Abstract
Although it’s been reported that women with premenstrual dysphoric disorder (PMDD) have increased negative mood, appetite (food cravings and food intake), alcohol intake and cognitive deficits premenstrually, few studies have examined these changes concurrently within the same group of women or compared to women without PMDD. Thus, to date, there is not a clear understanding of the full range of PMDD symptoms. The present study concurrently assessed mood and performance tasks in 29 normally cycling women (14 women who met DSM-IV criteria for PMDD and 15 women without PMDD). Women had a total of ten sessions: two practice sessions, 4 sessions during the follicular phase and 4 sessions during the late luteal phase of the menstrual cycle. Each session, participants completed mood and food-related questionnaires, a motor coordination task, performed various cognitive tasks and ate lunch. There was a significant increase in dysphoric mood during the luteal phase in women with PMDD compared to their follicular phase and compared to Control women. Further, during the luteal phase, women with PMDD showed impaired performance on the Immediate and Delayed Word Recall Task, the Immediate and Delayed Digit Recall Task and the Digit Symbol Substitution Test compared to Control women. Women with PMDD, but not Control women, also showed increased desire for food items high in fat during the luteal phase compared to the follicular phase and correspondingly, women with PMDD consumed more calories during the luteal phase (mostly derived from fat) compared to the follicular phase. In summary, women with PMDD experience dysphoric mood, a greater desire and actual intake of certain foods and show impaired cognitive performance during the luteal phase. An altered serotonergic system in women with PMDD may be the underlying mechanism for the observed symptoms; correspondingly, treatment with specific serotonin reuptake inhibitors (SSRIs) remains the preferred treatment at this time.
Keywords: Cognitive Performance, Food, PMDD, Mood, Women
INTRODUCTION
Premenstrual syndrome (PMS) is a recurrence of negative behavioral (e.g. fatigue), psychological (e.g. irritability) and physical symptoms (e.g. headaches) that occur during the luteal phase of the menstrual cycle and remit by the follicular phase (Dickerson et al., 2003). While the overwhelming majority of premenopausal women experience some level of premenstrual symptoms (Dickerson et al., 2003), only 8% of these women suffer from premenstrual symptoms to such a degree that it interferes with normal functioning and are diagnosed with premenstrual dysphoric disorder (PMDD; Bhatia & Bhatia, 2002; Wittchen et al., 2002). PMDD is characterized primarily by a cluster of mood symptoms, especially depression, tension, anxiety, irritability, and fatigue, with five or more symptoms present during the luteal phase (American Psychiatric Association, 1994). The diagnosis of PMDD can only be made by having women prospectively monitor their symptoms for at least two consecutive symptomatic menstrual cycles (Di Giulio & Reissing, 2006; Futterman & Rapkin, 2006). These symptoms have to occur during the last week of the luteal (premenstrual) phase, diminish with the onset of menses, and cease during the follicular phase. These dramatic cyclical changes in mood in women with PMDD have been well documented both clinically and in research studies (e.g. De Ronchi et al., 2005; Evans et al., 1998; Freeman et al. 1985; Halbreich et al. 1982; Landen et al., 2007; Rapkin et al. 1989).
In addition to increases in dysphoric mood during the luteal phase, women with PMDD also report impairments in cognitive abilities such as concentration, memory and motor coordination that interfere with their productivity and efficiency (American Psychiatric Association, 1994; Diener et al., 1992). However, the extent to which various aspects of psychomotor or cognitive performance are actually impaired during the luteal phase in women with PMDD has not been extensively studied and the results have been inconsistent, with studies reporting no differences (e.g. Rapkin et al., 1989) or only subtle differences on isolated tasks (e.g. Man et al., 1999; Posthuma et al., 1987; Resnick et al., 1998). For instance, although Keenan and colleagues (1992a, 1995) assessed a range of neuropsychological tasks, women with PMDD were only impaired on a verbal learning task compared to a group of control women and this impairment was not related to menstrual cycle phase. In another well-controlled study that evaluated a range of tasks, the only performance impairment observed was that women with PMDD showed more psychomotor slowing during the luteal phase compared to control women (Resnick et al., 1998). Another study (Morgan and Rapkin, 2002) that also assessed a full series of neurocognitive tasks reported no performance differences between women with PMDD and control women, despite a relatively large sample size. In a previous study conducted in our laboratory (Evans et al., 1998), we assessed changes in mood and performance as a function of menstrual cycle phase in women with confirmed PMDD after placebo or alprazolam administration. When placebo was administered, motor coordination (via a balance task) and performance on the Digit Symbol Substitution Task (DSST) were impaired in the luteal phase. Limitations of that study included only administering placebo one day in each phase and the lack of a control group. Regardless, one major distinction between the Evans et al. (1998) study and all the other studies mentioned above is that task performance was assessed multiple times over the day, not just once. The fact that most previous studies only assessed performance on a single occasion each phase may be one reason for the inconsistencies observed across studies. It is possible that most individuals might be able to perform relatively well if only required to do the task once for a brief period of time, whereas individuals may be less likely to sustain their performance if they required to perform for extended periods of time, particularly when experiencing PMDD symptoms during the luteal phase. Therefore, one goal of this study was to extend previous research by comprehensively assessing changes in cognitive performance in women with PMDD across the menstrual cycle and to include a control group of women without PMDD.
Lastly, women with PMDD also report changes in appetite, food intake and specific food cravings during the luteal phase (American Psychiatric Association, 1994), and these changes appear to be correlated with premenstrual mood changes, primarily depression (e.g. Both-Orthman et al., 1988; Dye & Blundell, 1997; Wurtman et al., 1989). Despite this, few studies have carefully assessed food cravings or food intake in women. In fact, most studies relied on retrospective reports of PMDD and retrospective food cravings, typically using a single question that did not specify food type (e.g. Both-Orthman et al., 1988; Bancroft et al., 1993). With respect to changes in food intake, the majority of studies have relied on retrospective food diaries, rather than measuring actual food intake. In fact, only two studies actually measured food intake in women with and without PMS while they resided as inpatients, and the foods provided to participants consisted of high carbohydrate (CHO) and high protein foods, with fat content held constant (Brzezinski et al., 1990; Wurtman et al., 1989). In both studies, only women with PMS showed a significant increase in food intake during the luteal phase compared to the follicular phase, and this was attributed to an increase in CHO intake. In another study (Evans et al., 1999) we assessed food cravings and food intake in 19 women with PMDD and showed that craving for foods, specifically those containing fat, were significantly increased in the luteal phase compared to the follicular phase, while desires for CHO alone did not change as a function of menstrual cycle phase. In that same study, when placebo was administered there was no corresponding increase in actual food consumption at lunch during the luteal phase. Unfortunately, limitations of that study included only administering placebo one day in each phase and the lack of a control group. Therefore, another goal of the present study was to carefully assess whether food cravings (across a range of food items), food intake, as well as macronutrient intake, vary between the luteal and follicular phases in these two groups of women.
Taken together, although PMDD is characterized by a spectrum of symptoms and complaints (mood, performance, appetite), the full spectrum has not been assessed in a comprehensive manner. Therefore, to expand on previous research in this area, the purpose of the present study was to concurrently assess the relationship between changes in mood, cognitive performance, food craving, as well as actual food consumption, as a function of menstrual cycle phase in women meeting DSM-IV criteria for PMDD compared to a matched group of Control women. All participants were prospectively monitored throughout the study, and menstrual cycle phase was verified via ovulation kits and hormone levels of estradiol and progesterone. Women were tested on four different days each phase, and on each day a variety of mood and performance tasks was assessed multiple times to simulate a modified workday and food cravings and food intake at lunch were measured. We hypothesized that women with PMDD would show impaired mood and cognitive task performance, along with increased cravings and intake of food and alcohol, in the late luteal phase compared to the follicular phase, or compared to Control women. This study aims to give a comprehensive description of PMDD that may aid in the development of treatments that address the full range of PMDD symptoms.
METHODS
Subjects
The 29 women (15 Control and 14 with PMDD) who participated in this study responded to an advertisement in a local newspaper for female volunteers suffering from premenstrual syndrome (see Table 1). Women had a mean age of 30, were predominately White, had a mean of 16 years of education, were normal weight (BMI ≤ 25) and had normal menstrual cycles. Overall, women were light drinkers and reported little or no other drug use. There were no significant differences on any of these demographic variables between PMDD and Control women.
Table 1.
Demographic characteristics of study participants.
| Control Women | PMDD women | |
|---|---|---|
| N | 15 | 14 |
| Age (yrs) | 30 (6.1) | 30 (6.7) |
| Race (Blk/Wht/Hisp) | 4/9/2 | 4/8/2 |
| Education (yrs) | 17 (2.5) | 16 (0.7) |
| Menstrual Cycle length (days) | 27 (2.6) | 29 (3.1) |
| BMI (kg/m2) | 22 (2.0) | 22 (2.1) |
| Cigarette smokers (#) | 0 | 2 |
| Users of other drugs (#) | 0 | 0 |
| Alcohol use (# drinks/week) | .33 (.30) | .22 (.29) |
Note: Data are presented as means (± SD) or as frequency. BMI: Body Mass Index.
