Abstract
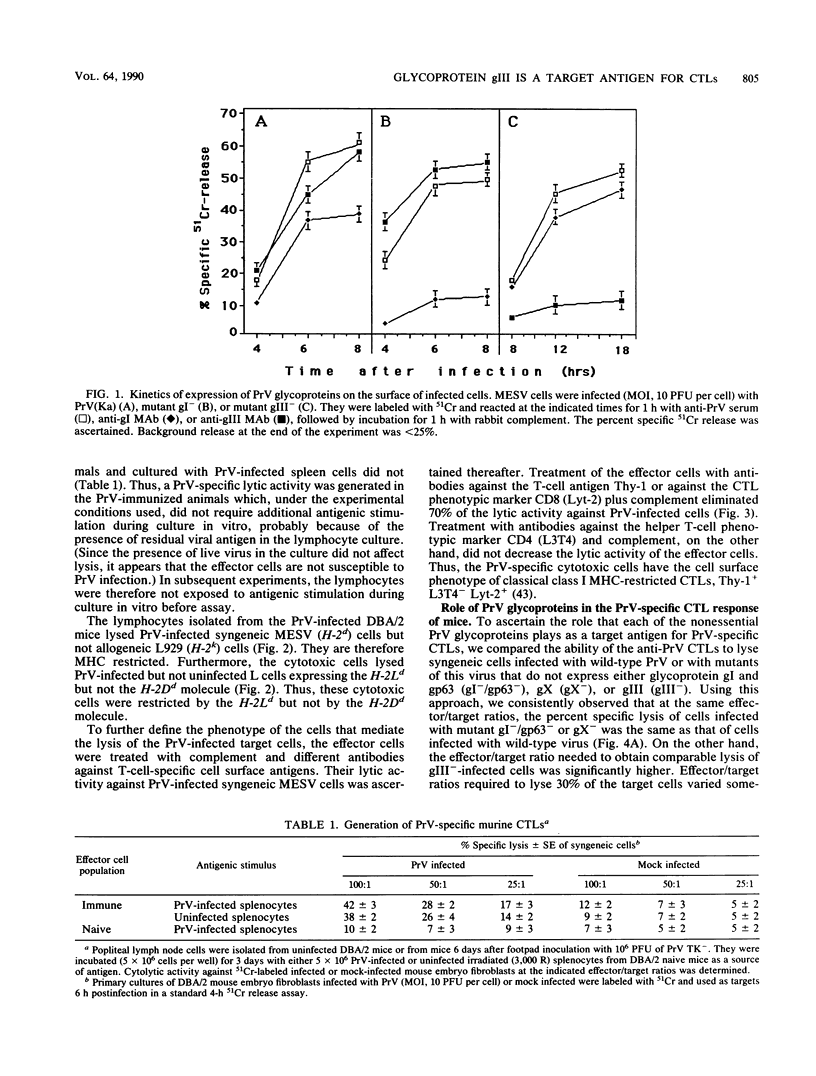
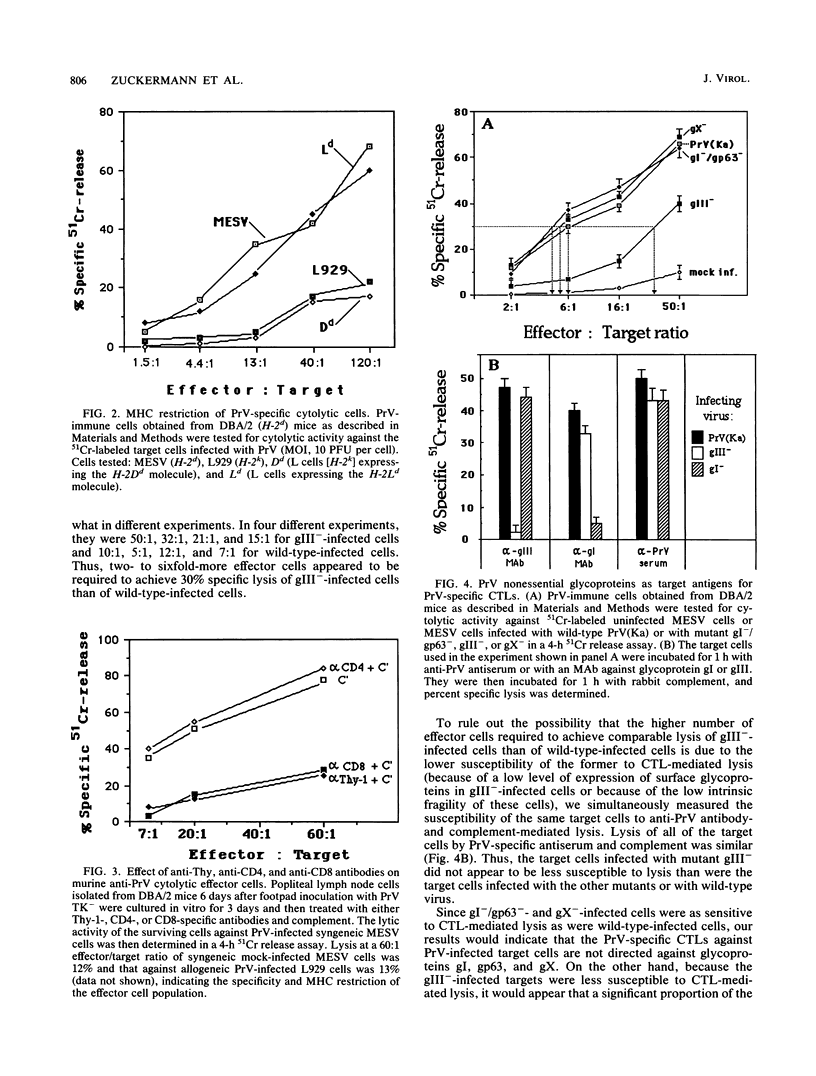
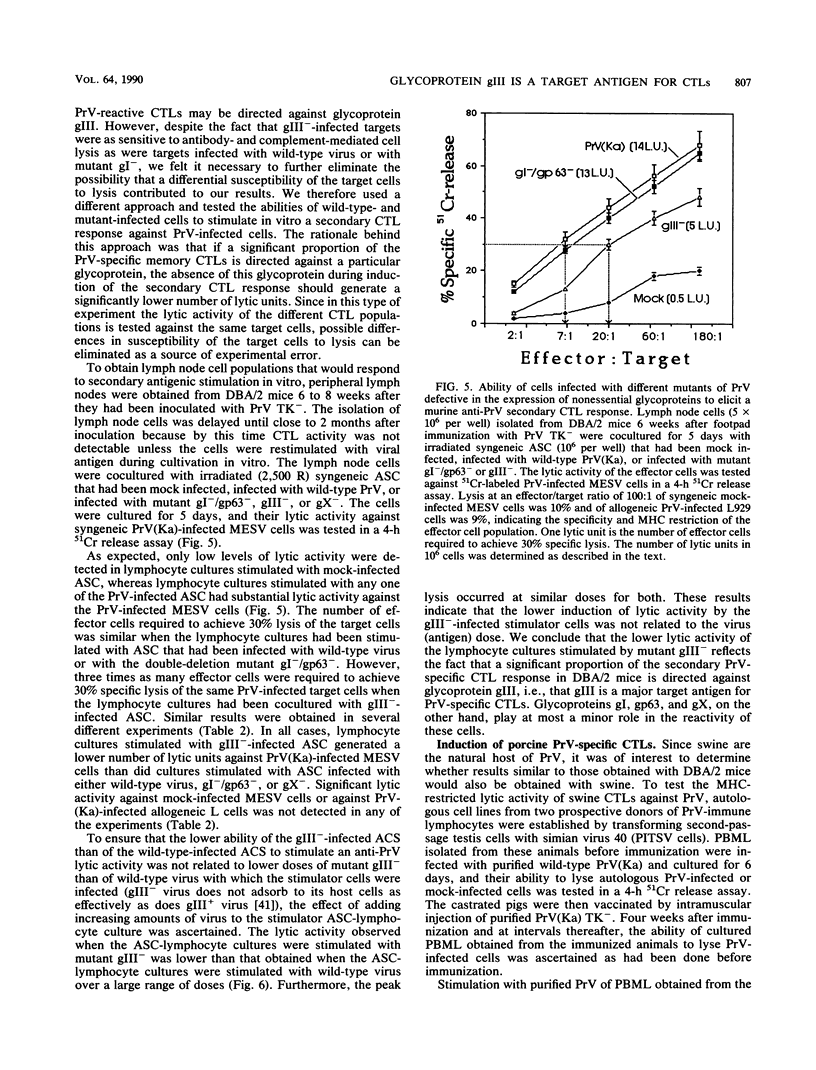
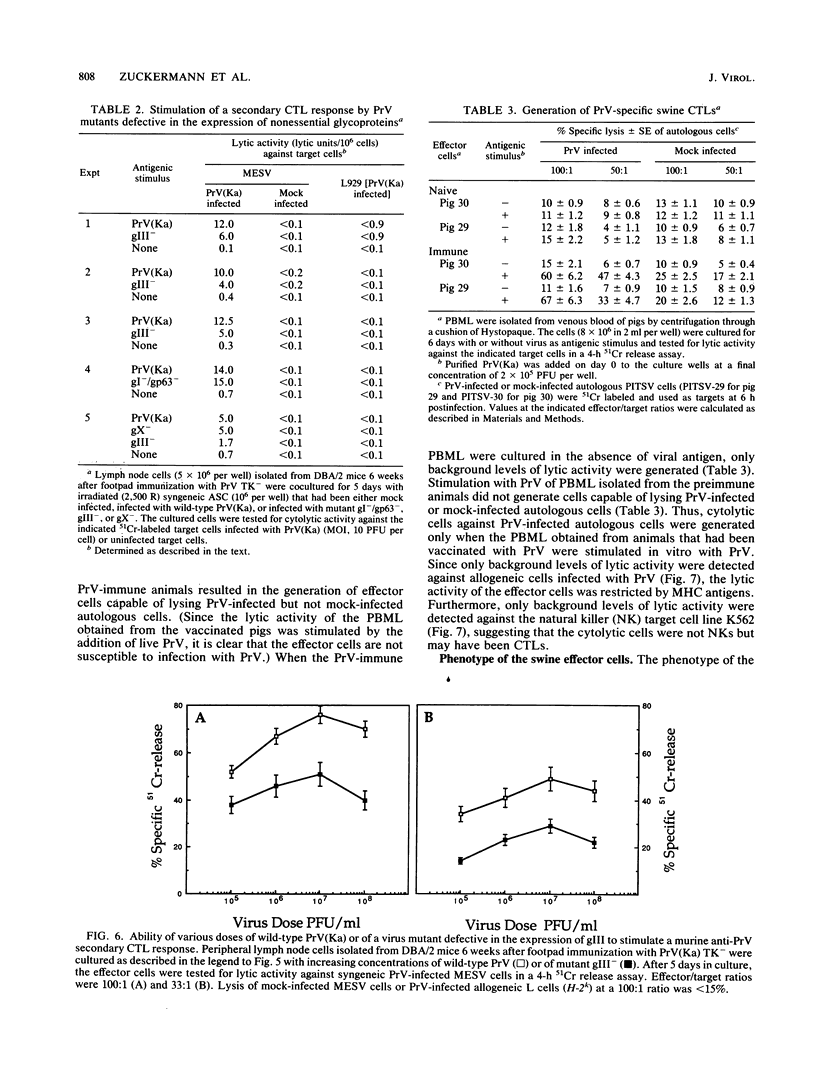
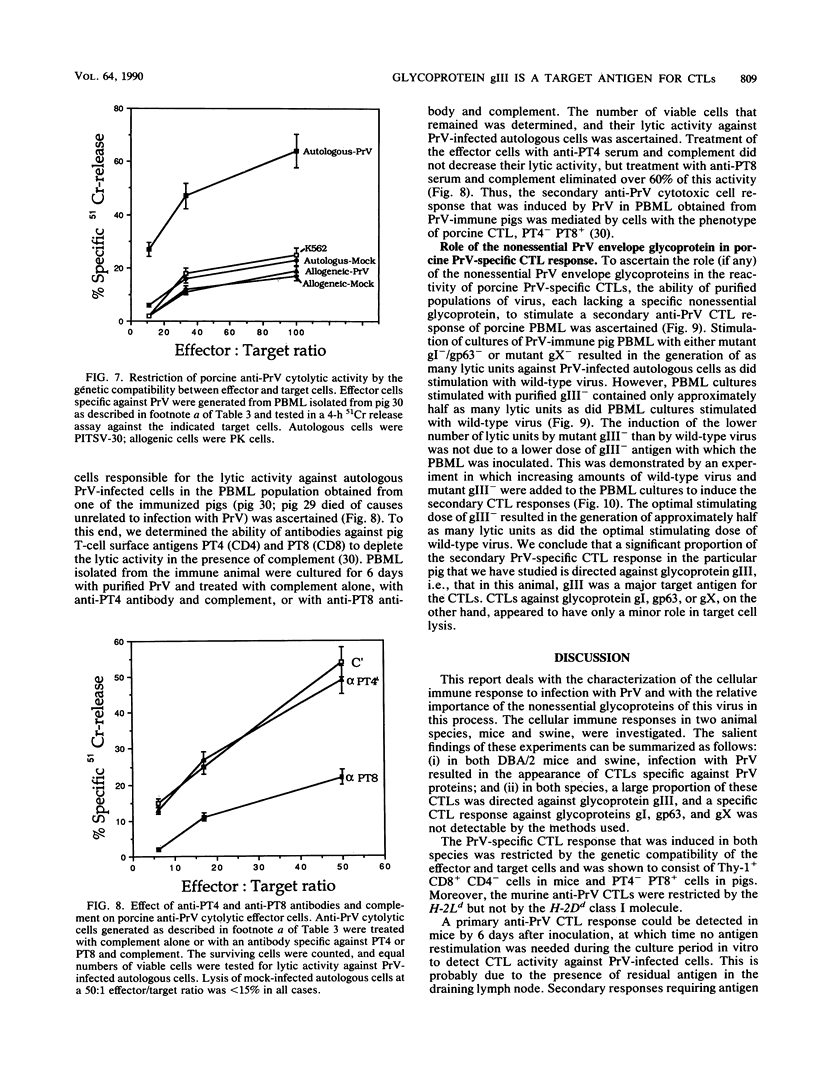
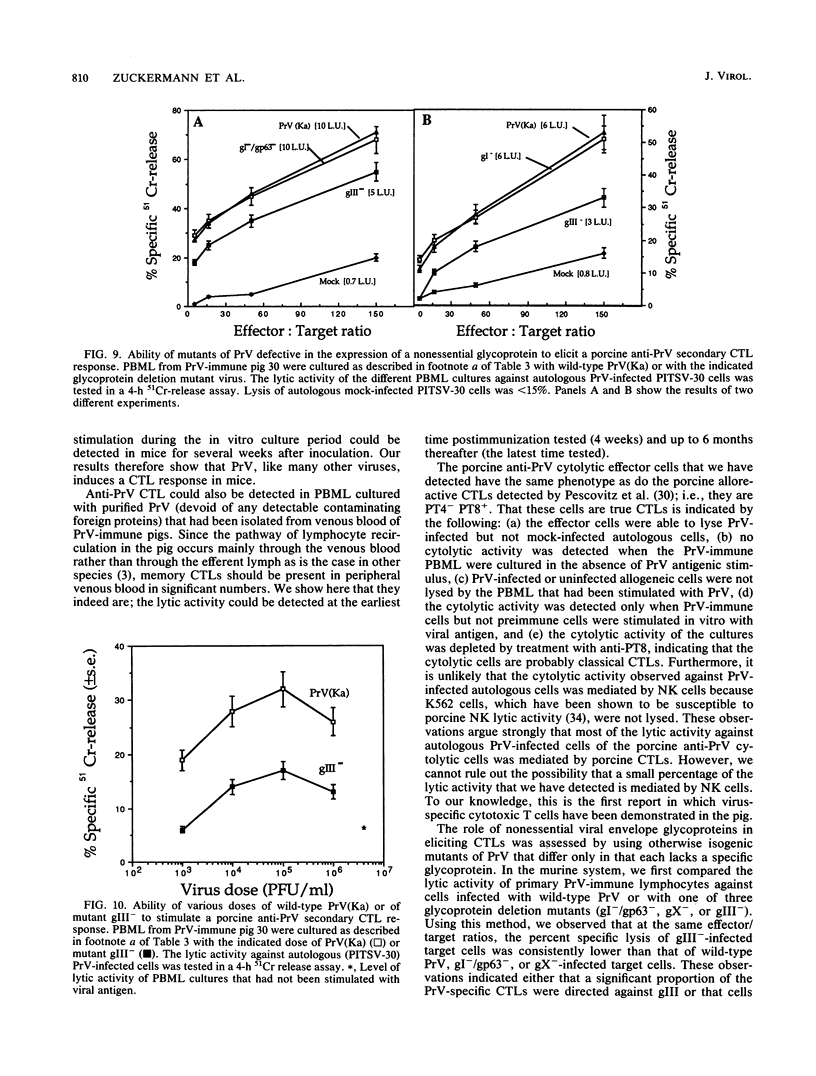
Pseudorabies virus (PrV) is the etiological agent of Aujeszky's disease, a disease that causes heavy economic losses in the swine industry. A rational approach to the generation of an effective vaccine against this virus requires an understanding of the immune response induced by it and of the role of the various viral antigens in inducing such a response. We have constructed mutants of PrV [strain PrV (Ka)] that differ from each other only in expression of the viral nonessential glycoproteins gI, gp63, gX, and gIII (i.e., are otherwise isogenic). These mutants were used to ascertain the importance of each of the nonessential glycoproteins in eliciting a PrV-specific cytotoxic T-lymphocyte (CTL) response in mice and pigs. Immunization of DBA/2 mice and pigs with a thymidine kinase-deficient (TK-) mutant of PrV elicits the formation of cytotoxic cells that specifically lyse syngeneic infected target cells. These PrV-specific cytolytic cells have the phenotype of major histocompatibility complex class I antigen-restricted CTLs. The relative number of CTLs specific for glycoproteins gI, gp63, gX, and gIII induced in mice vaccinated with a TK- mutant of PrV was ascertained by comparing their levels of cytotoxicity against syngeneic cells infected with either wild-type virus or gI-/gp63-, gX-, or gIII- virus deletion mutants. The PrV-specific CLTs were significantly less effective in lysing gIII(-)-infected targets than in lysing gI-/gp63-, gX-, or wild-type-infected targets. The in vitro secondary CTL response of lymphocytes obtained from either mice or pigs 6 or more weeks after immunization with a TK- mutant of PrV was also tested. Lymphocytes obtained from these animals were cultured with different glycoprotein-deficient mutants