1. Summary
The role of lipopolysaccharide (LPS) in the pathogenesis of Gram-negative septic shock is well established. The corresponding proinflammatory and immunostimulatory molecule(s) on the Gram-positive bacteria is less well understood, and their identification and characterization would be a key prerequisite in designing specific sequestrants of the Gram-positive endotoxin(s). We report in this paper the comparison of NF-κB-, cytokine- and chemokine-inducing activities of the TLR2 ligands, lipoteichoic acid (LTA), peptidoglycan (PGN), and lipopeptides, to LPS, a prototype TLR4 agonist, in murine macrophage cell-lines as well as in human blood. In murine cells, di- and triacyl liopopeptides are equipotent in their NF-κB inducing activity relative to LPS, but elicit much lower proinflammatory cytokines. However, both LPS and the lipopeptides potently induce the secretion of a pattern of chemokines that is suggestive of the engagement of a TLR4-independent TRIF pathway. In human blood, although the lipopeptides induce p38 MAP kinase phosphorylation and CD11b upregulation in granulocytes at ng/ml concentrations, they do not elicit proinflammatory cytokine production even at very high doses; LTA, however, activates neutrophils and induces cytokine secretion, although its potency is considerably less than that of LPS, presumably due to its binding to plasma proteins. We conclude that, in human blood, the pattern of immunostimulation and proinflammatory mediator production elicited by LTA parallels that of LPS.
Keywords: Sepsis, septic shock, lipoteichoic acid, peptidoglycan, lipopeptides, innate immunity
2. Introduction
Sepsis, or “blood poisoning” in lay terminology, and its sequel septic shock, a consequence of systemic inflammation leading to multiple organ failure [1], are common and serious clinical problems with no specific therapeutic options available as yet. While fewer than 100 cases were reported prior to 1920 [2], sepsis is now the thirteenth leading cause of overall mortality [3], and the number one cause of deaths in the non-cardiac intensive care unit [4] accounting for some 200,000 fatalities in the US annually [5]. While the incidence continues to rise in the US [6] and worldwide [7] due to increased invasive procedures, immunosuppression and cytotoxic chemotherapy, mortality has essentially remained unchanged at about 45% [8] despite tremendous strides in antimicrobial chemotherapy, due to the lack of specific therapy aimed at the pathophysiology of sepsis.
The primary trigger in the Gram-negative septic shock syndrome is endotoxin, a constituent of the outer membrane of enterobacterial Gram-negative bacteria. Endotoxins consist of a polysaccharide portion and a lipid called lipid A, and are therefore also called lipopolysaccharides (LPS). The polysaccharide portion consists of an O-antigen-specific polymer of repeating oligosaccharide units, the composition of which is highly varied among Gram-negative bacteria. A relatively well-conserved core hetero-oligosaccharide covalently bridges the O-antigen-specific chain with lipid A [9]. Total synthesis of the structurally highly conserved lipid A has been shown to be the active moiety of LPS [10, 11].
Septic shock is by no means an exclusive sequel of systemic Gram-negative infections [12, 13]. Owing to the increasing prevalence of nosocomial infections due to invasive procedures, immunosuppression and cancer chemotherapy, the incidence of septic shock due to Gram-positive organisms is on the rise [14–16], and is of particular concern in neutropenic individuals, a frequent attendant of ablative chemotherapy and radiotherapy [17, 18]. Because the shock state in systemic Gram-positive sepsis is clinically indistinguishable from that caused by Gram-negative bacteria [13, 19], it has been generally regarded that the initiation and progression of the systemic inflammatory response are pathophysiologically similar regardless of the causative organism. However, recent studies reporting differential gene expression and proinflammatory cytokine production [20–24] have cast doubt on the assumption that the patterns of innate immune activation by Gram-negative and -positive bacteria are equivalent. The prominent role of LPS in the pathogenesis of Gram-negative shock renders lipid A a logical therapeutic target in developing anti-endotoxin strategies, and we have made considerable progress in the structure-based design and development of LPS sequestrants [25–28]. The identification and characterization of proinflammatory and immunostimulatory molecule (s) in the Gram-positive bacterium would be a prerequisite if a similar strategy is to be employed successfully in designing specific sequestrants of the Gram-positive endotoxin(s).
