Abstract
Objective: To evaluate the accuracy of a scoring system combining zygote and embryo morphology in predicting the outcome of in vitro fertilization (IVF) treatment. Methods: In a study group, 117 consecutive IVF or intracytoplasmic sperm injection (ICSI) cycles with embryo transfer were carried out and 312 embryos were scored using a combined scoring system (CSS) of zygote and embryo morphology before transplantation. In a control group, a total of 420 IVF or ICSI cycles were carried out and 1176 embryos were scored using a cumulative embryo score (CES). The effects of the combined scoring system on the embryo implantation rate and pregnancy rate per cycle were analyzed. Results: Using the combined scoring system, the embryo implantation rate (27.6%) and the clinical pregnancy rate (48.7%) were significantly higher than those in the control group (20.8% and 38.6%, respectively). Also, the implantation rate of embryos scoring ≥70 (38.5%: 82 sacs/213 embryos) was significantly higher (P<0.001) than that of embryos scoring <70 (4%: 4 sacs/99 embryos). The pregnancy rate of patients with embryos scoring ≥70 using the combined scoring system (66.7%) was significantly higher (P<0.001) than that of patients with embryos scoring ≥20 using the cumulative embryo score (59.0%). Conclusion: The results suggest that selecting embryos with a high score (≥70) using the combined scoring system could increase the implantation rate and pregnancy rate, and that using a scoring system combining assessments of human zygotes and pre-implantation embryos might predict IVF outcomes more accurately than using a cumulative embryo score.
Keywords: Embryo score, Combined scoring system (CSS), Implantation, In vitro fertilization (IVF), Pregnancy
INTRODUCTION
The incidence of multiple pregnancies has increased dramatically over the past decade in many countries. This trend has been attributed largely to assisted reproduction (Hernandez, 2001; Schieve et al., 2002), since in vitro fertilization (IVF) centers frequently transfer multiple embryos to increase the pregnancy rate per cycle. To avoid the potential complications of high order multiples, the Chinese Ministry of Health Guidelines for IVF states that no more than two embryos should be transferred into women aged ≤35 years for the first embryo transfer attempt. Consequently, how to select the embryos best suited for transfer has become one of the most important challenges in the field of assisted reproductive technology.
A commonly used method of embryonic selection is to grade Day 2 or Day 3 embryos based on their morphological features such as cell number, blastomere size, shape and degree of fragmentation (Giorgetti et al., 1995; Ziebe et al., 1997; Hu et al., 1998). There is increasing evidence suggesting that a simple evaluation of cell number and morphology on Day 3 embryos might be insufficient to identify the best embryos (Rijnders and Jansen, 1998; Milki et al., 2002). Recent studies showed that pronuclear zygote morphology might predict the outcome of IVF (Scott et al., 2000; Ludwig et al., 2000; Zollner et al., 2002; 2003; Payne et al., 2005). However, the finding that there was no correlation between zygote and embryo morphology suggests that predicting the outcome of IVF using either zygote or embryo morphology alone would be unreliable (Rijnders and Jansen, 1998; de Placido et al., 2002). Although some investigations have been made to evaluate the correlation between different scoring methods and the predicted outcomes of IVF, the results are still controversial.
In the present study, a scoring system combining zygote and embryo morphology was used and its accuracy in predicting the outcome of IVF or intracytoplasmic sperm injection (ICSI) treatment was evaluated.
MATERIALS AND METHODS
Patients
Between June 2003 and April 2004, 537 IVF or ICSI cycles with embryo transfer were included in this trial. The trial was a prospective, randomized, single-center clinical trial to assess the efficacy of two embryo scoring systems in selecting the embryos best suited for transfer. All patients underwent the IVF/ICSI procedure at the Women’s Hospital, School of Medicine, Zhejiang University, China. The patients were randomly allocated to the study or control group at a ratio of about 1:3. One hundred and seventeen patients were assigned to the study group and 420 to the control group. Patients in the two groups were not selected on age, sperm parameters or infertility criteria. The study was approved by the Institution Review Board of School of Medicine, Zhejiang University, China. Informed consent was obtained from each patient.
