Abstract
Background: Bone marrow mesenchymal stem cell (MSC) transplantation is a promising strategy in the treatment of myocardial infarction (MI). However, the time for transplanting cells remains controversial. The aim of this study was to find an optimal time point for cell transplantation. Methods: MSCs were isolated and cultured from Sprague-Dawley (SD) rats. MI model was set up in SD rats by permanent ligation of left anterior descending coronary artery. MSCs were directly injected into the infarct border zone at 1 h, 1 week and 2 weeks after MI, respectively. Sham-operated and MI control groups received equal volume of phosphate buffered saline (PBS). At 4 weeks after MI, cardiac function was assessed by echocardiography; vessel density was analyzed on hematoxylin-eosin stained slides by light microscopy; the apoptosis of cardiomyocytes was evaluated by terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) assay; the expressions of proteins were analyzed by Western blot. Results: MSC transplantation improved cardiac function, reduced the apoptosis of cardiomyocytes and increased vessel density. These benefits were more obvious in 1-week group than in 1-h and 2-week groups. There are more obvious increases in the ratio of bcl-2/bax and the expression of vascular endothelial growth factor (VEGF) and more obvious decreases in the expression of cleaved-caspase-3 in 1-week group than those in other two groups. Conclusion: MSC transplantation was beneficial for the recovery of cardiac function. MSC transplantation at 1 week post-MI exerted the best effects on increases of cardiac function, anti-apoptosis and angiogenesis.
Keywords: Mesenchymal stem cells (MSCs), Transplantation, Myocardial infarction (MI), Apoptosis
INTRODUCTION
Myocardial infarction (MI) leads to an irreversible loss of cardiomyocytes and scar formation in the infarct area, which are major factors in the progression of heart failure (Braunwald and Bristow, 2000). Bone marrow mesenchymal stem cell (MSC) transplantation is considered to be an effective therapeutic approach for MI both in basic researches (Amado et al., 2005; Tang et al., 2006) and in clinical trials (Janssens et al., 2006; Meyer et al., 2006). The underlying mechanisms may involve myocardial regeneration, angiogenesis, apoptosis of myocardiocytes and inhibition of cardiac remodeling. However, the effects of cell transplantation after MI vary in different studies (Strauer et al., 2002; Schachinger et al., 2004; Wollert et al., 2004; Janssens et al., 2006), and this difference may partly result from different cell transplantation time as there is a time-dependent healing course post-MI (Koransky et al., 2002). The infarct area appears swelling at 15 h after MI and then softening at 24~48 h, granulation tissue outlines the infarct at 3~4 d, and scar tissues mature at 2~3 months. The time of cell delivery should be at the cross over the time point when the local microenvironment is ready to receive cells at the optimum of their development and function. However, the optimal time of delivery of donor cells remains unknown.
Growing evidence indicates that inhibition of cardiomyocyte apoptosis and stimulation of angiogenesis would contribute to improving cardiac function in postinfarction heart failure (Fukuda et al., 2006; Uemura et al., 2006). In this study, therefore, we focused on the angiogenesis and anti-apoptotic effects to investigate the optimal time for MSC transplantation in MI heart of Sprague-Dawley (SD) rats.
MATERIALS AND METHODS
Animals
SD rats were obtained from the Medical Institute Animal Center of Zhejiang University, China, for conducting the proposed study. The experiments were approved by the Animal Care and Use Committee of Zhejiang Provincal Medical Institute and were in compliance with the Guide for the Care and Use of Laboratory Animals as published by the US National Institutes of Health (1996).
Cell culture
MSCs were obtained from the femora and tibiae of male SD rats (80 g). The bones were dissected free and the proximal and distal ends were removed to reveal the marrow cavity, which was aspirated with 10 ml of Dulbecco’s modified Eagle’s medium (DMEM) through a 21G needle. The aspirant was layered over 1.073 g/ml percoll solution (GE Healthcare Bio-Sciences AB, Sweden) and centrifuged at 400×g for 25 min at room temperature. The mononuclear cells were recovered at the interface, resuspended in MSC growth medium (DMEM, 10% (v/v) fetal bovine serum, 100 U/ml penicillin G and 100 U/ml streptomycin; Gibco, USA), plated on 25 cm2 flasks, and incubated for 24 h at room temperature. The flasks were then washed with phosphate buffered saline (PBS) to leave an adherent layer of cells containing MSCs. The cultures were maintained at 37 °C in a 5% (v/v) CO2 incubator, and the medium was changed every 3~4 d.
