Summary
Mammalian spermatogenesis is a complex process in which male germ-line stem cells develop to ultimately form spermatozoa. Spermatogonial stem cells, or SSCs, are found in the basal compartment of the seminiferous epithelium. They self-renew to maintain the pool of stem cells throughout life, or they differentiate to generate a large number of germ cells. A balance between SSC self-renewal and differentiation in the adult testis is therefore essential to maintain normal spermatogenesis and fertility. Maintenance and self-renewal are tightly regulated by extrinsic signals from the surrounding microenvironment, called the spermatogonial stem cell niche. By physically supporting the SSCs and providing them with growth factors, the Sertoli cell is the main component of the niche. In addition, adhesion molecules that connect the SSCs to the basement membrane and cellular components of the interstitium between the seminiferous tubules are important regulators of the niche function. This review mainly focuses on glial cell line-derived neurotrophic factor (Gdnf), which is produced by Sertoli cells to maintain SSCs self-renewal, and the downstream signaling pathways induced by this crucial growth factor. Interactions between Gdnf and other signaling pathways that maintain self-renewal, as well as the role of novel SSC- and Sertoli cell-specific transcription factors, are also discussed.
Keywords: Spermatogonial stem cells, Sertoli cells, Niche, Testis, Gdnf, Transcription factors, Signaling pathways
1. Spermatogenesis and the spermatogonial stem cell niche
Mammalian spermatogenesis is a tightly regulated and continuous process in which spermatogonial stem cells (SSCs) develop to ultimately form spermatozoa. Spermatogenesis after puberty involves four distinct events: stem cell self-renewal that maintains the continuous production of germ cells throughout life, spermatogonial proliferation and differentiation that amplifies the number of premeiotic cells, meiosis in spermatocytes, and spermiogenesis, which is the morphological differentiation of round spermatids into spermatozoa. SSCs present the distinctive characteristic of being the only stem cells in the body that undergo self-renewal throughout life and transmit genetic information to the offspring (Dym, 1994; de Rooij and Russell, 2000; Brinster, 2002). SSCs reside in the basal part of the seminiferous epithelium, in contact with the basement membrane. Morphologically, they are undifferentiated single cells that are not connected by intercellular bridges like the more advanced germ cells (Huckins, 1971; Oakberg, 1971; Dym and Fawcett, 1971; de Rooij and Russell, 2000). According to the model of Huckins and Oakberg (Huckins and Oakberg, 1978), SSCs renew themselves or differentiate into two daughter cells called Apaired spermatogonia (Figure 1). Apaired spermatogonia further divide to form chains of 4, 8 or 16 Aaligned spermatogonia. The Aaligned cells will then differentiate into type A1 spermatogonia. The A1 spermatogonia resume division to form A2 to A4 spermatogonia. Next, A4 cells divide to form intermediate (In) spermatogonia, and In spermatogonia divide to produce type B spermatogonia. Finally, type B spermatogonia divide to form primary spermatocytes that will enter meiosis and further develop into haploid spermatids and sperm.
Figure 1. The first steps of mammalian spermatogenesis.
Diagram representing the first steps of spermatogenesis, in particular the different subtypes of A spermatogonia. The spermatogonial stem cell (SSC or As or Asingle spermatogonium is able to self-renew (curved arrow) or to differentiate into Apaired spermatogonia linked by an intercellular bridge (straight arrow). The Apaired spermatogonia subsequently proliferate into 4, 8, and 16 Aaligned spermatogonia, ultimately producing differentiating type A spermatogonia. The differentiating type A spermatogonia proliferate and differentiate to become spermatocytes that undergo meiosis, producing spermatids that will go through spermiogenesis. All spermatogenic cells differentiate as cohorts of units interconnected by intercellular bridges. Adapted from Kiger and Fuller, Male Germline Stem Cells. In: Stem Cell Biology, Cold Spring Harbor Laboratory Press, 2001 (Kiger and Fuller, 2001).
Stem-cell populations are established and maintained in specialized microenvironments called “niches”. This concept was proposed for the first time in 1978 by Schofield for the hematopoietic system (Schofield, 1978), and was later used to describe the intestinal crypt (Williams et al., 1992). Watt and Hogan pointed out that maintenance of the stem cell compartment ultimately depends on cell autonomous regulators modulated by external signals (Watt and Hogan, 2000). The external signals that control the stem cell's fate collectively make up the stem cell microenvironment or niche. The niche plays an important role in the decision that a stem cell makes to self-renew or differentiate, and involves a complex interplay of short- and long-range signals between the stem cells, their differentiating daughters, neighboring cells, and the extracellular matrix. Therefore, the niche constitutes a basic unit of tissue physiology (Scadden, 2006).
Much of our understanding of the spermatogonial stem cell niche comes from the study of Drosophila. The Drosophila testis is a closed tube with the germline stem cells located in the apical tip region. Approximately, 8–10 stem cells can be found clustered around the hub, which contains somatic cells producing specific growth factors (Figure 2A). One of these proteins is the cytokine-like ligand Unpaired (UPD), which ensures the self-renewal of the stem cells as long as they remain in the environment adjacent to the hub (Li and Xie, 2005; Tulina and Matunis, 2001; Kiger et al., 2000). UPD expressed by hub cells activates the Janus kinase–signal transducers and activators of transcription (JAK-STAT) pathway in Drosophila SSCs to specify stem cell self-renewal (Kiger et al., 2001) (Tulina and Matunis, 2001). In addition, centrosome positioning plays a key role in ensuring the correct mitotic spindle orientation (Yamashita et al., 2003), which is placed perpendicular to the interface with the hub during mitosis. Therefore, when the stem cells divide, one daughter cell will remain close to the hub, while the other will be displaced away from the hub, experiencing a weaker signal and initiating differentiation. As the germ cells differentiate, they will move toward the basal end of the tube.
Figure 2. Organization of the Drosophila testis and the seminiferous epithelium in mammals.
Figure 2A: Germinal proliferation center in Drosophila. In the apical tip region, the germline stem cells (S) are in contact with the hub cells (H). Each germline stem cell also associates with a pair of somatic cells called cyst progenitor cells (CP). When a germline stem cell or a cyst progenitor cell divides, the daughter cell that loses contact from the hub differentiates into a gonialblast (G) or a cyst cell (C) respectively. Two cyst cells will associate with one gonialblast and will never divide again. They will enclose the progeny of the gonialblast throughout spermatogenesis. From Fuller MT, Seminars in Developmental Biology, 1998 (Fuller, 1998).
Figure 2B: Seminiferous epithelium of the mammalian testis. The spermatogonial stem cells are a subpopulation of type A spermatogonia that reside in the basal compartment of the seminiferous epithelium, in contact with the basement membrane. As the germ cells proliferate and differentiate, they move toward the lumen of the seminiferous tubule. Adapted from Russell L, Mammalian Spermatogenesis, In: Histological and Histopathological Evaluation of the Testis, Cache River Press, 1990 (Russell, 1990).