of PrV, and their cytolytic activities against wild-type-infected targets were ascertained. The importance of each of the nonessential viral glycoproteins in eliciting CTLs was assessed from the effectiveness of each of the virus mutants to stimulate the secondary anti-PrV CTL response. Cultures of both murine or swine lymphocytes that had been stimulated with gIII- virus contained only approximately half as many lytic units as did those stimulated with either wild-type virus, a gX- virus mutant, or a gI-/gp63- virus mutant. Thus, a large proportion of the PrV-specific CTLs that are induced by immunization with PrV of both mice and pigs are directed against gIII. Furthermore, glycoproteins gI, gp63, and gX play at most a minor role in the CTL response of these animals to PrV.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Porat T., DeMarchi J. M., Lomniczi B., Kaplan A. S. Role of glycoproteins of pseudorabies virus in eliciting neutralizing antibodies. Virology. 1986 Oct 30;154(2):325–334. doi: 10.1016/0042-6822(86)90458-7. [DOI] [PubMed] [Google Scholar]
- Ben-Porat T., Kaplan A. S. A comparison of two populations of defective, interfering pseudorabies virus particles. Virology. 1976 Jul 15;72(2):471–479. doi: 10.1016/0042-6822(76)90175-6. [DOI] [PubMed] [Google Scholar]
- Binns R. M., Pabst R., Licence S. T. Lymphocyte emigration from lymph nodes by blood in the pig and efferent lymph in the sheep. Immunology. 1985 Jan;54(1):105–111. [PMC free article] [PubMed] [Google Scholar]
- Bonneau R. H., Jennings S. R. Modulation of acute and latent herpes simplex virus infection in C57BL/6 mice by adoptive transfer of immune lymphocytes with cytolytic activity. J Virol. 1989 Mar;63(3):1480–1484. doi: 10.1128/jvi.63.3.1480-1484.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerottini J. C., Brunner K. T. Cell-mediated cytotoxicity, allograft rejection, and tumor immunity. Adv Immunol. 1974;18:67–132. doi: 10.1016/s0065-2776(08)60308-9. [DOI] [PubMed] [Google Scholar]
