Abstract
The activation of Ca2+ entry through store-operated channels by agonists that deplete Ca2+ from the endoplasmic reticulum (ER) is a ubiquitous signaling mechanism, the molecular basis of which has remained elusive for the past two decades. Store-operated Ca2+-release-activated Ca2+ (CRAC) channels constitute the sole pathway for Ca2+ entry following antigen-receptor engagement. In a set of breakthrough studies over the past two years, stromal interaction molecule 1 (STIM1, the ER Ca2+ sensor) and Orai1 (a pore-forming subunit of the CRAC channel) have been identified. Here we review these recent studies and the insights they provide into the mechanism of store-operated Ca2+ channels (SOCCs).
Keywords: Store-operated Ca2+ entry (SOCE), Stromal interaction molecule (STIM), Orai
INTRODUCTION
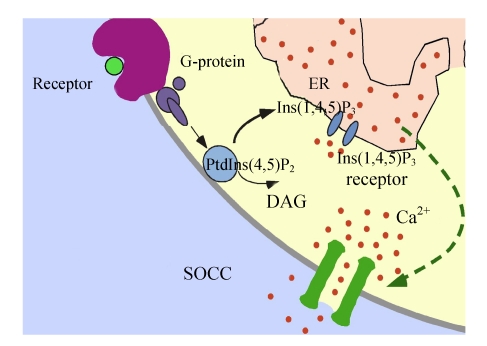
Ca2+ signals control a vast number of cellular functions ranging from short-term responses, such as contraction and secretion, to longer-term regulation of transcription, growth and cell division (Jiang et al., 2006). Eukaryotic cells can increase their cytoplasmic Ca2+ concentration in either of two ways: release from intracellular stores or Ca2+ influx into the cell (Bugaj et al., 2005). Endoplasmic reticulum (ER) serves to store the intracellular pool of Ca2+, but it is limited in its capacity to store and needs to be refilled when depleted. However, many key processes require sustained increases in intracellular Ca2+ and this is accomplished through Ca2+ entry into the cell. A variety of different Ca2+-permeable channels have been found to coexist in the plasma membrane (PM) (He et al., 2007; Demaurex et al., 2002). Voltage-gated Ca2+ channels are found in excitable cells like nerve and muscle but are largely excluded from non-excitable cells (Felix, 2005). Receptor-operated channels are also preponderant in excitable cells. As Fig.1 indicates, stimulation of diverse PM receptors converges on the activation of phosphatidylinositol (4,5)-bisphosphate [PtdIns(4,5)P2] hydrolysis, which results in the generation of diacylglycerol (DAG) and inositol (1,4,5)-trisphosphate [Ins(1,4,5)P3] and the subsequent activation of Ca2+ release from the ER via Ins(1,4,5)P3 receptors and Ca2+ influx across the PM. Depletion of ER Ca2+ activates PM-localized Ca2+ influx channels known as store-operated Ca2+ channels (SOCCs) (Parekh, 2003). SOCC apparently exists in all eukaryotes from yeast to humans (Liu et al., 2005; Mueller et al., 2007). A major function of this Ca2+ entry pathway is believed to be the maintenance of ER Ca2+ levels that are necessary for proper protein synthesis and folding. However, it has now been firmly established that store-operated Ca2+ entry (SOCE) is central to the physiology of eukaryotic cells. SOCE serves as the sole Ca2+ entry mechanism in a variety of non-excitable cells and plays an indispensable role in Ca2+ signaling and many other cellular processes ranging from proliferation to apoptosis.
Fig. 1.
Model for the activation mechanism of store-operated Ca2+ channels (SOCCs)
SOCCs are a family of Ca2+ permeable ion channels expressed by most cells. The signal for the activation of these ion channels is a decrease in the Ca2+ concentration in the endoplasmic reticulum (ER). Stimulation of diverse plasma membrane (PM) receptors converges on the activation of phosphatidylinositol (4,5)-bisphosphate [PtdIns(4,5)P2] hydrolysis, which results in the generation of diacylglycerol (DAG) and inositol (1,4,5)-trisphosphate [Ins(1,4,5)P3], and the subsequent activation of Ca2+ release from the ER via Ins(1,4,5)P3 receptor and Ca2+ influx across the PM. Depletion of ER Ca2+ activates PM-localized Ca2+ influx channels known as SOCCs
Although several biophysically distinct SOCCs have been reported, the best characterized are the Ca2+ release-activated Ca2+ (CRAC) channels. Over the years many genes have been claimed to code for the CRAC channel (Winslow et al., 2003; Prakriya et al., 2006). Recently two key players have been identified: stromal interaction molecule 1 (STIM1, the ER Ca2+ sensor) and Orai1 (the pore-forming CRAC channel subunit) (Huang et al., 2006; Yeromin et al., 2006). An RNAi-based screening approach has revealed a novel single membrane-spanning protein named STIM1 to be required for activation of SOCC. STIM1 is found to be dispersed on the ER membrane in a quiescent situation. Ca2+ store depletion stimulates redistribution of STIM1 to the PM. The redistribution is thought to transmit a store depletion signal to the CRAC channels in the PM. In addition, it has been suggested that Orai1 could function either as a component or a regulator of the CRAC channel (Gwack et al., 2007). Hence, SOCC is the product STIM1-mediated sensing of ER Ca2+ content leading to activation of PM-localized Orai1 channels. The following sections describe details of the discovery and functional elucidation of the mechanisms involved in this signaling pathway.