The exoskeleton of the Gram-positive organism, similar to that of Gram-negative bacteria, is comprised of underlying peptidoglycan (PGN), a super-sized polymer of β-1→4-linked N-acetylglucosamine-N-acetylmuramic acid glycan strands that are cross-linked by short peptides [29–31]. Unlike Gram-negative bacteria which bear lipopolysaccharide on the outer leaflet of the outer membrane, the external surface of the peptidoglycan layer is decorated with lipoteichoic acids (LTA) [32, 33]. LTA are anchored in the peptidoglycan substratum via a diacylglycerol moiety and bear a surface-exposed, polyanionic, 1–3-linked polyglycerophosphate appendage [34, 35] which varies in its subunit composition in LTAs from various Gram-positive bacteria; in S. aureus, the repeating subunit contains D-alanine and α-D-N-acetylglucosamine [36]. Lipoproteins are found in the bacterial cytoplasmic membrane and are also common constituents of the cell wall of both Gram-negative and Gram-positive bacteria [37–39]. The free amine of the N-terminus of lipoproteins are acylated with a S- (2,3-diacyloxypropyl)cysteinyl residue which constitutes the immunostimulatory moiety [40, 41] as shown by studies on synthetic peptides containing the diacylthioglycerol unit [42–44]. In contradistinction to enterobacterial LPS which is recognized by Toll-like receptor-4 (TLR4), PGN [45, 46], LTA [47, 48], lipopeptides, [49, 50] as well as some non-enterobacterial LPS [51, 52] signal via TLR2.
The discovery of the role of Toll -like receptors in the specific molecular recognition of Gram-negative versus -positive cell-wall components [53–55] have contributed enormously to our understanding of the recognition of pathogen-associated molecular patterns (‘PAMPs’) by TLRs, and of the signaling pathways underlying the effector responses of the innate immune system. Inasmuch as the development of TLR knockout mouse strains [56, 57] has revolutionized the analyses of the fine-specificities of individual PAMPs and of their recognition by target TLRs, many questions remain unaddressed. The paradigm of exquisite specificity of ligand recognition by conventional receptors does not lend itself very well to these pattern-recognition receptors; the plasticity of TLRs with respect to their recognition of diverse ligands, coupled with different signaling mechanisms employed by individual TLRs, as well as significant inter-species differences in terms of cellular responses to a given ligand are potential confounding variables, and the observation of a specific response should be cross-validated, whenever possible, in an appropriate ex vivo human model.
We report in this paper the comparison of NF-κB-, cytokine- and chemokine-inducing activities of the TLR2 ligands, LTA, PGN, and lipopeptides, to LPS (TLR4 agonist), in murine macrophage cell-lines as well as in human blood. In murine cells, di- and triacyl liopopeptides are equipotent in their NF-κB inducing activity relative to LPS, but elicit much lower proinflammatory cytokines. However, both LPS and the lipopeptides potently induce the secretion of a pattern of chemokines that is suggestive of the engagement of the MyD88-independent TRIF-Mal-TIRAP pathway. In human blood, although the lipopeptides induce p38 MAP kinase phosphorylation and CD11b upregulation in granulocytes at ng/ml concentrations, they do not elicit proinflammatory cytokine production even at very high doses. LTA, on the other hand, activates neutrophils and induces cytokine secretion, although with considerably less potency than LPS, presumably due to its binding to plasma proteins. However, the pattern of immunostimulation and proinflammatory mediator production elicited by LTA in human blood is qualitatively similar that of LPS.
3. Materials and Methods
3.1. Reagents
LTA from S. aureus from Sigma (St. Louis, MO) was found to be significantly contaminated with LPS (see Results and Fig. 1). All subsequent experiments were performed with LTA from S. aureus, extracted by the n-butanol procedure [58] and purified by delipidation and enzymatic deproteination (procured from InvivoGen, San Diego, CA). PGN from S. aureus, the synthetic diacylated lipopeptides FSL-1 and PAM2CSK4, and the triacylated PAM3CSK4 were also from InvivoGen (San Diego, CA). Smooth LPS and diphosphoryl lipid A from E. coli 0111:B4 was from List Biologicals (Campbell, CA). Human inflammation and chemokine, and murine inflammation multiplexed CBA kits, anti-CD11b antibody conjugated to phycoerythrin (PE), and anti-phospho-(T180/Y182)-p38 MAPK:PE conjugate, and corresponding isotype control antibody:PE conjugates were from Becton-Dickinson (San Jose, CA).
Fig. 1.
