that patients in these trials received intensive one-on-one mentoring and monitoring to support lifestyle changes, which could be difficult to provide in everyday clinical practice.
For patients diagnosed with type 2 diabetes, the current consensus guidelines from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) recommend that therapy should aim to maintain long-term glycosylated hemoglobin (Hb) A1c values of <7.0% and that an HbA1c in individual patients should be as close as possible to the normal value of <6.0% without risking significant hypoglycemia.[43] The American College of Endocrinology and the American Association of Clinical Endocrinologists (ACE/AACE) recommend a lower HbA1c target of ≤ 6.5%.[44]
The recommendations for strict control of HbA1c are based on data from the Diabetes Control and Complications Trial (DCCT) trial and the Epidemiology of Diabetes Interventions and Complications (EDIC) trial in patients with type 1 diabetes and from the UK Prospective Diabetes Study (UKPDS) in patients with type 2 diabetes.[45–48] These studies demonstrated that intensive therapy was associated with significant reductions in the risk for long-term diabetic comorbidities.[45–48] An epidemiologic analysis of UKPDS data suggested that each 1% reduction in HbA1c could result in a 21% reduction in diabetes-related death or any diabetes endpoint and a 37% decrease in microvascular complications (Figure 2).
Figure 2.
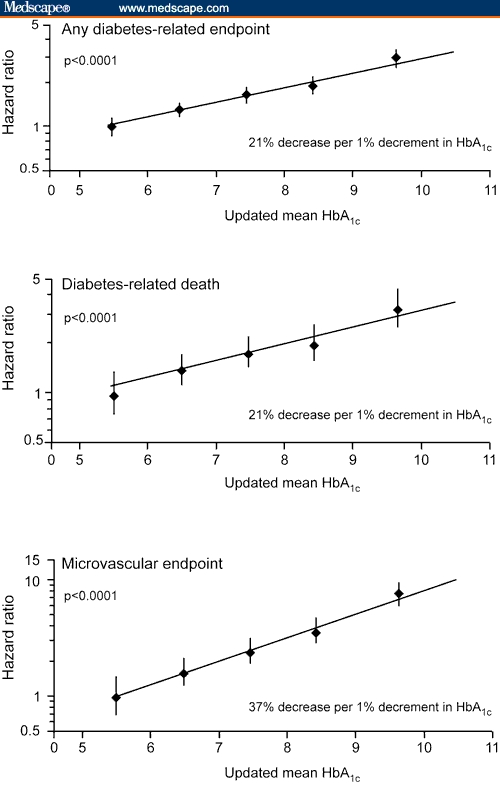
Benefits related to decreasing HbA1c. Figure adapted from Stratton et al,[48] copyright 2000, reproduced/amended with permission from the BMJ Publishing Group.
The current ADA/EASD guidelines recommend a stepwise approach to the management of type 2 diabetes.[43] Initial treatment should include lifestyle interventions to improve diet and increase daily exercise, which the ADA/EASD estimate may produce a 1% to 2% reduction in HbA1c levels (Table).[43, 49] However, although lifestyle interventions are important, many individuals find it hard to sustain effective weight loss and exercise strategies and most fail lifestyle interventions within 1 year.[43] Therefore, the guidelines recommend that metformin be initiated along with lifestyle changes when type 2 diabetes is diagnosed.[43] If lifestyle modification plus metformin fails to achieve an HbA1c ≤ 7% after 3 months, then the ADA/EASD recommend adding a basal insulin, sulfonyl urea, or glitazone.[43] If glycemic targets are not met by 3 months, physicians should intensify insulin treatment or add a third OAD or basal insulin in patients receiving combination OAD treatment. If these options do not control HbA1c levels, then intensive insulin treatment plus metformin (± glitazone) is recommended.[43] The Table shows estimates of the HbA1c-lowering capacity of insulin and other antidiabetic medications provided by the ADA/EASD, as well as their main advantages and disadvantages.