STORE-OPERATED Ca2+ ENTRY (SOCE)
The concept of SOCE was proposed in 1986. This idea originated from a series of experiments in parotid acinar cells investigating the relationships between Ca2+ release from internal stores, Ca2+ entry and store refilling. On the basis of this work it was suggested that the amount of Ca2+ in the stores controlled the extent of Ca2+ influx in non-excitable cells, a process originally called capacitate Ca2+ entry (Vanden Abeele et al., 2003). When stores were full, Ca2+ influx did not occur but, as the stores emptied, Ca2+ entry developed. It is important to note that SOCE does not necessarily refer to a single mechanism of Ca2+ entry, nor does store-operated entry necessarily refer to a single Ca2+ entry channel; instead, any channel that can be shown to exhibit Ca2+ store-dependent activity can be referred to as an SOCC. The most studied and best characterized SOCC current is the CRAC, which was first characterized in human T cells (Lewis and Cahalan, 1989) and mast cells (Hoth and Penner, 1992).
CRAC is non-voltage activated, inwardly rectifying and remarkably selective for Ca2+. Other SOCE currents that have been characterized generally exhibit less stringent ionic selectivity (Vig et al., 2006). The mechanism by which CRAC is activated, including the molecular identity of the CRAC channel, has long been the most sought after signaling paradigm in the store-operated entry field. Whether via CRAC channel or another SOCC, SOCE accomplishes several critical functions within the cell. First, Ca2+ that enters the cell via the SOCC pathway replenishes the ER Ca2+ stores following a release event, thus maintaining the ability of the ER to release Ca2+ into the cytoplasm in response to subsequent Ca2+ releasing stimuli. This is most notable in cells that respond to activation of the PtdIns(4,5)P2 pathway with Ca2+ influs oscillations, since ablation of Ca2+ entry by removing extracellular Ca2+ or by other pharmacological or molecular means will oftentimes preclude a cell’s ability to maintain Ca2+ influs oscillations (Parekh, 2003). Second, Ca2+ concentrations within the ER must be maintained at sufficient levels in order for the organelle to carry out many of its fundamental functions (Laporte et al., 2004). Thus, chronic depletion of ER Ca2+, as would occur in the absence of SOCE, can influence ER-dependent processes such as protein folding and trafficking, the ER stress response and apoptosis. Third, it should be noted that Ca2+ that enters the cell via the store-operated pathway first accesses the cytoplasm before entering the ER and, in many cases, results in sustained elevation in Ca2+ influs levels, which is significant for some signaling events, in particular T-lymphocyte activation (Aires et al., 2004). It is the increase in cytoplasmic Ca2+ resulting from SOCC, as opposed to that resulting from Ca2+ released from the ER, that is responsible for signaling. Thus, SOCE can influence many aspects of cell biology. It is vital that we understand the molecular compositions of all forms of SOCE so that we may fully appreciate its contribution to normal physiological as well as pathophysiological states of human health.
Now the quest to uncover the molecular identity of the CRAC channel and its mechanism of activation has reached a new important turning point with the recent discoveries that two proteins, STIM1 and Orai1, play obligatory roles in the activation of CRAC channel. Accumulating evidence indicates that STIM1 acts as a sensor of ER store content, while Orai1 may be the CRAC channel itself (Mignen et al., 2007; Gwack et al., 2007).
TRANSIENT RECEPTOR POTENTIAL CHANNELS (TRPCS) AND SOCCS
For some time now, a significant effort has been made to determine whether or not members of the TRPC family of ion channels are molecular components of the CRAC channel, just because it has been shown that TRPCs are activated in response to agonist-stimulated PtdIns(4,5)P2 hydrolysis in a variety of tissues. However, no TRPC has the same electrophysiological and pharmacological properties as CRAC channel. Although the exact function of TRPCs and how they are regulated have not been established, increasing data suggest that they are localized and regulated within Ca2+ signaling microdomains (Worley et al., 2007; Huang et al., 2006). TRPC monomers generate a wide variety of cation channels via homomeric and heteromeric interactions. In addition, interaction of TRPCs with regulatory and scaffolding proteins contributes to the diversity and segregation of the channels (Bollimuntha et al., 2005). Furthermore, there is clear evidence that TRPCs have a role in mediating agonist-stimulated SOCE and non-SOCE mechanisms. Furthermore, it is important to note that TRPCs may function in certain physiological contexts as SOCC, despite the fact that this activity is distinct from CRAC channels.
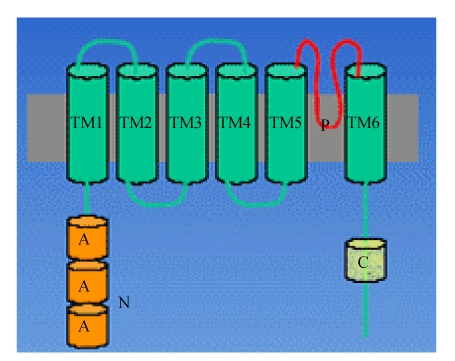
The TRPC family contains the mammalian genes most closely related to the Drosophila trp gene (Liu et al., 2007). Fig.2 indicates the quaternary structure of TRPC. The mammalian canonical TRPC family of cation channels consists of seven members (TRPC1~TRPC7) and the seven family members can be subdivided into subfamilies on the basis of their amino acid similarity (Fleming et al., 2007). While TRPC1 and TRPC2 are almost unique, TRPC4 and TRPC5 share about 64% amino acid identity. TRPC3, TRPC6 and TRPC7 form a structural and functional subfamily displaying 65%~78% identity at the amino acid level. All TRPC family members harbor an invariant sequence in the C-terminal tail, called the transient receptor potential (TRP) box (amino acid sequence: EWKFAR), as well as 3~4 NH2-terminal ankyrin repeats (Lepage and Boulay, 2007; Pigozzi et al., 2006).
Fig. 2.