MI model and MSC transplantation
Two-month old male SD rats were intubated under general anesthesia by using 4% (w/v) chloral hydrate (4 mg/kg, administered intraperitoneally) and ventilated with room air by using a small animal ventilator (Zhejiang University Apparatus). MI was induced by ligation of the left anterior descending coronary artery 2~3 mm from the tip of the left auricle with a 6-0 silk suture (Min et al., 1999). Successful performance of coronary occlusion was verified by blanching of the myocardium distal to the coronary ligation. The sham-operated group received the same procedure of thoracotomy without coronary ligation. The rats were divided randomly into 5 groups (10 rats in each group): sham-operated group, PBS group (rats received PBS at 1 h post-MI), 1-h group (rats received MSCs at 1 h post-MI), 1-week group (rats received MSCs at 1 week post-MI), 2-week group (rats received MSCs at 2 weeks post-MI). The second thoracotomy was needed in 1-week and 2-week groups. The 2×106 cells in 150 μl PBS were directly injected into the infarct border zone according to these time points. Sham-operated and MI control rats (PBS group) received the same volume of PBS injection.
Echocardiographic study
Echocardiography was performed blindly to assess the cardiac function 4 weeks after MI. The echocardiographic procedure was performed as previously described (Litwin et al., 1995; 1996). A commercially available echocardiographic system equipped with a 12-MHz probe (HP, SONOS5500) was used to obtain the measurements. Briefly, a 2D short-axis view of the left ventricle was obtained at the level of the papillary muscles, and M-mode tracings were recorded and analyzed to evaluate cardiac function.
Terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) analysis
The rats were euthanized and the hearts were removed after echocardiography. The excised heart was cut into 3 transverse sections and embedded in paraffin. Sections (5-μm thick) were cut and mounted on adhered slides. After deparaffinization and dehydrated, apoptotic cardiomyocytes were evaluated by TUNEL assay with an In Situ Cell Death Detection Kit (Roche, Germany) according to the manufacturer’s instructions. In brief, the sections were incubated in proteinase K working solution (20 μg/ml in 10 mmol/L Tris/HCl, pH 7.4~8) at 37 °C in a humidified atmosphere for 15 min, and then added with 50 μl TUNEL reaction mixture each and incubated for 60 min at 37 °C. After rinsed 3 times with PBS, the sections were added with 50 μl converter-peroxidase (POD) and incubated for 30 min at 37 °C, and then rinsed 3 times with PBS again and added with 100 μl diaminobenzidine (DAB) substrate. Finally, the sections were counterstained with hematoxylin and analyzed by light microscope. Percentage of TUNEL-positive cells was assessed in 5 randomly selected fields in the border zone of the ischemic region. A total of 20 sections were analyzed for each animal from the 5 groups.
Assessment of vessel density
The numeric density of arterioles (diameter>20 μm) was analyzed on hematoxylin-eosin stained slides (5 slides from each of the animals in each group) by light microscopy at 400× magnification. Five high-power fields each slide in the border zone of the ischemic region were randomly selected. The number of arterioles in each was averaged and expressed as the number of arterioles per unit area (0.2 mm2).
Western blot analysis
Frozen infarcted hearts were crushed, and then homogenized by vibration in RIPA lysis buffer containing 50 mmol/L Tris (pH 7.4), 150 mmol/L NaCl, 1 mmol/L ethylene diamine tetraacetic acid (EDTA), 0.1% (w/v) sodium dodecyl sulfate (SDS), 1% (w/v) Nonidet P-40 (NP-40), 1 mmol/L phenylmethanesulfonyl fluoride (PMSF) and 10 mmol/L NaF at 4 °C for 30 min. The homogenate was centrifuged at 12000×g at 4 °C for 20 min. The protein content of the supernatant was determined by the Bio-Rad DC protein assay (Bio-Rad, Hercules, CA, USA). Protein (50~150 μg per lane) was loaded and electrophoresis was carried out in sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Protein was transferred onto polyvinylidene difluoride (PVDF) immobilon-P membrane (Bio-Rad, CA, USA) using a transblot apparatus (Bio-Rad, CA, USA). The membranes were blocked in 10 mmol/L Tris-HCl (pH 8.0), 150 mmol/L NaCl and 0.05% (v/v) Tween 20 (TBST) with 5% (w/v) non-fat milk at room temperature, followed by overnight incubation at 4 °C with primary antibodies (1:1000 (v/v) for bcl-2, bax, cleaved-caspase-3, and β-actin, Cell Signal, USA; 1:1000 (v/v) for vascular endothelial growth factor (VEGF), Santa Cruz, USA). After washing with TBST, the membranes were incubated for 1 h with a horseradish peroxidase (HRP)-conjugated secondary antibody, and the labled proteins were detected by using the enhanced chemiluminescence reagents and exposed to the film (Kodak, USA).