In contrast to Drosophila, the anatomical site of the spermatogonial stem cell niche in mammals is less clearly defined. The mammalian testis contains seminiferous tubules lined by the seminiferous epithelium, which consists of cells of the germ line that proliferate and differentiate into sperm, with the differentiating cells developing toward the lumen (Figure 2B). The niche microenvironment is provided by the somatic Sertoli cells, the basement membrane and cellular components of the interstitial space between the seminiferous tubules. More specifically, these extrinsic signals include growth factors produced by Sertoli cells (Simon et al., 2007), adhesion molecules linking the SSCs to basement membrane components such as laminin (Orwig et al., 2002), and stimuli from the vascular network and interstitial cells (Yoshida et al., 2007). Spermatogonial stem cells are located at the periphery of the seminiferous epithelium, in contact with the basement membrane and Sertoli cells. Because the Sertoli cells are distributed evenly along the basement membrane and the length of the seminiferous tubules, it is difficult to pinpoint a specific location for the stem cell niche. However, Yoshida et al. recently demonstrated that Apaired and Aaligned spermatogonia, which are directly derived from SSCs, develop preferentially in the area of the seminiferous tubules adjacent to the vasculature and interstitial cells (Yoshida et al., 2007). It has been hypothesized that the true SSCs are localized in the region of the tubules that is not associated with the vasculature, but when they divide, the daughter cell that is closer to the vasculature may differentiate (Shetty and Meistrich, 2007). Therefore, circulating factors or factors produce by interstitial cells might be crucial for SSC regulation.
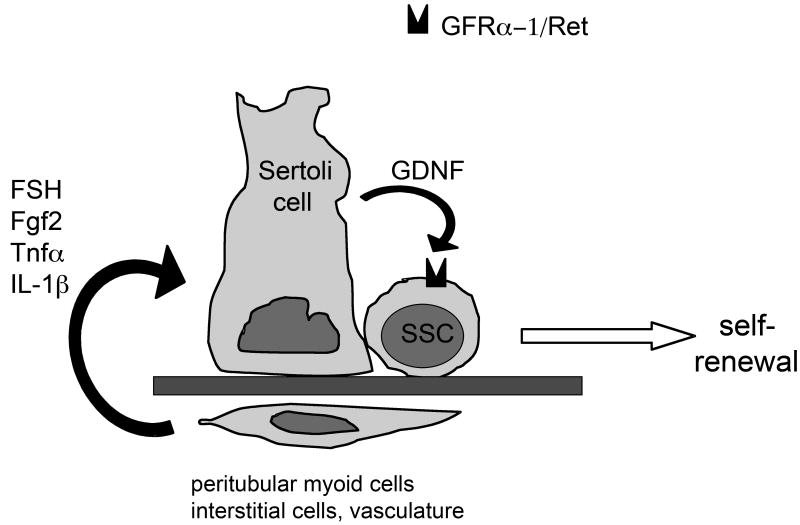
Our understanding of the mammalian spermatogonial stem cell niche has recently greatly progressed with the discovery that glial cell line-derived neurotrophic factor (Gdnf), a neurotropic factor secreted by glial cells in the brain, is also expressed in other organs during development, including ovary and testis (Moore et al., 1996; Hellmich et al., 1996; Trupp et al., 1995; Golden et al., 1999). Gdnf, secreted by Sertoli cells after birth, is so far the only known paracrine factor in the testis specifically responsible for the maintenance and self-renewal of SSCs in vivo and in vitro (Meng et al., 2000; Kubota et al., 2004b; Hofmann et al., 2005b). Other growth factors secreted by Sertoli cells, such as basic fibroblast growth factor (bFgf or Fgf2) and epidermal growth factor (Egf), while necessary for SSC proliferation in vitro (Kubota et al., 2004b), do not appear to be sufficient for self-renewal in vivo. In 2002, Tadokoro and colleagues established that the expression of Gdnf by Sertoli cells and the subsequent proliferation of SSCs were dependent on FSH (Tadokoro et al., 2002). The authors used male Sl/Sl d mutant mice, whose testes produce only undifferentiated type A spermatogonia, and abolished FSH stimulation of the Sertoli cells by injecting a gonadotropin-releasing hormone antagonist (Nal-Glu). As a result, Gdnf production in the testes of these mice was greatly decreased as well as the proliferation rate of undifferentiated spermatogonia. Conversely, primary cultures of Sertoli cells responded to FSH by an increase in Gdnf expression. Simon and colleagues recently confirmed this data by demonstrating that FSH increases the level of expression of Gdnf in the Sertoli cell line TM4 (Simon et al., 2007). In addition, Gdnf production by primary Sertoli cells is also dependent on Fgf2, tumor necrosis factor alpha (Tnfα) and interleukin-1beta (Il-1β) in vitro (Simon et al., 2007). Therefore, Gdnf production by Sertoli cells and SSCs self-renewal are controlled locally and systemically (Figure 3).
Figure 3. Regulation of GDNF expression in Sertoli cells.
In the mouse testis, the expression of GDNF by Sertoli cells is under control of FSH, FGF2, Tnfα and IL-1β, probably produced by interstitial cells. Therefore, SSCs self-renewal is controlled both systemically and locally.
2. Role of GDNF in the mammalian testis
Gdnf was originally identified in conditioned media of glioma cell line cultures (Lin et al., 1993). It promotes the survival and differentiation of dopaminergic neurons in midbrain embryonic cultures, without increasing the total number of neurons or astrocytes (Lin et al., 1993; Buj-Bello et al., 1995; Henderson et al., 1994). Gdnf also promotes the differentiation of several types of peripheral neurons (Zurn et al., 1996). Because it improves motor functions in animal models of parkinsonism, Gdnf has been seen as a promising therapy for Parkinson disease (Grondin and Gash, 1998). The role of Gdnf in embryonic development is not limited to the nervous system and it is also essential for kidney and gastrointestinal tract formation (Schuchardt et al., 1994; Moore et al., 1996; Hellmich et al., 1996). Cloning of Gdnf revealed that it is a distant member of the transforming growth factor beta (Tgf-β) superfamily (Lin et al., 1993). It signals through a multicomponent receptor complex comprising a glycosylphosphatidylinositol (GPI)-anchored cell surface molecule (Gdnf family receptor (Gfr) alpha-1, or Gfrα-1) and the Ret (rearranged during transfection) tyrosine kinase transmembrane protein (Jing et al., 1996; Treanor et al., 1996). The binding of Gdnf triggers the activation of multiple signaling pathways in responsive cells (Airaksinen and Saarma, 2002). As mentioned previously, Gdnf in the testis is produced by Sertoli cells, which establish the niche in which the SSCs reside (Viglietto et al., 2000; Meng et al., 2000; Meng et al., 2001a), and this activity extends throughout life (Chen et al., 2005). In Sertoli cells, the production of Gdnf is mainly under the influence of FSH, growth factors and cytokines (Tadokoro et al., 2002; Simon et al., 2007).
Mice lacking Gdnf die within the first day of birth with renal and neuronal abnormalities. The ureteric bud, kidney and enteric neurons are absent (Moore et al., 1996; Pichel et al., 1996; Sanchez et al., 1996). The Ret and Gfrα-1 receptors have also been knocked out and show the same phenotypes, indicating that Gdnf signals through the Gfrα-1/Ret-receptor complex and is essential for postnatal survival in the mouse (Schuchardt et al., 1994; Enomoto et al., 1998; Cacalano et al., 1998; Tomac et al., 2000). Interestingly, the testicular morphology of mice lacking Gdnf, Gfrα-1 and Ret is normal before birth. While Gdnf +/− mice survive to adulthood and are fertile, histological analysis of their testes has shown that spermatogenesis is disturbed (Meng et al., 2000). In particular, some seminiferous tubules are degenerated and contain spermatids in an abnormal position or phagocytosed by Sertoli cells. In older Gdnf +/- mice, the depletion of the germ cells often results in Sertoli cell–only seminiferous tubules without spermatogonia, while in other tubules the rate of spermatogonial proliferation is reduced. Since mice expressing one null allele for gdnf gradually lose their SSCs during testis development, one can conclude that Gdnf is an essential factor for spermatogonial stem cell maintenance.