Quaternary structure of TRPC
All members of the TRPC family are believed to share a common topology. The cytoplasmic N-terminus and C-terminus are separated by six predicted transmembrane domains (TM1~TM6), including a putative pore region (P) between TM5 and TM6. However, there is uncertainty as to the exact assignment of the first’s two transmembrane segments. The N-terminus is composed of three to four ankyrin (A) repeats, a predicted coiled-coil region and a putative caveolin binding region. The cytoplasmic C-terminus includes the TRP signature motif (EWKFAR), a highly conserved proline rich motif
TRPC1 is currently the strongest candidate component of SOCCs. Endogenous TRPC1 contributes to SOCE in several different cell types, including the salivary glands, smooth muscle, DT40 (a chicken B lymphocyte cell line) and endothelial cells (Homann et al., 2006; Venkatachalam et al., 2003). By contrast, heterologously expressed TRPC1 displays store-independent regulation of Ca2+ entry in some studies. Meanwhile, the notion that TRPC1 and TRPC4 may form or be part of SOCC is supported mainly by two different lines of experimental evidence. First, ectopic expression of TRPC1 or TRPC4 in several cell lines increases endogenous store-operated entry induced by either PLC-dependent or pharmacologically-induced depletion of ER Ca2+ stores (Alfonso et al., 2008). Second, the use of antisense constructs directed against TRPC1 or TRPC4, as well as genetic disruption of the corresponding genes, has efficiently reduced store-operated entry in a variety of cell types. In some instances, those protocols produced a significant reduction of the archetypical CRAC, leading to the conclusion that TRPC1 or TRPC4 is part of native CRAC channels (Ambudkar et al., 2007).
STIM PROTEINS AND SOCC
Roos et al.(2005) performed a large scale RNAi screen in drosophila S2 cells by targeting any protein with a transmembrane domain, including the TRPC family, as well as any protein that had been previously described as playing a role in SOCE. Of 170 genes targeted, only STIM was found to be needed for SOCE and activation of CRAC channel in S2 cells. In a parallel study, Liou et al.(2005) targeted a total of 2304 proteins. They identified two mammalian STIM homologues, STIM1 and STIM2, whose knockdown reduced store-operated entry. It should be noted that when Roos et al.(2005) applied siRNAs against STIM1 and STIM2 in Jurkat cells, STIM1 was essential for SOCE whereas loss of STIM2 had no effect.
The STIM protein was originally recognized and named STIM from a novel screening of cell surface expressed proteins on stromal cells by using a signal-sequence trap method to identify membrane proteins. This protein is identified as a type-1 (single-spanning) cell surface membrane protein and contains several conserved domains, including a sterile-alpha motif (SAM), a coiled-coil region, and an EF-hand domain (Ross et al., 2007). The gene is mapped to human chromosome 11p15.5 (Stathopulos et al., 2006). The STIM1 protein is proposed as a “sensor” of Ca2+ within stores; this sensor’s function is mediated via a single EF-hand Ca2+ binding motif located in its N-terminal ER luminal domain. STIM2 is the close mammalian homolog of STIM1. STIM1 and STIM2 are widely expressed and have almost identical EF-hand-containing N-terminal domains and trans-membrane sequences. The cytoplasmic C-terminal domains contain nearly identical coiled-coil regions; thereafter, their sequences deviate toward the C terminus (Soboloff et al., 2006a).
STIM1 is the Ca2+ sensor
It was clear that suppressed STIM1 expression prevents SOCE and eliminates the store-dependent activation of CRAC channels. Some reports showed that the STIM1 is a component of the Ca2+ influx channel (Soboloff et al., 2006b; Huang et al., 2006). In support of this opinion, Drosophila genome-wide search identified numerous potential ion channel genes as well as Ca2+-binding proteins with transmembrane domains, yet only STIM had a detectable effect on SOCE. Moreover, over-expression of STIM1 by nearly 100-fold in HEK293 cells enhanced SOCC influx by only 17% and did not induce detectable SOCC current, arguing that STIM1 is probably not the channel itself. A recent report concluded that STIM1 functions as a component that would facilitate assembly and regulate activity of SOCCs in different tissues, serving as an essential subunit (Spassova et al., 2006). Mechanistically STIM1 might function as a Ca2+ sensor or a coupler linking store depletion to activation of SOCCs, as shown in Fig.3. Some more recent study showed this function being mediated via the EF-hand Ca2+-binding domain on the N-terminal ER luminal portion of STIM1 (Hewavitharana et al., 2007; Li et al., 2007). Decreased ER Ca2+ results in a profound intracellular redistribution of STIM1 from a uniform ER pattern to spatially discrete areas termed puncta. Mutations in acidic residues within the Ca2+-binding pocket of the EF-hand domain of STIM1, which presumably lower the affinity for Ca2+, produce constitutive Ca2+ entry that is independent of store depletion. The EF-hand-mutated STIM1 protein is already distributed in puncta, exactly mimicking the store-depleted mode. These results are highly consistent with the premise that the EF-hand is indeed the luminal Ca2+ sensor for SOCCs (Baba et al., 2006; Luik et al., 2006). However, without physical evidence of actual Ca2+-binding, it must be considered that the EF-hand domain of STIM1 might not bind Ca2+ and could serve another purpose.
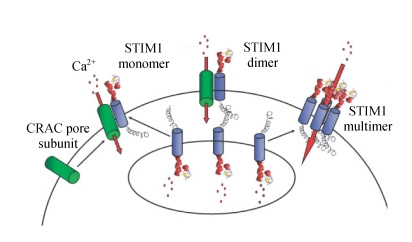
Fig. 3.