Statistical analysis
Data were expressed as mean±SEM. Statistical significance between groups was assessed by a two-way analysis of variance (ANOVA) followed by Student-Newmen-Keuls (SNK), using SPSS 11.5. P<0.05 was considered statistically significant.
RESULTS
Morphology of MSCs
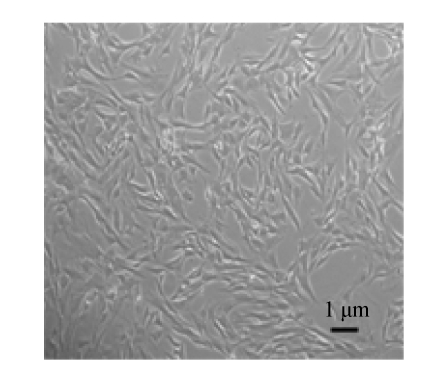
We isolated and cultured MSCs from the bone marrows of male SD rats as described above. Passage 3 MSCs were attached to culture dishes and the majority of MSCs displayed a spindle-like shape (Fig.1). Surface molecular markers of Passage 3 MSCs were examined with flow cytometry analysis, and most adherent cells were found to express CD44 and CD90 but not CD45 (Xie et al., 2006).
Fig. 1.
The shape of Passage 3 MSCs under phasecontrast microscope
Effect of MSC transplantation on cardiac function
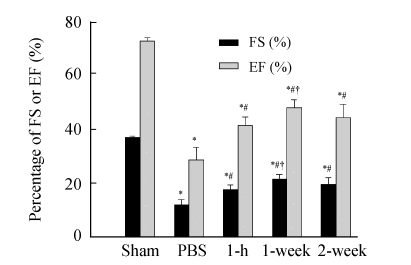
Cardiac function assessment was performed 4 weeks after MI. The results showed that fractional shortening (FS) and ejection fraction (EF) were significantly decreased in all MI groups compared with sham-operated group (P<0.01). FS and EF were significantly increased in three MSC transplantation groups compared with PBS group (P<0.01). Compared with 1-h and 2-week groups, FS and EF were significantly increased in 1-week group (P<0.05) (Fig.2).