As mentioned above, mice lacking Gdnf die neonatally, and therefore the effects of the loss of both alleles initially could not be determined. Similarly, the ablation of Ret or Gfrα-1 is neonatally lethal, preventing the analysis of a lack of receptor activation on SSC maintenance and self-renewal. To overcome the problem of neonatal mortality, Naughton and colleagues transplanted Gdnf, Gfrα-1 and Ret deficient neonatal testes under the back skin of immunodeficient mice, and subsequently monitored the development of the grafted testes (Naughton et al., 2006). This strategy revealed that any disruption of Gdnf-mediated Ret signaling results in a lack of spermatogonial stem cell self-renewal and induces the progressive loss of spermatogenesis by germ cell depletion. In comparison, normal spermatogenesis and maintenance of SSC populations was observed in the grafted WT testes. Thus, Gdnf, Ret and Gfra-1 are all crucial for SSC maintenance, emphasizing the essential role of the Gdnf/Ret/Gfra-1 signaling pathway in SSCs.
In 2000, Meng and colleagues over-expressed gdnf in transgenic mice under the testis-specific human translation elongation factor–1a (EF-1a) promoter (Abdallah et al., 1991; Meng et al., 2000). These mice accumulated undifferentiated spermatogonia in their seminiferous tubules, confirming the critical role of Gdnf for SSCs and their direct progeny, the Apaired spermatogonia. Mice over-expressing Gdnf are infertile and develop testicular tumors resembling seminoma in adulthood (Meng et al., 2001b). However the usefulness of these mice to study the etiology of these tumors is controversial since the precursor lesion seems different in mouse and man (Sariola and Meng, 2003). In summary, these results clearly established Gdnf as a growth factor produced by Sertoli cells, which is crucial for SSC self-renewal and maintenance within the testis stem cell niche.
Recent in vitro work has further emphasized the importance of Gdnf for SSC proliferation and self-renewal in culture. Spermatogonial stem cells have been difficult to isolate, due to their low number and the fact that none of the surface markers known so far is specific. Although expressed by both the stem cell and its direct progeny, the Apaired spermatogonia, Gfrα-1 is an adequate marker for purifying SSCs from testes using antibody selection, since the isolated cells successfully repopulate an infertile testis (Buageaw et al., 2005). Using gravity sedimentation on a BSA gradient (STAPUT method) followed by magnetic beads isolation with a Gfrα-1 antibody, we are able to isolate cell populations containing up to 98% SSCs (Hofmann et al., 2005b). However, while the purity is high, the number of cells recovered is low, and the method does not allow accurate protein investigation due to the paucity of the material recovered. Others have been successful at enriching SSCs using antibodies to Thy-1 (thymus cell antigen 1), producing an enrichment that was sufficient for most of their studies (Kubota et al., 2004a). In addition, SSCs can be expanded in long-term cultures, provided that they are grown onto feeder layers in the presence of Gdnf and other growth factors such as Egf, Fgf2 and the soluble form of the GFRα-1 receptor (Ogawa et al., 2003; Kubota et al., 2004b; Kanatsu-Shinohara et al., 2005). After expansion in culture for a period of 3-6 months, the cells were transplanted into an infertile testis and re-established spermatogenesis, confirming that SSCs maintain their ability to self-renew in vitro and that GDNF is a determining factor in this process.
3. Signaling pathways driven by Gdnf, and target molecules
3.1. Src signaling pathway
The functional receptor for Gdnf is the Ret tyrosine kinase (REarranged during Transfection) originally discovered by Takahashi and colleagues who showed a novel gene rearrangement and oncogenic activation in a transfection assay of NIH 3T3 fibroblasts with lymphoma DNA (Takahashi et al., 1985). Using serum-free short-term cultures of SSCs and a spermatogonial stem cell line (Hofmann et al., 2005a), our group recently elucidated some of the pathways induced by Gdnf in these cells. Spermatogonial stem cells express Gfrα-1 and Ret, and several kinases from the Src family co-precipitate with Ret after Gdnf stimulation (Braydich-Stolle et al., 2007). Four Src family kinases have been so far implicated in SSC proliferation through Ret activation: Src, Yes, Lyn and Fyn, which are all inhibited by the pharmacological inhibitor SU6656. Although the function of these kinases overlap, it is believed that Src and Yes play a predominant role in the immediate response of primary SSCs to Gdnf. Further, we demonstrated that Src activates a PI3K/Akt signaling pathway (Figure 4). Ultimately the Src-PI3K/Akt pathway leads to N-myc expression and promotes SSC proliferation. Thus, we identified a critical nuclear target of Gdnf/Ret signaling in SSCs (Figure 5). Subsequently, the groups of T. Shinohara and R. Brinster established the in vivo relevance of this signaling axis for SSC self-renewal by using germ cell transplantations after down-regulating Akt and Src expression by RNA interference or pharmacological inhibitors (Lee et al., 2007; Oatley et al., 2007).
Figure 4. GDNF/Src signaling in spermatogonial stem cells.
This model shows how GDNF can promote cell cycle progression via activation of Src kinase(s) and a PI3K/Akt pathway to increase N-myc gene expression. All 4 kinases depicted are inhibited by SU6656 and are involved in spermatogonial proliferation. p60-Src and c-Yes are necessary for the early response to GDNF, while the addition of Lyn and Fyn might be important for proliferation associated with differentiation. From Braydich-Stolle et al, Dev Biol, 2007 (Braydich-Stolle et al., 2007).
Figure 5. Immunocytochemistry study showing the up-regulation of nuclear N-Myc expression after stimulation by GDNF.
a: C18-4 spermatogonial stem cell line with 10% Nu serum in the culture media, negative control (no first antibody); b: C18-4 cells with 10% Nu serum in the culture media, showing a weak, basal expression of N-Myc; c: C18-4 cells with 10% Nu serum and 100 ng/ml GDNF in the culture media, showing an increase in staining intensity for N-Myc in comparison to the basal expression; d: cluster of primary spermatogonial stem cells grown for 3 days in presence of GDNF (100 ng/ml) and showing strong up-regulation of N-myc only in some cells (arrows). Because GDNF activates N-Myc expression in a subpopulation of undifferentiated spermatogonia, it suggests that N-Myc activation is relevant for spermatogonial stem cell self-renewal only. From Braydich-Stolle et al, Dev Biol, 2007 (Braydich-Stolle et al., 2007).
3.2. Ras signaling pathway
The Ret receptor is a tyrosine kinase transmembrane receptor that, in response to Gdnf activation, autophosphorylates at many tyrosine residues on its intracellular domain. These residues can serve as intracellular docking sites to many different SH2 domain-containing proteins, including Src. While Src interacts with Ret at Tyr 981 (Encinas et al., 2004), Tyr 1062 serves as a docking site to most other effectors and triggers the activation of the Ras signaling pathway in the developing enteric nervous system, the developing kidney, and also in neuroblastoma (Worby et al., 1996; Jijiwa et al., 2004; Hayashi et al., 2000). Therefore, we examined whether Gdnf can activate Ras signaling in SSCs (He et al., 2008). Our data demonstrated that Ret activation by Gdnf induces the binding and activation of the protein adaptors Shc and Grb2 in these cells. Further, we showed that Gdnf rapidly and transiently activates the Ras/ERK1/2 pathway, which ultimately leads to the phosphorylation and activation of transcription factors such as Creb-1, Atf-1, and Crem-1. Finally, the Gdnf/Ret/Ras axis up-regulates the transcription of the immediate-early gene c-fos, the cell cycle activator cyclin A, as well as cdk2 (Figure 6). Cyclin A is a key regulatory protein that is involved in control of the S phase of the cell cycle and associates with Cdk2 in mammalian cells (Cardoso et al., 1993). In addition, the A-type cyclins are predominantly expressed during spermatogenesis (Sweeney et al., 1996). Cyclin A1 is highly expressed in pachytene spermatocytes and required for meiosis, and Cyclin A2 is mostly present in type A spermatogonia, including the SSCs (Ravnik and Wolgemuth, 1996; Ravnik and Wolgemuth, 1999). Therefore, like in other cell types, Creb and c-Fos enhance the expression of cyclin A and favors the G1/S cell cycle transition in Gfrα-1 positive spermatogonia (Desdouets et al., 1995; Sunters et al., 2004). Taken together, our studies of the effects of Gdnf on SSCs indicate that multiple signaling pathways are responsible for their self-renewal and maintenance.