Model of STIM1 function
Upon store depletion, STIM1 located in the ER unbinds Ca2+ and translocates to the plasma membrane to activate CRAC channel subunits that are already in the plasma membrane (left), form junctions between the Ca2+ store and the plasma membrane (middle) or assemble to form functional CRAC channels (right) (Zhang et al., 2005)
The precise location of STIM1 after store depletion is crucial in understanding its role in the SOCC process. One hypothesis is that STIM1 is transported to and inserted into the PM upon Ca2+ store depletion (Zhang et al., 2005). In contrast, others have suggested that STIM1 may aggregate underneath the PM without being inserted into the membrane (Wu et al., 2006). A recent study reinforced this argument by reporting little surface staining of EYFP (enhanced yellow fluorescent protein) antibody using an immunofluorescence method in HEK293 cells transfected with EYFP-STIM1 (Smyth et al., 2007). However, the result would be hard to reconcile with the electrophysiological experiments showing that extracellular application of antibody against the N-terminus of STIM1 blocks SOCE in HEK293 cells (Spassova et al., 2006). Although a most recent report concluded that STIM1 puncta formed in the ER near the PM (Spassova et al., 2006), quantitative comparisons of STIM1 fractions in the ER have not been made between, before and after store depletion.
Three preliminary models have been proposed to explain how STIM1 might work based upon its cellular localization patterns. All the three models have in common the fact that STIM1 is located in the ER membrane prior to store depletion. The first model suggests that STIM1 in the ER is combined with STIM1 in the PM following store depletion. This model of course requires that some STIM1 is found in the PM and that this pool of STIM1 is necessary for activation of CRAC channel (Luik et al., 2006). The second model contends that most or all STIM1 is confined to the ER membrane prior to store depletion. After store depletion, however, STIM1 is somehow transported to and inserted into the PM. It is not clear what mechanism would be involved in the transport of STIM1 to the PM, as accumulating evidence suggests that the most likely mechanism, vesicle trafficking, is not involved in CRAC channel. Both the first and second models are supported by cell surface biotinylation experiments that detected STIM1. However, these data must be interpreted with caution since STIM1 may have been co-precipitated with a cell surface protein such as the channel itself rather than being directly biotinylated. In studies employing transfection of HEK293 cells with STIM1 and Orai1 (discussed in the following section), no STIM1 was detected in the PM by antibody binding, despite the generation of huge STIM1- and Orai1-dependent CRAC-like currents. These latter findings favor a third model which places STIM1 only in the ER membrane. Following store depletion, the puncta would be very close to the cell surface, but not actually inserted into the PM. In this scenario the STIM1 would interact with additional components of the CRAC pathway, such as the channels themselves, when brought into the puncta. This model does not exclude the possibility that STIM1 under certain circumstances can be found in the PM. It does indicate, however, that this putative PM pool of STIM1 is not essential for activation of CRAC channel and SOCE (Wu et al., 2006).
STIM2 is an inhibitor of STIM1
STIM1 and STIM2 are clearly closely related to each other with respect to their primary amino acid sequence and their predicted secondary structure and domain organization. Both proteins are predicted to be single-pass transmembrane proteins, with an exoplasmic N-terminal region and a cytoplasmic C-terminal region. The sequences of the two STIM proteins diverge significantly from C-terminal region to the coiled-coils region; STIM1 and STIM2 contain unique proline-rich regions that include serine/threonine residues. Human STIM2 maps to chromosome 4p15.1 (Soboloff et al., 2006b; Wissenbach et al., 2007). While STIM1 is expressed in both the ER and PM, STIM2 is expressed only intracellularly (Wissenbach et al., 2007).
Despite sharing close structural homology with STIM1, STIM2 has a very different role in the control of Ca2+ entry. The effects of over-expressed STIM1 and STIM2 on SOCC were compared by using stably expressing clonal HEK293 lines. STIM2 expression in HEK293 cells results in almost complete inhibition in SOCE, with good correlation between STIM2 expression and suppression of SOCC. The recent study revealed that expression of STIM2 has a powerful inhibitory effect on SOCC activation in other cells, including PC12 pheochromocytoma cells, A7r5 smooth muscle cells and Jurkat T cells (Soboloff et al., 2006b).
There are two models for STIM1-mediated SOCC activation involving: (1) “insertion” of STIM1 into the PM after store depletion and (2) “interaction” of the ER STIM1 with the PM to activate the channel. In an insertion model, binding of STIM1 to STIM2 could prevent STIM1 transfer into the PM. The N-terminal half of the cytoplasmic region of both STIM molecules is predicted to be almost exclusively a-helical, most of which is predicted to form coiled-coils. The homotypic and heterotypic interactions between STIM proteins are mediated by these cytoplasmic coiled-coil regions (Dziadek and Johnstone, 2007). SOCC activation involving ER-PM interactions is compatible with the “conformational-coupling” model supported by evidence that close interactions, but not ER-PM fusion, are involved in SOCC activation. In the store-replete resting state, SOCCs are closed and STIM proteins are distributed throughout the ER. STIM1 is also present in the PM, where it is required for SOCC activation. The effect of STIM2 is depicted as dependent on its ratio with STIM1 (Soboloff et al., 2006b). After store emptying, STIM1 and STIM2 become aggregated and organized within puncta close to the PM. When the STIM2/STIM1 ratio is low, functional coupling to activate SOCC occurs; this is depicted as C-terminal interactions between ER STIM1 and PM STIM1 associated with the channel. When the STIM2/STIM1 ratio is high, puncta contains more STIM2 depicted as interfering with successful conformational coupling to activate SOCC. Thus, expression and localization of STIM2 within puncta may be a key regulatory control process in the activation of SOCC. STIM2 may exert an important level of control over the activation of SOCC and hence the mediation of longer-term Ca2+ signals regulating transcription, cell growth and proliferation.