All women were medically and psychiatrically (except for a diagnosis of PMDD in the PMDD women) healthy based on a detailed medical history and clinical interview. None of the participants were pregnant (based on urine pregnancy tests), taking oral contraceptives, hormones or any other prescription medication. The Structured Clinical Interview for DSM-IV (SCID I, First et al., 1995) was conducted by a trained clinical interviewer when the women were in the follicular phase of the menstrual cycle to rule out women with a current Axis I psychiatric disorder (including substance abuse or eating disorders), except for PMDD in the PMDD group.
To initially screen for PMDD, women completed the Premenstrual Assessment Form (Halbreich et al., 1982), a retrospective self-report questionnaire (composite scores on 18 factors) regarding changes in mood, behavior and physical symptoms for the previous three menstrual cycles. To accurately determine the presence or absence of PMDD, all women filled out the Daily Ratings Form (Endicott et al., 1986) each evening for at least two menstrual cycles before starting the study (see below for details). The criterion for PMDD women was defined as an average increase of at least 2 points (i.e., 30% increase on a 6-point scale; NIH guidelines) for the 5 days immediately preceding the onset of menstruation (late luteal phase) compared to the five postmenstrual days (i.e., days 6–10 after the onset of menstruation or the follicular phase). That is, women had to show premenstrual symptoms during the late luteal phase and a symptom-free period of at least 5 days during the follicular phase of the menstrual cycle. Those women with an average score of 3 or greater during the follicular phase (suggestive of other mood disorders) were excluded. The criterion for Control women was defined as an average increase of less than 1 point on the Daily Ratings Forms for the 5 days immediately preceding the onset of menstruation (late luteal phase) compared to the five postmenstrual days.
The study was approved by the Institutional Review Board of the New York State Psychiatric Institute. Participants gave their written informed consent before beginning the study and were paid for their participation. Participants were told that the purpose of the study was to investigate alterations in a woman’s mood, behavior and ability to perform certain tasks during different phases of the menstrual cycle and to compare women with and without PMDD.
Design and General Procedures
The women participated as outpatients at the New York State Psychiatric Institute for a total of 10 sessions. Data were collected on a range of subject-rated, observer rated and performance measures over a 4 h time course (see Evans et al., 1998). Desires for specific foods across the menstrual cycle and food intake at lunch were measured as well. All participants had two practice sessions (usually during the late follicular or early luteal phase of the menstrual cycle) to familiarize them with the routines to be followed and to provide training on the performance tasks. These data were not analyzed. Participants then started the testing phase, which consisted of eight testing sessions: Four sessions were scheduled during the late luteal phase (1–5 days before the onset of menstruation) and the other four sessions were scheduled during the follicular phase (6–10 days after the onset of menstruation). Sessions were conducted over two different cycles (2 sessions premenstrually and 2 sessions postmenstrually during each cycle) and the menstrual cycle phase that participants were started in was counterbalanced between the two groups and across women (i.e., half of the women in each group started testing in the follicular phase). Sessions were scheduled based upon the changes in mood premenstrually in the PMDD group, menstrual cycle length, the onset of menstruation and ovulation. Mood, menstrual cycle length and onset of menstruation were obtained from the Daily Ratings Form that participants filled out each evening. To determine ovulation, participants provided daily urine samples to determine the time of ovulation using OvuQuick® (QUIDEL Corp., San Diego, CA; Martini et al, 1994) during the midfollicular phase. This test is simple to use and is 96–99% accurate at detecting luteinizing hormone (LH) in urine. In the event that a woman began menstruating earlier than expected, failed to ovulate, or a session could not be scheduled for other reasons, missed sessions were rescheduled during the correct phase of the next menstrual cycle.
Experimental Session
Participants reported to the laboratory at approximately 9:00 a.m. and remained until approximately 2:30 p.m. They were instructed not to eat breakfast before reporting to the laboratory and to refrain from using alcohol and all psychoactive drugs (with the exception of tobacco and caffeinated products) the day before and the day of an experimental session. Upon arrival each session, a urine specimen was collected and analyzed for the presence of illicit drugs, a breath alcohol test was conducted to test for the presence of alcohol in expired air. When possible, blood was drawn for hormone assays in the morning before the tasks began (between 9–10 am). Weekly, urine pregnancy tests were performed. Participants first filled out a Food Desirability Questionnaire (see Evans et al., 1999) and then selected their lunch for that day (see below for details). After selecting lunch, they were served a light breakfast approximately 45 min before the beginning of the session. Breakfast typically consisted of a bagel, cereal or waffles, juice and a caffeinated beverage (for those women who regularly consumed caffeine). Participants were given the same breakfast on all subsequent sessions. Throughout the session, participants completed an assessment battery of various computerized questionnaires and performance tasks at specified times, described below. Approximately 3.5 h after the beginning of the session, women were given 30 min to eat the lunch items they had selected earlier that morning. Between breakfast and lunch the women were only allowed to consume water. Upon completion of the study, women with PMDD were informed about possible treatment options and given referrals if interested.
Measures
Mood-Related Measures
Daily Ratings Form (DRF)
This modified rating scale (Endicott et al., 1986) was completed each evening to accurately diagnose whether a woman has clinically meaningful premenstrual mood changes and to determine when a woman was menstruating. Participants were provided stamped envelopes and were required to mail these forms in daily to ensure the prospective nature of these ratings and eliminate the bias of referring to ratings from previous days. The form consists of 21 items describing problems with mood, behavior and physical symptoms. Three additional items determined if any of these problems interfered with work or school, social activities or interpersonal relationships. Women rated the severity of each of these symptoms on a 6-point scale, from 1 (“not at all”) to 6 (“extreme”). The measure used to determine the level of premenstrual symptoms each day was the mean score of all 24 items. Women also indicated their alcohol intake each day and if they were spotting or menstruating.
Beck Depression Inventory II (BDI II)
This 21-item self-report questionnaire was completed once at the beginning of each session (Beck et al., 1996). A score of 16 or greater is indicative of clinical depression.
State-Trait Anxiety Inventory (STAI)
The State component of this self-report questionnaire (Spielberger et al., 1970) consists of 20 items rating state anxiety. The State Anxiety Inventory was completed at baseline, 0.5, 1, 2, 3 and 4 h during the session.
Profile of Mood States (POMS)
For this 72-item Profile of Mood States questionnaire (POMS; McNair et al. 1971), 10 scales were analyzed (see Evans et al. 2000 for details). Participants rated each item on a 5-point scale from 0 (“not at all”) to 4 (“extremely”) by pressing keys on a keypad. To have all subscales on a similar 5-point scale, total scores for each subscale were divided by the number of items used to determine the subscale score. The POMS was completed at baseline, 0.5, 1, 2, 3 and 4 h during the session. Data were split into two POMS subscales: positive POMS subscales (Positive Mood, Elation, Vigor, Friendliness, Arousal) and negative POMS subscales (Tension-Anxiety, Depression-Dejection, Anger-Hostility, Fatigue, Confusion).
Observer-Rated Questionnaire
Observer ratings were completed by trained research assistants who were blind to study group. The participant was rated on a 5-point scale, from 0 (normal) to 4 (extreme impairment or disruption) on seven dimensions representative of mood changes associated with PMDD. Observers were instructed to base their ratings on observation of the participant’s behavior rather than on the participant’s verbal reports or ratings. Observer ratings were completed at baseline, 0.5, 1, 2, 3 and 4 h during the session. Data were split into two POMS subscales: Positive Mood consisted of friendly, cooperative and talkative and Negative Mood consisted of tired, short-tempered, confused and clumsy.
Performance Measures
All of the performance measures described below, with the exception of the Word Recall task, were conducted at baseline, 0.5, 1, 2, 3, and 4 h during each session (see Evans et al., 2000 for details).
Word Recall/Recognition Task
One hour after the onset of each session, participants studied a list of 12 common nouns (from a pool of 1000 nouns derived from Thorndike & Lorge, 1944) for 90 s, then they had to write as many of the words as they could remember. Delayed free recall was tested at 2 h and 4 h. For the recognition test, participants had to identify from this list the 12 words they had been shown 4 h earlier. The four dependent measures were immediate word recall, delayed word recall (2 and 4 h) and delayed word recognition.
Digit-Recall Task
For the 3 min digit-recall task, an 8-digit number was displayed on the computer screen. Participants were instructed to correctly enter each number while it was on the screen and again after it had disappeared from the screen. They were also told that they would be asked to re-enter and recognize one of the numbers near the end of the performance battery. The three primary measures were immediate digit recall, delayed digit recall and delayed digit recognition.
Digit Symbol Substitution Test (DSST)
In each trial, a randomly generated number (1–9), appears at the bottom of the screen, indicating which of the arrays displayed at the top of the screen should be reproduced. Participants were instructed to press the keys in a 3-row by 3-column keypad that corresponded to the pattern associated with the randomly generated number. The two dependent measures were total attempts to complete arrays and total arrays correctly completed.
