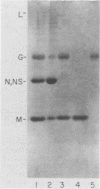
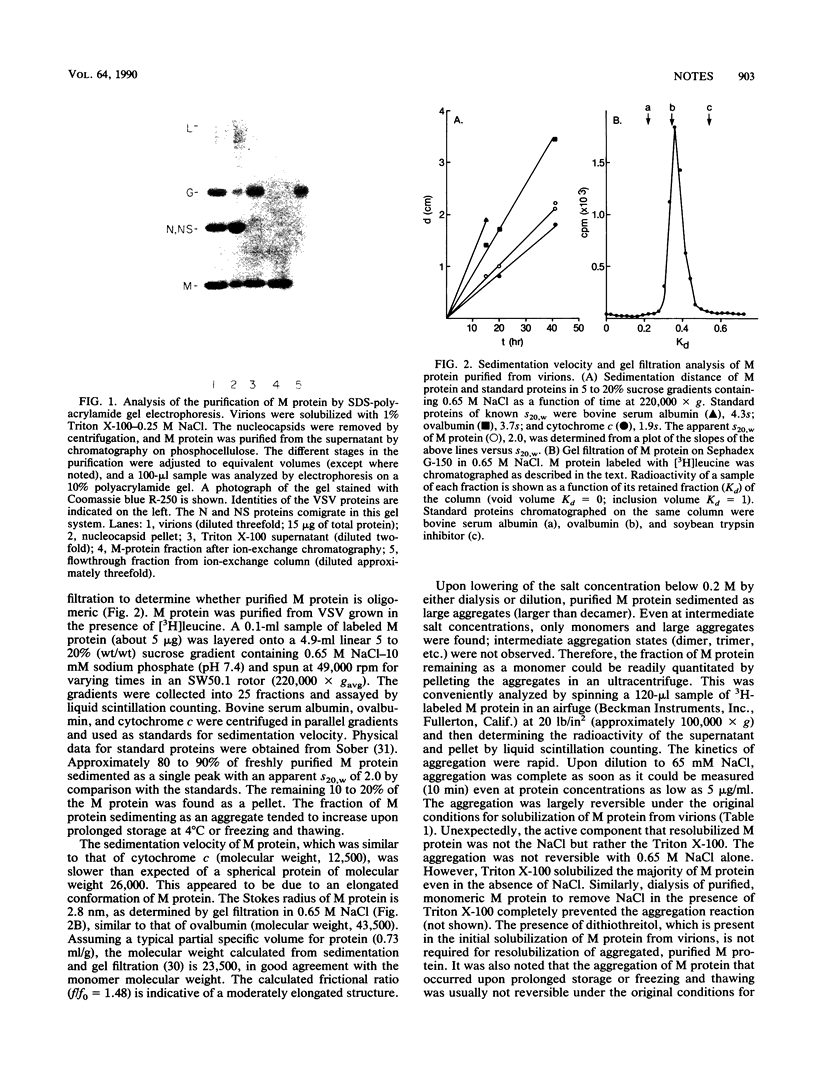
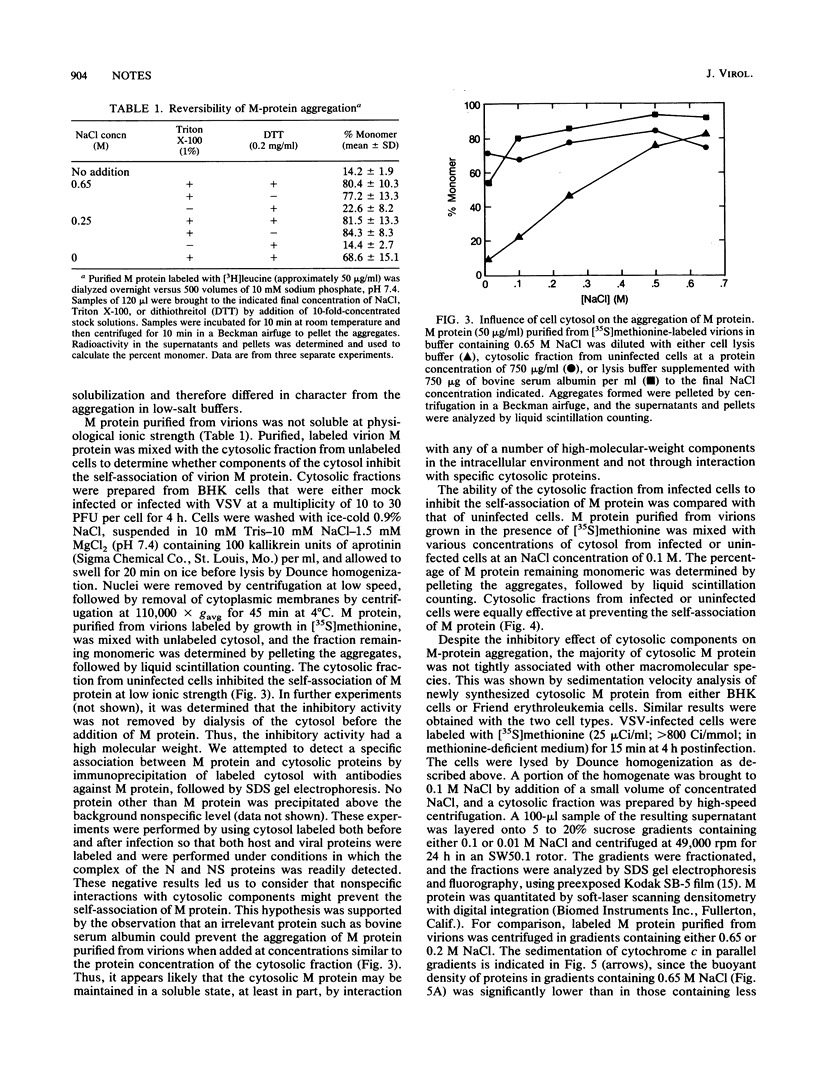
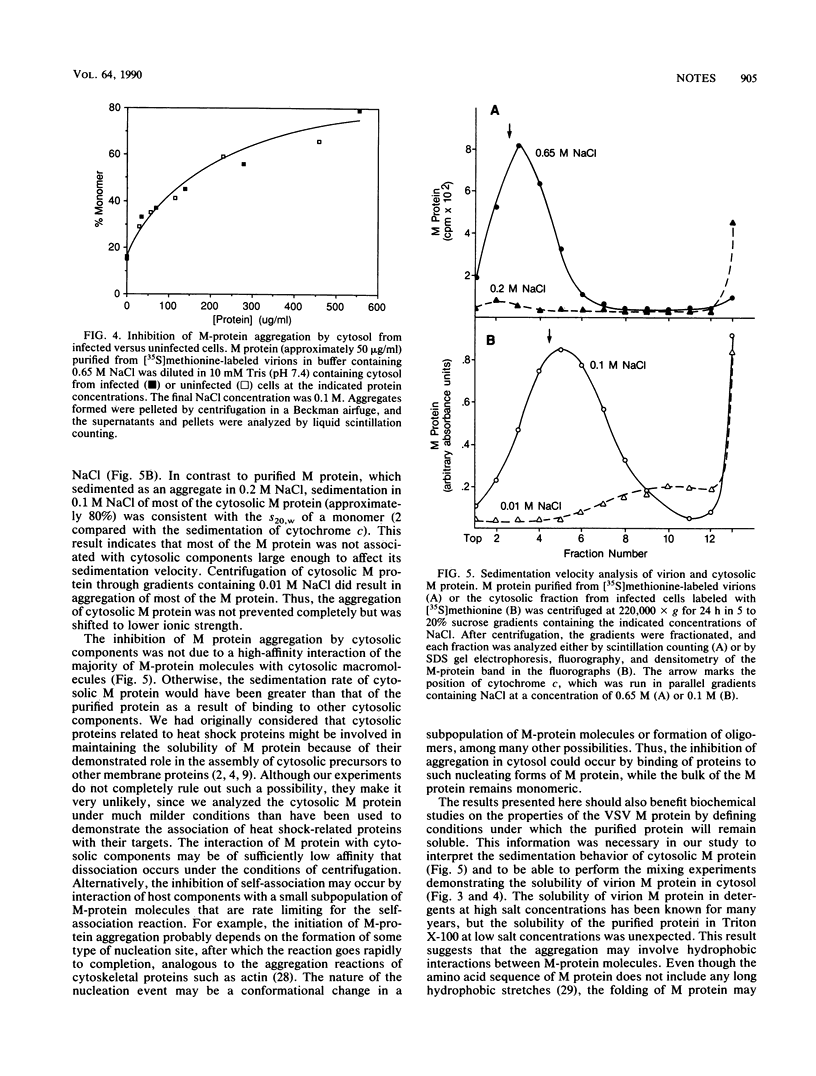
Abstract
The peripheral membrane M protein of vesicular stomatitis virus purified by detergent extraction of virions and ion-exchange chromatography was determined to be a monomer in the absence of detergent at high salt concentrations. Reduction of the ionic strength below 0.2 M resulted in a rapid aggregation of M protein. This self-association was reversible by the detergent Triton X-100 even in low salt. However, aggregation was not reversible by high salt concentration alone. M protein is initially synthesized as a soluble protein in the cytosol of infected cells, thus raising the question of how the solubility of M protein is maintained at physiological ionic strength. Addition of radiolabeled M protein purified from virions to unlabeled cytosol from either infected or uninfected cells inhibited the self-association reaction. Cytosolic fractions from infected or uninfected cells were equally effective at preventing the self-association of M protein. Self-association could also be prevented by an irrelevant protein such as bovine serum albumin. Sedimentation velocity analysis indicated that most of the newly synthesized M protein is monomeric, suggesting that the solubility of M protein in the cytosol is maintained by either low-affinity interaction with macromolecules in the cytosol or interaction of a small population of M-protein molecules with cytosolic components.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson P. H., Moyer S. A., Summers D. F. Assembly of vesicular stomatitis virus glycoprotein and matrix protein into HeLa cell plasma membranes. J Mol Biol. 1976 Apr 15;102(3):613–631. doi: 10.1016/0022-2836(76)90338-7. [DOI] [PubMed] [Google Scholar]
- Brugge J. S. Interaction of the Rous sarcoma virus protein pp60src with the cellular proteins pp50 and pp90. Curr Top Microbiol Immunol. 1986;123:1–22. doi: 10.1007/978-3-642-70810-7_1. [DOI] [PubMed] [Google Scholar]