ORAI PROTEINS AND SOCC
Recently the second protein component of CRAC channel has been identified and named as Orai (in Greek mythology, the Orai is the keeper of the gate of heaven), which is a PM four-transmembrane spanning protein (Feske et al., 2006). This revelation came from a combination of elegant studies including genome wide RNAi screening (drosophila genome wide RNA interference screen) and modified linkage analysis identifying (a modified linkage analysis with single-nucleotide polymorphism arrays in the cells from patients with one form of hereditary severe combined immune deficiency (SCID) syndrome, who are defective in SOCE and CRAC channel function). The naturally occurring mutation in Orai1 led to elimination of CRAC, as did Orai1 knockdown. Expression of wild type Orai1 restored CRAC channel activity to normal levels in cells taken from immune-deficient patients. Initially the Orai1 protein was not recognized as being related to other known channel proteins, and hence it was unclear whether it was the channel moiety of SOCC or whether it was yet another protein involved in coupling or regulating of SOCCs. In 2006, by using site-directed mutagenesis, Dr. Cahalan’s group showed that a point mutation (E180D) in the conserved S1-S2 loop of drosophila Orai (olf186-F) transforms the ion selectivity properties of CRAC from being Ca2+-selective with inward rectification to being selective for monovalent cations and outwardly rectifying (Yeromin et al., 2006). Moreover, Dr. Rao’s group proved that mutations within putative pore regions of human Orai1 also change the biophysical properties of SOCE (Prakriya et al., 2006). All these results suggest that human Orai1 and drosophila homologue Orai are essential pore-forming subunits of the SOCC. It is now clear that the Orai1 protein fulfills all the criteria of being the SOCC moiety itself. Unlike STIM1, Orai1 apparently resides only at the PM, the N-terminus and C-terminus of the tetra spanning membrane protein both exist within the cytoplasm (Gwack et al., 2007). In addition to Orai1 there are two other Orai family members, Orai2 and Orai3, expressed in mammals (DeHaven et al., 2007). Hence, heteromeric combinations of these proteins may result in channels with distinct regulatory and/or coupling processes.
Orai1 co-expressed with STIM1 is sufficient to reconstitute SOCC
Orai1, like STIM1, is an essential part of the signaling sequence that links empty stores to activation of SOCE, but where does it fit? Orai1 is predicted to have four membrane spanning regions (M1~M4) and is incorporated into the PM with its M3-M4 loop in the extracellular space and its N-terminus and C-terminus in the cytosol (Gwack et al., 2007; Vig et al., 2006). Orai1 has no obvious similarity to any known channel, but CRAC is also an unusual current; so, is Orai1 the missing SOCE channel? Recently, two independent groups have indicated this to be the case by demonstrating that mutations within putative pore regions of Orai or Orai1 change the biophysical properties of SOCE (Yeromin et al., 2006; Feske et al., 2006). In addition, several reports have demonstrated that Orai1 co-expressed in STIM1-expressing cells resulted in a massive and rapid increase in SOCC (Hewavitharana et al., 2007; Takahashi et al., 2007). This increase is not seen when STIM1 alone is transfected into cells. However, when Orai1 alone is transfected, there is a clear inhibition of CRAC in Jurkat, RBL cells and HEK293 cells (Prakriya et al., 2006; DeHaven et al., 2007). These findings illustrate several important points: (1) They demonstrate that STIM1 and Orai1 are the only components necessary to produce large CRAC currents when over-expressed, indicating that there are no other rate-limiting components of the system. Interestingly the CRAC currents observed when STIM1 and Orai1 are co-expressed do not completely recapitulate all of the canonical characteristics of CRAC; (2) A significant question is why over-expression of Orai1 results in substantially lower SOCC in HEK293 cells or decreased CRAC channel activity in RBL cells. Some report (Hewavitharana et al., 2007; Prakriya et al., 2006) explained that over-expression of Orai1 alters coupling stoichiometry, consistent with theories previously presented by Putney (2005). Thus, this inhibitory action of Orai1 provides potentially useful information on the functional coupling stoichiometry that exists between the two proteins; (3) The observation that over-expression of these two proteins produces such a dramatic increase in CRAC current density argues that Orai1 may be the CRAC pore-forming unit. If Orai1 was merely another part of the activation machinery, then over-expression of STIM1 plus Orai1 might result in a small increase in current density, but only to the point at which the endogenous channels are all in the open state. Especially in HEK293 cells which have nearly undetectable endogenous CRAC, the likelihood of the cells expressing enough endogenous channels to accommodate such large increases in current density seems unlikely (Gwack et al., 2007; Vig et al., 2006).
How does Ca2+ dissociation from the EF-hand of STIM1 cause the pore of Orai1 to open? Co-immuno-precipitation experiments suggest that association of the two proteins is promoted by Ca2+ release from intracellular stores (Yeromin et al., 2006). A study is to resolve whether STIM1 acts only from within the ER membrane or whether it also becomes incorporated into the PM where, with Orai1, it might contribute to the formation of the channel. The latter might explain the ability of extracellular antibodies to the N-terminus of STIM1 to attenuate SOCE (Li et al., 2007). For now, the most parsimonious mechanism suggests that STIM1 anchored within the ER membrane interacts, after store depletion, with N-terminus of Orai1 in the PM, as shown in Fig.4 (Lewis, 2007). Which region of the Orai1 N-terminus participates in SOCE? The N-terminal cytosolic domain of Orai1 has several unique features, including five positively charged arginine residues and two proline-rich domains. Huang et al.(2006) showed that the STIM1 C-terminus, especially the ezrin-radixin-moesin (ERM) domain, activates native SOCC. ERM domains bind with positively charged amino acid clusters in the juxta-membrane cytoplasmic domains of CD44, CD43 and ICAM-2. Moreover, proline-rich domains serve as binding sites in various types of proteins. For example, the proline-rich domain in Group 1 metabotropic glutamate receptors mediates the interaction with Homer proteins. Thus, the proline-rich domain of the Orai1 N-terminus may participate in SOCE (Takahashi et al., 2007).
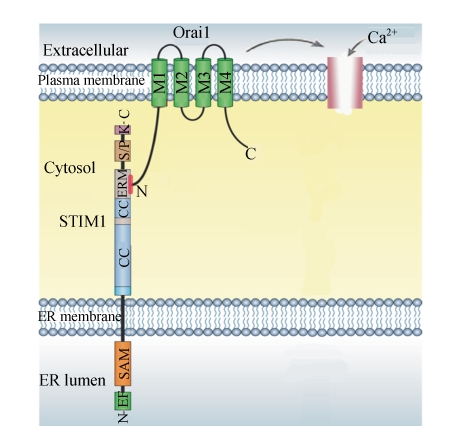
Fig. 4.