The ACE/AACE recommendations are stratified by initial HbA1c level and differentiate between therapy-naive patients and previously treated patients.[44] Of note, the ACE/AACE recommendations include 2 new drug classes that target the incretin system: glucagon-like-peptide 1 (GLP-1) mimetics, such as exenatide (Byetta, Amylin Pharmaceuticals, Inc., and Eli Lilly and Company), and dipeptidyl-peptidase-4 (DPP-4) inhibitors, such as sitagliptin (Januvia, Merck & Co., Inc). The ACE/AACE guidelines recommend lifestyle modification in all patients. In treatment-naive patients with an HbA1c between 6% and 7%, metformin, thiazolidinediones (TZDs) (orglitazones), sulfonylureas, DPP-4 inhibitors, or alpha-glucosidase inhibitors are recommended.[44] Combination therapy is recommended if patients do not achieve glycemic goals at 2 to 3 months, and there is the option to add an incretin mimetic to oral therapy.[44] Combination treatment with oral antidiabetic agents and/or insulin treatment is recommended in treatment-naive people with initial HbA1c levels between 7% to 8%.[44] Insulin therapy should be initiated in patients on maximum combination therapy whose HbA1c levels are 6.5% to 8.5%,[43] although some clinicians may question the benefit of adding insulin if patients are achieving an HbA1c level of 6.5%. Basal-bolus insulin therapy should be considered for patients with HbA1c levels of > 8.5%.[43] Treatment-naive patients with initial HbA1c levels over 10% should be started on insulin.[44]
Regarding the recommendations for TZDs in the ADA and ACA/AACE guidelines, a recent meta-analysis of 42 rosiglitazone (Avandia, Glaxo Smith Kline) trials reported a significantly increased risk for myocardial infarction (odds ratio, 1.43 [95% CI, 1.03 to 1.98; P = .03]) with rosiglitazone treatment versus the control group.[50] However, an interim analysis of the Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycemia in Diabetes (RECORD) study showed an increase in heart failure in the rosiglitazone group versus the control group (hazard ratio, 2.15[95% CI, 1.30 to 3.57]) but no statistically increased risk for myocardial infarction or death from the cardiovascular effects.[51] In response, the FDA asked for a new boxed warning to be included on prescribing information to highlight that rosiglitazone may cause myocardial is chemia or congestive heart failure in some patients, it should not be co-administered with insulin, and it is not recommended for patients with heart disease who are taking nitrates.[52] Given this concern about TZDs, both physicians and guideline committees may be reconsidering the recommendations for using these drugs in patients with type 2 diabetes.
The new drug classes that target the incretin system, such as GLP-1 mimetics, offer further treatment options to patients who do not achieve glycemic control with OAD monotherapy or combination therapy. GLP-1 is secreted by the small intestine in response to such stimuli as food in take and physical exercise.[53] GLP-1 stimulates secretion of insulin; inhibits the endogenous conversion of glucagon to glucose in the liver[53]; and reduces gastric emptying, appetite, and food intake via the central nervous system, which could be beneficial in terms of weight control.[53] Two GLP-1 agonists have been developed: Exenatide is a GLP-1 mimetic that has been approved as an adjunctive treatment for type 2 diabetes in the United States and Europe, and liraglutide (Novo Nordisk) is a human GLP-1 analog that is currently undergoing phase 3 trials. Exenatide is approved for use as a twice-daily regimen, while liraglutide is formulated for once-daily administration.[54] In addition, a long-acting release version of exenatide is being developed that may allow once-weekly subcutaneous injection.[55] GLP-1 mimetics/analogs are particularly interesting because they improve glycemic control in association with weight loss. For example, exenatide therapy reduced HbA1c by 0.75% to 0.8% as monotherapy or as part of a combination regimen and patients experienced dose-dependent weight loss of 1.6 to 2.8 kg from baseline in a 30-week trial.[54] Similarly, liraglutide monotherapy or combination therapy with OADs significantly reduced HbA1c levels by 0.6% to 0.8% and resulted in weight loss of 1.2 to 2.9 kg compared with control groups.[54,56]
GLP-1 is degraded by the enzyme DPP-4, so DPP-4 inhibitors are another possible diabetes treatment. Sitagliptin (Januvia, Merck), an oral, once-daily DPP-4 inhibitor, is approved by the FDA for monotherapy or combination therapy with metformin or a TZD.[54] Trials have shown that sitaglipt in monotherapy can reduce HbA1c levels by 0.79% to 0.94%, although in patients with an HbA1c level ≥ 8.5%, reductions of 1.5% have been recorded.[54] The addition of sitaglipt in to metformin can reduce HbA1c level by 0.65%.[54] In contrast to GLP-1 analogs, HbA1c reductions with DPP-4 inhibitors were not associated with weight loss.
