Abstract
Objective: To explore the effects of down-regulated tryptase expression in mast cells on the synthesis and release of interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) of vascular endothelial cells. Methods: Tryptase-siRNA (small-interfering RNA) vector was constructed to inhibit tryptase expression in P815 cells. The medium of P815 cells treated by the tryptase-siRNA (RNAi-P815 group) or pure vector (P815 group) was collected and used to culture bEnd.3 cells. The messenger RNAs (mRNAs) of IL-6 and TNF-α in bEnd.3 cells and their protein levels in the medium were measured by reverse transcription polymerase chain reaction (RT-PCR) and enzyme-linked immunosorbent assay (ELISA), respectively. Results: IL-6 and TNF-α mRNAs in bEnd.3 cells cultured in RNAi-P815-conditioned medium decreased significantly compared to those in P815-conditioned medium. Consistently, IL-6 and TNF-α protein levels in the medium of bEnd.3 of RNAi-P815 group were lower than those of P815 group. Conclusion: Reduced tryptase expression significantly inhibited the synthesis and release of IL-6 and TNF-α in vascular endothelial cells. RNA interference targeting tryptase expression may be a new anti-inflammatory strategy for vascular diseases.
Keywords: Tryptase, RNA interference, Interleukin-6 (IL-6), Tumor necrosis factor-alpha (TNF-α), Endothelial cells
INTRODUCTION
Tryptase secreted by mast cells is a protease with multiple biological functions. As a proinflammatory factor (Kelley et al., 2000), it may promote vascular inflammation by simulating the proliferation of vascular smooth muscle cells, inducing foam cells and injuring vascular endothelial cells. Tryptase may reduce the efflux of cholesterol from macrophages by depleting high density lipoprotein (HDL)3 and thus decrease the formation of foam cells during atherogenesis (Lee et al., 2002). Vascular endothelial cell is the barrier between blood and vessel wall. Tryptase decreases the barrier function and increases the permeability of endothelial monolayer (Sendo et al., 2003). The dysfunction of vascular endothelial cells plays an important role in vascular inflammation. Tryptase may injure the cells by increasing inflammatory mediators such as monocyte chemoattractant protein-1 (MCP-1) and interleukin-8 (IL-8) (Kinoshita et al., 2005).
Interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) are important proinflammatory mediators in pathogenesis of vascular diseases. We have shown that tryptase up-regulated synthesis and release of IL-6 and TNF-α in endothelial cells (Mangsur et al., 2006). Besides, tryptase stimulates the expression of stem cell factor, which promotes the accumulation of mast cells in inflammatory reaction (Lu et al., 2006). The present study evaluated the effects of RNAinterference (RNAi) down-regulated tryptase expression in mast cells on the expression and release of IL-6 and TNF-α in vascular endothelial cells.
MATERIALS AND METHODS
Tryptase-siRNA (small-interfering RNA) vector construction
According to tryptase gene sequence, two siRNA for tryptase were designed. The sequences from the 139th and 518th sites were GTGAGCCTGAGATTTAAAT and CTTATCCTCTGAAGCAAGT, respectively. For each siRNA sequence, two DNA template single chains were synthesized. The template single chain included sense and antisense strands of siRNA combined with a 9-deoxynucleotides loop, a BamHI restriction site and a HindIII restriction site at each end. The correspondent DNA chains were annealed at 95 °C for 10 min to insert into siRNA vector. The control vectors encoding green fluorescent protein (GFP) and ampicillin resistance genes were digested by BamHI and HindIII. Inserted siRNA segment was ligated into siRNA vector with T4 ligase. The ligated product then transfected Esherichia coli DH5α. Several clones were selected to extract the plasmids. Positive clones were identified by polymerase chain reaction (PCR) followed by 2% (w/v) agarose gel electrophoresis.
P815 cells transfection
P815 is a mouse mastocytoma cell line. The 2×105 P815 cells were plated in 6-well plates and cultured with Dulbecco’s modified Eagle’s medium (DMEM) containing 10% (v/v) fetal bovine serum (FBS) at 37 °C. The cells were maintained in 500 μl medium per well for 24 h before transfection. The cells were then divided into 4 groups with 3 wells per group. They were treated with Site 139 siRNA vector, Site 518 siRNA vector, control vector, and phosphate buffer solution (PBS), respectively. Vectors, Lipofectamine 2000 (Invitrogen, USA) and serum-free DMEM medium were mixed to form DNA-Lipofectamine 2000 transfection mixture. The 100 μl transfection mixture was added into each well. Six hours after transfection, the medium was replaced with regular medium. The cells were observed by a fluorescent microscope 48 h after transfection. Percentage rate of the positive cells in 10 fields were counted to evaluate transfection efficiency.