- Carroll A. R., Wagner R. R. Role of the membrane (M) protein in endogenous inhibition of in vitro transcription by vesicular stomatitis virus. J Virol. 1979 Jan;29(1):134–142. doi: 10.1128/jvi.29.1.134-142.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirico W. J., Waters M. G., Blobel G. 70K heat shock related proteins stimulate protein translocation into microsomes. Nature. 1988 Apr 28;332(6167):805–810. doi: 10.1038/332805a0. [DOI] [PubMed] [Google Scholar]
- Clinton G. M., Burge B. W., Huang A. S. Effects of phosphorylation and pH on the association of NS protein with vesicular stomatitis virus cores. J Virol. 1978 Aug;27(2):340–346. doi: 10.1128/jvi.27.2.340-346.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David A. E. Assembly of the vesicular stomatitis virus envelope: incorporation of viral polypeptides into the host plasma membrane. J Mol Biol. 1973 May 5;76(1):135–148. doi: 10.1016/0022-2836(73)90085-5. [DOI] [PubMed] [Google Scholar]
- Davis N. L., Arnheiter H., Wertz G. W. Vesicular stomatitis virus N and NS proteins form multiple complexes. J Virol. 1986 Sep;59(3):751–754. doi: 10.1128/jvi.59.3.751-754.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De B. P., Thornton G. B., Luk D., Banerjee A. K. Purified matrix protein of vesicular stomatitis virus blocks viral transcription in vitro. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7137–7141. doi: 10.1073/pnas.79.23.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshaies R. J., Koch B. D., Werner-Washburne M., Craig E. A., Schekman R. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature. 1988 Apr 28;332(6167):800–805. doi: 10.1038/332800a0. [DOI] [PubMed] [Google Scholar]
- Dubovi E. J., Wagner R. R. Spatial relationships of the proteins of vesicular stomatitis virus: induction of reversible oligomers by cleavable protein cross-linkers and oxidation. J Virol. 1977 May;22(2):500–509. doi: 10.1128/jvi.22.2.500-509.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faaberg K. S., Peeples M. E. Association of soluble matrix protein of Newcastle disease virus with liposomes is independent of ionic conditions. Virology. 1988 Sep;166(1):123–132. doi: 10.1016/0042-6822(88)90153-5. [DOI] [PubMed] [Google Scholar]
- Hewitt J. A. Studies on the subunit composition of the M-protein of Sendai virus. FEBS Lett. 1977 Sep 15;81(2):395–397. doi: 10.1016/0014-5793(77)80562-0. [DOI] [PubMed] [Google Scholar]