Ideally, insulin therapy should mimic healthy pancreatic insulin secretion. This has been made easier with the introduction of long-acting and rapid-acting insulin analogs.[57] Long-acting insulin analogs, such as insulin detemir (Levemir, Novo Nordisk) and insulin glargine (Lantus, sanofi-aventis), provide a more physiologic time-action profile and a longer duration of action than conventional insulin formulations.[58] Improved predictability of response from one injection to another and reduced peak effect with these analogs can also decrease the risk for hypoglycemic episodes, particularly nocturnal episodes.[59] In addition, insulin detemir treatment is associated with less weight gain than NPH insulin.[60–63]
Individuals with diabetes may need rapid-acting insulin to control postprandial glucose. Rapid-acting insulin analogs, such as insulin aspart (NovoLog, NovoRapid, NovoNordisk), insulin lispro (Humalog, Eli Lilly], and insulin glulisine (Apidra, sanofi-aventis), allow individuals greater freedom to inject insulin directly before meals. In addition, these analogs have a shorter duration of action than unmodified human soluble insulin, which may explain why they are associated with fewer hypoglycemic episodes.[64]
Rapid-acting and intermediate-acting insulin analogs are also available in “premix” form that produces a biphasic absorption profile and can help to simplify dosing. In a recent trial, 63 newly diagnosed patients with type 2 diabetes were initiated on metformin and biphasic insulin aspart (NovoLog Mix 70/30, NovoMix 30, Novo Nordisk).[65] This treatment decreased mean HbA1c levels by approximately 5% (10.8% to 5.9%; P < .0001), and 100% of patients achieved an HbA1c <7%.[65] In addition to novel insulin analogs, modern insulin injection devices, such as the FlexPen (Novo Nordisk), offer patients improved convenience and flexibility in insulin dosing and may therefore lead to improved compliance compared with vial and syringe delivery.[66]
The Future of Diabetes Therapy
The epidemic of obesity and sedentary lifestyles, which affects younger as well as older people, means that type 2 diabetes is changing from a disease of late-middle-aged and elderly individuals to one that can strike any age group. In addition, developing countries with populations that are more genetically susceptible to diabetes are likely to see dramatic increases in the incidence of diabetes in young people, as traditional lifestyles and diets change. In this environment, many individuals will have to live with diabetes for longer periods, and in these individuals, controlling the comorbidities associated with diabetes will be the key to ensuring long-term quality of life, limiting disability, and avoiding early death.
One way to address the diabetes epidemic is to target the major causes of the disease through educational programs, incentives, and policies to encourage healthy diets and increased levels of physical activity. These programs should be aimed at all sectors of society and particularly groups in which obesity and diabetes are most prevalent. In addition, although such medical interventions as antiobesity drugs have yet to show convincing benefits, future developments in antiobesity medications may make them a more positive option for individuals who are unable to make appropriate lifestyle changes.
While lifestyle changes are the best and most cost-effective way to prevent diabetes, many individuals find it very difficult to make the long-term changes required for risk reduction. There is good evidence that intensive diabetes therapy with the optimal combination of treatments early in the disease process, with the goal of achieving HbA1c levels as close to normal as possible, has a very positive long-term impact on microvascular and macrovascular comorbidities. These long-term benefits of intensive therapy are particularly important for younger individuals to minimize their risk for retinopathy, heart disease, and other comorbidities in the prime of life. Therefore, it is important to base diabetes treatment on strict HbA1c goals and to intensify or change treatment when these goals are not met. In addition to current OAD treatments, there are a number of new treatment options, such as GLP-1 mimetics and DPP-4 inhibitors, that can provide treatment-intensification options for patients who are unable to achieve glycemic control with OAD monotherapy or combination therapy. However, as soon as maximum doses of OAD combination therapy no longer achieve glycemic goals, insulin therapy should be initiated.