Analysis of RNAi effect
To analyze the effect of tryptase-siRNA on tryptase gene expression, we measured tryptase mRNA level in P815 cells. At 48 h after transfection, total RNA was isolated using Trizol (Invitrogen), and cDNA was synthesized with reverse transcription polymerase chain reaction (RT-PCR) Kit (Invitrogen) as described by the manufacturer. The PCR primers for tryptase and β-actin messenger RNAs (mRNAs) are shown in Table 1. PCR cycling conditions included 30 cycles of 94 °C for 50 s, 55 °C for 50 s, and 72 °C for 50 s. The PCR products were visualized in a 1.5% (w/v) agarose gel containing 5 μg/ml ethidium bromide.
Table 1.
The PCR primers for mRNAs
| Genes | Size (bp) | Sequence |
|
| Sense | Anti-sense | ||
| Tryptase | 301 | 5′-agtaagtggccctggcaggtgagcc-3′ | 5′-ggtccccatagtatactgctc-3′ |
| β-actin | 490 | 5′-gtcgtaccacaggcattgtgatgg-3′ | 5′-gcaatgcctgggtacatggtgg-3′ |
| IL-6 | 155 | 5′-tggagtcacagaaggagtggctaag-3′ | 5′-tctgaccacagtgaggaatgtccac-3′ |
| TNF-α | 428 | 5′-gtgacaagcctgtagccca-3′ | 5′-aaagtagacctgcccggac-3′ |
| GAPDH | 226 | 5′-acagccgcatcttcttgtgcagtg-3′ | 5′-ggccttgactgtgccgttgaattt-3′ |
Preparation of conditioned medium
Site 139 siRNA vector was selected to prepare P815 conditional medium based on its stronger inhibitory effect on tryptase synthesis. P815 cells treated with tryptase-siRNA were maintained in DMEM medium containing 10% (v/v) FBS for 48 h. The cells were then washed with PBS for 3 times and cultured in serum-free DMEM medium for additional 48 h. A separate portion of P815 cells were cultured without tryptase-siRNA treatment. The culture media were collected, centrifuged, filtered and named as RNAi-P815-conditioned and P815-conditioned media, respectively.
Culturing bEnd.3 cells with the conditioned media
bEnd.3 is a vascular endothelial cell line. The cells were obtained from American Type Culture Collection (ATCC, USA). They were cultured with DMEM medium containing 10% (v/v) FBS at 37 °C and 5% (v/v) CO2, and then were planted into 6-well plates at 2×105 per well. The cells were divided into 3 groups with 3 wells each, and were treated with 2 ml RNAi-P815-conditioned, 2 ml P815-conditioned and 2 ml unconditioned control media for 24 h, respectively.
Measurement of released tryptase and TNF-α in conditioned media
Tryptase in the conditioned media was detected by enzyme-linked immunosorbent assay (ELISA) (Lu et al., 2003). Microplate was coated with primary tryptase antibody (1.0 μg/ml, produced by our lab) at 4 °C for 18 h. After blocking with 10% (v/v) bovine serum albumin (BSA) for 1 h, tryptase samples and standards were applied into wells and incubated at 4 °C for 12 h. The cells were then incubated with biotinylated antibody (produced by our lab) at 4 °C for 2 h followed by enzyme mixture (Shenggong, China) at 37 °C for 60 min. The reaction was developed with teramethylbenzidine (TMB) for 15 min and read at 450 nm. TNF-α in the conditioned media was detected by ELISA using mouse TNF-α ELISA Kit (Jingmei, China) according to the manufacturer’s instruction.
mRNA level measurement by RT-PCR
After bEnd.3 cells were cultured with the conditioned media for 24 h, total RNA was isolated by Trizol (Invitrogen). The cDNA was synthesized with RT-PCR Kit (Invitrogen) as described by the manufacturer. The PCR primers for IL-6, TNF-α and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) are shown in Table 1. PCR cycling conditions are: IL-6, 94 °C for 50 s, 55 °C for 50s, 72 °C for 50 s, and 26 cycles; TNF-α, 94 °C for 50 s, 61 °C for 50 s, 72 °C for 50 s, and 30 cycles. The PCR fragments were visualized in a 1.5% (w/v) agarose gel containing 5 μg/ml ethidium bromide.