N-terminus of Orai1 anchored with STIM1 activates SOCE
STIM1 is localized primarily in the ER membrane, with a small fraction in the plasma membrane. The organization of the major predicted domains is shown, including an unpaired EF-hand and sterile-alpha motif (SAM) domains on the ER luminal side, and overlapping coiled-coil (CC) and ezrin-radixin-moesin (ERM) domains, and serine-proline-rich (S/P) and lysine-rich (K) domains on the cytosolic side. Orai1 is a plasma membrane protein with four membrane-spanning regions and intracellular N-terminus and C-terminus. It is impossible that the N-terminus of Orai1, anchored with ezrin-radixin-moesin (ERM) domain of STIM1 C-terminus, activates SOCE (Lewis, 2007)
As discussed above, STIM1 and STIM2 are close in structure but STIM2 has an opposing inhibitory action on SOCC-mediated Ca2+ entry. Orai1 and STIM2 co-expression resulted in a substantial increase in constitutive Ca2+ entry. STIM2 expressed alone does not visibly change its ER distribution in response to store depletion (Soboloff et al., 2006b). However, some experts suspect that a many-fold increase in STIM2 expressed throughout the ER might lead to the presence of some STIM2 in puncta, independently of altered luminal Ca2+ (Lewis, 2007). Thus, at high levels of STIM2 expression, while it is normally an inhibitor of SOCC, STIM2 may mimic the action of STIM1 and, through interaction with over-expressed Orai1, result in significant constitutive SOCC activation (Hewavitharana et al., 2007).
Orai2, Orai3 and SOCCs
Mercer et al.(2006) reported that Orai2 exhibits properties similar to Orai1; over-expression of Orai2 alone resulted in inhibition of SOCE in HEK293 cells, whereas its co-expression with STIM1 resulted in significantly augmented entry and CRAC. The magnitude of the currents with Orai2 was somewhat less than that with Orai1. Orai3, on the other hand, did not synergistically enhance Ca2+ entry when co-expressed with STIM1 (Mercer et al., 2006; Gross et al., 2007). However, Orai3 was shown to restore Ca2+ entry in cells in which entry was reduced due to RNAi mediated knockdown of endogenous Orai1, demonstrating that Orai3 is able to function in the Ca2+ permeation pathway (Gwack et al., 2007). Thus, in transient transfection experiments, the rank order of efficacy of Orai family members appeared to be Orai1>Orai2>Orai3. However, some report demonstrated that co-expression of Orai2 or Orai3 with STIM1 produced only a marginal effect on SOCC (Mercer et al., 2006; DeHaven et al., 2007).
CONCLUSION
From the new information on the function of the STIM and Orai proteins, we can now rather definitively conclude that the two proteins are both necessary and sufficient to mediate the process of store-operated channel function. STIM1 is a sensor of ER Ca2+, a transduction of this signal to the PM, and a opening of a highly selective channel located in the PM, and Orai1 co-expressed with STIM1 is sufficient to reconstitute SOCC. While these revelations answer the basic questions on store-operated channel function, there are many other questions that arise. For example, recent information indicates that the STIM1 protein can interact with and cause the activation of TRPC1 channels (López et al., 2006; Huang et al., 2006). However, the relationship between the three Orai proteins and the six mammalian TRPCs will be an interesting one to examine. Does STIM1 function as a universal Ca2+ sensor, capable of activating mechanisms of store-operated entry other than CRAC? And whether Orai1 is indeed the CRAC channel, then how do molecular determinants within the protein sequence explain the electrophysiological properties of CRAC, such as permeation and ionic selectivity? Answers to these questions and many more will significantly enhance our understanding of cellular Ca2+ signaling and its contribution to normal human health and disease.
References
- 1.Aires V, Adote S, Hichami A, Moutairou K, Boustani ES, Khan NA. Modulation of intracellular calcium concentrations and T cell activation by prickly pear polyphenols. Mol Cell Biochem. 2004;260(1-2):103–110. doi: 10.1023/B:MCBI.0000026061.57326.28. [DOI] [PubMed] [Google Scholar]
- 2.Alfonso S, Benito O, Alicia S, Angélica Z, Patricia G, Diana K, Luis V. Regulation of the cellular localization and function of human transient receptor potential channel 1 by other members of the TRPC family. Cell Calcium. 2008;43(4):375–387. doi: 10.1016/j.ceca.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Ambudkar IS, Ong HL, Liu X, Bandyopadhyay B, Cheng KT. TRPC1: the link between functionally distinct store-operated calcium channels. Cell Calcium. 2007;42(2):213–223. doi: 10.1016/j.ceca.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Baba Y, Hayashi K, Fujii Y, Mizushima A, Watarai H, Wakamori M, Numaga T, Mori Y, Iino M, Hikida M, et al. Coupling of STIM1 to store-operated Ca2+ entry through its constitutive and inducible movement in the endoplasmic reticulum. Proc Natl Acad Sci USA. 2006;103(45):16704–16709. doi: 10.1073/pnas.0608358103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bollimuntha S, Cornatzer E, Singh BB. Plasma membrane localization and function of TRPC1 is dependent on its interaction with beta-tubulin in retinal epithelium cells. Vis Neurosci. 2005;22(2):163–170. doi: 10.1017/S0952523805222058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bugaj V, Alexeenko V, Zubov A, Glushankova L, Nikolaev A, Wang Z, Kaznacheyeva E, Bezprozvanny I, Mozhayeva GN. Functional properties of endogenous receptor- and store-operated calcium influx channels in HEK293 cells. J Biol Chem. 2005;280(17):16790–16797. doi: 10.1074/jbc.M500192200. [DOI] [PubMed] [Google Scholar]