Although evidence that early initiation of insulin therapy can be beneficial in terms of reducing diabetic comorbidities in the long-term is convincing, numerous patient and physician attitudes can delay early adoption. Patients are concerned about needles, possible weight gain, and hypoglycemic episodes.[67] Patients may also underestimate the disease severity and see signs of disease progression as a failure to follow lifestyle guidelines rather than an indication that they might require more intensive therapy.[67] Physicians may delay prescribing insulin because they are aware of the patient's worries, are concerned about possible difficulties in prescribing insulin and the need for patient referral, or have doubts about efficacy or cost.[67] However, physicians can do much to address concerns by demonstrating to their patients that modern insulin injection devices, with fine, coated needles, make injections easier, more comfortable, and more manageable.[66] Physicians can also explain that new insulin analogs and dosing regimens can reduce the number of daily insulin injections and minimize the possibility of hypoglycemic episodes. In addition, if weight gain is a concern, certain treatment choices, such as the basal analog insulin detemir, are associated with less weight gain than conventional NPH insulin. In addition, an inhaled insulin product was developed (Exubera recombinant inhaled insulin powder, Pfizer, Inc.), although it has been with drawn from the market due to lower-than-expected sales.
Conclusion
Type 2 diabetes is changing. Due to lifestyle changes, the disease is becoming more prevalent and is affecting greater numbers of younger individuals. In addition, while in the past type 2 diabetes was considered to be a disparate disease, many clinicians now regard it as one of several metabolic disorders, including the metabolic syndrome and prediabetes. Given that the chronic inflammatory responses that underlie the metabolic syndrome, prediabetes, and type 2 diabetes are induced in part by excessive intake of nutrients and sedentary lifestyles, disease prevention programs that emphasize lifestyle improvements will become increasingly important. In addition, insulin itself may have anti-inflammatory and antiatherogenic effects, as well as effects on glucose metabolism.[16,68] Therefore, starting patients who are unable to produce adequate levels of insulin on insulin therapy may be beneficial in terms of reducing proinflammatory and proatherogenic signaling, in addition to improving glycemic control.
Treatment for diabetes is also changing. Historically, the emphasis was on controlling day-to-day symptoms of hyper- and hypoglycemia. However, the DCCT, EDIC, and UKPDS trials[45] made it clear that strict glycemic control from disease outset, with specific HbA1c targets, can minimize long-term diabetic comorbidities. Current treatment guidelines, such as those from the ADA/EASD[43] and ACE/AACE,[44] recommend strict HbA1c targets and intensifying or changing treatment when these targets are not met.
There are a number of promising new therapeutic agents that target the incretin system, such as GLP-1 mimetics and DPP-4 inhibitors, which could provide additional treatment options for patients not achieving appropriate glycemic control with OAD therapy. However, insulin therapy should be initiated as soon as maximum OAD therapy no longer adequately controls glycemia. With insulin analogs, insulin premixes, possibly inhaled insulin, and improved insulin-delivery devices, insulin is easier to administer and control, meaning that patients are less likely to experience hypoglycemic episodes. Given the changing nature of type 2 diabetes and the increasing numbers of younger individuals who are being affected by the disease, it is important that patients achieve and maintain optimal glycemic control to ensure long-term quality of life.
Table 1.
The HbA1c-Lowering Capacities of Antidiabetic Medications and Their Advantages and Disadvantages
| Interventions | Expected Decrease in HbA1c (%) | Advantages | Disadvantages |
|---|---|---|---|
| Lifestyle modifications | 1–2 | Low cost, many benefits | Most patients fail within 1 year |
| Insulin | ≥ 2.5 | No dose limit, inexpensive, improved lipids | Hypoglycemia, weight gain |
| Inhaled insulin | 1.5 | No injection | Risk for pulmonary complications |
| Metformin | 1.5 | Weight neutral, inexpensive | GI distress, lactic acidosis |
| Sulfonylureas | 1.5 | Inexpensive | Hypoglycemia, weight gain |
| Thiazolidinediones(glitazones) | 0.8–1.0 | Improved lipid profile | Weight gain, edema, anemia, possible CV risks, expensive |
| GLP mimetic/analogs | 0.6 | Weight loss | GI side effects, injection, expensive |
| DPP-4 inhibitors | 0.5–0.9 | No need for dose adjustment | Limited HbA1c lowering |
| Alpha-glucosidase inhibitors | 0.5–0.8 | Weight neutral | Frequent GI side effects, three-times daily dosing, expensive |
Acknowledgements
The author would like to thank Dr. Mike Lappin and Dr. Catherine Jones (Watermeadow Medical, Witney, United Kingdom) for their assistance in preparing this manuscript. Manuscript preparation was supported by Novo Nordisk Inc.