IVF procedure and embryo evaluation
All patients were stimulated with recombinant human follicle stimulating hormone (FSH) (Gonal F; Serono, Germany) after pituitary function was down-regulated with gonadotropin releasing hormone (GnRH-agonist) starting on the 21st day of the cycle preceding IVF treatment. Follicular development was monitored using serial vaginal ultrasound and serum E2 levels. Human chorionic gonadotrophin (hCG) was administered when two or more follicles reached 18 mm in mean diameter. Oocytes were transvaginally retrieved under ultrasound guidance 34~36 h after triggering ovulation.
The oocyte-corona-cumulus complexes were cultured in human tubal fluid (HTF) (Irvine Science, USA) supplemented with 10% (v/v) synthetic serum substitute (SSS). IVF or ICSI was carried out using motile spermatozoa prepared by density gradient centrifugation. About 17 h after insemination, the oocytes were checked for pronuclei and polar bodies (Zhang et al., 2007). The zygotes were scored according to the Z-score scoring system (Scott et al., 2000; Lan et al., 2003). Briefly, Z-1 zygotes have equal numbers of nucleoli aligned at the pronuclear junction. Z-2 zygotes have equal numbers and sizes of nucleoli, which are equally scattered in the two nuclei. Z-3 zygotes have equal numbers and sizes of nucleoli in the two nuclei, with one nucleus having aligned nucleoli at the pronuclear junction and the other having scattered nucleoli in itself. Zygotes with unequal numbers and/or sizes of nucleoli are also considered as Z-3. Z-4 zygotes are those with pronuclei that are not aligned, are of grossly different sizes or are not located in the central part of the zygote. Regularly fertilized oocytes were cultured individually in droplets of P-1 medium (Irvine Science, USA) under oil at 37 °C in a humidified atmosphere with 5% (v/v) CO2. Embryos were evaluated on Day 2 and Day 3 of culture, and were scored using either a new combined scoring system (CSS) or a traditional cumulative embryo score (CES) system. The CSS is a modification of the graduated embryo scoring system (Fisch et al., 2001; 2003) and is the combination of evaluations of early development at three intervals: the presence of nucleolar alignment along the pronuclear axis 16~18 h after insemination, the presence of regular and symmetrical cleavage 25~29 h after insemination, and cell number and fragmentation on Day 3 of culture. A detailed description of CSS is shown in Table 1. The grading and scoring system of CES is shown in Table 2. A scale of 1 to 5 was used for CES embryo grading, and then each grade was converted into an embryo score on a scale of 0 to 4. The CES was the embryo score multiplied by the total number of blastomeres within the embryo. The mean cumulative embryo score (MCES) was the sum of each cumulative embryo score divided by the total number of embryos transferred. In the study group, embryos with the highest CSS score were chosen for embryo transfer on Day 3 of culture. In the control group, embryos with the highest CES score were transferred. Pregnancy was confirmed by serum hCG measurement 14 d after embryo transfer. Clinical pregnancy was defined as the presence of a gestational sac on ultrasound scans.
Table 1.
Combined scoring system (CSS) of embryos
| Evaluation | Time after insemination (h) | Development milestone | Score |
| 1 | 16~18 | Z-1 zygote | 20 |
|
Z-2 zygote |
10 |
||
| 2 | 25~27 | Cleavage regular and symmetrical | 20 |
| Fragmentation*: Absent | 30 | ||
| <20% | 25 | ||
| >20% |
0 |
||
| 3 | 64~67 | Cell number and grade**: 7I; 8I; 9I; 8II; compacting I | 30 |
| 7II; 9II; 10III; 11III |
15 |
||
| Total score | 100 |
If the embryo was not cleaved at 25~27 h, grading of fragmentation should be carried out during the 64~67 h evaluation.
Grade I: Symmetrical blastomeres and no fragmentation; Grade II: Slightly uneven blastomeres and <20% fragmentation; Grade III: Uneven blastomeres and >20% fragmentation
Table 2.