- Knipe D. M., Baltimore D., Lodish H. F. Separate pathways of maturation of the major structural proteins of vesicular stomatitis virus. J Virol. 1977 Mar;21(3):1128–1139. doi: 10.1128/jvi.21.3.1128-1139.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lenard J., Mancarella D. A., Wilson T., Reidler J. A., Keller P. M., Elson E. L. The m protein of vesicular stomatitis virus: variability in lipid-protein interaction compatible with function. Biophys J. 1982 Jan;37(1):26–28. doi: 10.1016/S0006-3495(82)84582-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyles D. S. Glycoproteins of Sendai virus are transmembrane proteins. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5621–5625. doi: 10.1073/pnas.76.11.5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyles D. S., Puddington L., McCreedy B. J., Jr Vesicular stomatitis virus M protein in the nuclei of infected cells. J Virol. 1988 Nov;62(11):4387–4392. doi: 10.1128/jvi.62.11.4387-4392.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancarella D. A., Lenard J. Interactions of wild-type and mutant M protein of vesicular stomatitis virus with viral nucleocapsid and envelope in intact virions. Evidence from [125I]iodonaphthyl azide labeling and specific cross-linking. Biochemistry. 1981 Nov 24;20(24):6872–6877. doi: 10.1021/bi00527a020. [DOI] [PubMed] [Google Scholar]
- Morrison T. G., McQuain C. Assembly of viral membranes. I. Association of vesicular stomatitis virus membrane proteins and membranes in a cell-free system. J Virol. 1977 Feb;21(2):451–458. doi: 10.1128/jvi.21.2.451-458.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T. G. Site of synthesis of membrane and nonmembrane proteins of vesicular stomatitis virus. J Biol Chem. 1975 Sep 10;250(17):6955–6962. [PubMed] [Google Scholar]
- Newcomb W. W., Tobin G. J., McGowan J. J., Brown J. C. In vitro reassembly of vesicular stomatitis virus skeletons. J Virol. 1982 Mar;41(3):1055–1062. doi: 10.1128/jvi.41.3.1055-1062.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odenwald W. F., Arnheiter H., Dubois-Dalcq M., Lazzarini R. A. Stereo images of vesicular stomatitis virus assembly. J Virol. 1986 Mar;57(3):922–932. doi: 10.1128/jvi.57.3.922-932.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden J. R., Pal R., Wagner R. R. Mapping regions of the matrix protein of vesicular stomatitis virus which bind to ribonucleocapsids, liposomes, and monoclonal antibodies. J Virol. 