Divided-Attention Task (DAT)
This 10-min task consists of concurrent pursuit-tracking and vigilance tasks (Miller et al., 1988). For the central tracking component, participants tracked a randomly moving circle on a computer screen with a cross-hair controlled by movement of the mouse, and were instructed to keep the cross-hair within the circle. The peripheral-vigilance task required a response (click on the mouse) when a small black square appeared at any of the four corners of the screen. The four primary measurements were tracking speed, false alarms, tracking distance and correct detections (hits).
Repeated Acquisition of Response Sequences
At the start of the 3-min learning task, four buttons were illuminated, and participants were instructed to learn a 10-response sequence of button presses (Kelly et al., 1992). The 10-response sequence remained the same throughout the 3-min task, but a new random sequence was generated when the task occurred again. The two dependent measures were the number of sequences completed and the number of errors.
Balance Task
This task assessed the participant’s ability to stand upright for a maximum of 30 s on each foot (Evans et al., 1994). The score was the total number of seconds the participant was able to balance (maximum of 60 s).
Food-Related Measures
Food Desirability Questionnaire
Participants completed the Food Desirability Questionnaire (Evans et al., 1999) before other assessments each session to avoid any influence from other factors including breakfast or selecting lunch. This locally-derived questionnaire consists of 38 food and beverage items. Participants were instructed to rate how much they would like to eat each item that day on a 5-point scale, from 0 (“not at all”) to 4 (“extremely”). For data analyses, ratings for individual items were grouped according to food type to derive six different food categories (see Evans et al., 1999 for details): (1) savory carbohydrate (CHO)/fat foods (e.g. potato chips); (2) sweet CHO/fat foods (e.g. chocolate candy); (3) protein/fat foods (e.g. hamburger); (4) CHO alone foods (e.g. bread); (5) beverages (e.g. soda); and (6) alcohol.
Food Selection and Consumption Procedure
After completing the Food Desirability Questionnaire, but before eating breakfast, participants selected their lunch for that day. A binder containing the original pictures or wrappers of all available food items was placed in front of the participant. These food items consisted of a wide variety of single-serving standard calorie and low-calorie food items including: grain/cereal items (e.g. cornflakes, bagel), fruit/salad items (e.g. banana), hot drinks, cold drinks, snack items (e.g. 1 oz bag of Wise® potato chips, 1.5 oz box of Sun-Maid® raisins), condiments (e.g. sugar, salad dressing), sandwich items (e.g. cheese slices, 2.6 oz can of Bumble Bee® tuna), frozen meal items (e.g. Stouffer’s® fish filet with macaroni and cheese, Stouffer’s® Lean Cuisine chicken breast in wine sauce). Participants were instructed to peruse the food binder and to mark on a food sheet each food item and the quantity desired for lunch that day (e.g. two slices of cheese). There was no restriction on the number of food items or quantity of any item than a participant could order, but no changes could be made when lunch was served. After the third assessment battery, a research assistant presented the participant with a tray containing all of the food items selected for lunch. Participants were never told that their food intake was being measured. They were instructed that they did not have to eat all of their lunch, but that they could not hoard food, share food with others, or throw food or wrappers away; all uneaten food and wrappers had to remain on the tray. To ensure this, participants were observed as they ate lunch via a one-way mirror. After the completion of lunch, the trash was removed. Research assistants measured the amount of food consumed and then calculated total food intake at lunch [(total energy intake, g-intake of carbohydrate, fat, and protein, percent of energy intake derived from each macronutrient estimated as kcal from g-intake using Atwater factors (McLaren, 1976) based on the caloric and macronutrient information provided by the manufacturers].
Other Measures
Vital Signs
Heart rate and blood pressure were measured each session at baseline, 0.5, 1, 2, 3, and 4 h using a Sentry II vital signs monitor (Model 6100; NBS Medical Services, Costa Mesa, CA).
Hormone Assays
Each session, venous blood samples (approximately 6 ml) for estradiol and progesterone were drawn into tubes containing SST® gel and clot activator. Samples were centrifuged within 30 min of collection, yielding approximately 3 ml of plasma, and stored frozen until the time of analysis. Estradiol and progesterone levels were determined by Dr. Michel Ferin at the College of Physicians and Surgeons of Columbia University, Department of Obstetrics and Gynecology (New York, NY). Estradiol and progesterone were measured by a commercial solid-phase, chemiluminescent immunoassay (Immulite, Diagnostic Products Co., DPC, Los Angeles, CA). For estradiol, the assay sensitivity was 4 pg/ml and the intra- and interassay coefficients of variation were 4.3 and 10.5%. For progesterone, the assay sensitivity was 0.2 ng/ml and the intra- and interassay coefficients of variation were 4.8 and 9.1%.
Data Analysis
Analyses were based on the 29 women who completed the entire study. For hormone levels, no hormone samples were obtained from three PMDD women and three Control women due to technical difficulties or refusal to have their blood drawn. However, all of the women had normal cycles based on the urinary ovulation kits. Because the pattern of the missing values was not related to PMDD status or menstrual cycle phase, i.e., data were missing at random (Little & Rubin, 1987), plausible estimates of the missing values were calculated using multiple imputation (Rubin & Schenker, 1991).
The results from the two practice days were not included in the data analyses. For all measures assessed during the eight testing sessions, separate three-factor repeated measures analyses of variance were conducted. The three factors were group (PMDD vs. Control), phase (late luteal vs. follicular) and session days (days 3 – 10). Since there were little or no effects of session days across any of the measures, only main effects of group and phase and group × phase interaction effects are reported in the results. For all measures that were assessed multiple times within each session (DAT, DSST, Repeated Acquisition Task, Digit Recall, State STAI, POMS, Balance, Observer-Ratings and Vital Signs), peak measurement during each session was used. The direction of the peak effect was based on the hypothesized direction of effects in PMDD women during the luteal phase.
For each measure, planned contrasts were used to compare: (1) PMDD women in their luteal phase to their follicular phase; (2) Control women in their luteal phase to their follicular phase; (3) PMDD women to Control women during their luteal phase; and (4) PMDD women to Control women during their follicular phase. For all analyses, results were considered statistically significant if p ≤ 0.05, using Huynh-Feldt corrections as a conservative measure to control for potentially uncorrelated within-subject data.
RESULTS
Hormone Levels
All women had ovulatory menstrual cycles that ranged from 23 to 33 days. When examining hormone levels as a function of group and phase, estradiol levels were not significantly different in the luteal phase compared to the follicular phase in the PMDD and Control women (96.35 ± 8.99 pg/ml vs. 74.28 ± 10.68 pg/ml; p ≥ 0.05). Regarding progesterone levels, there was a main effect of phase [F(1,21) = 113.82, p = 0.0001]. Specifically, progesterone levels in the luteal phase were significantly higher than in the follicular phase in both the PMDD and Control women (6.96 ± 0.62 ng/ml vs. 0.58 ± 0.05 ng/ml; p ≤ 0.05), with no difference between the two groups.
Mood Questionnaires
Figure 1 documents the mood changes based on the DRF and BDI scores as a function of menstrual cycle phase and PMDD status. Although both groups reported significantly greater dysphoric mood on their DRFs during the luteal phase [phase effect: F(1,27) = 101.85, p = 0.0001], PMDD women reported significantly greater dysphoric mood on their DRFs than the Control women overall [group effect: F(1,27) = 48.21, p = 0.0001]. In particular, dysphoric mood on the DRFs was greatest in the PMDD women in their luteal phase than Control women in their luteal phase [group × phase interaction: F(1,27) = 45.46, p = 0.0001]. There were no significant differences in self-reported alcohol use on the DRFs as a function of menstrual cycle phase or between groups (p ≥ 0.05).
Figure 1.
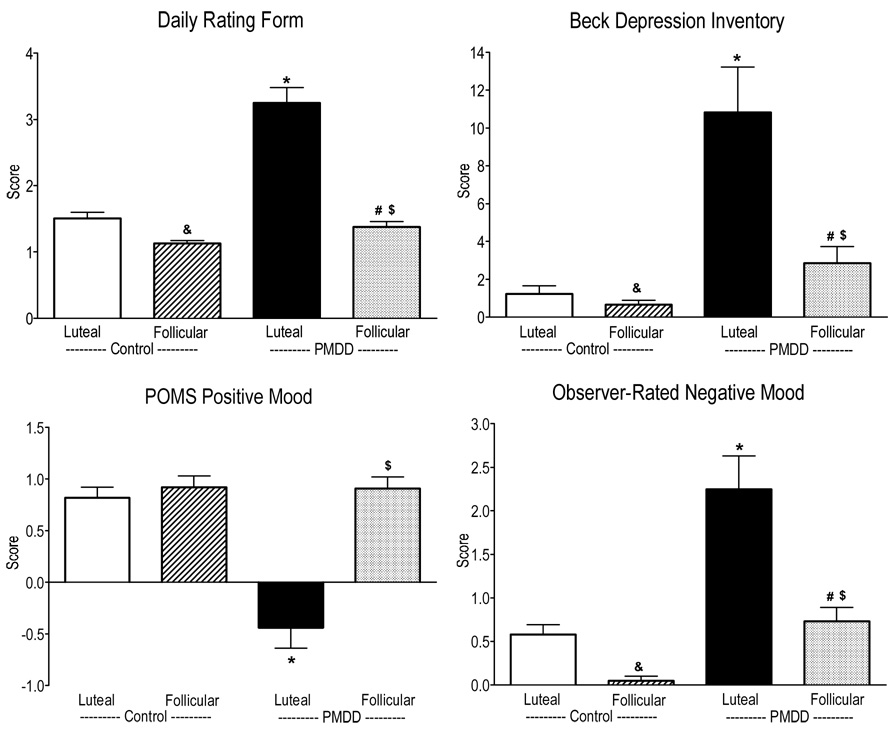
Scores on the Daily Ratings Form, the Beck Depression Inventory, POMS Positive Mood and Observer-Rated Negative Mood averaged over the sessions as a function of menstrual cycle phase and PMDD status. * denotes a significant difference between PMDD women in their luteal phase and Control women in their luteal phase (p ≤ 0.05). # denotes a significant difference between PMDD women in their follicular phase and Control women in their follicular phase (p ≤ 0.05). $ denotes a significant difference between PMDD women in their luteal phase compared to their follicular phase (p ≤ 0.05). & denotes a significant difference between Control women in their luteal phase compared to their follicular phase (p ≤ 0.05). Error bars represent 1 SEM.