Protein level measurement by ELISA
After 24 h conditioned culturing, the media of bEnd.3 cells were collected, centrifuged and filtrated. IL-6 and TNF-α in the filtrated medium were detected using mouse IL-6 and TNF-α ELISA Kits (Jingmei, China) according to the manufacturer’s instruction. The final concentration of TNF-α was counted by subtracting TNF-α in the conditioned media.
Statistical analysis
Data analysis was performed using Student’s t-test. Difference was significant if P value <0.01.
RESULTS
Efficiency of P815 cells transfection
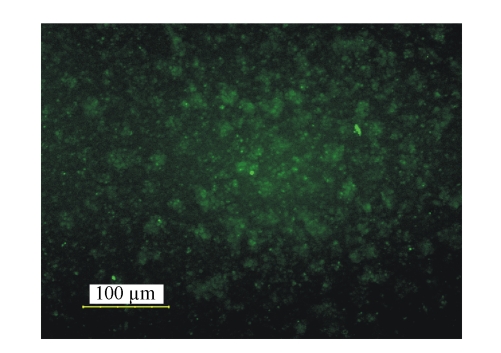
Forty-eight hours after siRNA transfection, (51.8±4.0)% of P815 cells were positive for green fluorescence (Fig.1).
Fig. 1.
Positive transfected cells under fluorescent microscope
P815 cells were transfected with tryptase-siRNA or control vectors containing GFP. Forth-eight hours later, the cells were observed under fluorescent microscope. The cells emitting green fluorescence were considered positive
Decrease of tryptase expression in P815 cells by tryptase-siRNA
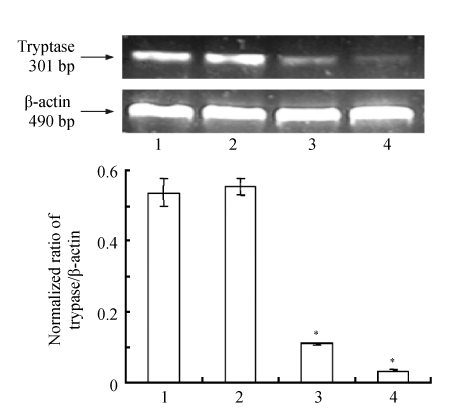
To evaluate the effect of tryptase-siRNA on tryptase expression, tryptase mRNA in P815 cells was observed using β-actin as internal control. Tryptase mRNA in P815 cells transfected with Site 139 and Site 518 siRNA vectors decreased to (6.3±0.6)% and (20.6±1.7)%, respectively, compared to non-transfected cells (Fig.2). The inhibitory effect of Site 139 siRNA vector was greater than that of Site 518 siRNA vector. Therefore, Site 139 siRNA vector was selected for the remaining experiments.
Fig. 2.
The effect of tryptase-siRNA on tryptase mRNA expression
P815 cells were transfected with tryptase-siRNA or control vectors. Tryptase mRNA was extracted from the cells after 48 h and examined by RT-PCR [cingulum of mRNA (upper) and normalized ratio of mRNA expression (lower)]. Lanes 1~4 represent tryptase in P815 cells maintained in vector-free DMEM medium, control vector, Site 518 siRNA vector and Site 319 siRNA vector, respectively. Data were presented as mean±SD; n=3; * P<0.01 Lane 3 or 4 vs Lane 1 or 2
Decrease of tryptase released in the conditioned media by tryptase-siRNA
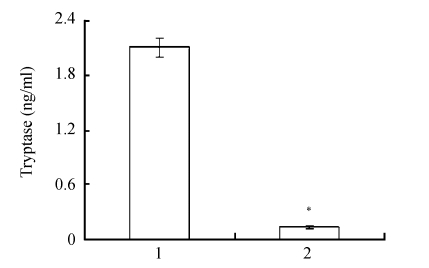
Compared to the P815-conditioned medium, tryptase in the RNAi-P815-conditioned medium was significantly lower (Fig.3).
Fig. 3.