Figure 6. GDNF/Ras signaling in spermatogonial stem cells.
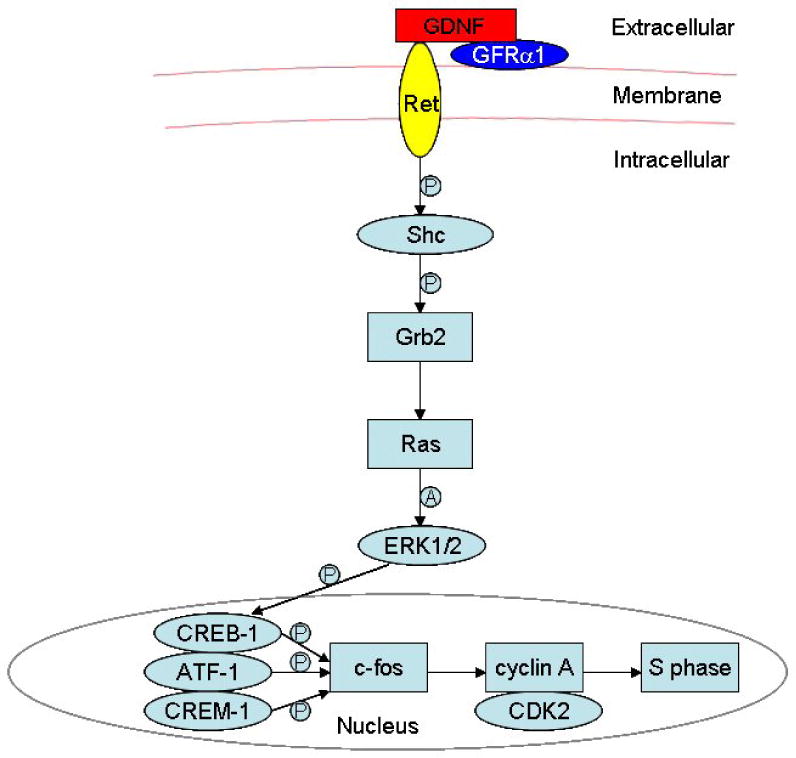
The schematic diagram demonstrates intracellular signaling events in the Ras/ERK1/2 pathway as well as the downstream cascades activated by GDNF in spermatogonial stem cells. “P” indicates “phosphorylate”, and “A” denotes “activate”. From He et al, Stem Cells, 2008 (He et al., 2008).
3.3. Other Gdnf target molecules
In order to establish which genes are differentially regulated by Gdnf, we recently performed microarray analysis of spermatogonial stem cells under the influence of Gdnf in vitro (Hofmann et al., 2005b). We identified components of two additional signaling pathways that are modulated by Gdnf. Numb, a regulator of the Notch pathway, was up-regulated by Gdnf (Braydich-Stolle et al., 2005). Since Notch signaling is involved in germ cell differentiation (Hayashi et al., 2001; Hayashi et al., 2004), down-regulation of this pathway by Gdnf could enhance self-renewal by suppressing differentiation signals. However, the effect of Gdnf on Numb expression was not confirmed in a subsequent microarray study that also attempted to identify Gdnf targets in SSCs (Oatley et al., 2006), and the reason for this difference is not clear. We also found that Gdnf was able to up-regulate the expression of fibroblast growth factor receptor-2 (Fgfr2). This suggests that Gdnf might increase the responsiveness of SSCs to Fgf2, and that a cooperation of both growth factors is essential for their proliferation in vitro (Hofmann et al., 2005b). Fgf2 amplifies the effects of GDNF, while the addition of other growth factors does not further stimulate proliferation, indicating that the combination Gdnf/Fgf2 is the limiting factor. Fgfr2 is also expressed by human spermatogonial stem cells and may play a role analogous to Ret in regulating their clonal expansion and fate (Goriely et al., 2003). Gdnf and Fgf2 are part of a cocktail of factors used recently by several investigators to establish long-term cultures of gonocytes and spermatogonial stem cells (Kanatsu-Shinohara et al., 2003; Kubota et al., 2004b). Thus, these findings confirm that the cooperation between Gdnf and Fgf2 is likely essential to maintain spermatogonial stem cell self-renewal.
B cell CLL/lymphoma 6, member B (Bcl6b) is another target of Gdnf that was recently identified in SSCs by microarray analysis (Oatley et al., 2006). Bcl6b is a member of the POZ (poxvirus and zinc finger) family of transcriptional repressors. Inhibition of Bcl6b in SSCs in vitro by RNA interference indicated that this protein has a critical role in SSC maintenance. Furthermore, like N-Myc, Bcl6b is a nuclear target of the Src signaling pathway (Oatley et al., 2007). In addition, mice with a targeted disruption of Bcl6b have an increased incidence of Sertoli cell-only tubules, confirming the in vitro results and the role of this protein for SSC maintenance.
4. Etv5 and other transcription factors
Several knockouts targeting transcription factors have recently resulted in mice exhibiting a progressive loss of germ cells leading to adult infertility (Chen et al., 2005; Falender et al., 2005; Buaas et al., 2004; Costoya et al., 2004). One of these factors, Etv5 (Ets Variant Gene 5), belongs to the Pea3 group of the Ets family of proteins, which are characterized by a highly conserved DNA-binding ETS domain (Sharrocks et al., 1997). Etv5 is expressed in numerous developing organs of different species (Raible and Brand, 2001; Paratore et al., 2002; Liu et al., 2003; Ouyang et al., 1999), and is over-expressed in several human tumors, in particular breast and endometrial carcinoma (Chotteau-Lelievre et al., 2004; Planaguma et al., 2005). Mice with a targeted disruption of Etv5 (Etv5-/-) undergo a first wave of spermatogenesis during juvenile life, but show a progressive loss of spermatogenesis, with disappearance of all germ cells and a Sertoli cell-only phenotype by 10 weeks of age (Chen et al., 2005). The initial testicular development in Etv5-/- mice is similar to that of the wild type, indicating that the major effects of Etv5 deficiency occur postnatally (Schlesser et al., 2007). Etv5 is expressed in Sertoli cells and germ cells (Chen et al., 2005; Oatley et al., 2007), but its exact mode of action in both cell types is still unknown. Microarray and RT-PCR analysis of Etv5-/-Sertoli cells detected a 9- to 25-fold reduction in several chemokines and a tenfold reduction in matrix metalloproteinase-12 (MMP-12) expression. Therefore, Etv5 in Sertoli cells might be responsible for attracting and maintaining the stem cells inside their niches, in addition to balance their self-renewal and differentiation. This phenotype provided the first evidence that a single transcription factor can regulate a stem cell niche in a vertebrate animal. Etv5 expression by Sertoli cells is up-regulated by Fgf2 and Egf, but interestingly, neither testosterone nor FSH seem to have any influence on this process, at least in vitro (Simon et al., 2007). Therefore, Etv5 expression might be regulated exclusively by local soluble factors (Figure 7).