- 7.DeHaven WI, Smyth JT, Boyles RR, Putney JWJr. Calcium inhibition and calcium potentiation of Orai1, Orai2 and Orai3 calcium release-activated calcium channels. J Biol Chem. 2007;282(24):17548–17556. doi: 10.1074/jbc.M611374200. [DOI] [PubMed] [Google Scholar]
- 8.Demaurex N, Arnaudeau S, Opas M. Measurement of intracellular Ca2+ concentration. Methods Cell Biol. 2002;70:453–474. doi: 10.1016/S0091-679X(02)70014-9. [DOI] [PubMed] [Google Scholar]
- 9.Dziadek MA, Johnstone LS. Biochemical properties and cellular localisation of STIM proteins. Cell Calcium. 2007;42(2):123–132. doi: 10.1016/j.ceca.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Felix R. Molecular regulation of voltage-gated Ca2+ channels. J Recept Signal Transduct. 2005;25(2):57–71. doi: 10.1081/RRS-200068102. [DOI] [PubMed] [Google Scholar]
- 11.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441(7090):179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 12.Fleming I, Rueben A, Popp R, Fisslthaler B, Schrodt S, Sander A, Haendeler J, Falck JR, Morisseau C, Hammock BD, et al. Epoxyeicosatrienoic acids regulate Trp channel dependent Ca2+ signaling and hyperpolarization in endothelial cells. Arterioscler Thromb Vasc Biol. 2007;27(12):2612–2618. doi: 10.1161/ATVBAHA.107.152074. [DOI] [PubMed] [Google Scholar]
- 13.Gross SA, Wissenbach U, Philipp SE, Freichel M, Cavalié A, Flockerzi V. Murine ORAI2 splice variants form functional Ca2+ release-activated Ca2+ (CRAC) channels. J Biol Chem. 2007;282(27):19375–19384. doi: 10.1074/jbc.M701962200. [DOI] [PubMed] [Google Scholar]
- 14.Gwack Y, Srikanth S, Feske S, Cruz-Guilloty F, Oh-hora M, Neems DS, Hogan PG, Rao A. Biochemical and functional characterization of Orai proteins. J Biol Chem. 2007;282(22):16232–16243. doi: 10.1074/jbc.M609630200. [DOI] [PubMed] [Google Scholar]
- 15.He LL, Zhang Y, Chen YH, Yamada Y, Yang J. Functional modularity of the beta-subunit of voltage-gated Ca2+ channels. Biophys J. 2007;93(3):834–845. doi: 10.1529/biophysj.106.101691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hewavitharana T, Deng X, Soboloff J, Gill DL. Role of STIM and Orai proteins in the store-operated calcium signaling pathway. Cell Calcium. 2007;42(2):173–182. doi: 10.1016/j.ceca.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Homann V, Kinne-Saffran E, Arnold WH, Gaengler P, Kinne RK. Calcium transport in human salivary glands: a proposed model of calcium secretion into saliva. Histochem Cell Biol. 2006;125(5):583–591. doi: 10.1007/s00418-005-0100-2. [DOI] [PubMed] [Google Scholar]
- 18.Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355(6358):353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 19.Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nat Cell Biol. 2006;8(9):1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- 20.Jiang QJ, Xu G, Mao FF, Zhu YF. Effects of combination of irbesartan and perindopril on calcineurin expression and sarcoplasmic reticulum Ca2+-ATPase ativity in rat cardiac pressure-overload hypertrophy. J Zhejiang Univ Sci B. 2006;7(3):228–234. doi: 10.1631/jzus.2006.B0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laporte R, Hui A, Laher I. Pharmacological modulation of sarcoplasmic reticulum function in smooth muscle. Pharmacol Rev. 2004;56(4):439–513. doi: 10.1124/pr.56.4.1. [DOI] [PubMed] [Google Scholar]
- 22.Lepage PK, Boulay G. Molecular determinants of TRP channel assembly. Biochem Soc Trans. 2007;35(Pt 1):81–83. doi: 10.1042/BST0350081. [DOI] [PubMed] [Google Scholar]
- 23.Lewis RS. The molecular choreography of a store-operated calcium channel. Nature. 2007;446(7133):284–287. doi: 10.1038/nature05637. [DOI] [PubMed] [Google Scholar]
- 24.Lewis RS, Cahalan MD. Mitogen-induced oscillations of cytosolic Ca2+ and transmembrane Ca2+ current in human leukemic T cells. Cell Regul. 1989;1:99–112. doi: 10.1091/mbc.1.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z, Lu J, Xu P, Xie X, Chen L, Xu T. Mapping the interacting domains of STIM1 and Orai1 in Ca2+ release-activated Ca2+ channel activation. J Biol Chem. 2007;282(40):29448–29456. doi: 10.1074/jbc.M703573200. [DOI] [PubMed] [Google Scholar]
- 26.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JEJr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15(13):1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu L, Li Y, Wang R, Yin C, Dong Q, Hing H, Kim C, Welsh MJ. Drosophila hygrosensation requires the TRP channels water witch and nanchung. Nature. 2007;450(7167):294–298. doi: 10.1038/nature06223. [DOI] [PubMed] [Google Scholar]
- 28.Liu X, Bandyopadhyay BC, Singh BB, Groschner K, Ambudkar IS. Molecular analysis of a store-operated and 2-acetyl-sn-glycerol-sensitive non-selective cation channel. Heteromeric assembly of TRPC1-TRPC3. J Biol Chem. 2005;280(22):21600–21606. doi: 10.1074/jbc.C400492200. [DOI] [PubMed] [Google Scholar]
- 29.López JJ, Salido GM, Pariente JA, Rosado JA. Interaction of STIM1 with endogenously expressed human canonical TRP1 upon depletion of intracellular Ca2+ stores. J Biol Chem. 2006;281(38):28254–28264. doi: 10.1074/jbc.M604272200. [DOI] [PubMed] [Google Scholar]