Tryptase in the conditioned media
The medium collected from P815 cells transfected with tryptase-siRNA vector was used as RNAi-P815-conditioned medium. Medium collected from P815 cells without vector transfection was named as P815-conditioned medium. Lanes 1 and 2 represent tryptase in P815-conditioned medium and in RNAi-P815-conditioned medium, respectively. Data were expressed as mean±SD; n=3; * P<0.01 Lane 2 vs Lane 1
Effect of tryptase down-regulation on the expressions of IL-6 and TNF-α mRNAs in bEnd.3 cells
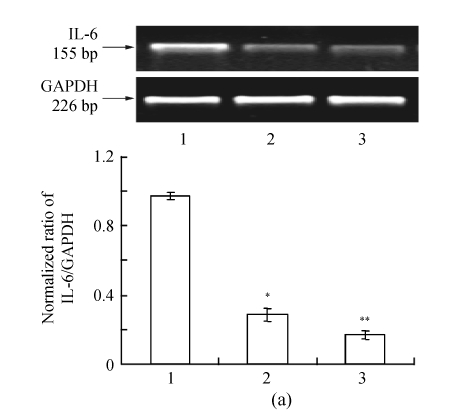
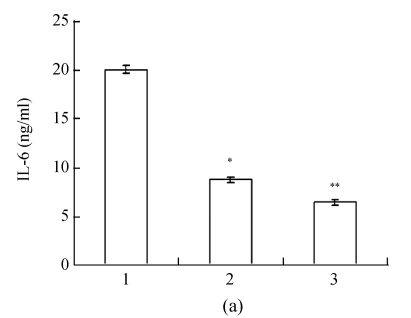
The IL-6 and TNF-α mRNA expressions were significantly lower in bEnd.3 cells cultured in RNAi-P815-conditioned medium compared to those in P815-conditioned medium (Fig.4, n=3, P<0.01). IL-6 and TNF-α mRNAs in RNAi-P815 group decreased to (29.4±3.2)% and (10.6±1.4)%, respectively.
Fig. 4.
The effect of conditioned culturing on IL-6 (a) and TNF-α (b) mRNA expressions
bEnd.3 cells were cultured with conditioned media from P815 cells for 24 h. Total RNA was extracted from bEnd.3 cells. IL-6 and TNF-α mRNAs were observed by RT-PCR [cingulum of mRNA (upper) and normalized ratio of mRNA expression (lower)]. Lanes 1~3 represent IL-6 and TNF-α mRNA expressions in bEnd.3 cells cultured in the P815-conditioned, RNAi-P815-conditioned and vector-free DMEM media, respectively. Data were expressed as mean±SD; n=3; * P<0.01 Lane 2 vs Lane 1; ** P<0.01 Lane 3 vs Lane 1
Roles of tryptase down-regulation on IL-6 and TNF-α concentrations in culture media
The result of ELISA was consistent to that of RT-PCR. IL-6 and TNF-α concentrations in RNAi-P815 group were significantly lower than those in P815 group (Fig.5, n=3, P<0.01). They were decreased to (43.6±2.1)% and (35.8±2.9)% in RNAi-P815 group, respectively.
Fig. 5.
The effect of conditioned culturing on IL-6 (a) and TNF-α (b) protein concentrations
bEnd.3 cells were cultured in the media conditioned by P815 cells for 24 h. Protein levels of IL-6 and TNF-α were determined by ELISA. The concentration of TNF-α was counted by subtracting TNF-α in the conditioned media. Lanes 1~3 represent IL-6 and TNF-α protein expressions in bEnd.3 cells cultured in the P815-conditioned, RNAi-P815-conditioned and vector-free DMEM media, respectively. Data were expressed as mean±SD; n=3; * P<0.01 Lane 2 vs Lane 1; ** P<0.01 Lane 3 vs Lane 1
DISCUSSION
In vascular system, tryptase affects vascular relaxation and contraction, cell mitogenesis, intracellular Ca2+, NO, and von Willebrand factor release (Macfarlane et al., 2001). Tryptase promotes vascular inflammation by increasing neutrophil recruitment. Tryptase stimulates endothelial cells to synthesize and release IL-1β and IL-8 that induce leukocyte infiltration (van Haelst et al., 2001; Compton et al., 1998). Tryptase can also activate peripheral blood mononuclear cells to produce and secrete IL-6 and TNF-α (Malamud et al., 2003), so as to affect the occurrence and development of inflammation in vascular system. The proteinase-activated receptor 2 (PAR-2) on multiplicate cells can be activated by tryptase to react on its physiological roles. We found that tryptase up-regulated the synthesis and release of endothelial IL-6 and TNF-α by activating PAR-2 in concentration- and time-dependent manner (Mangsur et al., 2006). In the present study, mRNA and protein expressions of IL-6 and TNF-α in bEnd.3 cells were inhibited by decreased extracellular tryptase concentration. The results further demonstrate that tryptase may stimulate IL-6 and TNF-α release from vascular endothelial cells.