Figure 7. Regulation of Etv5 expression in Sertoli cells.
In the mouse testis, the expression of the transcription factor Etv5 in Sertoli cells is under the control of Fgf2 and Egf, probably produced by interstitial cells. Genes regulated by Etv5 might include chemokines important for SSC homing.
Another transcription factor that regulates SSC self-renewal is Taf4b. Taf4b is a component of the RNA Polymerase II basal transcription apparatus and is germ cell specific (Falender et al., 2005). In the mouse, Taf4b is expressed in gonocytes in the postnatal testes, and in spermatogonia and spermatids in the adult testes. It is not expressed in Sertoli cells. Targeted disruption of taf4b induces a phenotype similar to the etv5-/-phenotype, including normal testis histology at birth, the completion of the first wave of spermatogenesis and transient fertility. However, the SSCs start to disappear at soon as 3 days after birth, which leads to a progressive loss of the germ cells, the appearance of Sertoli cell-only seminiferous tubules and testicular atrophy after 12 weeks. The deficiency produced in the taf4b knockout testis reflects a block that is cell-autonomous and inherent to germ cells, since the taf4b -/- Sertoli cells support spermatogenesis of transplanted wild type spermatogonia (Falender et al., 2005).
Plzf (Promyelocytic Leukaemia Zinc-Finger) is another SSC-intrinsic factor crucial for self-renewal. It is a transcriptional repressor that inhibits stem cell differentiation and helps maintaining their presence in the niche. Plzf was originally identified in hematopoietic stem cells, but is also crucial for the patterning of the axial skeleton and the limbs (Reid et al., 1995; Barna et al., 2000). Plzf expression is high in undifferentiated multipotential hematopoietic progenitor cells, and low in differentiated cells (Reid et al., 1995). Plzf is crucial for the regulation of SSC self-renewal since both the naturally occurring mutant lacking plzf (Luxoid) and plzf knockout mice lose progressively their spematogonia as they age (Buaas et al., 2004; Costoya et al., 2004). Because Plzf in the WT animals represses SSC differentiation, its loss in the Luxoid mutants or knockout mice shifts the balance toward differentiation at the cost of self-renewal. In addition, germ cells of the testes of the Luxoid mutants are not able to repopulate the testes of recipient W/Wv mice, suggesting that the defect is inherent to the stem cells. Interestingly, Plzf directly represses the transcription of the receptor c-kit, which is characteristic of spermatogonial differentiation (Filipponi et al., 2007). Also, undifferentiated spermatogonia isolated from plzf -/- mice exhibit a marked increase in c-kit expression. Therefore, Plzf might maintain the pool of spermatogonial stem cells through direct repression of c-kit expression.
5. Conclusion
The spermatogonial stem cell niche is the product of the combined effects of extrinsic factors that maintain the balance between stem cell self-renewal and differentiation in the basal part of the seminiferous epithelium. These extrinsic factors modulate intrinsic factors such as kinases, second messengers and transcription factors that ultimately control the behavior of the stem cells. Many of these signals and cross-talk between the somatic cells and stem cells are still unknown, although it is evident that some, like GDNF, have essential roles in embryonic development and are not restricted to the testis. In addition, recent work has demonstrated that the decline in spermatogenesis with aging reflects a degradation of the stem cell niche, rather than defects inherent to the stem cells (Ryu et al., 2006). Therefore, a thorough understanding of the niche and its experimental modulation may help devise new treatments for male infertility or possibly lead to the development of novel male contraceptives.
Acknowledgments
The author is supported by NIH grants R01-HD044543 and K02-HD054607
List of abbreviations
- Akt
thymoma viral proto-oncogene 1
- ATF
activating transcription factor
- Bcl6b
B-cell CLL/lymphoma 6, member B
- Cdk2
cyclin-dependent kinase 2
- Creb
cAMP responsive element binding protein
- Crem
cAMP responsive element modulator
- Egf
epidermal growth factor
- Erk
Mapk3, mitogen-activated protein kinase 3
- Ets family
erythroblastosis virus E26 family
- Etv5
ets variant gene 5
- Fgf2
(basic) fibroblast growth factor
- FSH
follicule-stimulating hormone
- Gdnf
glial cell line-derived neurotrophic factor
- Gfrα-1
glial cell line-derived neurotrophic factor family receptor alpha 1
- GPI
glycosylphosphatidylinositol
- Il-1β
interleukin-1 beta
- N-Myc
myelocytomatosis protooncogene, neural tissue
- Pea3
polyoma virus enhancer activator 3
- PI3K
phosphatidylinositol 3-kinase
- Plzf
promyelocytic leukemia zinc finger protein
- POZ domain
poxvirus and zinc finger domain
- Ras
Rat sarcoma virus protooncogene
- Ret
Ret (rearranged during transfection) protooncogene
- SH2 domain
Src homology 2 domain
- SSC
spermatogonial stem cell
- Taf4B
TATA box-binding protein (TBP)-associated factor, subunit 4B
- Tgf-β
transforming growth factor-beta
- Tnfα
tumor necrosis factor alpha
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This PDF receipt will only be used as the basis for generating PubMed Central (PMC) documents. PMC documents will be made available for review after conversion (approx. 2-3 weeks time). Any corrections that need to be made will be done at that time. No materials will be released to PMC without the approval of an author. Only the PMC documents will appear on PubMed Central -- this PDF Receipt will not appear on PubMed Central.