1986 Jun;58(3):860–868. doi: 10.1128/jvi.58.3.860-868.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K., Dubois-Dalcq M. E., Schubert M., Lazzarini R. A. A mutated membrane protein of vesicular stomatitis virus has an abnormal distribution within the infected cell and causes defective budding. J Virol. 1987 May;61(5):1332–1341. doi: 10.1128/jvi.61.5.1332-1341.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso R. W., Moyer S. A. Viral proteins required for the in vitro replication of vesicular stomatitis virus defective interfering particle genome RNA. Virology. 1988 Feb;162(2):369–376. doi: 10.1016/0042-6822(88)90477-1. [DOI] [PubMed] [Google Scholar]
- Pepinsky R. B., Vogt V. M. Identification of retrovirus matrix proteins by lipid-protein cross-linking. J Mol Biol. 1979 Jul 15;131(4):819–837. doi: 10.1016/0022-2836(79)90203-1. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Cooper J. A. Actin and actin-binding proteins. A critical evaluation of mechanisms and functions. Annu Rev Biochem. 1986;55:987–1035. doi: 10.1146/annurev.bi.55.070186.005011. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Gallione C. J. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus G and M proteins determined from cDNA clones containing the complete coding regions. J Virol. 1981 Aug;39(2):519–528. doi: 10.1128/jvi.39.2.519-528.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Wilson T., Lenard J. Interaction of wild-type and mutant M protein vesicular stomatitis virus with nucleocapsids in vitro. Biochemistry. 1981 Mar 3;20(5):1349–1354. doi: 10.1021/bi00508a048. [DOI] [PubMed] [Google Scholar]
- Zakowski J. J., Petri W. A., Jr, Wagner R. R. Role of matrix protein in assembling the membrane of vesicular stomatitis virus: reconstitution of matrix protein with negatively charged phospholipid vesicles. Biochemistry. 1981 Jun 23;20(13):3902–3907. doi: 10.1021/bi00516a037. [DOI] [PubMed] [Google Scholar]
- Zakowski J. J., Wagner R. R. Localization of membrane-associated proteins in vesicular stomatitis virus by use of hydrophobic membrane probes and cross-linking reagents. J Virol. 1980 Oct;36(1):93–102. doi: 10.1128/jvi.36.1.93-102.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]