Vascular endothelial cell dysfunction is an early pathological event in vascular inflammation (Gibbons, 1997; Takase et al., 1998). IL-6 and TNF-α secreted by mononuclear cells, vascular smooth muscle cells and vascular endothelial cells play an important role in mediating inflammation (Ridker et al., 2000a; 2000b). One of the mechanisms by which TNF-α mediates inflammation is to activate nuclear factor-κB (NF-κB) (Barnes and Karin, 1997; Monaco et al., 2004). NF-κB regulates productions of many proinflammatory substances such as vascular endothelial growth factor (VEGF), interleukin-1 (IL-1), IL-6, IL-8, vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1). These proinflammatory molecules stimulate the proliferation and migration of vascular smooth muscle cells (Warner and Libby, 1989; Eickelberg et al., 1997), increase inflammatory infiltration in vessel wall (Smalley et al., 1996; Hou et al., 2000), and induce new release of inflammatory mediators (Ridker et al., 2000b). Thus, inhibiting tryptase expression in mast cells may be a new therapeutic strategy for vascular inflammation.
Several studies have shown the effects of tryptase on cardiovascular diseases including atherosclerosis (Libby, 2002; Chung et al., 2007). In the present study, we inhibited tryptase expression in P815 cells using siRNA vectors targeting tryptase and then used RNAi-P815-conditioned and P815-conditioned media to culture bEnd.3 cells. Tryptase secreted by P815 cells was significantly lower in RNAi-P815-conditioned medium than in P815-conditioned medium. Expression and secretion of IL-6 and TNF-α in bEnd.3 cells decreased significantly after being cultured in RNAi-P815-conditioned medium. The result suggests that trypase inhibition may down-regulate the expression and secretion of IL-6 and TNF-α in vascular endothelial cells. Decreased tryptase expression induced by RNAi may inhibit vascular inflammation by decreasing downstream proinflammatory factors.
Footnotes
Project (No. 30470689) supported by the National Natural Science Foundation of China
References
- 1.Barnes PJ, Karin M. Nuclear factor-κB—a pivotal transcription factor in chronic inflammatory diseases. The New England Journal of Medicine. 1997;336(15):1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 2.Chung CP, Avalos I, Raggi P, Stein CM. Atherosclerosis and inflammation: insights from rheumatoid arthritis. Clinical Rheumatology. 2007;26(8):1228–1233. doi: 10.1007/s10067-007-0548-7. [DOI] [PubMed] [Google Scholar]
- 3.Compton SJ, Cairns JA, Holgate ST, Walls AF. The role of mast cell tryptase in regulating endothelial cell proliferation, cytokine release, and adhesion molecule expression: tryptase induces expression of mRNA for IL-1β and IL-8 and stimulates the selective release of IL-8 from human umbilical vein endothelial cells. The Journal of Immunology. 1998;161:1939–1946. [PubMed] [Google Scholar]
- 4.Eickelberg O, Roth M, Block LH. Effects of amlodipine on gene expression and extracellular matrix formation in human vascular smooth muscle cells and fibroblasts: implication for vascular protection. International Journal of Cardiology. 1997;62:S31–S37. doi: 10.1016/S0167-5273(97)00239-8. [DOI] [PubMed] [Google Scholar]
- 5.Gibbons GH. Endothelial function as a determinant of vascular function and structure: a new therapeutic target. American Journal of Cardiology. 1997;79(12):3–8. doi: 10.1016/S0002-9149(97)00122-7. [DOI] [PubMed] [Google Scholar]
- 6.Hou YQ, Hafezi-Moghadam A, Ley K. Role of vascular cell adhesion molecule-1 and fibronectin connecting segment-1 in monocyte rolling and adhesion on early atherosclerotic lesions. Circulation Research. 2000;87:153–159. doi: 10.1161/01.res.87.2.153. [DOI] [PubMed] [Google Scholar]
- 7.Kelley JL, Chi DS, Abou-Auda W, Smith JK, Krishnaswamy G. The molecular role of mast cells in atherosclerotic cardiovascular disease. Molecular Medicine Today. 2000;6(8):304–308. doi: 10.1016/S1357-4310(00)01747-0. [DOI] [PubMed] [Google Scholar]