Fig. 2.
Comparison of cardiac function among 5 groups
FS: Fractional shortening; EF: Ejection fraction; Sham: Sham-operated rats without coronary ligation; PBS: MI rats that received PBS injection; 1-h, 1-week and 2-week: Rats receiving cell transplantation at 1 h, 1 week and 2 weeks post-MI, respectively. * P<0.01 vs Sham group; # P<0.01 vs PBS group; † P<0.05 vs 1-h and 2-week groups (n=10)
Effect of MSC transplantation on cardiomyocyte apoptosis
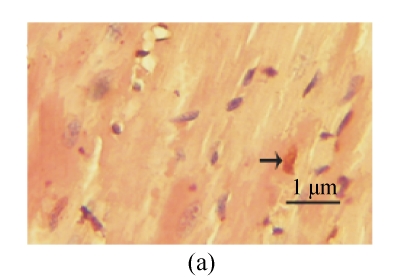
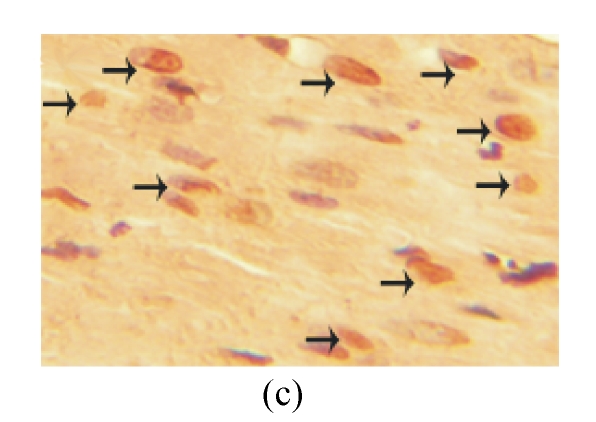
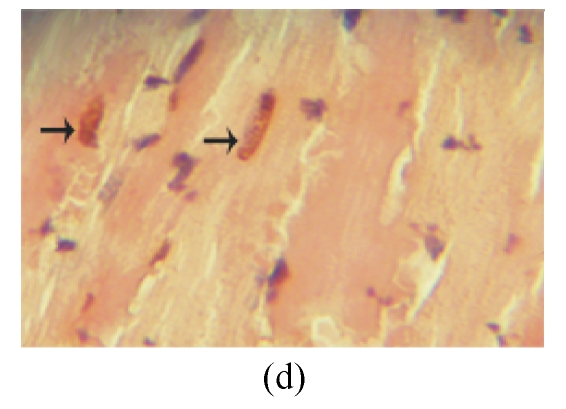
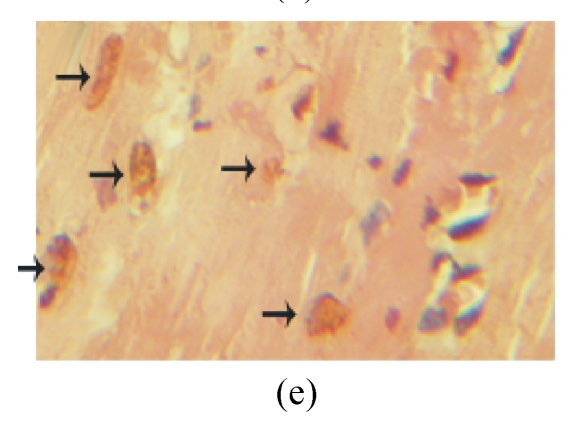
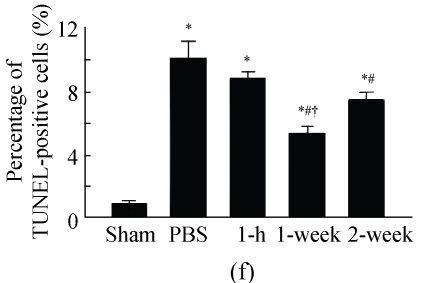
TUNEL labeling was performed 4 weeks after MI (Figs.3a~3e). The results show that the number of TUNEL-positive cells increased significantly in PBS group compared with sham-operated group (PBS group: (10.2±1.0)%, sham-operated group: (0.9±0.2)%; P<0.01, Fig.3f). Compared with PBS group, transplantation of MSCs at 1 week and 2 weeks significantly reduced the apoptosis of cardiomyocytes (1 week: (5.4±0.4)%, 2 weeks: (7.5±0.5)%; P<0.01, Fig.3f), and the reduction in 1-week group was more obvious than that in 2-week group (P<0.05, Fig.3f). However, there was no significant difference between PBS group and 1-h group.
Fig. 3.