- Charpentier B., Michelson S., Martin B. Definition of human cytomegalovirus-specific target antigens recognized by cytotoxic T cells generated in vitro by using an autologous lymphocyte system. J Immunol. 1986 Jul 1;137(1):330–336. [PubMed] [Google Scholar]
- Glorioso J., Kees U., Kümel G., Kirchner H., Krammer P. H. Identification of herpes simplex virus type 1 (HSV-1) glycoprotein gC as the immunodominant antigen for HSV-1-specific memory cytotoxic T lymphocytes. J Immunol. 1985 Jul;135(1):575–582. [PubMed] [Google Scholar]
- Gutekunst D. E. Cellular immunity shown in pseudorabies virus-infected pigs by leukocyte migration-inhibition procedure. Am J Vet Res. 1979 Jan;40(1):66–68. [PubMed] [Google Scholar]
- Gutekunst D. E., Pirtle E. C. Humoral and cellular immune responses in swine after vaccination with inactivated pseudorabies virus. Am J Vet Res. 1979 Oct;40(10):1343–1346. [PubMed] [Google Scholar]
- Haffer K., Gustafson D. P., Kanitz C. L. Detection of pseudorabies virus antibodies: time of appearance by two serotests. Am J Vet Res. 1980 Aug;41(8):1317–1318. [PubMed] [Google Scholar]
- Hampl H., Ben-Porat T., Ehrlicher L., Habermehl K. O., Kaplan A. S. Characterization of the envelope proteins of pseudorabies virus. J Virol. 1984 Nov;52(2):583–590. doi: 10.1128/jvi.52.2.583-590.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hany M., Oehen S., Schulz M., Hengartner H., Mackett M., Bishop D. H., Overton H., Zinkernagel R. M. Anti-viral protection and prevention of lymphocytic choriomeningitis or of the local footpad swelling reaction in mice by immunization with vaccinia-recombinant virus expressing LCMV-WE nucleoprotein or glycoprotein. Eur J Immunol. 1989 Mar;19(3):417–424. doi: 10.1002/eji.1830190302. [DOI] [PubMed] [Google Scholar]