Similar to the DRF scores, Figure 1 shows that depression scores on the BDI were significantly greater during the luteal phase than the follicular phase in both groups [phase effect: F(1,27) = 14.46, p = 0.0007] and PMDD women had significantly greater depression scores than Control women overall [group effect: F(1,27) = 21.78, p = 0.0001]. Further, during the luteal phase, BDI scores were higher in PMDD women compared to Control women [group × phase interaction: F(1,27) = 10.91, p = 0.003]. On the State Anxiety Inventory, PMDD women had significantly greater State anxiety scores during their luteal phase than during their follicular phase [phase effect: F(1,27) = 18.15, p = 0.0002] and compared to Control women in their luteal phase [group × phase interaction: F(1,27) = 13.19, p = 0.001].
On the POMS, as shown in Figure 1, peak scores on the positive POMS subscales were significantly lower in PMDD women compared to Control women during the luteal phase [group × phase interaction: F(1,27) > 6.30, p < 0.02]. Correspondingly, peak scores on the negative POMS subscales were significantly higher during the luteal phase compared to the follicular phase in both groups [phase effect: F(1,27) > 10.09, p < 0.004], although these scores were higher in PMDD women compared to Control women during the luteal phase [group × phase interaction: F(1,27) > 6.65, p < 0.02].
Figure 1 also shows that peak observer-rated Negative Mood scores were significantly higher in the luteal phase compared to the follicular phase in both groups of women [phase effect: F(1,27) = 21.93, p = 0.0001], although these scores were substantially higher in PMDD women compared to Control women [group × phase interaction: F(1,27) = 4.96, p = 0.04]. Even in the follicular phase, PMDD women had significantly higher observer-rated Negative Mood scores than Control women (p ≤ 0.05). Correspondingly, peak observer-rated Positive Mood scores were significantly lower in the luteal phase compared to the follicular phase in both groups of women [phase effect: F(1,27) = 10.70, p = 0.003], with scores substantially lower in PMDD women compared to Control women [group × phase interaction: [F(1,27) = 4.08, p = 0.05].
Together, these results show that measures of negative mood were higher in PMDD women in their luteal phase compared to Control women in their luteal phase, and, for some measures, were higher compared to the follicular phase in the PMDD women as well.
Psychomotor Performance and Memory
Figure 2 documents the scores of the Immediate Digit Recall, Delay Digit Recognition, 4-h Delayed Word Recall and total attempts on the DSST as a function of menstrual cycle phase and PMDD status. There was a trend for PMDD women in their luteal phase to have worse immediate digit recall [group × phase interaction: F(1,27) = 3.24, p = 0.08] compared to Control women in their luteal phase. PMDD women had significantly worse delayed digit recognition [group × phase interaction: F(1,27) = 4.74, p = 0.04] compared to Control women in their luteal phase. On the word recall and recognition task, PMDD women in the luteal phase had significantly worse 4-h delayed word recall than Control women in their luteal phase [phase × group interaction: F(1,27) = 6.26, p = 0.02]. Further, planned contrasts showed that immediate word recall, 2-h delayed word recall and delayed word recognition were also significantly worse in PMDD women in their luteal phase than Control women in their luteal phase (p ≤ 0.05). On the DSST, PMDD women in their luteal phase scored significantly lower on the number of attempts to complete arrays than Control women in their luteal phase [group × phase interaction: F(1,27) = 7.56, p = 0.01]. On the DAT, there was a trend for both PMDD and Control women to have worse tracking speed during the luteal phase than the follicular phase [phase effect: F(1,27) = 3.89, p = 0.06]. There were no significant differences on any of the other performance measures as a function of group or menstrual cycle phase (p ≥ 0.05).
Figure 2.
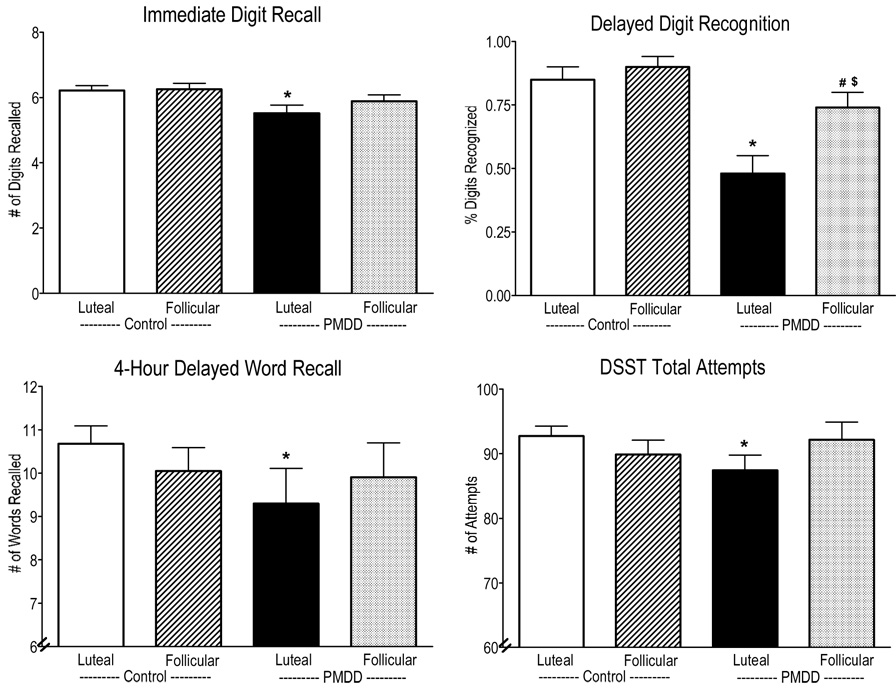
Ratings of the Immediate Digit Recall, Delayed Digit Recognition, 4-hour Delayed Word Recall and DSST total attempts averaged over the sessions as a function of menstrual cycle phase and PMDD status. See Figure 1 for details.
Overall, for most of the cognitive tasks, PMDD women in their luteal phase had worse performance than Control women in their luteal phase.
Food-related Questionnaires and Food Intake
Figure 3 documents scores on the Food Desirability Questionnaire as a function of menstrual cycle phase and PMDD status. Desire for foods containing savory fat/CHO, sweet fat/CHO and protein/fat was greater in the luteal phase than the follicular phase [phase effect: F(1,27) = 20.16, p = 0.0001]. Specifically, PMDD women in their luteal phase had a greater desire for savory fat/CHO, sweet fat/CHO and protein/fat compared to their follicular phase (p ≤ 0.05) and compared to Control women in their luteal phase (p ≤ 0.05). In contrast, desire for CHO alone foods did not vary as a function of menstrual cycle phase or group. While the overall desire for alcohol was relatively low in both groups, the PMDD group did show an increased desire for alcohol compared to the Control group (p ≤ 0.05).
Figure 3.
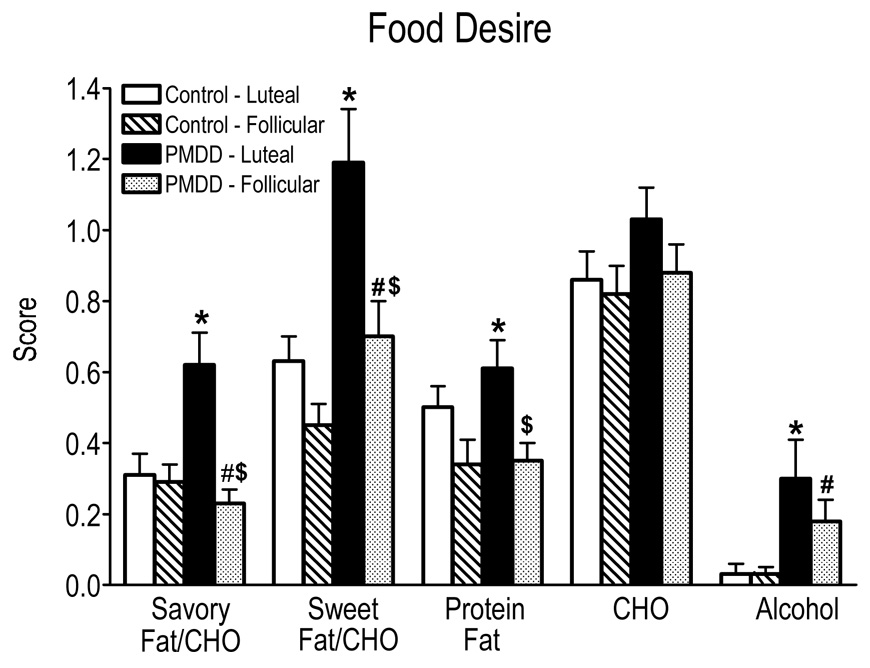
Ratings of food desires for each of the six food groups averaged over the sessions as a function of menstrual cycle phase and PMDD status. CHO = carbohydrates. See Figure 1 for details.