The apoptosis of cardiomyocytes was tested by TUNEL assay. (a) Sham-operated group; (b) PBS group; (c) 1-h group; (d) 1-week group; (e) 2-week group; (f) Percentage of TUNEL-positive cells. The percentage of apoptotic cells was determined in 5 random microscopic fields totally at least 1000 cells/section. A total of 20 sections were analyzed for each rat from the 5 groups. * P<0.01 vs Sham group; # P<0.01 vs PBS group; † P<0.05 vs 1-h and 2-week groups. The black arrows indicate the apoptotic cells (brown nucleus staining)
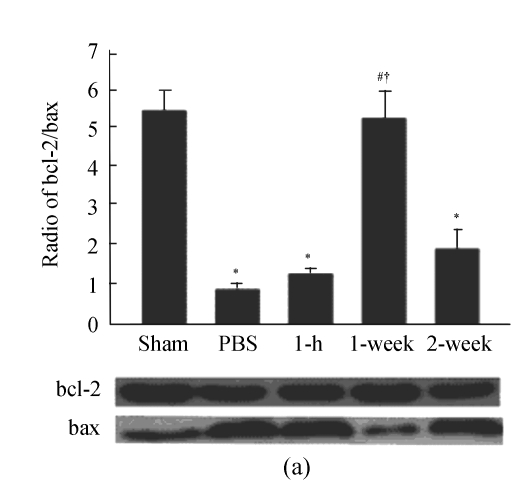
We further examined the expression of apoptotic protein including cleaved-caspase-3, bcl-2 and bax in infarct heart. The results show that the ratio of bcl-2/bax was significantly decreased and cleaved-caspase-3 was increased in PBS group compared with sham-operated group (P<0.01). Compared with PBS group, the ratio of bcl-2/bax was increased in 1-week group, and cleaved-caspase-3 was decreased in 1-week and 2-week groups (P<0.01). However, there was no significant difference in the ratio of bcl-2/bax and cleaved-caspase-3 between PBS and 1-h groups (Fig.4).
Fig. 4.
Apoptotic proteins were analyzed by Western blot. (a) bcl-2 and bax; (b) Cleaved-caspase-3. β-actin was the internal control. * P<0.01 vs Sham group; # P<0.01 vs PBS group; † P<0.01 vs 1-h and 2-week groups (n=3)
Effect of MSC transplantation on vessel density
The vessel density was significantly decreased after MI compared to sham-operated group. The results also show that MSC transplantation increased vessel density, and that the vessel density was significantly greater in 1-week group than in 1-h and 2-week groups (P<0.05, Fig.5a).
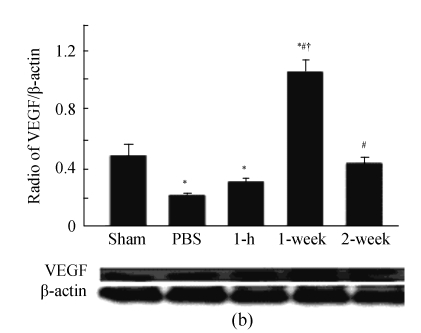
Fig. 5.
(a) Quantitative analysis of vessel density in peri-infarct area at 4 weeks post-MI (n=10). Vessel density was assessed in 5 randomly selected fields in each section and expressed as number of arterioles per unit area (0.2 mm2) (400× magnification); (b) Western blot analysis of the expression of VEGF (n=3). β-actin was the internal control. * P<0.05 vs Sham group; # P<0.05 vs PBS group; † P<0.05 vs 1-h and 2-week groups
VEGF, which could be secreted by MSCs (Uemura et al., 2006), is an important protein for angiogenesis (Koransky et al., 2002). We further examined its expression in infarct heart, and found that it was significantly greater in 1-week group than in other groups (P<0.05, Fig.5b). However, there was no significant difference between PBS and 1-h groups.
DISCUSSION
The present study shows: (1) MSC transplantation could improve the cardiac function, reduce the apoptosis of cardiomyocytes, increase the bcl-2/bax ratio, inhibit the activation of cleaved-caspase-3, and increase vessel density and the expression of VEGF; (2) MSC transplantation at 1 week post-MI exerted the best effect on the increase of cardiac function, anti-apoptosis and angiogenesis.
Apoptosis, with a preferential localization of apoptotic cardiomyocytes at the borders of the infarcted area, may be as important as necrosis in determining the cardiomyocyte loss after acute MI (Kajstura et al., 1996; Olivetti et al., 1996). Apoptosis takes place at 3 to 120 h after acute MI and lasts at least 60 d (Piro et al., 2000; Saraste et al., 1997), and apoptosis at late post-acute MI (>10 d) suggests a possible relation with the progression of left ventricular dysfunction (Baldi et al., 2002). Therefore, in this study we analyzed the apoptosis of cardiomyocytes at 4 weeks post-acute MI.