References
- Abdallah B, Hourdry J, Krieg P, Denis H, Mazabraud A. Germ cell-specific expression of a gene encoding eukaryotic translation elongation factor 1 alpha (eEF-1 alpha) and generation of eEF-1 alpha retropseudogenes in Xenopus laevis. Proc Natl Acad Sci U S A. 1991;88:9277–9281. doi: 10.1073/pnas.88.20.9277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airaksinen M, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- Barna M, Hawe N, Niswander L, Pandolfi P. Plzf regulates limb and axial skeletal patterning. Nat Genet. 2000;25:166–172. doi: 10.1038/76014. [DOI] [PubMed] [Google Scholar]
- Braydich-Stolle L, Kostereva N, Dym M, Hofmann M. Role of Src family kinases and N-Myc in spermatogonial stem cell proliferation. Dev Biol. 2007;304:34–45. doi: 10.1016/j.ydbio.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braydich-Stolle L, Nolan C, Dym M, Hofmann M. Role of glial cell line-derived neurotrophic factor in germ-line stem cell fate. Ann N Y Acad Sci. 2005;1061:94–99. doi: 10.1196/annals.1336.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster R. Germline stem cell transplantation and transgenesis. Science. 2002;296:2174–2176. doi: 10.1126/science.1071607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buaas F, Kirsh A, Sharma M, Mclean DJ, Morris J, Griswold M, De Rooij DG, Braun R. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet. 2004;36:647–652. doi: 10.1038/ng1366. [DOI] [PubMed] [Google Scholar]
- Buageaw A, Sukhwani M, Ben-Yehudah A, Ehmcke J, Rawe V, Pholpramool C, Orwig K, Schlatt S. GDNF family receptor alpha1 phenotype of spermatogonial stem cells in immature mouse testes. Biol Reprod. 2005;73:1011–1016. doi: 10.1095/biolreprod.105.043810. [DOI] [PubMed] [Google Scholar]
- Buj-Bello A, Buchman V, Horton A, Rosenthal A, Davies A. GDNF is an age-specific survival factor for sensory and autonomic neurons. Neuron. 1995;15:821–828. doi: 10.1016/0896-6273(95)90173-6. [DOI] [PubMed] [Google Scholar]
- Cacalano G, Farinas I, Wang L, Hagler K, Forgie A, Moore M, Armanini M, Phillips H, Ryan A, Reichardt L, Hynes M, Davies A, Rosenthal A. GFRalpha1 is an essential receptor component for GDNF in the developing nervous system and kidney. Neuron. 1998;21:53–62. doi: 10.1016/s0896-6273(00)80514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso M, Leonhardt H, Nadal-Ginard B. Reversal of terminal differentiation and control of DNA replication: cyclin A and Cdk2 specifically localize at subnuclear sites of DNA replication. Cell. 1993;74:979–992. doi: 10.1016/0092-8674(93)90721-2. [DOI] [PubMed] [Google Scholar]
- Chen C, Ouyang W, Grigura V, Zhou Q, Carnes K, Lim H, Zhao G, Arber S, Kurpios N, Murphy T, Cheng A, Hassell J, Chandrashekar V, Hofmann M, Hess R, Murphy KM. ERM is required for transcriptional control of the spermatogonial stem cell niche. Nature. 2005;436:1030–1034. doi: 10.1038/nature03894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotteau-Lelievre A, Revillion F, Lhotellier V, Hornez L, Desbiens X, Cabaret V, De Launoit Y, Peyrat J. Prognostic value of ERM gene expression in human primary breast cancers. Clin Cancer Res. 2004;10:7297–7303. doi: 10.1158/1078-0432.CCR-04-0593. [DOI] [PubMed] [Google Scholar]
- Costoya JA, Hobbs R, Barna M, Cattoretti G, Manova K, Sukhwani M, Orwig K, Wolgemuth D, Pandolfi P. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat Genet. 2004;36:653–659. doi: 10.1038/ng1367. [DOI] [PubMed] [Google Scholar]
- De Rooij DG, Russell L. All you wanted to know about spermatogonia but were afraid to ask. J Androl. 2000;21:776–798. [PubMed] [Google Scholar]
- Desdouets C, Matesic G, Molina C, Foulkes N, Sassone-Corsi P, Brechot C, Sobczak-Thepot J. Cell cycle regulation of cyclin A gene expression by the cyclic AMP-responsive transcription factors CREB and CREM. Mol Cell Biol. 1995;15:3301–3309. doi: 10.1128/mcb.15.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dym M. Spermatogonial stem cells of the testis [comment] Proc Natl Acad Sci U S A. 1994;91:11287–11289. doi: 10.1073/pnas.91.24.11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dym M, Fawcett D. Further observations on the numbers of spermatogonia, spermatocytes, and spermatids connected by intercellular bridges in the mammalian testis. Biol Reprod. 1971;4:195–215. doi: 10.1093/biolreprod/4.2.195. [DOI] [PubMed] [Google Scholar]
- Encinas M, Crowder RJ, Milbrandt J, Johnson EJ. Tyrosine 981, a novel ret autophosphorylation site, binds c-Src to mediate neuronal survival. J Biol Chem. 2004;279:18262–18269. doi: 10.1074/jbc.M400505200. [DOI] [PubMed] [Google Scholar]
- Enomoto H, Araki T, Jackman A, Heuckeroth R, Snider WD, Johnson EJ, Milbrandt J. GFR alpha1-deficient mice have deficits in the enteric nervous system and kidneys. Neuron. 1998;21:317–324. doi: 10.1016/s0896-6273(00)80541-3. [DOI] [PubMed] [Google Scholar]
- Falender A, Freiman RN, Geles K, Lo K, Hwang K, Lamb D, Morris P, Tjian R, Richards J. Maintenance of spermatogenesis requires TAF4b, a gonad-specific subunit of TFIID. Genes Dev. 2005;19:794–803. doi: 10.1101/gad.1290105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipponi D, Hobbs R, Ottolenghi S, Rossi P, Jannini EA, Pandolfi P, Dolci S. Repression of kit expression by Plzf in germ cells. Mol Cell Biol. 2007;27:6770–6781. doi: 10.1128/MCB.00479-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller M. Genetic control of cell proliferation and differentiation in Drosophila spermatogenesis. Semin Cell Dev Biol. 1998;9:433–444. doi: 10.1006/scdb.1998.0227. [DOI] [PubMed] [Google Scholar]
- Golden J, Demaro JA, Osborne PA, Milbrandt J, Johnson EJ. Expression of neurturin, GDNF, and GDNF family-receptor mRNA in the developing and mature mouse. Exp Neurol. 1999;158:504–528. doi: 10.1006/exnr.1999.7127. [DOI] [PubMed] [Google Scholar]
- Goriely A, Mcvean G, Rojmyr M, Ingemarsson B, Wilkie AO. Evidence for selective advantage of pathogenic FGFR2 mutations in the male germ line. Science. 2003;301:643–646. doi: 10.1126/science.1085710. [DOI] [PubMed] [Google Scholar]
- Grondin R, Gash D. Glial cell line-derived neurotrophic factor (GDNF): a drug candidate for the treatment of Parkinson's disease. J Neurol. 1998;245:P35–42. doi: 10.1007/pl00007744. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Ichihara M, Iwashita T, Murakami H, Shimono Y, Kawai K, Kurokawa K, Murakumo Y, Imai T, Funahashi H, Nakao A, Takahashi M. Characterization of intracellular signals via tyrosine 1062 in RET activated by glial cell line-derived neurotrophic factor. Oncogene. 2000;19:4469–4475. doi: 10.1038/sj.onc.1203799. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Kageyama Y, Ishizaka K, Xia G, Kihara K, Oshima H. Requirement of Notch 1 and its ligand jagged 2 expressions for spermatogenesis in rat and human testes. J Androl. 2001;22:999–1011. doi: 10.1002/j.1939-4640.2001.tb03441.x. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Yamada T, Kageyama Y, Negishi T, Kihara K. Expression failure of the Notch signaling system is associated with the pathogenesis of maturation arrest in male infertility patients. Fertil Steril. 2004;81(3):697–699. doi: 10.1016/j.fertnstert.2003.08.026. [DOI] [PubMed] [Google Scholar]