- Marchioli C. C., Yancey R. J., Jr, Petrovskis E. A., Timmins J. G., Post L. E. Evaluation of pseudorabies virus glycoprotein gp50 as a vaccine for Aujeszky's disease in mice and swine: expression by vaccinia virus and Chinese hamster ovary cells. J Virol. 1987 Dec;61(12):3977–3982. doi: 10.1128/jvi.61.12.3977-3982.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchioli C., Yancey R. J., Jr, Timmins J. G., Post L. E., Young B. R., Povendo D. A. Protection of mice and swine from pseudorabies virus-induced mortality by administration of pseudorabies virus-specific mouse monoclonal antibodies. Am J Vet Res. 1988 Jun;49(6):860–864. [PubMed] [Google Scholar]
- Martin S., Courtney R. J., Fowler G., Rouse B. T. Herpes simplex virus type 1-specific cytotoxic T lymphocytes recognize virus nonstructural proteins. J Virol. 1988 Jul;62(7):2265–2273. doi: 10.1128/jvi.62.7.2265-2273.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S., Moss B., Berman P. W., Laskey L. A., Rouse B. T. Mechanisms of antiviral immunity induced by a vaccinia virus recombinant expressing herpes simplex virus type 1 glycoprotein D: cytotoxic T cells. J Virol. 1987 Mar;61(3):726–734. doi: 10.1128/jvi.61.3.726-734.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S., Rouse B. T. The mechanisms of antiviral immunity induced by a vaccinia virus recombinant expressing herpes simplex virus type 1 glycoprotein D: clearance of local infection. J Immunol. 1987 May 15;138(10):3431–3437. [PubMed] [Google Scholar]
- Martin S., Wardley R. C., Donaldson A. I. Functional antibody responses in pigs vaccinated with live and inactivated Aujeszky's disease virus. Res Vet Sci. 1986 Nov;41(3):331–335. [PubMed] [Google Scholar]
- Martin S., Wardley R. C., Donaldson A. I. Serological response of pigs infected with Aujeszky's disease virus. Res Vet Sci. 1983 Sep;35(2):227–233. [PubMed] [Google Scholar]
- Martin S., Wardley R. C. Local humoral and cellular responses in Aujeszky's disease virus infection in pigs. Res Vet Sci. 1987 Mar;42(2):170–174. [PubMed] [Google Scholar]
- McGregor S., Easterday B. C., Kaplan A. S., Ben-Porat T. Vaccination of swine with thymidine kinase-deficient mutants of pseudorabies virus. Am J Vet Res. 1985 Jul;46(7):1494–1497. [PubMed] [Google Scholar]