Embryo grading and score for cumulative embryo score (CES) system
| Grade | Score | Morphology |
| 1 | 4 | ≥5 cells; blastomeres of equal size; no cytoplasmic fragments |
| 2 | 3 | ≥5 cells; blastomeres of equal size; <30% cytoplasmic fragments |
| 3 | 2 | ≥5 cells; blastomeres of distinctly unequal size; no cytoplasmic fragments |
| 4 | 1 | ≥5 cells; blastomeres of equal or unequal size; 30%~50% cytoplasmic fragments if equal or 1%~50% if unequal |
| 5 | 0 | <5 cells of any size, or any pre-embryos with >50% cytoplasmic fragments |
Statistical analysis
The SPSS 10.0 statistical package was used for data analysis. All P-values were two-sided, and P<0.05 was considered statistically significant. One-way analysis of variance (ANOVA) and the Kruskal-Wallis test were used to evaluate the relationship between CSS score, CES score and zygote score. The accuracy of predictions of pregnancy rate and implantation rate based on combined scores was analyzed using a χ 2 test.
RESULTS
General characteristics
The study group included 117 patients who underwent IVF or ICSI and had at least one embryo transferred. Of 1365 oocytes retrieved, 973 were normally fertilized (fertilization rate 71.4%) and reached the pronuclear (PN) stage after 16~18 h. A total of 312 embryos were transferred, resulting in 2 chemical pregnancies and 57 clinical pregnancies including 33 singletons, 19 twins and 5 triplets. The mean number of embryos transferred was 2.7±0.6 and the implantation rate was 27.6% (86 sacs/312 embryos). The overall pregnancy rate was 50.4% and the clinical pregnancy rate was 48.7%. The control group included 420 patients and had a fertilization rate of 71.8%. The mean number of embryos transferred was 2.8±0.6, and the implantation rate was 20.8% (245 sacs/1176 embryos). The clinical pregnancy rate was 38.6% (162/420). The clinical pregnancy rate and the implantation rate of the study group were significantly higher than those of the control group (P<0.05 and P<0.025, respectively). The general characteristics of the study and control groups are summarized in Table 3.
Table 3.
General characteristics of the study and control groups
| Patients | Age (years) | Fertilization rate (%) | Number of embryo transferred | Implantation rate (%) | Clinical pregnancy rate (%) | |
| Study group | 117 | 32.8±3.5 | 71.40 | 2.7±0.6 | 27.60 | 48.70 |
| Control group |
420 |
32.5±2.8 |
71.80 |
2.8±0.6 |
20.80 |
38.60 |
| P value | >0.05 | >0.05 | >0.05 | >0.05 | <0.025 | <0.05 |
Relationship between zygote score and embryo score
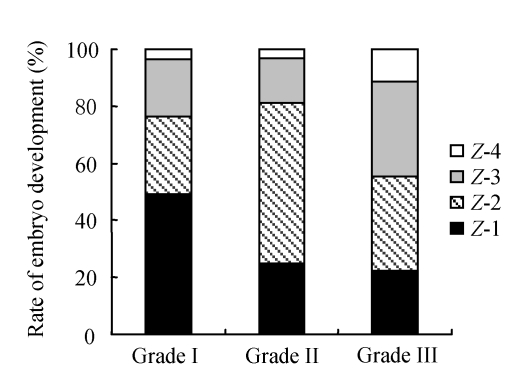
The relationship between zygote score and embryo score is shown in Table 4. It was noted that the zygote score did not always correlate with the Day 3 embryo score (CES). Only 49.1% of Z-1 (top quality) zygotes formed Grade 1 embryos. In contrast, poor quality zygotes could also become high quality embryos (Fig.1).
Table 4.
Relationship between zygote score and embryo score
| Zygote score | CSS* | CES** |
| Z-1 | 80.8±18.0 | 32 (24, 32) |
| Z-2 | 59.9±21.5 | 24 (18, 32) |
| Z-3 | 54.8±24.6 | 24 (16, 32) |
|
Z-4 |
42.0±32.7 |
16 (0, 26.5) |
| P value | <0.001 | 0.049 |
Mean±SD;
Median (the 25th percentiles, the 75th percentiles)
Fig. 1.