- He Z, Jiang J, Kokkinaki M, Golestaneh N, Hofmann M, Dym M. GDNF Up-regulates c-fos Transcription via the Ras/ERK1/2 Pathway to Promote Mouse Spermatogonial Stem Cell Proliferation. Stem Cells. 2008;26:266–278. doi: 10.1634/stemcells.2007-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmich H, Kos L, Cho E, Mahon K, Zimmer A. Embryonic expression of glial cell-line derived neurotrophic factor (GDNF) suggests multiple developmental roles in neural differentiation and epithelial-mesenchymal interactions. Mech Dev. 1996;54:95–105. doi: 10.1016/0925-4773(95)00464-5. [DOI] [PubMed] [Google Scholar]
- Henderson C, Phillips H, Pollock R, Davies AM, Lemeulle C, Armanini M, Simmons L, Moffet B, Vandlen R, Simpson L et al. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 1994;266:1062–1064. doi: 10.1126/science.7973664. . C. T. S. L. [DOI] [PubMed] [Google Scholar]
- Hofmann MC, Braydich-Stolle L, Dym M. Isolation of male germ-line stem cells; influence of GDNF. Dev Biol. 2005;279:114–124. doi: 10.1016/j.ydbio.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckins C. The spermatogonial stem cell population in adult rats. I. Their morphology, proliferation and maturation. Anat Rec. 1971;169:533–557. doi: 10.1002/ar.1091690306. [DOI] [PubMed] [Google Scholar]
- Huckins C, Oakberg E. Morphological and quantitative analysis of spermatogonia in mouse testes using whole mounted seminiferous tubules, I. The normal testes. Anat Rec. 1978;192:519–528. doi: 10.1002/ar.1091920406. [DOI] [PubMed] [Google Scholar]
- Jijiwa M, Fukuda T, Kawai K, Nakamura A, Kurokawa K, Murakumo Y, Ichihara M, Takahashi M. A targeting mutation of tyrosine 1062 in Ret causes a marked decrease of enteric neurons and renal hypoplasia. Mol Cell Biol. 2004;24:8026–8036. doi: 10.1128/MCB.24.18.8026-8036.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing S, Wen D, Yu Y, Holst P, Luo Y, Fang M, Tamir R, Antonio L, Hu Z, Cupples R, Louis J, Hu S, Altrock BW, Fox G. GDNF-induced activation of the ret protein tyrosine kinase is mediated by GDNFR-alpha, a novel receptor for GDNF. Cell. 1996;85:1113–1124. doi: 10.1016/s0092-8674(00)81311-2. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Miki H, Inoue K, Ogonuki N, Toyokuni S, Ogura A, Shinohara T. Long-term culture of mouse male germline stem cells under serum-or feeder-free conditions. Biol Reprod. 2005;72:985–991. doi: 10.1095/biolreprod.104.036400. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, Shinohara T. Long-Term Proliferation in Culture and Germline Transmission of Mouse Male Germline Stem Cells. Biol Reprod. 2003;69:612–616. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- Kiger A, Jones D, Schulz C, Rogers M, Fuller M. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science. 2001;294:2542–2545. doi: 10.1126/science.1066707. [DOI] [PubMed] [Google Scholar]
- Kiger A, White-Cooper H, Fuller MT. Somatic support cells restrict germline stem cell self-renewal and promote differentiation. Nature. 2000;407:750–754. doi: 10.1038/35037606. [DOI] [PubMed] [Google Scholar]
- Kiger A, Fuller MT. Male germline stem cells. In: Marshak DR, Gardner RK, Gottlieb D, editors. Stem Cell Biology. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2001. pp. 149–188. [Google Scholar]
- Kubota H, Avarbock M, Brinster R. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci U S A. 2004b;101:16489–16494. doi: 10.1073/pnas.0407063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota H, Avarbock M, Brinster R. Culture conditions and single growth factors affect fate determination of mouse spermatogonial stem cells. Biol Reprod. 2004a;71:722–731. doi: 10.1095/biolreprod.104.029207. [DOI] [PubMed] [Google Scholar]
- Lee J, Kanatsu-Shinohara M, Inoue K, Ogonuki N, Miki H, Toyokuni S, Kimura T, Nakano T, Ogura A, Shinohara T. Akt mediates self-renewal division of mouse spermatogonial stem cells. Development. 2007;134:1853–1859. doi: 10.1242/dev.003004. [DOI] [PubMed] [Google Scholar]
- Li L, Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol. 2005;21:605–631. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- Lin L, Doherty D, Lile J, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Liu Y, Jiang H, Crawford H, Hogan B. Role for ETS domain transcription factors Pea3/Erm in mouse lung development. Dev Biol. 2003;261:10–24. doi: 10.1016/s0012-1606(03)00359-2. [DOI] [PubMed] [Google Scholar]
- Meng X, De Rooij DG, Westerdahl K, Saarma M, Sariola H. Promotion of seminomatous tumors by targeted overexpression of glial cell line-derived neurotrophic factor in mouse testis. Cancer Res. 2001b;61:3267–3271. [PubMed] [Google Scholar]
- Meng X, Lindahl M, Hyvonen M, Parvinen M, De Rooij DG, Hess M, Raatikainen-Ahokas A, Sainio K, Rauvala H, Lakso M, Pichel JG, Westphal H, Saarma M, Sariola H. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489–1493. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- Meng X, Pata I, Pedrono E, Popsueva A, De Rooij DG, Janne M, Rauvala H, Sariola H. Transient disruption of spermatogenesis by deregulated expression of neurturin in testis. Mol Cell Endocrinol. 2001a;184:33–39. doi: 10.1016/s0303-7207(01)00649-9. [DOI] [PubMed] [Google Scholar]
- Moore M, Klein R, Farinas I, Sauer H, Armanini M, Phillips H, Reichardt L, Ryan A, Carver-Moore K, Rosenthal A. Renal and neuronal abnormalities in mice lacking. GDNF Nature. 1996;382:76–79. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- Naughton C, Jain S, Strickland A, Gupta A, Milbrandt J. Glial Cell-Line Derived Neurotrophic Factor (GDNF)-Mediated RET Signaling Regulates Spermatogonial Stem Cell Fate. Biol Reprod. 2006;74:314–321. doi: 10.1095/biolreprod.105.047365. [DOI] [PubMed] [Google Scholar]
- Oakberg E. Spermatogonial stem-cell renewal in the mouse. Anat Rec. 1971;169:515–531. doi: 10.1002/ar.1091690305. [DOI] [PubMed] [Google Scholar]
- Oatley J, Avarbock M, Brinster R. Glial cell line-derived neurotrophic factor regulation of genes essential for self-renewal of mouse spermatogonial stem cells is dependent on Src family kinase signaling. J Biol Chem. 2007;282:25842–25851. doi: 10.1074/jbc.M703474200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatley J, Avarbock M, Telaranta A, Fearon D, Brinster R. Identifying genes important for spermatogonial stem cell self-renewal and survival. Proc Natl Acad Sci U S A. 2006;103:9524–9529. doi: 10.1073/pnas.0603332103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Ohmura M, Yumura Y, Sawada H, Kubota Y. Expansion of murine spermatogonial stem cells through serial transplantation. Biol Reprod. 2003;68:316–322. doi: 10.1095/biolreprod.102.004549. [DOI] [PubMed] [Google Scholar]
- Orwig K, Shinohara T, Avarbock M, Brinster R. Functional analysis of stem cells in the adult rat testis. Biol Reprod. 2002;66:944–949. doi: 10.1095/biolreprod66.4.944. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Jacobson N, Bhattacharya D, Gorham JD, Fenoglio D, Sha W, Murphy T, Murphy KM. The Ets transcription factor ERM is Th1-specific and induced by IL-12 through a Stat4-dependent pathway. Proc Natl Acad Sci U S A. 1999;96:3888–3893. doi: 10.1073/pnas.96.7.3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paratore C, Brugnoli G, Lee H, Suter U, Sommer L. The role of the Ets domain transcription factor Erm in modulating differentiation of neural crest stem cells. Dev Biol. 2002;250:168–180. doi: 10.1006/dbio.2002.0795. [DOI] [PubMed] [Google Scholar]