- McMichael A. J., Michie C. A., Gotch F. M., Smith G. L., Moss B. Recognition of influenza A virus nucleoprotein by human cytotoxic T lymphocytes. J Gen Virol. 1986 Apr;67(Pt 4):719–726. doi: 10.1099/0022-1317-67-4-719. [DOI] [PubMed] [Google Scholar]
- Mettenleiter T. C., Lukàcs N., Rziha H. J. Pseudorabies virus avirulent strains fail to express a major glycoprotein. J Virol. 1985 Oct;56(1):307–311. doi: 10.1128/jvi.56.1.307-311.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettenleiter T. C., Lukàcs N., Thiel H. J., Schreurs C., Rziha H. J. Location of the structural gene of pseudorabies virus glycoprotein complex gII. Virology. 1986 Jul 15;152(1):66–75. doi: 10.1016/0042-6822(86)90372-7. [DOI] [PubMed] [Google Scholar]
- Mettenleiter T. C., Schreurs C., Zuckermann F., Ben-Porat T., Kaplan A. S. Role of glycoprotein gIII of pseudorabies virus in virulence. J Virol. 1988 Aug;62(8):2712–2717. doi: 10.1128/jvi.62.8.2712-2717.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettenleiter T. C., Schreurs C., Zuckermann F., Ben-Porat T. Role of pseudorabies virus glycoprotein gI in virus release from infected cells. J Virol. 1987 Sep;61(9):2764–2769. doi: 10.1128/jvi.61.9.2764-2769.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettenleiter T. C., Zsak L., Kaplan A. S., Ben-Porat T., Lomniczi B. Role of a structural glycoprotein of pseudorabies in virus virulence. J Virol. 1987 Dec;61(12):4030–4032. doi: 10.1128/jvi.61.12.4030-4032.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash A. A., Jayasuriya A., Phelan J., Cobbold S. P., Waldmann H., Prospero T. Different roles for L3T4+ and Lyt 2+ T cell subsets in the control of an acute herpes simplex virus infection of the skin and nervous system. J Gen Virol. 1987 Mar;68(Pt 3):825–833. doi: 10.1099/0022-1317-68-3-825. [DOI] [PubMed] [Google Scholar]
- Pescovitz M. D., Lunney J. K., Sachs D. H. Murine anti-swine T4 and T8 monoclonal antibodies: distribution and effects on proliferative and cytotoxic T cells. J Immunol. 1985 Jan;134(1):37–44. [PubMed] [Google Scholar]