Figure 4 documents the total number of calories ordered and consumed during lunch, including the macronutrient content as a function of menstrual cycle phase and PMDD status. Overall, there was a significant increase in the number of calories ordered [phase effect: F(1,27) = 9.99, p = 0.004] and eaten [phase effect: F(1,27) = 4.65, p = 0.04] in the luteal phase compared to the follicular phase. This was due to the fact that PMDD women tended to order more (p ≤ 0.06) and significantly eat more (p ≤ 0.05) calories in their luteal phase than in their follicular phase and compared to Control women in their luteal phase. Specifically, in the luteal phase PMDD women ordered and ate approximately 100 more calories at lunch compared to their follicular phase. This represented a caloric increase of 16% during the luteal phase in PMDD women, whereas Control women only increased their lunch consumption by 3%. The bottom panel of Figure 4 shows that when broken down by macronutrient content, fat [phase effect: F(1,27) = 8.40, p = 0.008] and protein [group × phase interaction: F(1,27) = 5.37, p = 0.03] intake were significantly greater during the luteal phase than during the follicular phase. This again was due to PMDD women eating significantly more fat (64 calories; p ≤ 0.05) and tending to eat more protein (p ≤ 0.06) in the luteal phase compared to their follicular phase and compared to Control women in their luteal phase.
Figure 4.
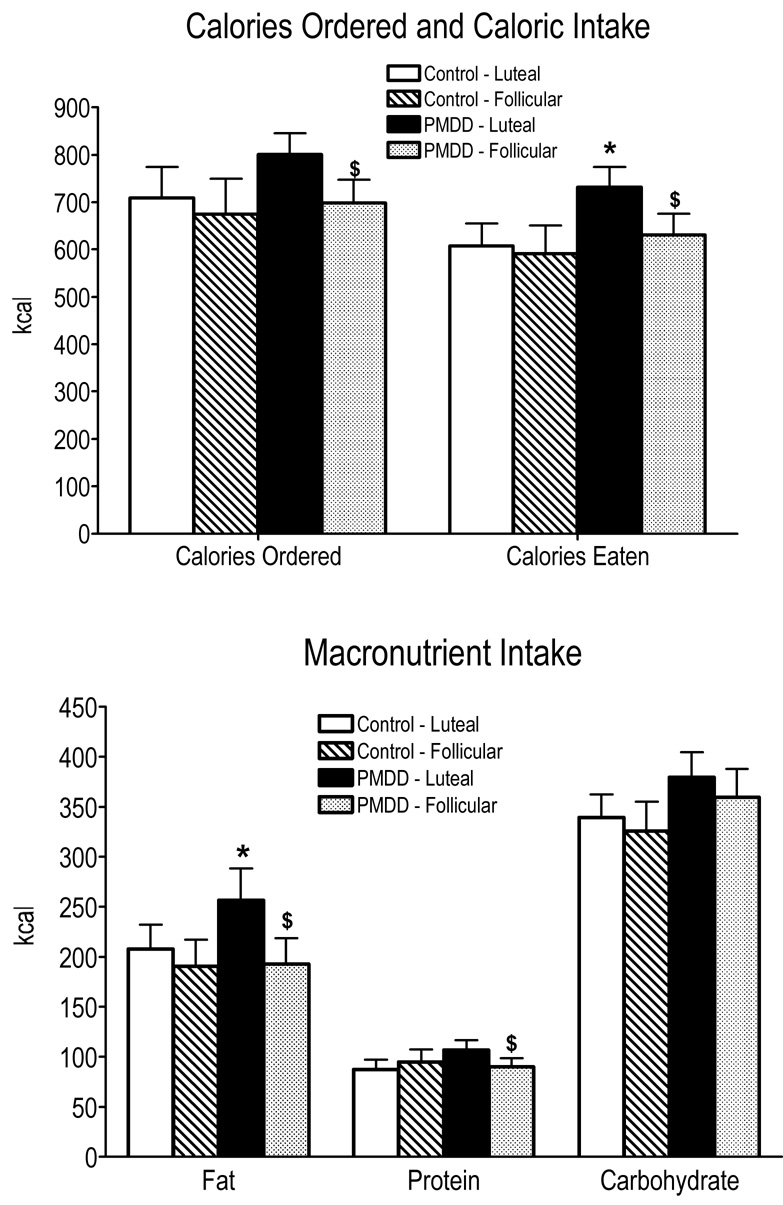
Number of calories ordered and consumed and macronutrient content of calories consumed averaged over the sessions as a function of menstrual cycle phase and PMDD status. See Figure 1 for details.
Taken together, the data show that PMDD women in their luteal phase desired, ordered, and ate more calories (primarily fat) than in their follicular phase or compared to Control women in their luteal phase.
Vital Signs
Peak systolic pressure was significantly higher in the luteal phase than in the follicular phase [phase effect: F(1,27) = 4.70, p = 0.04]; this was due to a trend in systolic pressure being higher in the PMDD women in their luteal phase compared to their follicular phase (p = 0.08). There were no significant differences in peak heart rate or diastolic pressure as a function of group or menstrual cycle phase.
DISCUSSION
As hypothesized, the current study found that women with PMDD in their luteal phase: (1) had increased dysphoric mood; (2) had impaired cognitive performance; (3) had an increased desire for food items high in fat (both savory and sweet); and (4) ate more calories (particularly fat) at lunch compared to when they were in their follicular phase and/or compared to women without PMDD.
In the present study, women were carefully prospectively screened and those women in the PMDD group met full DSM-IV criteria for PMDD, i.e., they experienced substantial mood changes, physical symptoms and changes in appetite during the luteal phase, which resolved during the follicular phase. In contrast, Control women experienced minimal changes in these symptoms across the menstrual cycle. DRF scores and measures related to anxiety (e.g. the State Anxiety Inventory, Tension-Anxiety scores on the POMS) and depression (Depression-Dejection scores on the POMS and BDI scores) were significantly increased in the luteal phase of women with PMDD compared to the follicular phase and compared to Control women. These results are consistent with previous studies documenting dysphoric mood during the luteal phase in women with PMDD (Evans et al., 1998; Keenan et al., 1995; Man et al., 1999; Rapkin et al., 1989; Resnick et al., 1998).
In addition to examining mood changes in women with PMDD, this study concurrently assessed cognitive task performance multiple times over approximately 5 hours. Performance on some (e.g. Word Recall, Digit Recall, DSST), but not all, cognitive tasks was impaired during the luteal phase in PMDD women compared to their follicular phase and compared to Control women in their luteal phase. These data extend our previous findings showing that women with PMDD had some performance impairment during the luteal phase (Evans et al., 1998).
Several other studies in women with PMDD have reported modest performance impairment on isolated tasks or no differences in performance. For instance, Keenan et al. (1992b, 1995) showed that verbal recall, but not performance on other tasks, was impaired in women with PMDD compared to women without PMDD, irrespective of menstrual cycle phase. In another study, women with PMDD showed more psychomotor slowing during the luteal phase compared to the follicular phase (Resnick et al., 1998). Although Man et al. (1999) showed that working memory was impaired during the luteal phase, this was true for both PMDD and Control women. Conversely, attention, memory, cognitive flexibility and overall mental agility was not impaired in PMDD women in their luteal phase compared to Control women during their luteal phase (Morgan & Rapkin, 2002). While the studies mentioned above all tested a range of cognitive tasks and included a control group, they only tested task performance on a single occasion each phase. This may have limited their ability to detect differences in performance. The strength of the present study compared to previous studies included the fact that all women had extensive training on the tasks, both groups of women were tested on several days each phase, and task performance was assessed multiple times within each day to simulate a modified workday. Even under these conditions, PMDD women did not show impairment on all tasks during the luteal phase and when impairment was observed, it was relatively modest. Taken together, these data suggest that women with PMDD experience significant dysphoric mood in the luteal phase, accompanied by subtle impairment in performance, particularly memory skills. This mild cognitive impairment during the luteal phase could be exacerbated by the concomitant administration of alcohol, or medications to treat premenstrual symptoms. For instance, although some studies (e.g. Berger & Presser, 1994; Freeman et al., 1995), showed that alprazolam was effective in reducing premenstrual mood symptoms, alprazolam also impairs cognitive performance and memory and has abuse liability (see Evans et al., 1998), which limited its acceptance as a treatment for PMDD.