Footnotes
Reader Comments on: The Changing Shape of Type 2 Diabetes See reader comments on this article and provide your own.
Readers are encouraged to respond to the author at OzDoc@aol.com or to George Lundberg, MD, Editor in Chief of The Medscape Journal of Medicine, for the editor's eyes only or for possible publication as an actual Letter in the Medscape Journal via email: glundberg@medscape.net
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Misra A, Misra R, Wijesuriya M, Banerjee D. The metabolic syndrome in South Asians: Continuing escalation & possible solutions. Indian J Med Res. 2007;125:345–354. [PubMed] [Google Scholar]
- 3.Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genetics. 1962;14:353–362. [PMC free article] [PubMed] [Google Scholar]
- 4.Speakman JR. Thrifty genes for obesity and the metabolic syndrome-time to call off the search? Diab Vasc Dis Res. 2006;3:7–11. doi: 10.3132/dvdr.2006.010. [DOI] [PubMed] [Google Scholar]
- 5.Narayan KM, Boyle JP, Geiss LS, Saaddine JB, Thompson TJ. Impact of recent increase in incidence on future diabetes burden: U.S., 2005–2050. Diabetes Care. 2006;29:2114–2116. doi: 10.2337/dc06-1136. [DOI] [PubMed] [Google Scholar]
- 6.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290:1884–1890. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control. National Diabetes Fact Sheet. 2005 United States. Available at: http:/www.cdc.gov/diabetes/pubs/pdf/ndfs_2005.pdf Accessed May 30, 2007.
- 8.Hoy WE, Kondalsamy-Chennakesavan S, Wang Z, et al. Aus Diab Study Group. Quantifying the excess risk for proteinuria, hypertension and diabetes in Australian Aborigines: comparison of profiles in three remote communities in the Northern Territory with those in the Aus Diab study. Aust N Z J Public Health. 2007;31:177–183. doi: 10.1111/j.1753-6405.2007.00038.x. [DOI] [PubMed] [Google Scholar]
- 9.Harris MI. Diabetes in America. 2nd ed. Washington, DC: Government Printing Office; Summary. [Google Scholar]
- 10.Zinman B. Type 2 diabetes mellitus: magnitude of the problem and failure to achieve glycemic control. Endocrinol Metab Clin North Am. 2006;35(Suppl 1):3–5. doi: 10.1016/s0889-8529(07)70003-8. [DOI] [PubMed] [Google Scholar]
- 11.Day C. Metabolic syndrome, or what you will: Definitions and epidemiology. Diab Vasc Dis Res. 2007;4:32–38. doi: 10.3132/dvdr.2007.003. [DOI] [PubMed] [Google Scholar]
- 12.Eckel RH. Mechanisms of the components of the metabolic syndrome that predispose to diabetes and atherosclerotic CVD. Proc Nutr Soc. 2007;66:82–95. doi: 10.1017/S0029665107005320. [DOI] [PubMed] [Google Scholar]
- 13.Theuma P, Fonseca VA. Inflammation, insulin resistance, and atherosclerosis. Metab Syndr Relat Disord. 2004;2:105–113. doi: 10.1089/met.2004.2.105. [DOI] [PubMed] [Google Scholar]
- 14.Mohanty P, Hamouda W, Garg R, Aljada A, Ghanim H, Dandona P. Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. JClin Endocrinol Metab. 2000;85:2970–2973. doi: 10.1210/jcem.85.8.6854. [DOI] [PubMed] [Google Scholar]
- 15.Aljada A, Ghanim H, Mohanty P, Syed T, Bandyopadhyay A, Dandona P. Glucose intake induces an increase in activator protein 1 and early growth response 1 binding activities, in the expression of tissue factor and matrix metalloproteinase in mononuclear cells, and in plasma tissue factor and matrix metalloproteinase concentrations. Am J Clin Nutr. 2004;80:51–57. doi: 10.1093/ajcn/80.1.51. [DOI] [PubMed] [Google Scholar]