Relationship between zygote score and embryo grade
Grade I: Symmetrical blastomeres and no fragmentation; Grade II: Slightly uneven blastomeres and <20% fragmentation; Grade III: Uneven blastomeres and >20% fragmentation
CSS and IVF outcome
Scores of ≥70 using CSS and of ≥20 using CES were used as thresholds to identify embryos with high implantation potential. Of the 312 transferred embryos, 150 (48.1%) scored ≥70 using CSS and 234 (75.0%) scored ≥20 using CES. The implantation rate in patients who had one or more embryos scoring ≥70 with CSS was 38.5% (82 sacs/213 embryos). This was much higher (P<0.001) than the implantation rate (4%, 4 sacs/99 embryos) in patients who received embryos all scoring <70 (Table 5). The pregnancy rate of patients receiving one or more transferred embryos scoring ≥70 using CSS (66.7%, 54/81) was also higher than that of patients receiving transferred embryos scoring <70 (8.3%, 3/36) (P<0.001) (Table 5).
Table 5.
Embryo score and IVF outcome
| Patients n | Ongoing pregnancy rate | Implantation rate | |
| CSS≥70 | |||
| ≥1 embryo | 81 | 66.7% (54/81) | 38.5% (82/213)* |
| 0 embryo | 36 | 8.3% (3/36) | 4% (4/99) |
| CES≥20 | |||
| ≥1 embryo | 106 | 50.9% (54/106) | 29.1% (83/285) |
| 0 embryo | 11 | 27.3% (3/11) | 11.1% (3/27) |
Sacs/embryos
Statistical measures of the two embryo grading system
Statistical measures of the two embryo grading systems were compared (Table 6). Sensitivity values for one or more embryos transferred in the ‘CSS≥70’ and ‘CES≥20’ groups were similar (94.7%), although more patients had an embryo transferred in the ‘CES≥20’ group. The specificity value for one or more embryos transferred was 55% in the ‘CSS≥70’ group but only 13.3% in the ‘CES≥20’ group. The positive and negative values of ‘CSS≥70’ group (66.7% and 91.7%, respectively) are both higher than those of ‘CES≥20’ group (50.9% and 72.7%, respectively).
Table 6.
Statistical measures of the two embryo grading systems
| Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) | |
| CSS≥70 | 94.7 | 55 | 66.7 | 91.7 |
| CES≥20 | 94.7 | 13.3 | 50.9 | 72.7 |
DISCUSSION
To increase IVF success rates and reduce unacceptably high multiple pregnancy rates, it is extremely important to select one or two embryos with high viability for uterine transfer in IVF. However, widespread acceptance of such a strategy is hindered by the lack of reliable predictive factors for selecting good quality embryos. Embryo scores are still accepted as the best predictor of pregnancy from IVF (Fisch et al., 2003; Laasch and Puscheck, 2004), but different scoring methods have been shown to give different results. In this study, a prospective CSS was designed for selection of the most viable embryos in the first fresh embryo transfer cycle. The new system achieved increased implantation and clinical pregnancy rates. The data presented in this paper show that combining morphological analysis of human zygotes and pre-implantation embryos might be useful in predicting IVF outcomes.
The quality of embryos has traditionally been evaluated based on the cleavage rate and blastomere morphology. However, these criteria by themselves lack sufficient sensitivity and specificity in the prediction of pregnancy rates. Multiple-embryo transfers were carried out to achieve an acceptable pregnancy rate, leading to a dramatically increased multiple-gestation rate. To lower the multiple-gestation rate following IVF, blastocyst transfer was performed to generate high implantation rates. However, blastocyst culture can only be performed in patients meeting certain selection criteria and is dependent on sufficient oocytes being available. More effort is required for blastocyst transfer than for Day 2 or Day 3 embryo transfer. For this reason, many clinics routinely perform Day 2 or Day 3 embryo transfers.