Another noted feature of PMDD is reported increases in appetite, either food cravings or food intake, during the luteal phase (e.g. Both-Orthman et al., 1988; Dye & Blundell, 1997; Wurtman et al., 1989). In the present study, women with PMDD desired and ate more foods, particularly those food items high in fat, in their luteal phase compared to their follicular phase and compared to Control women. Specifically, lunch intake in women with PMDD increased by 100 calories (16%) in the luteal phase (compared to an increase of only 16 calories in Control women) than the follicular phase at lunch, with the majority of those calories derived from fat. However, there were no differences in desire or actual intake of foods predominantly consisting of carbohydrates as a function of menstrual cycle phase or PMDD status. These findings extend those of our previous study (Evans et al., 1999) showing that women with PMDD had increased cravings for foods high in fat and protein, but not carbohydrates alone, during the luteal phase. However, in our previous study increases in food intake in the luteal phase were not observed, perhaps because that study was limited to a single non-drug day each phase (Evans et al., 1999).
Similar to the present study, Cross et al. (2001) found that women with PMS reported increased intake of fat and simple sugars premenstrually, but not carbohydrate-specific intake. Earlier studies found that women with PMS in their luteal phase generally had an increased self-reported desire of “sweets” compared to their follicular phase (Morton et al., 1953; Smith & Sauder, 1969). However, a more recent study found minimal changes in self-reported food intake or macronutrient content as a function of PMS status or menstrual cycle phase (Bryant et al., 2006). Unfortunately, many of those studies examined women with PMS, not PMDD, and, for the most part, relied on self-reported food desires and food intake.
Only two studies have measured actual food intake in women with severe PMS while residing in a laboratory. In one of those studies (Wurtman et al., 1989), daily food intake (meals and snacks) increased by 21% (500 calories) in PMS women during the luteal phase compared to the follicular phase. Similar increases in daily food intake were obtained in a subsequent study by the same laboratory (Brzenzinski et al., 1990), whereas treatment with d-fenfluramine, a drug that increases serotonin levels, decreased food intake back to follicular phase levels and also improved mood. Unlike the present study, these two studies provided high carbohydrate and high protein foods, but held the fat content of foods relatively constant, which may have limited the ability to see greater increases in fat intake. Presumably, greater increases in food intake would have been observed in the present study if we had been able to measure food intake over the entire day. It should be noted that these luteal phase increases in daily food intake (approximately a 500 calorie increase) among women with PMDD are somewhat lower than what other studies have observed following the administration of certain drugs. Specifically, in well-controlled residential studies, the benzodiazepine alprazolam (Haney et al., 1997) increased daily food intake by 1,000 calories, whereas smoked marijuana or the cannabinoid agonist, dronabinol, increased daily food intake by 500 to 1,000 calories (Haney et al., 2004, 2007).
Although treatments for PMS and PMDD have focused on hormone-based interventions or serotonin specific reuptake inhibitors (SSRIs) to improve the dysphoric mood symptoms, to our knowledge, no pharmacological studies have been conducted to specifically treat the symptoms of increased appetite and food cravings in women with PMDD. With respect to hormonal interventions, one study found that danazol increased weight gain and craving for sweets (Halbreich et al., 1991) in women with PMS, while an oral contraceptive containing drospirenone and ethinyl estradiol significantly improved increased appetite and food cravings compared to baseline (Freeman et al., 2001). The effectiveness of SSRI treatment on food cravings and appetite in women with PMDD has been inconsistent (Eriksson et al., 1995; Yonkers et al., 1996; Su et al., 1997). For example, sertraline did not alter food cravings or appetite compared to placebo or desipramine (Freeman et al., 1999a) or when full- and half-cycle treatments of sertraline were compared (Freeman et al., 1999b). In fact, several studies have shown that long-term treatment with SSRIs can result in significant weight gain (e.g. Fogelson, 1991; Harvey & Bouwer, 2000). Consequently, women with PMDD may need to be cautioned about the risks of increased food cravings and food intake, particularly in combination with specific drugs or medications (e.g. marijuana), and pharmacological treatments for PMDD should focus on those medications that are less prone to these side effects.
The mechanism underlying PMS/PMDD symptoms may be hormonal or serotonergic. Although there are no differences in estrogen and progesterone levels between women with and without PMS (Rubinow et al., 1988), levels of progesterone (Redei & Freeman, 1995) or estrogen (Seippel & Backstrom, 1998) have been shown to correlate with the severity of PMS symptoms. Despite this, the overwhelming majority of double-blind studies have failed to show that progesterone is effective for the treatment of PMS or PMDD (e.g. Sampson, 1979; Maddocks et al., 1986). In contrast, SSRIs are currently the most widely used effective treatments for the mood disturbances associated with PMDD (see Rapkin, 2003 for review). In addition to mood, the serotonergic system has also been implicated in other symptoms associated with PMDD, including food cravings (Rapkin, 1992; Dye & Blundell, 1997) and cognitive deficits (e.g. Chamberlain et al., 2006; Constant et al., 2005). For example, in women with premenstrual complaints, serotonergic stimulation improved memory deficits (Schmitt et al., 2005). More recently, studies have shown that neuroactive steroids also vary across the menstrual cycle, that some of their effects are mediated by progesterone receptors, and that women with PMDD may have reduced levels of these neuroactive steroids (see Rupprecht, 2003 for review). The relationship between these neuroactive steroids and the serotonergic system may explain why SSRIs are effective for treating PMDD (Steiner et al., 1995). Although the present study was not designed to address the neurochemical mechanism underlying the various symptoms of PMDD, taken together, increases in negative mood, food cravings and appetite, and cognitive deficits, each have been separately linked to serotonin deficits, which may also involve alterations in neuroactive steroid function. Thus, medications that alter the serotonin system and/or neuroactive steroids to concurrently treat the full range of PMDD symptomology appear to be promising and warrant further examination.
With respect to alcohol use, several clinical studies and surveys suggest that women with moderate to severe PMS tend to drink more premenstrually, purportedly to self-medicate their dysphoric symptoms, and may be at increased risk for developing alcoholism (e.g. Podolsky, 1963; Price et al., 1987; McLeod et al., 1994). However, relatively few studies have supported this when alcohol consumption has been prospectively monitored across the menstrual cycle in this population. Tobin et al. (1994) failed to show any change in prospective self-reported alcohol intake across the menstrual cycle in women with PMS and there was no correlation with dysphoric symptoms. In the present study, although women with PMDD reported a greater desire for alcohol in their luteal phase compared to the follicular phase or compared to Control women, this increase was quite small, and there was no evidence that women in either group altered their alcohol consumption as a function of menstrual cycle phase. The findings in the current study replicate our previous study in women with PMDD (Evans et al., 1999). The failure to show a greater increase in alcohol craving or alcohol intake is most likely due to the fact that the women in the present study were all light drinkers (less than one drink per day). However, Mello et al. (1990) reported that among female social drinkers who varied in their drinking levels (from light to heavy drinkers), those who experiences greater premenstrual symptoms based on the Premenstrual Assessment Form (Halbreich et al., 1982) actually consumed more alcohol during the luteal phase. Therefore, there may be fluctuations in alcohol consumption across the menstrual cycle phase in women, with moderate to heavy drinkers with PMDD being at greater risk to increase their drinking during the luteal phase relative to light drinkers.
The present study had a number of procedural strengths and concurrently measured changes in mood, performance and appetite (both desires and intake). First, all women were prospectively tracked throughout the study and women assigned to the PMDD group met full DSM-IV criteria for PMDD. Second, this study compared PMDD women to a Control group of women. Third, all women were tested on four separate days during both the luteal and follicular phases of the menstrual cycle, with menstrual cycle phase objectively confirmed. Fourth, most assessments, including a range of cognitive performance tasks, were measured multiple times each day. Lastly, actual food intake, including macronutrient content, was measured. Despite these strengths, the main limitation was the small sample size, which may have reduced our power to detect more robust cognitive impairment. However, this still represents one of the first carefully controlled studies to evaluate the spectrum of PMDD symptoms, including mood, cognitive performance and food intake concurrently in women with PMDD.