- Petrovskis E. A., Timmins J. G., Armentrout M. A., Marchioli C. C., Yancey R. J., Jr, Post L. E. DNA sequence of the gene for pseudorabies virus gp50, a glycoprotein without N-linked glycosylation. J Virol. 1986 Aug;59(2):216–223. doi: 10.1128/jvi.59.2.216-223.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovskis E. A., Timmins J. G., Gierman T. M., Post L. E. Deletions in vaccine strains of pseudorabies virus and their effect on synthesis of glycoprotein gp63. J Virol. 1986 Dec;60(3):1166–1169. doi: 10.1128/jvi.60.3.1166-1169.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovskis E. A., Timmins J. G., Post L. E. Use of lambda gt11 to isolate genes for two pseudorabies virus glycoproteins with homology to herpes simplex virus and varicella-zoster virus glycoproteins. J Virol. 1986 Oct;60(1):185–193. doi: 10.1128/jvi.60.1.185-193.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto A., Ferguson F. Characteristics of Yorkshire swine natural killer cells. Vet Immunol Immunopathol. 1988 Dec;20(1):15–29. doi: 10.1016/0165-2427(88)90022-0. [DOI] [PubMed] [Google Scholar]
- Platt K. B. The evaluation of a lectin-agarose based subunit vaccine and complementary diagnostic antigen for Aujeszky's disease (pseudorabies) in the pig. Vet Microbiol. 1984 Feb;9(1):35–51. doi: 10.1016/0378-1135(84)90077-4. [DOI] [PubMed] [Google Scholar]
- Puddington L., Bevan M. J., Rose J. K., Lefrançois L. N protein is the predominant antigen recognized by vesicular stomatitis virus-specific cytotoxic T cells. J Virol. 1986 Nov;60(2):708–717. doi: 10.1128/jvi.60.2.708-717.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddehase M. J., Koszinowski U. H. Significance of herpesvirus immediate early gene expression in cellular immunity to cytomegalovirus infection. Nature. 1984 Nov 22;312(5992):369–371. doi: 10.1038/312369a0. [DOI] [PubMed] [Google Scholar]