It has been shown that there is a relationship between the morphology of the zygote at 16~18 h post insemination and its ability to continue development (Scott et al., 2000; Rossi-Ferragut et al., 2003; Payne et al., 2005). Moreover, a correlation between pronuclear zygote morphology and aneuploidy in in vitro-fertilized embryos has been reported (Edirisinghe et al., 2005). Several scoring systems were developed to score the zygotes. The Z-score is based on the status of the nuclei and nucleoli within the zygote. When an oocyte is activated by the entry of spermatozoa, a series of events ensue. The male and female pronuclei are brought together by the centriole (the microtubule organizing center) and the microtubules arising from it. One component of the Z-score is the alignment of the pronuclei by 16~18 h post insemination. Embryos that fail to progress to this stage rarely form blastocysts (Scott et al., 2000). The other component of the Z-score relates to the size, number and distribution of nucleoli. The nucleoli are the sites where rRNA is synthesized and are active sites within the oocyte and sperm pronuclei. At fertilization, rRNA synthesis resumes with concomitant changes in the nucleoli as they reform and grow (Tesarik and Kopecny, 1989; 1990). Synthesis of rRNA is essential for meiotic competence. It is important for the zygote to initiate rRNA synthesis in a timely sequence. The Z-score system was incorporated into the CSS. Results showed that only 49.1% of Z-1 (top quality) zygotes formed Grade 1 embryos while poor quality zygotes could also become high quality embryos, suggesting that static analysis of zygotes alone might not predict accurately the further development of embryos. The present results suggest that a combination of scores might be more reliable for selecting embryos for transfer.
Early embryo cleavage (24~29 h after insemination) was also identified as a strong positive predictor of IVF outcomes (Zaninovic et al., 2000; Petersen et al., 2001; Fenwick et al., 2002). The speed of embryonic development and morphology of blastomeres were considered important aspects of developmental competence. In the present study, embryos were assessed by combining evaluation of zygote morphology and Day 2 and Day 3 embryo morphology. The results of this study showed that this system was highly predictive of IVF outcomes. An overall pregnancy rate of 50.4% was obtained, together with an implantation rate of 27.6%.
Because embryo development is a dynamic process, a single and static observation will inevitably miss-identify some embryos that appear normal at some time points, but will not result in a live birth. It is hypothesized that multi-step grading systems could be used to better monitor developmental status and therefore be more predictive of IVF outcomes. Results of this study support this hypothesis. The implantation rate of the group receiving one or more transferred embryos scoring ≥70 in CSS was 38.5% (82 sacs/213 embryos). This was significantly higher (P<0.001) than that of the group receiving no embryo scoring ≥70 (4%, 4 sacs/99 embryos). The pregnancy rate of the group with one or more transferred embryos scoring ≥70 in the CSS (66.7%, 54/81) was also significantly higher (P<0.001) than that of the group with no embryos scoring ≥70 (8.3%, 3/36).
Day 3 embryo scoring by the CES system has been commonly used in embryo selection. However, the results show that the specificity value for the patients with one or more embryos scoring ≥70 using CSS was 55%, whereas the specificity value for the patients with one or more embryos scoring ≥20 using CES was only 13.3%. The specificity of a test reflects its false-positive rate. A false-positive case occurs when a good-looking embryo does not result in pregnancy following IVF. The results of this study showed that the specificity values and the pregnancy rates in the CES group were lower than those in the CSS group, suggesting that a simple evaluation of Day 3 embryos might not be sufficient to distinguish good quality embryos and that CSS might be a better scoring system for predicting IVF outcomes.
In conclusion, the present study showed that a new CSS was more helpful in selecting embryos of high quality for transfer in IVF. Application of this scoring system could improve the implantation rate and reduce the IVF-induced multiple-pregnancy rate. Further studies to refine the scoring system might increase its predictive value in IVF. A recent study, using multifactorial analyses, attempted to identify variables in the embryo scoring model that are independently predictive of treatment outcomes (Holte et al., 2007). This approach might be helpful in developing a model with greater accuracy in the prediction of treatment outcomes.