MSCs have been shown to have an anti-apoptotic effect (Uemura et al., 2006) due to their paracrine effect. MSCs could secret a great number of anti-apoptotic cytokines (Silva et al., 2003; Wang et al., 2004; Kemp et al., 2005; Nagaya et al., 2005; Uemura et al., 2006), such as VEGF, stem cells derived factor (SDF), hetapocyte growth factor (HGF), insulin-like growth factor-1 (IGF-1), platelet-derived growth factor (PDGF), and so on. In this study, we demonstrated that MSC transplantation reduced the apoptosis of cardiomyocytes, and the best benefits were observed in 1-week transplantation group. This might be explained as followings: (1) In our study, increased VEGF expression was observed more obviously in 1-week group, so we supposed that 1 week post-MI, when scar formation has not occurred and the inflammation is reduced (Virag and Murry, 2003), may provide a suitable environment to favor the paracrine function of the implanted MSCs; (2) We also found that the vessel density was higher in 1-week group as compared to PBS group, 1-h and 2-week groups. It might be associated with increased VEGF expression, which facilitated greater angiogenesis and further reduced the apoptosis of cardiomyocytes. However, further studies are needed to clarify this phenomenon.
Our study also indicates that 1 h post-MI may not be the ideal time for cell transplantation. After 1 h post-MI, massive myocardial necrosis, leukocytes and mast cells rapidly infiltrate into the ischemic myocardium (Virag and Murry, 2003), which may do harm for the survival of the implanted cells. By 1 week, the acute inflammatory reaction is nearly complete, and scar formation has not occurred yet, so this may be a positive stimulation for the implanted cells. By 2 weeks, scar tissues begin to form (Virag and Murry, 2003) and adverse ventricular remodeling begins (Virag and Murry, 2003), which may also inhibit the survival and function of the implanted cells. Similarly, Li et al.(2001) transplanted embryonic rat cardiomyocytes into the cryoinjuried heart and found that the transplantation at 2 weeks after cryoinjury was better than the one immediately followed or at 4 weeks post-MI.
The effects of cell transplantation after MI vary in different clinical trials (Janssens et al., 2006; Schachinger et al., 2004; Strauer et al., 2002; Wollert et al., 2004), among which, cardiac function improved from approximately 2% to 9%. Different transplanted cell types (Assmus et al., 2002; Stamm et al., 2003; Wollert et al., 2004), cell quantity, survival ability and delivering time may contribute to these differences. Although the healing course after MI in humans is not the same as the one in rats, this optimal time point for cell transplantation in rats may provide a suggestion for the application in human.
Transplantation of stem cells after MI leads to an engraftment of the implanted cells in the peri-infarct area (Uemura et al., 2006). These transplanted cells can differentiate into multiple phenotypes of myocardium, and thereby contribute to myocardial regeneration (Shim et al., 2004). However, other studies (Nygren et al., 2004; Garbade et al., 2005) have suggested that phenotypic changes may occur as a consequence of cell fusion rather than transdifferentiation. The contribution of fusion in cardiac regeneration was still unknown (Yoon et al., 2005). Further studies will be done to illuminate whether there is an optimal time point for differentiation or fusion.
In conclusion, MSC transplantation was beneficial for the recovery of cardiac function in experimental rats. MSC transplantation at 1 week post-MI exerted the best effects on cardiac function, anti-apoptosis and angiogenesis, suggesting that 1 week post-MI may be the optimal choice for MSC transplantation in rats.
Acknowledgments
We thank Xing Zhang and Jiang Cao (Center Lab, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, China) for technical support.