ACKNOWLEDGMENTS
This research was supported by Grants R01 DA009114 and K02 DA00465 from the National Institute on Drug Abuse. The authors gratefully acknowledge the assistance the research and clinical staff.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: U.S. Department of Health and Human Services; 1994. [Google Scholar]
- Bancroft J, Williamson L, Warner P, Rennie D, Smith SK. Perimenstrual complaints in women complaining of PMS, menorrhagia, and dysmenorrhea: toward a dismantling of the premenstrual syndrome. Psychosom. Med. 1993;55:133–145. doi: 10.1097/00006842-199303000-00001. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck Depression Inventories -IA and -II in Psychiatric Outpatients. J. Pers. Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Berger CP, Presser B. Alprazolam in the treatment of two subsamples of patients with late luteal phase dysphoric disorder: a double-blind, placebo-controlled crossover study. Obstet. Gynecol. 1994;84:379–385. [PubMed] [Google Scholar]
- Bhatia SC, Bhatia SK. Diagnosis and treatment of premenstrual dysphoric disorder. Am. Fam. Physician. 2002;66:1239–1248. [PubMed] [Google Scholar]
- Both-Orthman B, Rubinow DR, Hoban MC, Malley J, Grover GN. Menstrual cycle phase-related changes in appetite in patients with premenstrual syndrome and in control subjects. Am. J. Psychiatry. 1988;145:628–631. doi: 10.1176/ajp.145.5.628. [DOI] [PubMed] [Google Scholar]
- Bryant M, Truesdale KP, Dye L. Modest changes in dietary intake across the menstrual cycle: implications for food intake research. Br. J. Nutr. 2006;96:888–894. doi: 10.1017/bjn20061931. [DOI] [PubMed] [Google Scholar]
- Brzezinski AA, Wurtman JJ, Wurtman RJ, Gleason R, Greenfield J, Nader T. d-Fenfluramine suppresses the increased calorie and carbohydrate intakes and improves the mood of women with premenstrual depression. Obstet. Gynecol. 1990;76:296–301. [PubMed] [Google Scholar]
- Chamberlain SR, Muller U, Robbins TW, Sahakian BJ. Neuropharmacological modulation of cognition. Curr. Opin. Neurol. 2006;19:607–612. doi: 10.1097/01.wco.0000247613.28859.77. [DOI] [PubMed] [Google Scholar]
- Constant EL, Adam S, Gillain B, Seron X, Bruyer R, Seghers A. Effects of sertraline on depressive symptoms and attentional and executive functions in major depression. Depress. Anxiety. 2005;21:78–89. doi: 10.1002/da.20060. [DOI] [PubMed] [Google Scholar]
- Cross GB, Marley J, Miles H, Willson K. Changes in nutrient intake during the menstrual cycle of overweight women with premenstrual syndrome. Br. J. Nutr. 2001;85:475–482. doi: 10.1079/bjn2000283. [DOI] [PubMed] [Google Scholar]
- De Ronchi D, Ujkaj M, Boaron F, Muro A, Piselli M, Quartesan R. Symptoms of depression in late luteal phase dysphoric disorder: a variant of mood disorder? J. Affect. Disord. 2005;86:169–174. doi: 10.1016/j.jad.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Dickerson LM, Mazyck PJ, Hunter MH. Premenstrual syndrome. Am. Fam. Physician. 2003;67:1743–1752. [PubMed] [Google Scholar]
- Diener D, Greenstein FL, Turnbough PD. Cyclical variation in digit-span and visual-search performance in women differing in the severity of their premenstrual symptoms. Percept. Mot. Skills. 1992;74:67–76. doi: 10.2466/pms.1992.74.1.67. [DOI] [PubMed] [Google Scholar]
- Di Giulio G, Reissing ED. Premenstrual dysphoric disorder: prevalence, diagnostic considerations, and controversies. J. Psychosom. Obstet. Gynaecol. 2006;27:201–210. doi: 10.1080/01674820600747269. [DOI] [PubMed] [Google Scholar]
- Dye L, Blundell JE. Menstrual cycle and appetite control: implications for weight regulation. Hum. Reprod. 1997;12:1142–1151. doi: 10.1093/humrep/12.6.1142. [DOI] [PubMed] [Google Scholar]
- Endicott J, Nee J, Cohen J, Halbreich U. Premenstrual changes: patterns and correlates of daily ratings. J. Affect. Disord. 1986;10:127–135. doi: 10.1016/0165-0327(86)90035-2. [DOI] [PubMed] [Google Scholar]
- Eriksson E, Hedberg MA, Andersch B, Sundblad C. The serotonin reuptake inhibitor paroxetine is superior to the noradrenaline reuptake inhibitor maprotiline in the treatment of premenstrual syndrome. Neuropsychopharmacology. 1995;12:167–176. doi: 10.1016/0893-133X(94)00076-C. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW, Fischman MW. Food "cravings" and the acute effects of alprazolam on food intake in women with premenstrual dysphoric disorder. Appetite. 1999;32:331–349. doi: 10.1006/appe.1998.0222. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Levin FR, Foltin RW, Fischman MW. Mood and performance changes in women with premenstrual dysphoric disorder: acute effects of alprazolam. Neuropsychopharm. 1998;19:499–516. doi: 10.1016/S0893-133X(98)00064-5. [DOI] [PubMed] [Google Scholar]
- Evans SM, Levin FR, Fischman MW. Increased sensitivity to alprazolam in females with a paternal history of alcoholism. Psychopharmacology. 2000;150:150–162. doi: 10.1007/s002130000421. [DOI] [PubMed] [Google Scholar]
- Evans SM, Troisi JR, II, Griffiths RR. Tandospirone and alprazolam: Comparison of behavioral effects and abuse liability in humans. J. Pharmacol. Exp. Ther. 1994;271:683–694. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I disorders - Patient Edition (SCID-I/P, version 2.0) New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- Fogelson DL. Weight gain during fluoxetine treatment. Journal of Clinical Psychopharmacology. 1991;11:220–221. [PubMed] [Google Scholar]
- Freeman EW, Kroll R, Rapkin A, Pearlstein T, Brown C, Parsey K, Zhang P, Patel H, Foegh M PMS/PMDD Research Group. Evaluation of a unique oral contraceptive in the treatment of premenstrual dysphoric disorder. J. Womens Health Gend. Based Med. 2001;10:561–569. doi: 10.1089/15246090152543148. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Rickels K, Arredondo F, Kao LC, Pollack SE, Sondheimer SJ. Full- or half-cycle treatment of severe premenstrual syndrome with a serotonergic antidepressant. J. Clin. Psychopharmacol. 1999b;19:3–8. doi: 10.1097/00004714-199902000-00002. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Rickels K, Sondheimer SJ, Polansky M. A double-blind trial of oral progesterone, alprazolam, and placebo in the treatment of severe premenstrual syndrome. JAMA. 1995;274:51–57. [PubMed] [Google Scholar]
- Freeman EW, Rickels K, Sondheimer SJ, Polansky M. Differential response to antidepressants in women with premenstrual syndrome/premenstrual dysphoric disorder: a randomized controlled trial. Arch. Gen. Psychiatry. 1999a;56:932–939. doi: 10.1001/archpsyc.56.10.932. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Sondheimer S, Weinbaum PJ, Rickels K. Evaluating premenstrual symptoms in medical practice. Obstet Gynecol. 1985;65:500–505. [PubMed] [Google Scholar]
- Futterman LA, Rapkin AJ. Diagnosis of premenstrual disorders. J. Reprod. Med. 2006;51:349–358. [PubMed] [Google Scholar]
- Halbreich U, Endicott J, Schacht S. Premenstrual syndromes: A new instrument for their assessment. J. Psychiatr. Treat. Eval. 1982;4:161–164. [Google Scholar]
- Halbreich U, Rojansky N, Palter S. Elimination of ovulation and menstrual cyclicity (with danazol) improves dysphoric premenstrual syndromes. Fertil. Steril. 1991;56:1066–1069. [PubMed] [Google Scholar]
- Haney M, Comer SD, Fischman MW, Foltin RW. Alprazolam increases food intake in humans. Psychopharmacology. 1997;132:311–314. doi: 10.1007/s002130050350. [DOI] [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, Comer SD, Reed SC, Foltin RW. Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse. Psychopharmacology. 2007 doi: 10.1007/s00213-007-1020-8. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, Nasser J, Bennett A, Zubaran C, Foltin RW. Marijuana withdrawal in humans: effects of oral THC or divalproex. Neuropsychopharmacology. 2004;29:158–170. doi: 10.1038/sj.npp.1300310. [DOI] [PubMed] [Google Scholar]
- Harvey BH, Bouwer CD. Neuropharmacology of paradoxic weight gain with selective serotonin reuptake inhibitors. Clin. Neuropharmacol. 2000;23:90–97. doi: 10.1097/00002826-200003000-00006. [DOI] [PubMed] [Google Scholar]
- Keenan PA, Lindamer LA, Jong SK. Psychological aspects of premenstrual syndrome. II: Utility of standardized measures. Psychoneuroendocrinology. 1992a;17:189–194. doi: 10.1016/0306-4530(92)90057-e. [DOI] [PubMed] [Google Scholar]