Footnotes
Project (No. 021107137) supported by the Department of Science and Technology of Zhejiang Province, China
References
- 1.de Placido GD, Wilding M, Strina I, Alviggi E, Alviggi C, Mollo A, Varicchio MT, Tolino A, Schiattarella C, Dale B. High outcome predictability after IVF using a combined score for zygote and embryo morphology and growth rate. Hum Reprod. 2002;17(9):2402–2409. doi: 10.1093/humrep/17.9.2402. [DOI] [PubMed] [Google Scholar]
- 2.Edirisinghe WR, Jemmott R, Smith C, Allan J. Association of pronuclear Z score with rates of aneuploidy in in vitro-fertilised embryos. Reprod Fertil Dev. 2005;17(5):529–534. doi: 10.1071/RD04065. [DOI] [PubMed] [Google Scholar]
- 3.Fenwick J, Platteau P, Murdoch AP, Herbert M. Time from insemination to first cleavage predicts developmental competence of human preimplantation embryos in vitro. Hum Reprod. 2002;17(2):407–412. doi: 10.1093/humrep/17.2.407. [DOI] [PubMed] [Google Scholar]
- 4.Fisch JD, Rodriguez H, Ross R, Overby G, Sher G. The Graduated Embryo Score (GES) predicts blastocyst formation and pregnancy rate from cleavage-stage embryos. Hum Reprod. 2001;16(9):1970–1975. doi: 10.1093/humrep/16.9.1970. [DOI] [PubMed] [Google Scholar]
- 5.Fisch JD, Sher G, Adamowicz M, Keskintepe L. The graduated embryo score predicts the outcome of assisted reproductive technologies better than a single day 3 evaluation and achieves results associated with blastocyst transfer from day 3 embryo transfer. Fertil Steril. 2003;80(6):1352–1358. doi: 10.1016/j.fertnstert.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Giorgetti C, Terriou P, Auquier P, Hans E, Spach JL, Salzmann J, Roulier R. Embryo score to predict implantation after in vitro fertilization: based on 957 single embryo transfers. Hum Reprod. 1995;10(9):2427–2431. doi: 10.1093/oxfordjournals.humrep.a136312. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez ER. Avoiding multiple pregnancies: sailing uncharted seas. Hum Reprod. 2001;16(4):615–616. doi: 10.1093/humrep/16.4.615. [DOI] [PubMed] [Google Scholar]
- 8.Holte J, Berglund L, Milton K, Garello C, Gemmarelli G, Revelli A, Bergh T. Construction of an evidence-based integrated morphology cleavage embryo score for implantation potential of embryos scored and transferred on day 2 after oocyte retrieval. Hum Reprod. 2007;22(2):548–557. doi: 10.1093/humrep/del403. [DOI] [PubMed] [Google Scholar]
- 9.Hu Y, Maxson WS, Hoffman DI, Ory SJ, Eager S, Dupre J, Lu C. Maximising pregnancy rates and limiting high-order multiple conceptions by determining the optimal number of embryos to transfer based on quality. Fertil Steril. 1998;69(4):650–657. doi: 10.1016/S0015-0282(98)00024-7. [DOI] [PubMed] [Google Scholar]
- 10.Laasch C, Puscheck E. Cumulative embryo score, not endometrial thickness, is best for pregnancy prediction in IVF. J Assist Reprod Genet. 2004;21(2):47–50. doi: 10.1023/B:JARG.0000025937.43936.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lan K, Huang F, Lin Y, Kung F, Hsieh C, Huang H, Tan P, Chang SY. The predictive value of using a combined Z-score and day 3 embryo morphology score in the assessment of embryo survival on day 5. Hum Reprod. 2003;18(6):1299–1306. doi: 10.1093/humrep/deg239. [DOI] [PubMed] [Google Scholar]
- 12.Ludwig M, Schopper B, AI-Hasani S, Diedrich K. Clinical use of a pronuclear stage score following intracytoplasmic sperm injection: impact on pregnancy rates under the conditions of German embryo protection law. Hum Reprod. 2000;15(2):325–329. doi: 10.1093/humrep/15.2.325. [DOI] [PubMed] [Google Scholar]