- Pichel J, Shen L, Sheng H, Granholm A, Drago J, Grinberg A, Lee E, Huang S, Saarma M, Hoffer BJ, Sariola H, Westphal H. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382:73–76. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- Planaguma J, Abal M, Gil-Moreno A, Diaz-Fuertes M, Monge M, Garcia A, Baro T, Xercavins J, Reventos J, Alameda F. Up-regulation of ERM/ETV5 correlates with the degree of myometrial infiltration in endometrioid endometrial carcinoma. J Pathol. 2005;207:422–429. doi: 10.1002/path.1853. [DOI] [PubMed] [Google Scholar]
- Raible F, Brand M. Tight transcriptional control of the ETS domain factors Erm and Pea3 by Fgf signaling during early zebrafish development. Mech Dev. 2001;107:105–117. doi: 10.1016/s0925-4773(01)00456-7. [DOI] [PubMed] [Google Scholar]
- Ravnik S, Wolgemuth D. The developmentally restricted pattern of expression in the male germ line of a murine cyclin A, cyclin A2, suggests roles in both mitotic and meiotic cell cycles. Dev Biol. 1996;173:69–78. doi: 10.1006/dbio.1996.0007. [DOI] [PubMed] [Google Scholar]
- Ravnik S, Wolgemuth D. Regulation of meiosis during mammalian spermatogenesis: the A-type cyclins and their associated cyclin-dependent kinases are differentially expressed in the germ-cell lineage. Dev Biol. 1999;207:408–418. doi: 10.1006/dbio.1998.9156. [DOI] [PubMed] [Google Scholar]
- Reid A, Gould A, Brand N, Cook M, Strutt P, Li J, Licht J, Waxman S, Krumlauf R, Zelent A. Leukemia translocation gene, PLZF, is expressed with a speckled nuclear pattern in early hematopoietic progenitors. Blood. 1995;86:4544–4552. [PubMed] [Google Scholar]
- Russell LD. Mammalian spermatogenesis. In: Russell LD, Ettlin RA, Hikim AP, Clegg ED, editors. Histological and Histopathological Evaluation of the Testis. Cache River Press; Clearwater, FL: 1990. pp. 119–161. [Google Scholar]
- Ryu B, Orwig K, Oatley J, Avarbock M, Brinster R. Effects of aging and niche microenvironment on spermatogonial stem cell self-renewal. Stem Cells. 2006;24:1505–1511. doi: 10.1634/stemcells.2005-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez M, Silos-Santiago I, Frisen J, He B, Lira S, Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking. GDNF Nature. 1996;382:70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- Sariola H, Meng X. GDNF-induced seminomatous tumours in mouse--an experimental model for human seminomas? APMIS. 2003;111:192–6. doi: 10.1034/j.1600-0463.2003.11101231.x. discussion 196. [DOI] [PubMed] [Google Scholar]
- Scadden D. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- Schlesser H, Simon L, Hofmann M, Murphy KM, Murphy T, Hess R, Cooke P. Effects of ETV5 (Ets Variant Gene 5) on Testis and Body Growth, Time Course of Spermatogonial Stem Cell Loss, and Fertility in Mice. Biol Reprod. 2007 doi: 10.1095/biolreprod.107.062935. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- Schuchardt A, D'agati V, Larsson-Blomberg L, Costantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367:380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- Sharrocks A, Brown A, Ling Y, Yates P. The ETS-domain transcription factor family. Int J Biochem Cell Biol. 1997;29:1371–1387. doi: 10.1016/s1357-2725(97)00086-1. [DOI] [PubMed] [Google Scholar]
- Shetty G, Meistrich M. The missing niche for spermatogonial stem cells: Do blood vessels point the way? Cell Stem Cell. 2007;1:361–363. doi: 10.1016/j.stem.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Simon L, Ekman G, Tyagi G, Hess R, Murphy KM, Cooke P. Common and distinct factors regulate expression of mRNA for ETV5 and GDNF, Sertoli cell proteins essential for spermatogonial stem cell maintenance. Exp Cell Res. 2007;313:3090–3099. doi: 10.1016/j.yexcr.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Sunters A, Thomas DP, Yeudall W, Grigoriadis A. Accelerated cell cycle progression in osteoblasts overexpressing the c-fos proto-oncogene: induction of cyclin A and enhanced CDK2 activity. J Biol Chem. 2004;279:9882–9891. doi: 10.1074/jbc.M310184200. [DOI] [PubMed] [Google Scholar]
- Sweeney C, Murphy M, Kubelka M, Ravnik SE, Hawkins C, Wolgemuth D, Carrington M. A distinct cyclin A is expressed in germ cells in the mouse. Development. 1996;122:53–64. doi: 10.1242/dev.122.1.53. [DOI] [PubMed] [Google Scholar]
- Tadokoro Y, Yomogida K, Ohta H, Tohda A, Nishimune Y. Homeostatic regulation of germinal stem cell proliferation by the GDNF/FSH pathway. Mech Dev. 2002;113:29–39. doi: 10.1016/s0925-4773(02)00004-7. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Ritz J, Cooper G. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell. 1985;42:581–588. doi: 10.1016/0092-8674(85)90115-1. [DOI] [PubMed] [Google Scholar]
- Tomac A, Grinberg A, Huang S, Nosrat C, Wang Y, Borlongan C, Lin S, Chiang Y, Olson L, Westphal H, Hoffer B. Glial cell line-derived neurotrophic factor receptor alpha1 availability regulates glial cell line-derived neurotrophic factor signaling: evidence from mice carrying one or two mutated alleles. Neuroscience. 2000;95:1011–1023. doi: 10.1016/s0306-4522(99)00503-5. [DOI] [PubMed] [Google Scholar]
- Treanor J, Goodman L, De SF, Stone D, Poulsen K, Beck C, Gray C, Armanini M, Pollock R, Hefti F, Phillips H, Goddard A, Moore M, Buj-Bello A, Davies A, Asai N, Takahashi M, Vandlen R, Henderson C, Rosenthal A. Characterization of a multicomponent receptor for GDNF. Nature. 1996;382:80–83. doi: 10.1038/382080a0. [DOI] [PubMed] [Google Scholar]
- Trupp M, Ryden M, Jornvall H, Funakoshi H, Timmusk T, Arenas E, Ibanez C. Peripheral expression and biological activities of GDNF, a new neurotrophic factor for avian and mammalian peripheral neurons. J Cell Biol. 1995;130:137–148. doi: 10.1083/jcb.130.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulina N, Matunis E. Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling. Science. 2001;294:2546–2549. doi: 10.1126/science.1066700. [DOI] [PubMed] [Google Scholar]
- Viglietto G, Dolci S, Bruni P, Baldassarre G, Chiariotti L, Melillo RM, Salvatore G, Chiappetta G, Sferratore F, Fusco A, Santoro M. Glial cell line-derived neutrotrophic factor and neurturin can act as paracrine growth factors stimulating DNA synthesis of Ret-expressing spermatogonia. Int J Oncol. 2000;16:689–694. doi: 10.3892/ijo.16.4.689. [DOI] [PubMed] [Google Scholar]
- Watt F, Hogan B. Out of Eden: stem cells and their niches. Science. 2000;287:1427–1430. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- Williams E, Lowes A, Williams D, Williams G. A stem cell niche theory of intestinal crypt maintenance based on a study of somatic mutation in colonic mucosa. Am J Pathol. 1992;141:773–776. [PMC free article] [PubMed] [Google Scholar]
- Worby C, Vega Q, Zhao Y, Chao H, Seasholtz A, Dixon J. Glial cell line-derived neurotrophic factor signals through the RET receptor and activates mitogen-activated protein kinase. J Biol Chem. 1996;271:23619–23622. doi: 10.1074/jbc.271.39.23619. [DOI] [PubMed] [Google Scholar]
- Yamashita Y, Jones D, Fuller M. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science. 2003;301:1547–1550. doi: 10.1126/science.1087795. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Sukeno M, Nabeshima Y. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science. 2007;317:1722–1726. doi: 10.1126/science.1144885. [DOI] [PubMed] [Google Scholar]
- Zurn A, Winkel L, Menoud A, Djabali K, Aebischer P. Combined effects of GDNF, BDNF, and CNTF on motoneuron differentiation in vitro. J Neurosci Res. 1996;44:133–141. doi: 10.1002/(SICI)1097-4547(19960415)44:2<133::AID-JNR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]