- Robbins A. K., Dorney D. J., Wathen M. W., Whealy M. E., Gold C., Watson R. J., Holland L. E., Weed S. D., Levine M., Glorioso J. C. The pseudorabies virus gII gene is closely related to the gB glycoprotein gene of herpes simplex virus. J Virol. 1987 Sep;61(9):2691–2701. doi: 10.1128/jvi.61.9.2691-2701.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. K., Watson R. J., Whealy M. E., Hays W. W., Enquist L. W. Characterization of a pseudorabies virus glycoprotein gene with homology to herpes simplex virus type 1 and type 2 glycoprotein C. J Virol. 1986 May;58(2):339–347. doi: 10.1128/jvi.58.2.339-347.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. K., Whealy M. E., Watson R. J., Enquist L. W. Pseudorabies virus gene encoding glycoprotein gIII is not essential for growth in tissue culture. J Virol. 1986 Sep;59(3):635–645. doi: 10.1128/jvi.59.3.635-645.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs C., Mettenleiter T. C., Zuckermann F., Sugg N., Ben-Porat T. Glycoprotein gIII of pseudorabies virus is multifunctional. J Virol. 1988 Jul;62(7):2251–2257. doi: 10.1128/jvi.62.7.2251-2257.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. C., Mengeling W. L. A skin test for pseudorabies virus infection in swine. Can J Comp Med. 1977 Oct;41(4):364–368. [PMC free article] [PubMed] [Google Scholar]
- Swain S. L. T cell subsets and the recognition of MHC class. Immunol Rev. 1983;74:129–142. doi: 10.1111/j.1600-065x.1983.tb01087.x. [DOI] [PubMed] [Google Scholar]
- Todd D., Hull J., McNair J. Antigenically important proteins of Aujeszky's disease (pseudorabies) virus identified by immunoblotting. Arch Virol. 1987;96(3-4):215–224. doi: 10.1007/BF01320961. [DOI] [PubMed] [Google Scholar]
- Wathen L. M., Platt K. B., Wathen M. W., Van Deusen R. A., Whetstone C. A., Pirtle E. C. Production and characterization of monoclonal antibodies directed against pseudorabies virus. Virus Res. 1985 Dec;4(1):19–29. doi: 10.1016/0168-1702(85)90017-6. [DOI] [PubMed] [Google Scholar]