- 30.Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol. 2006;174(6):815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mercer JC, Dehaven WI, Smyth JT, Wedel B, Boyles RR, Bird GS, Putney JWJr. Large store-operated calcium selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, STIM1. J Biol Chem. 2006;281(34):24979–24990. doi: 10.1074/jbc.M604589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mignen O, Thompson JL, Shuttleworth TJ. STIM1 regulates Ca2+ entry via arachidonate-regulated Ca2+-selective (ARC) channels without store depletion or translocation to the plasma membrane. J Physiol. 2007;579(Pt 3):703–715. doi: 10.1113/jphysiol.2006.122432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mueller P, Quintana A, Griesemer D, Hoth M, Pieters J. Disruption of the cortical actin cytoskeleton does not affect store operated Ca2+ channels in human T-cells. FEBS Lett. 2007;581(18):3557–3562. doi: 10.1016/j.febslet.2007.06.068. [DOI] [PubMed] [Google Scholar]
- 34.Parekh AB. Store-operated Ca2+ entry: dynamic interplay between endoplasmic reticulum, mitochondria and plasma membrane. J Physiol. 2003;547(Pt 2):333–348. doi: 10.1113/jphysiol.2002.034140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pigozzi D, Ducret T, Tajeddine N, Gala JL, Tombal B, Gailly P. Calcium store contents control the expression of TRPC1, TRPC3 and TRPV6 proteins in LNCaP prostate cancer cell line. Cell Calcium. 2006;39(5):401–415. doi: 10.1016/j.ceca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443(7108):230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 37.Putney JWJr. Capacitative calcium entry: sensing the calcium stores. J Cell Biol. 2005;169(3):381–389. doi: 10.1083/jcb.200503161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169(3):435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ross K, Whitaker M, Reynolds NJ. Agonist-induced calcium entry correlates with STIM1 translocation. J Cell Physiol. 2007;211(3):569–576. doi: 10.1002/jcp.20993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smyth JT, DeHaven WI, Bird GS, Putney JWJr. Role of the microtubule cytoskeleton in the function of the store-operated Ca2+ channel activator STIM1. J Cell Sci. 2007;120(Pt 21):3762–3771. doi: 10.1242/jcs.015735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, Gill DL. Orai1 and STIM reconstitute store-operated calcium channel function. J Biol Chem. 2006;281(30):20661–20665. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- 42.Soboloff J, Spassova MA, Hewavitharana T, He LP, Xu W, Johnstone LS, Dziadek MA, Gill DL. STIM2 is an inhibitor of STIM1-mediated store-operated Ca2+ entry. Curr Biol. 2006;16(14):1465–1470. doi: 10.1016/j.cub.2006.05.051. [DOI] [PubMed] [Google Scholar]
- 43.Spassova MA, Soboloff J, He LP, Xu W, Dziadek MA, Gill DL. STIM1 has a plasma membrane role in the activation of store-operated Ca2+ channels. Proc Natl Acad Sci USA. 2006;103(11):4040–4045. doi: 10.1073/pnas.0510050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stathopulos PB, Li GY, Plevin MJ, Ames JB, Ikura M. Stored Ca2+ depletion-induced oligomerization of stromal interaction molecule 1 (STIM1) via the EF-SAM region: an initiation mechanism for capacitive Ca2+ entry. J Biol Chem. 2006;281(47):35855–35862. doi: 10.1074/jbc.M608247200. [DOI] [PubMed] [Google Scholar]
- 45.Takahashi Y, Murakami M, Watanabe H, Hasegawa H, Ohba T, Munehisa Y, Nobori K, Ono K, Iijima T, Ito H. Essential role of the N-terminus of murine Orai1 in store-operated Ca2+ entry. Biochem Biophys Res Commun. 2007;356(1):45–52. doi: 10.1016/j.bbrc.2007.02.107. [DOI] [PubMed] [Google Scholar]
- 46.Vanden Abeele F, Shuba Y, Roudbaraki M, Lemonnier L, Vanoverberghe K, Mariot P, Skryma R, Prevarskaya N. Store-operated Ca2+ channels in prostate cancer epithelial cells: function, regulation and role in carcinogenesis. Cell Calcium. 2003;33(5-6):357–373. doi: 10.1016/S0143-4160(03)00049-6. [DOI] [PubMed] [Google Scholar]
- 47.Venkatachalam K, Zheng F, Gill DL. Regulation of canonical transient receptor potential (TRPC) channel function by diacylglycerol and protein kinase C. J Biol Chem. 2003;278(31):29031–29040. doi: 10.1074/jbc.M302751200. [DOI] [PubMed] [Google Scholar]
- 48.Vig M, Beck A, Billingsley JM, Lis A, Parvez S, Peinelt C, Koomoa DL, Soboloff J, Gill DL, Fleig A, et al. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr Biol. 2006;16(20):2073–2079. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Winslow MM, Neilson JR, Crabtree GR. Calcium signalling in lymphocytes. Curr Opin Immunol. 2003;15(3):299–307. doi: 10.1016/S0952-7915(03)00050-5. [DOI] [PubMed] [Google Scholar]
- 50.Wissenbach U, Philipp SE, Gross SA, Cavalié A, Flockerzi V. Primary structure, chromosomal localization and expression in immune cells of the murine ORAI and STIM genes. Cell Calcium. 2007;42(4-5):439–446. doi: 10.1016/j.ceca.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 51.Worley PF, Zeng W, Huang GN, Yuan JP, Kim JY, Lee MG, Muallem S. TRPC channels as STIM1-regulated store-operated channels. Cell Calcium. 2007;42(2):205–211. doi: 10.1016/j.ceca.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174(6):803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443(7108):226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437(7060):902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]