- 16.Dandona P, Mohanty P, Hamouda W, et al. Inhibitory effect of a two day fast on reactive oxygen species (ROS) generation by leucocytes and plasma ortho-tyrosine and meta-tyrosine concentrations. J Clin Endocrinol Metab. 2001;86:2899–2902. doi: 10.1210/jcem.86.6.7745. [DOI] [PubMed] [Google Scholar]
- 17.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 18.Wu Y. Overweight and obesity in China. BMJ. 2006;333:362–363. doi: 10.1136/bmj.333.7564.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou B-F. Effect of body mass index on all-cause mortality and incidence of cardiovascular diseases – Report for meta-analysis of prospective studies on optimal cut-off points of body mass index in Chinese adults. Biomed Environ Sci. 2002;15:245–252. [PubMed] [Google Scholar]
- 20.Foreyt JP. Cultural competence in the prevention and treatment of obesity: Latino Americans. Permanente J. 2003;7:42–45. [Google Scholar]
- 21.Mazur RE, Marquis GS, Jensen HH. Diet and food insufficiency among Hispanic youths: acculturation and socioeconomic factors in the third National Health and Nutrition Examination Survey. Am J Clin Nutr. 2003;78:1120–1127. doi: 10.1093/ajcn/78.6.1120. [DOI] [PubMed] [Google Scholar]
- 22.Musaiger AO, al-Roomi KA. Prevalence of risk factors for cardiovascular diseases among men and women in an Arab Gulf community. Nutr Health. 1997;11:149–157. doi: 10.1177/026010609701100302. [DOI] [PubMed] [Google Scholar]
- 23.Rocchini AP. Childhood obesity and a diabetes epidemic. N Engl J Med. 2002;346:854–855. doi: 10.1056/NEJM200203143461112. [DOI] [PubMed] [Google Scholar]
- 24.Zimmet P, Alberti KGMM, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 25.Weinstock RS. Treating type 2 diabetes mellitus: a growing epidemic. Mayo Clin Proc. 2003;78:411–413. doi: 10.4065/78.4.411. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control. National Diabetes Surveillance System. 2007 Available at: http://www.cdc.gov/diabetes/statistics/prev/national/tnumage.htm Accessed May 30, 2007.
- 27.Pinhas-Hamiel O, Zeitler P. The global spread of type 2 diabetes mellitus in children and adolescents. J Pediatr. 2005;146:693–700. doi: 10.1016/j.jpeds.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 28.Pinhas-Hamiel O, Zeitler P. Acute and chronic complications of type 2 diabetes mellitus in children and adolescents. Lancet. 2007;369:1823–1831. doi: 10.1016/S0140-6736(07)60821-6. [DOI] [PubMed] [Google Scholar]
- 29.Yokoyama H, Okudaira M, Otani T, et al. Existence of early-onset NIDDM Japanese demonstrating severe diabetic complications. Diabetes Care. 1997;20:844–847. doi: 10.2337/diacare.20.5.844. [DOI] [PubMed] [Google Scholar]
- 30.Dominiczak MH. Obesity, glucose intolerance and diabetes and their links to cardiovascular disease. Implications for laboratory medicine. Clin Chem Lab Med. 2003;41:1266–1278. doi: 10.1515/CCLM.2003.194. [DOI] [PubMed] [Google Scholar]
- 31.Festa A, Williams K, D'Agostino R, Jr, Wagenknecht LE, Haffner SM. The natural course of beta-cell function in nondiabetic and diabetic individuals: the Insulin Resistance Atherosclerosis Study. Diabetes. 2006;55:1114–1120. doi: 10.2337/diabetes.55.04.06.db05-1100. [DOI] [PubMed] [Google Scholar]