- Wathen M. W., Wathen L. M. Characterization and mapping of a nonessential pseudorabies virus glycoprotein. J Virol. 1986 Apr;58(1):173–178. doi: 10.1128/jvi.58.1.173-178.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathen M. W., Wathen L. M. Isolation, characterization, and physical mapping of a pseudorabies virus mutant containing antigenically altered gp50. J Virol. 1984 Jul;51(1):57–62. doi: 10.1128/jvi.51.1.57-62.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton J. L., Southern P. J., Oldstone M. B. Analyses of the cytotoxic T lymphocyte responses to glycoprotein and nucleoprotein components of lymphocytic choriomeningitis virus. Virology. 1988 Feb;162(2):321–327. doi: 10.1016/0042-6822(88)90471-0. [DOI] [PubMed] [Google Scholar]
- Wittmann G., Bartenbach G., Jakubik J. Cell-mediated immunity in Aujeszky disease virus infected pigs. I. Lymphocyte stimulation. Arch Virol. 1976;50(3):215–222. doi: 10.1007/BF01320575. [DOI] [PubMed] [Google Scholar]
- Wittmann G., Leitzke I., Höhn U. Zellvermittelte Zytotoxizität und Lymphozytenstimulierung bei Aujeszkyscher Krankheit. I. Bei experimentell infizierten Schweinen. Zentralbl Veterinarmed B. 1985 Feb;32(2):101–115. [PubMed] [Google Scholar]
- Wittmann G., Leitzke I., Höhn U. Zellvermittelte Zytotoxizität und Lymprozytenstimulierung bei Aujeszkyscher Krankheit. II. Nach Vakzinierung von Schweinen und nachfolgender Infektion. Zentralbl Veterinarmed B. 1985 Mar;32(3):181–196. [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R., Mackett M., Lefrancois L., Lyles D. S., Moss B. Recognition of cloned vesicular stomatitis virus internal and external gene products by cytotoxic T lymphocytes. J Exp Med. 1986 Jun 1;163(6):1529–1538. doi: 10.1084/jem.163.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckermann F., Zsak L., Reilly L., Sugg N., Ben-Porat T. Early interactions of pseudorabies virus with host cells: functions of glycoprotein gIII. J Virol. 1989 Aug;63(8):3323–3329. doi: 10.1128/jvi.63.8.3323-3329.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