- 13.Milki AA, Hinckley MD, Gebhart J, Dasig D, Westphal LM, Behr B. Accuracy of day 3 criteria for selecting the best embryos. Fertil Steril. 2002;77(6):1191–1195. doi: 10.1016/S0015-0282(02)03104-7. [DOI] [PubMed] [Google Scholar]
- 14.Payne JF, Raburn DJ, Couchman GM, Price TM, Jamison MG, Walmer DK. Relationship between pre-embryo pronuclear morphology (zygote score) and standard day 2 or 3 embryo morphology with regard to assisted reproductive technique outcomes. Fertil Steril. 2005;84(4):900–909. doi: 10.1016/j.fertnstert.2005.04.047. [DOI] [PubMed] [Google Scholar]
- 15.Petersen CG, Mauri AL, Ferreira R, Baruffi RL, Franco-Junior JG. Embryo selection by the first cleavage parameter between 25 and 27 hours after ICSI. J Assist Reprod Genet. 2001;18(4):211–214. doi: 10.1023/A:1009460013579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rijnders PM, Jansen CA. The predictive value of day 3 embryo morphology regarding blastocyst formation, pregnancy and implantation rate after day 5 transfer following in vitro fertilization and intracytoplasmic sperm injection. Hum Reprod. 1998;13(10):2869–2873. doi: 10.1093/humrep/13.10.2869. [DOI] [PubMed] [Google Scholar]
- 17.Rossi-Ferragut LM, Iaconelli AJr, Aoki T, Rocha CC, dos Santos DR, Pasqualotto FF, Borges EJr. Pronuclear and morphological features as a cumulative score to select embryos in ICSI (intracytoplasmic sperm injection) cycles according to sperm origin. J Assist Reprod Genet. 2003;20(1):1–7. doi: 10.1023/A:1021286119979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schieve LA, Meikle SF, Ferre C, Peterson HB, Jeng G, Wilcox LS. Low and very low birth weight in infants conceived with the use of assisted reproductive technology. N Engl J Med. 2002;346(10):731–737. doi: 10.1056/NEJMoa010806. [DOI] [PubMed] [Google Scholar]
- 19.Scott L, Alvero R, Leondires M, Bradley M. The morphology of human pronuclear embryos is positively related to blastocyst development and implantation. Hum Reprod. 2000;15(11):2394–2403. doi: 10.1093/humrep/15.11.2394. [DOI] [PubMed] [Google Scholar]
- 20.Tesarik J, Kopecny V. Development of human male pronucleus: ultrastructure and timing. Gamete Res. 1989;24(2):135–149. doi: 10.1002/mrd.1120240203. [DOI] [PubMed] [Google Scholar]
- 21.Tesarik J, Kopecny V. Assembly of the nuclear precursor’s bodies in human male pronuclei is correlated with an early RNA synthetic activity. Exp Cell Res. 1990;191(1):153–156. doi: 10.1016/0014-4827(90)90050-K. [DOI] [PubMed] [Google Scholar]
- 22.Zaninovic N, Veeck LL, Clarke R, Rosenwaks Z. Early assessment of human pre-embryos as an indicator for potential blastocyst development. Fertil Steril. 2000;74(3):S80. doi: 10.1016/S0015-0282(00)00938-9. [DOI] [Google Scholar]
- 23.Zhang Y, Xu CM, Zhu YM, Dong MY, Qian YL, Jin F, Huang HF. Preimplantation genetic diagnosis for Down syndrome pregnancy. J Zhejiang Univ Sci B. 2007;8(7):515–521. doi: 10.1631/jzus.2007.B0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ziebe S, Peterson K, Lindenberg S, Andersen AG, Gabrielsen A, Anderson AN. Embryo morphology of cleavage stage: how to select the best embryos for transfer after in-vitro fertilization. Hum Reprod. 1997;12(7):1545–1549. doi: 10.1093/humrep/12.7.1545. [DOI] [PubMed] [Google Scholar]
- 25.Zollner U, Zollner KP, Hartl G, Dietl J, Steck T. The use of a detailed zygote score after IVF/ICSI to obtain good quality blastocysts: the German experience. Hum Reprod. 2002;17(5):1327–1333. doi: 10.1093/humrep/17.5.1327. [DOI] [PubMed] [Google Scholar]
- 26.Zollner U, Zollner KP, Steck T, Dietl J. Pronuclear scoring. Time for international standardization. J Reprod Med. 2003;48(5):365–369. [PubMed] [Google Scholar]