- 32.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999;104:787–794. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 34.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 35.Twigg SM, Kamp MC, Davis TM, Neylon EK, Flack JR. Prediabetes: a position statement from the Australian Diabetes Society and Australian Diabetes Educators Association. Med J Aust. 2007;186:461–465. doi: 10.5694/j.1326-5377.2007.tb00998.x. [DOI] [PubMed] [Google Scholar]
- 36.The DECODE Study Group on behalf of the European Diabetes Epidemiology Group. Glucose tolerance and mortality: comparison of WHO and American Diabetic Association diagnostic criteria. Lancet. 1999;354:617–621. [PubMed] [Google Scholar]
- 37.Tominaga M, Eguchi H, Manaka H, Igarashi K, Kato T, Sekikawa A. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care. 1999;22:920–924. doi: 10.2337/diacare.22.6.920. [DOI] [PubMed] [Google Scholar]
- 38.Cooke D, Bloom S. The obesity pipeline: current strategies in the development of anti-obesity drugs. Nat Rev Drug Discov. 2006;5:919–931. doi: 10.1038/nrd2136. [DOI] [PubMed] [Google Scholar]
- 39.Rimonabant Regulatory Update in the United States. Available at: http://en.sanofi-aventis.com/press/ppc_17004.asp#3 Accessed July 17, 2007
- 40.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lindström J, Ilanne-Parikka P, Peltonen M, et al. Finnish Diabetes Prevention Study Group. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet. 2006;368:1673–1679. doi: 10.1016/S0140-6736(06)69701-8. [DOI] [PubMed] [Google Scholar]
- 42.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 43.Nathan DM, Buse JB, Davidson MB, et al. Management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2006;29:1963–1972. doi: 10.2337/dc06-9912. [DOI] [PubMed] [Google Scholar]
- 44.Jellinger PS, Davidson JA, Blonde L, et al. ACE/AACE Diabetes Road Map Task Force. Road maps to achieve glycemic control in type 2 diabetes mellitus: ACE/AACE Diabetes Road Map Task Force. Endocr Pract. 2007;13:260–268. doi: 10.4158/EP.13.3.260. [DOI] [PubMed] [Google Scholar]
- 45.The Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications(DCCT/EDIC) Research Group. Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA. 2002;287:2563–2569. doi: 10.1001/jama.287.19.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.UKProspective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 48.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nathan DM. Finding new treatments for diabetes–how many, how fast… how good? N Engl J Med. 2007;356:437–440. doi: 10.1056/NEJMp068294. [DOI] [PubMed] [Google Scholar]
- 50.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 51.Home PD, Pocock SJ, Beck-Nielsen H, et al. RECORD Study Group. Rosiglitazone evaluated for cardiovascular outcomes–an interim analysis. N Engl JMed. 2007;357:28–38. doi: 10.1056/NEJMoa073394. [DOI] [PubMed] [Google Scholar]
- 52.FDA. Information for healthcare professionals on rosiglitazone maleate (marketed as Avandia, Avandamet, and Avandaryl). Available at: http://www.fda.gov/cder/drug/InfoSheets/HCP/rosiglitazone200707HCP.htm Accessed April 8, 2008.