- Keenan PA, Lindamer LA, Jong SK. Menstrual phase - independent retrieval deficit in women with PMS. Biol. Psychiatry. 1995;38:369–377. doi: 10.1016/0006-3223(94)00303-K. [DOI] [PubMed] [Google Scholar]
- Keenan PA, Stern RA, Janowsky DS, Pedersen CA. Psychological aspects of premenstrual syndrome I: Cognition and memory. Psychoneuroendocrinology. 1992b;17:179–187. doi: 10.1016/0306-4530(92)90056-d. [DOI] [PubMed] [Google Scholar]
- Kelly TH, Foltin RW, King L, Fischman MW. Behavioral response to diazepam in a residential laboratory. Biol. Psychiatry. 1992;31:808–822. doi: 10.1016/0006-3223(92)90312-n. [DOI] [PubMed] [Google Scholar]
- Landen M, Nissbrandt H, Allgulander C, Sorvik K, Ysander C, Eriksson E. Placebo-controlled trial comparing intermittent and continuous paroxetine in premenstrual dysphoric disorder. Neuropsychopharmacology. 2007;32:153–161. doi: 10.1038/sj.npp.1301216. [DOI] [PubMed] [Google Scholar]
- Little RJA, Rubin DB. Statistical analysis with missing data. New York: John Wiley Press; 1987. [Google Scholar]
- Maddocks S, Hahn P, Moller F, Reid RL. A double-blind placebo-controlled trial of progesterone vaginal suppositories in the treatment of premenstrual syndrome. Am. J. Obstet. Gynecol. 1986;154:573–581. doi: 10.1016/0002-9378(86)90604-6. [DOI] [PubMed] [Google Scholar]
- Man MS, MacMillan I, Scott J, Young AH. Mood, neuropsychological function and cognitions in premenstrual dysphoric disorder. Psychol. Med. 1999;29:727–733. doi: 10.1017/s0033291798007715. [DOI] [PubMed] [Google Scholar]
- Martini MC, Lampe JW, Slavin JL, Kurzer MS. Effect of the menstrual cycle on energy and nutrient intake. Am. J. Clin. Nutr. 1994;60:895–899. doi: 10.1093/ajcn/60.6.895. [DOI] [PubMed] [Google Scholar]
- McLaren DS. Nutrition and its disorders. 2nd ed. New York: Churchill Livingstone; 1976. [Google Scholar]
- McLeod DR, Foster GV, Hoehn-Saric R, Svikis DS, Hipsley PA. Family history of alcoholism in women with generalized anxiety disorder who have premenstrual syndrome: patient reports of premenstrual alcohol consumption and symptoms of anxiety. Alcohol Clin. Exp. Res. 1994;18:664–670. doi: 10.1111/j.1530-0277.1994.tb00928.x. [DOI] [PubMed] [Google Scholar]
- McNair D, Lorr M, Droppleman LF. Profile of Mood States. San Diego: Educational and Industrial Testing Services; 1971. [Google Scholar]
- Mello NK, Mendelson JH, Lex BW. Alcohol use and premenstrual symptoms in social drinkers. Psychopharmacology. 1990;101:448–455. doi: 10.1007/BF02244221. [DOI] [PubMed] [Google Scholar]
- Miller TP, Taylor JL, Tinklenberg JR. A comparison of assessment techniques measuring the effects of methylphenidate, secobarbital, diazepam and diphenhydramine in abstinent alcoholics. Neuropsychobiology. 1988;19:90–96. doi: 10.1159/000118441. [DOI] [PubMed] [Google Scholar]
- Morgan M, Rapkin A. Cognitive flexibility, reaction time, and attention in women with premenstrual dysphoric disorder. J. Gend. Specif. Med. 2002;5:28–36. [PubMed] [Google Scholar]
- Morton JH, Additon H, Addison RG, Hunt L, Sullivan JJ. A clinical study of premenstrual tension. Am. J. Obstet. Gynecol. 1953;65:1182–1191. doi: 10.1016/0002-9378(53)90358-5. [DOI] [PubMed] [Google Scholar]
- Podolsky E. The woman alcoholic and premenstrual tension. J. Am. Med. Womens Assoc. 1963;18:816–818. [PubMed] [Google Scholar]
- Posthuma BW, Bass MH, Shelley BB, Nisker JA. Detecting changes in functional ability in women with premenstrual syndrome. Am. J. Obstet. Gynecol. 1987;156:275–278. doi: 10.1016/0002-9378(87)90267-5. [DOI] [PubMed] [Google Scholar]
- Price WA, DiMarzio LR, Eckert JL. Correlation between PMS and alcoholism among women. Ohio Med. 1987;83:201–202. [PubMed] [Google Scholar]
- Rapkin AJ. The role of serotonin in premenstrual syndrome. Clin. Obstet. Gynecol. 1992;35:629–636. doi: 10.1097/00003081-199209000-00022. [DOI] [PubMed] [Google Scholar]
- Rapkin AJ, Chang LC, Reading AE. Mood and cognitive style in premenstrual syndrome. Obstet. Gynecol. 1989;74:644–649. [PubMed] [Google Scholar]
- Rapkin A. A review of treatment of premenstrual syndrome & premenstrual dysphoric disorder. Psychoneuroendocrinology. 2003;28:39–53. doi: 10.1016/s0306-4530(03)00096-9. [DOI] [PubMed] [Google Scholar]
- Redei E, Freeman EW. Daily plasma estradiol and progesterone levels over the menstrual cycle and their relation to premenstrual symptoms. Psychoneuroendocrinology. 1995;20:259–267. doi: 10.1016/0306-4530(94)00057-h. [DOI] [PubMed] [Google Scholar]
- Resnick A, Perry W, Parry B, Mostofi N, Udell C. Neuropsychological performance across the menstrual cycle in women with and without premenstrual dysphoric disorder. Psychiatry Research. 1998;77:147–158. doi: 10.1016/s0165-1781(97)00142-x. [DOI] [PubMed] [Google Scholar]
- Rubin DB, Schenker N. Multiple imputation in health-care data bases: an overview and some applications. Stat. Med. 1991;10:585–598. doi: 10.1002/sim.4780100410. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Hoban MC, Grover GN, Galloway DS, Roy-Byrne P, Andersen R, Merriam GR. Changes in plasma hormones across the menstrual cycle in patients with menstrually related mood disorder and in control subjects. Am. J. Obstet. Gynecol. 1988;158:5–11. doi: 10.1016/0002-9378(88)90765-x. [DOI] [PubMed] [Google Scholar]
- Rupprecht R. Neuroactive steroids: mechanisms of action and neuropsychopharmacological properties. Psychoneuroendocrinology. 2003;28:139–168. doi: 10.1016/s0306-4530(02)00064-1. [DOI] [PubMed] [Google Scholar]
- Sampson GA. Premenstrual syndrome: a double-blind controlled trial of progesterone and placebo. Br. J. Psychiatry. 1979;135:209–215. doi: 10.1192/bjp.135.3.209. [DOI] [PubMed] [Google Scholar]
- Schmitt JA, Jorissen BL, Dye L, Markus CR, Deutz NE, Riedel WJ. Memory function in women with premenstrual complaints and the effect of serotonergic stimulation by acute administration of an alpha-lactalbumin protein. J. Psychopharmacol. 2005;19:375–384. doi: 10.1177/0269881105053288. [DOI] [PubMed] [Google Scholar]
- Seippel L, Bäckström T. Luteal-phase estradiol relates to symptom severity in patients with premenstrual syndrome. J. Clin. Endocrinol. Metab. 1998;83:1988–1992. doi: 10.1210/jcem.83.6.4899. [DOI] [PubMed] [Google Scholar]
- Smith SL, Sauder C. Food cravings, depression, and premenstrual problems. Psychosom. Med. 1969;31:281–287. doi: 10.1097/00006842-196907000-00001. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. STAI Manual for the State-Trait Anxiety Inventory (“Self-Evaluation Questionnaire”) Palo Alto, CA: Consulting Psychologists Press, Inc; 1970. [Google Scholar]
- Steiner M, Steinberg S, Stewart D, Carter D, Berger C, Reid R, Grover D, Streiner D Canadian Fluoxetine/Premenstrual Dysphoria Collaborative Study Group. Fluoxetine in the treatment of premenstrual dysphoria. N. Engl. J. Med. 1995;332:1529–1534. doi: 10.1056/NEJM199506083322301. [DOI] [PubMed] [Google Scholar]
- Su TP, Schmidt PJ, Danaceau MA, Tobin MB, Rosenstein DL, Murphy DL, Rubinow DR. Fluoxetine in the treatment of premenstrual dysphoria. Neuropsychopharmacology. 1997;16:346–356. doi: 10.1016/S0893-133X(96)00245-X. [DOI] [PubMed] [Google Scholar]
- Thorndike EL, Lorge I. The Teacher’s Word Book of 30,000 Words. Columbia University, NY, Teachers College; 1944. [Google Scholar]
- Tobin MB, Schmidt PJ, Rubinow DR. Reported alcohol use in women with premenstrual syndrome. Am. J. Psychiatry. 1994;151:1503–1504. doi: 10.1176/ajp.151.10.1503. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Becker E, Lieb R, Krause P. Prevalence, incidence and stability of premenstrual dysphoric disorder in the community. Psychol. Med. 2002;32:119–132. doi: 10.1017/s0033291701004925. [DOI] [PubMed] [Google Scholar]
- Wurtman JJ, Brzezinski A, Wurtman RJ, Laferrere B. Effect of nutrient intake on premenstrual depression. Am. J. Obstet. Gynecol. 1989;161:1228–1234. doi: 10.1016/0002-9378(89)90671-6. [DOI] [PubMed] [Google Scholar]
- Yonkers KA, Halbreich U, Freeman E, Brown C, Pearlstein T. Sertraline in the treatment of premenstrual dysphoric disorder. Psychopharmacol. Bull. 1996;32:41–46. [PubMed] [Google Scholar]