- 8.Kinoshita M, Okada M, Hara M, Furukawa Y, Matsumori A. Mast cell tryptase in mast cell granules enhances MCP-1 and IL-8 production in human endothelial cells. Arteriosclerosis Thrombosis and Vascular Biology. 2005;25(9):1858–1863. doi: 10.1161/01.ATV.0000174797.71708.97. [DOI] [PubMed] [Google Scholar]
- 9.Lee M, Sommerhoff CP, von Eckardstein A, Zettl F, Fritz H, Kovanen PT. Mast cell tryptase degrades HDL and blocks its function as an acceptor of cellular cholesterol. Artesriosclerosis Thrombosis and Vascular Biology. 2002;22(12):2086–2091. doi: 10.1161/01.ATV.0000041405.07367.B5. [DOI] [PubMed] [Google Scholar]
- 10.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 11.Lu C, Wang XW, Zhao FD, Yin LH. Establishment of a new experimental detective assay of human serum tryptase. Fudan University Journal of Medical Sciences. 2003;30(1):66–67. (in Chinese) [Google Scholar]
- 12.Lu C, Ma YJ, Mangsur M, Zhao FD, Yin LH. Effects of mast cell tryptase on the expression of stem cell factor in endothelial cell line and its mechanism. Fudan University Journal of Medical Sciences. 2006;33(3):291–294. (in Chinese) [Google Scholar]
- 13.Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacological Reviews. 2001;53:245–282. [PubMed] [Google Scholar]
- 14.Malamud V, Vaaknin A, Abramsky O, Mor M, Burgess LE, Ben-Yehudah A, Lorberboum-Golski H. Tryptase activates peripheral blood mononuclear cells causing the synthesis and release of TNF-α, IL-6 and IL-1β: possible relevance to multiple sclerosis. Journal of Neuroimmunology. 2003;138(1-2):115–122. doi: 10.1016/S0165-5728(03)00090-0. [DOI] [PubMed] [Google Scholar]
- 15.Mangsur M, Ma YJ, Lu C, Zhao FD, Yin LH. Effect of tryptase on the expression of IL-6 and TNF-α in microvascular endothelial cell. Fudan University Journal of Medical Sciences. 2006;33(2):217–221. (in Chinese) [Google Scholar]
- 16.Monaco C, Andreakos E, Kiriakidis S, Mauri C, Bicknell C, Foxwell B, Cheshire N, Paleolog E, Feldmann M. Canonical pathway of nuclear factor κB activation selectively regulates proinflammatory and prothrombotic responses in human atherosclerosis. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(15):5634–5639. doi: 10.1073/pnas.0401060101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 18.Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, Braunwald E. Elevation of tumor necrosis factor-α and increased risk of recurrent coronary events after myocardial infarction. Circulation. 2000;101:2149–2153. doi: 10.1161/01.cir.101.18.2149. [DOI] [PubMed] [Google Scholar]
- 19.Sendo T, Sumimura T, Itoh Y, Goromaru T, Aki K, Yano T, Oike M, Ito Y, Mori S, Nishibori M, et al. Involvement of proteinase-activated receptor-2 in mast cell tryptase-induced barrier dysfunction in bovine aortic endothelial cells. Cellular Signalling. 2003;15:773–781. doi: 10.1016/s0898-6568(03)00014-7. [DOI] [PubMed] [Google Scholar]
- 20.Smalley DM, Lin JHC, Curtis ML, Kobari Y, Stemerman MB, Pritchard KA. Native LDL increases endothelial cell adhesiveness by inducing intercellular adhesion molecule-1. Arteriosclerosis, Thrombosis, and Vascular Biology. 1996;16:585–590. doi: 10.1161/01.atv.16.4.585. [DOI] [PubMed] [Google Scholar]
- 21.Takase B, Uehata A, Akima T, Nagai T, Nishioka T, Hamabe A, Satomura K, Ohsuzu F, Kurita A. Endothelium-dependent flow-mediated vasodilation in coronary and brachial arteries in suspected coronary artery disease. American Journal of Cardiology. 1998;82(12):1535–1539. doi: 10.1016/S0002-9149(98)00702-4. [DOI] [PubMed] [Google Scholar]
- 22.van Haelst PL, Timmer JR, Grijns HJ, Kauffman HF, Gans RO, van Doormaal JJ. No long-lasting or intermittent mast cell activation in acute coronary syndromes. International Journal of Cardiology. 2001;78(1):75–80. doi: 10.1016/S0167-5273(00)00475-7. [DOI] [PubMed] [Google Scholar]
- 23.Warner SJ, Libby P. Human vascular smooth muscles: target for and source of tumor necrosis factor. The Journal of Immunology. 1989;142:100–109. [PubMed] [Google Scholar]