- 53.Geelhoed-Duijvestijn PH. Incretins: a new treatment option for type 2 diabetes? Neth J Med. 2007;65:60–64. [PubMed] [Google Scholar]
- 54.Triplitt CL. New technologies and therapies in the management of diabetes. Am J Manag Care. 2007;13(Suppl 2):S47–54. [PubMed] [Google Scholar]
- 55.Kim D, MacConell L, Zhuang D, et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care. 2007;30:1487–1493. doi: 10.2337/dc06-2375. [DOI] [PubMed] [Google Scholar]
- 56.Madsbad S, Schmitz O, Ranstam J, Jakobsen G, Matthews DR NN2211-1310International Study Group. Improved glycemic control with no weight increase in patients with type 2 diabetes after once-daily treatment with the long-acting glucagon-like peptide 1 analog liraglutide (NN2211): a 12-week, double-blind, randomized, controlled trial. Diabetes Care. 2004;27:1335–1342. doi: 10.2337/diacare.27.6.1335. [DOI] [PubMed] [Google Scholar]
- 57.Levy P. Insulin analogues or premixed insulin analogs in combination with oral agents for treatment of type 2 diabetes. Med Gen Med. 2007;9:12. [PMC free article] [PubMed] [Google Scholar]
- 58.Heise T, Heinemann L. Rapid and long-acting analogues as an approach to improve insulin therapy: an evidence-based medicine assessment. Curr Pharm Des. 2001;7:1303–1325. doi: 10.2174/1381612013397375. [DOI] [PubMed] [Google Scholar]
- 59.Meneghini LF, Rosenberg KH, Koenen C, Merilainen MJ, Luddeke HJ. Insulin detemir improves glycemic control with less hypoglycaemia and no weight gain in patients with type 2 diabetes who were insulin naive or treated with NPH or insulin glargine: clinical practice experience from a German subgroup of the PREDICTIVE study. Diabetes Obes Metab. 2007;9:418–427. doi: 10.1111/j.1463-1326.2006.00674.x. [DOI] [PubMed] [Google Scholar]
- 60.Hermansen K, Fontaine P, Kukolja KK, Peterkova V, Leth G, Gall MA. Insulin analogues (insulin detemir and insulin aspart) versus traditional human insulins (NPH insulin and regular human insulin) in basal-bolus therapy for patients with type 1 diabetes. Diabetologia. 2004;47:622–629. doi: 10.1007/s00125-004-1365-z. [DOI] [PubMed] [Google Scholar]
- 61.Haak T, Tiengo A, Draeger E, Suntum M, Waldhausl W. Lower within-subject variability of fasting blood glucose and reduced weight gain with insulin detemir compared to NPH insulin in patients with type 2 diabetes. Diabetes Obes Metab. 2005;7:56–64. doi: 10.1111/j.1463-1326.2004.00373.x. [DOI] [PubMed] [Google Scholar]
- 62.Philis-Tsimikas A, Charpentier G, Clauson P, Ravn GM, Roberts VL, Thorsteinsson B. Comparison of once-daily insulin detemir with NPH insulin added to a regimen of oral antidiabetic drugs in poorly controlled type 2 diabetes. Clin Ther. 2006;28:1569–1581. doi: 10.1016/j.clinthera.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 63.Raslova K, Tamer SC, Clauson P, Karl D. Insulin detemir results in less weight gain than NPH insulin when used in basal-bolus therapy for type 2 diabetes mellitus, and this advantage increases with baseline body mass index. Clin Drug Investig. 2007;27:279–285. doi: 10.2165/00044011-200727040-00007. [DOI] [PubMed] [Google Scholar]
- 64.Heinemann L. Overcoming obstacles: new management options. Eur J Endo. 2004;151:T23–27. doi: 10.1530/eje.0.151t023. [DOI] [PubMed] [Google Scholar]
- 65.Lingvay I, Kaloyanova PF, Adams-Huet B, Salinas K, Raskin P. Insulin as initial therapy in type 2 diabetes: effective, safe, and well accepted. J Investig Med. 2007;55:62–68. doi: 10.2310/6650.2007.06036. [DOI] [PubMed] [Google Scholar]
- 66.Korytkowski M, Bell D, Jacobsen C, Suwannasari R Flex Pen Study Team. Amulticenter, randomized, open-label, comparative, two-period crossover trial of preference, efficacy, and safety profiles of a prefilled, disposable pen and conventional vial/syringe for insulin injection in patients with type 1 or 2 diabetes mellitus. Clin Ther. 2003;25:2836–2848. doi: 10.1016/s0149-2918(03)80337-5. [DOI] [PubMed] [Google Scholar]
- 67.Philips P. Type 2 diabetes – failure, blame and guilt in the adoption of insulin therapy. Rev Diabet Stud. 2005;2:35–39. doi: 10.1900/RDS.2005.2.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation. 2005;111:1448–1454. doi: 10.1161/01.CIR.0000158483.13093.9D. [DOI] [PubMed] [Google Scholar]