Footnotes
Project (No. 2004QN018) supported by the Health Bureau of Zhejiang Province, China
References
- 1.Amado LC, Saliaris AP, Schuleri KH, St John M, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci USA. 2005;102(32):11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, Grunwald F, Aicher A, Urbich C, Martin H, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI) Circulation. 2002;106(24):3009–3017. doi: 10.1161/01.CIR.0000043246.74879.CD. [DOI] [PubMed] [Google Scholar]
- 3.Baldi A, Abbate A, Bussani R, Patti G, Melfi R, Angelini A, Dobrina A, Rossiello R, Silvestri F, Baldi F, et al. Apoptosis and post-infarction left ventricular remodeling. J Mol Cell Cardiol. 2002;34(2):165–174. doi: 10.1006/jmcc.2001.1498. [DOI] [PubMed] [Google Scholar]
- 4.Braunwald E, Bristow MR. Congestive heart failure: fifty years of progress. Circulation. 2000;102(20 Suppl. 4):IV14–IV23. doi: 10.1161/01.cir.102.suppl_4.iv-14. [DOI] [PubMed] [Google Scholar]
- 5.Fukuda S, Kaga S, Zhan L, Bagchi D, Das DK, Bertelli A, Maulik N. Resveratrol ameliorates myocardial damage by inducing vascular endothelial growth factor-angiogenesis and tyrosine kinase receptor Flk-1. Cell Biochem Biophys. 2006;44(1):43–49. doi: 10.1385/CBB:44:1:043. [DOI] [PubMed] [Google Scholar]
- 6.Garbade J, Schubert A, Rastan AJ, Lenz D, Walther T, Gummert JF, Dhein S, Mohr FW. Fusion of bone marrow-derived stem cells with cardiomyocytes in a heterologous in vitro model. Eur J Cardiothorac Surg. 2005;28(5):685–691. doi: 10.1016/j.ejcts.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 7.Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, Kalantzi M, Herbots L, Sinnaeve P, Dens J, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367(9505):113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 8.Kajstura J, Cheng W, Reiss K, Clark WA, Sonnenblick EH, Krajewski S, Reed JC, Olivetti G, Anversa P. Apoptotic and necrotic myocyte cell deaths are independent contributing variables of infarct size in rats. Lab Invest. 1996;74(1):86–107. [PubMed] [Google Scholar]
- 9.Kemp KC, Hows J, Donaldson C. Bone marrow-derived mesenchymal stem cells. Leuk Lymphoma. 2005;46(11):1531–1544. doi: 10.1080/10428190500215076. [DOI] [PubMed] [Google Scholar]
- 10.Koransky ML, Robbins RC, Blau HM. VEGF gene delivery for treatment of ischemic cardiovascular disease. Trends Cardiovasc Med. 2002;12(3):108–114. doi: 10.1016/S1050-1738(01)00158-X. [DOI] [PubMed] [Google Scholar]
- 11.Li RK, Mickle DA, Weisel RD, Rao V, Jia ZQ. Optimal time for cardiomyocyte transplantation to maximize myocardial function after left ventricular injury. Ann Thorac Surg. 2001;72(6):1957–1963. doi: 10.1016/S0003-4975(01)03216-7. [DOI] [PubMed] [Google Scholar]
- 12.Litwin SE, Katz SE, Weinberg EO, Lorell BH, Aurigemma GP, Douglas PS. Serial echocardiographic-Doppler assessment of left ventricular geometry and function in rats with pressure-overload hypertrophy. Chronic angiotensin-converting enzyme inhibition attenuates the transition to heart failure. Circulation. 1995;91(10):2642–2654. doi: 10.1161/01.cir.91.10.2642. [DOI] [PubMed] [Google Scholar]
- 13.Litwin SE, Katz SE, Morgan JP, Douglas PS. Long-term captopril treatment improves diastolic filling more than systolic performance in rats with large myocardial infarction. J Am Coll Cardiol. 1996;28(3):773–781. doi: 10.1016/0735-1097(96)00215-X. [DOI] [PubMed] [Google Scholar]
- 14.Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, Hecker H, Schaefer A, Arseniev L, Hertenstein B, et al. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months’ follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006;113(10):1287–1294. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 15.Min JY, Sandmann S, Meissner A, Unger T, Simon R. Differential effects of mibefradil, verapamil, and amlodipine on myocardial function and intracellular Ca(2+) handling in rats with chronic myocardial infarction. J Pharmacol Exp Ther. 1999;291(3):1038–1044. [PubMed] [Google Scholar]
- 16.Nagaya N, Kangawa K, Itoh T, Iwase T, Murakami S, Miyahara Y, Fujii T, Uematsu M, Ohgushi H, Yamagishi M, et al. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005;112(8):1128–1135. doi: 10.1161/CIRCULATIONAHA.104.500447. [DOI] [PubMed] [Google Scholar]
- 17.Nygren JM, Jovinge S, Breitbach M, Sawen P, Roll W, Hescheler J, Taneera J, Fleischmann BK, Jacobsen SE. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004;10(5):494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]
- 18.Olivetti G, Quaini F, Sala R, Lagrasta C, Corradi D, Bonacina E, Gambert SR, Cigola E, Anversa P. Acute myocardial infarction in humans is associated with activation of programmed myocyte cell death in the surviving portion of the heart. J Mol Cell Cardiol. 1996;28(9):2005–2016. doi: 10.1006/jmcc.1996.0193. [DOI] [PubMed] [Google Scholar]
- 19.Piro FR, di Gioia CR, Gallo P, Giordano C, d′Amati G. Is apoptosis a diagnostic marker of acute myocardial infarction? Arch Pathol Lab Med. 2000;124(6):827–831. doi: 10.5858/2000-124-0827-IAADMO. [DOI] [PubMed] [Google Scholar]
- 20.Saraste A, Pulkki K, Kallajoki M, Henriksen K, Parvinen M, Voipio-Pulkki LM. Apoptosis in human acute myocardial infarction. Circulation. 1997;95(2):320–323. doi: 10.1161/01.cir.95.2.320. [DOI] [PubMed] [Google Scholar]
- 21.Schachinger V, Assmus B, Britten MB, Honold J, Lehmann R, Teupe C, Abolmaali ND, Vogl TJ, Hofmann WK, Martin H, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one-year results of the TOPCARE-AMI trial. J Am Coll Cardiol. 2004;44(8):1690–1699. doi: 10.1016/j.jacc.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Shim WS, Jiang S, Wong P, Tan J, Chua YL, Tan YS, Sin YK, Lim CH, Chua T, Teh M, et al. Ex vivo differentiation of human adult bone marrow stem cells into cardiomyocyte-like cells. Biochem Biophys Res Commun. 2004;324(2):481–488. doi: 10.1016/j.bbrc.2004.09.087. [DOI] [PubMed] [Google Scholar]
- 23.Silva WAJr, Covas DT, Panepucci RA, Proto-Siqueira R, Siufi JL, Zanette DL, Santos AR, Zago MA. The profile of gene expression of human marrow mesenchymal stem cells. Stem Cells. 2003;21(6):661–669. doi: 10.1634/stemcells.21-6-661. [DOI] [PubMed] [Google Scholar]
- 24.Stamm C, Westphal B, Kleine HD, Petzsch M, Kittner C, Klinge H, Schumichen C, Nienaber CA, Freund M, Steinhoff G. Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet. 2003;361(9351):45–46. doi: 10.1016/S0140-6736(03)12110-1. [DOI] [PubMed] [Google Scholar]
- 25.Strauer BE, Brehm M, Zeus T, Kostering M, Hernandez A, Sorg RV, Kogler G, Wernet P. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106(15):1913–1918. doi: 10.1161/01.CIR.0000034046.87607.1C. [DOI] [PubMed] [Google Scholar]
- 26.Tang J, Xie Q, Pan G, Wang J, Wang M. Mesenchymal stem cells participate in angiogenesis and improve heart function in rat model of myocardial ischemia with reperfusion. Eur J Cardiothorac Surg. 2006;30(2):353–361. doi: 10.1016/j.ejcts.2006.02.070. [DOI] [PubMed] [Google Scholar]
- 27.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98(11):1414–1421. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 28.US National Institutes of Health. Guide for the Care and Use of Laboratory Animals. US National Institutes of Health; 1996. No. 85-23. [Google Scholar]
- 29.Virag JI, Murry CE. Myofibroblast and endothelial cell proliferation during murine myocardial infarct repair. Am J Pathol. 2003;163(6):2433–2440. doi: 10.1016/S0002-9440(10)63598-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang PP, Wang JH, Yan ZP, Hu MY, Lau GK, Fan ST, Luk JM. Expression of hepatocyte-like phenotypes in bone marrow stromal cells after HGF induction. Biochem Biophys Res Commun. 2004;320(3):712–716. doi: 10.1016/j.bbrc.2004.05.213. [DOI] [PubMed] [Google Scholar]
- 31.Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364(9429):141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 32.Xie XJ, Wang JA, Cao J, Zhang X. Differentiation of bone marrow mesenchymal stem cells induced by myocardial medium under hypoxic conditions. Acta Pharmacol Sin. 2006;27(9):1153–1158. doi: 10.1111/j.1745-7254.2006.00436.x. [DOI] [PubMed] [Google Scholar]
- 33.Yoon YS, Lee N, Scadova H. Myocardial regeneration with bone-marrow-derived stem cells. Biol Cell. 2005;97(4):253–263. doi: 10.1042/BC20040099. [DOI] [PubMed] [Google Scholar]