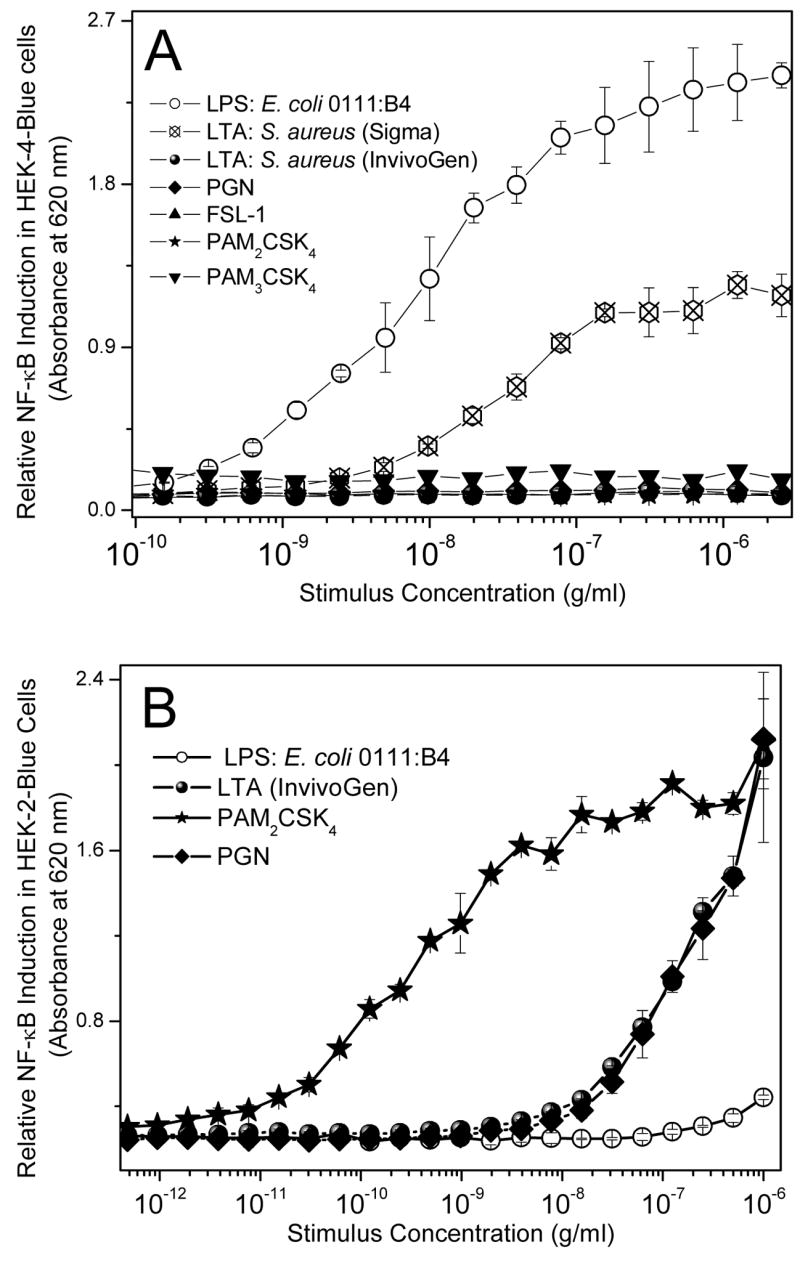
(A) NF-κB induction was measured in HEK-4-Blue™ cells treated overnight with 1:2 serially diluted doses of TLR ligands of interest. Secreted alkaline phosphatase was measured colorimetrically. (B) NF-κB induction in HEK-2-Blue™ cells treated overnight with 1:2 serially diluted doses of the various ligands was similarly ascertained via a colorimetric assay.
3.2. Cell lines
LPS-responsive murine J774.A1 and LPS-nonresponsive 23ScCR (C57BL/10ScNCr background) cell lines were from ATCC and were maintained in RPMI 1640 supplemented with 10% fetal bovine serum (FBS). HEK-Blue™-4 cells (HEK293 cells stably transfected with TLR4, MD2, CD14, as well as secreted alkaline phosphatase (sAP) under the control of a promoter inducible by NF-κB and AP-1) and HEK-Blue™-2 cells (HEK293 cells stably transfected with TLR2, MD2, CD14, and sAP) were from InvivoGen and were maintained in HEK-Blue™ Selection medium containing zeocin and normocin. 23ScCR cells stably transfected with pnifty2 (InvivoGen), an NF-κB reporter gene under the control of ELAM1 promoter, and driving the expression of sAP were generated as follows: 23 ScCR (107 cells) were nucleofected with 1 μg of purified pnifty2 plasmid using an Amaxa Nucleofector (Gaithersburg, MD). After a two-week long culture in medium containing 10 μg/ml zeocin, a single colony persisted and expanded, which was subsequently subcloned and maintained in HEK-Blue™ Selection medium. This stably transfected cell line (trans-ScCR) responded robustly and consistently over the course of several passages to murine TNF-α in producing sAP which was quantitated as described above.
3.3. NF-κB induction
The induction of NF-κB was quantified using HEK-Blue-2™ cells. In experiments designed to verify that the Gram-positive stimuli were not contaminated with trace amounts of LPS, HEK-Blue™-4 cells were used. Stable expression of secreted alkaline phosphatase (sAP) under control of NF-κB/AP-1 promoters is inducible by LPS, and extracellular sAP in the supernatant is proportional to NF-κB induction. HEK- Blue-2™ or -4™ cells were incubated at a density of ~105 cells/ml in a volume of 80 μl/well, in 384-well, flat-bottomed, cell culture-treated microtiter plates until confluency was achieved, and subsequently graded concentrations of stimuli. sAP was assayed spectrophotometrically using an alkaline phosphatase-specific chromogen (present in HEK-detection medium as supplied by the vendor) at 620 nm.
3.4. Measurement of nitric oxide release in murine macrophages
Nitric oxide (NO) was measured as total nitrite in murine macrophage J774.A1 cells using the Griess reagent system [59, 60] as described previously [26, 61]. J774.A1 cells were grown in RPMI-1640 cell-culture medium containing L-glutamine and sodium bicarbonate and supplemented with 10% fetal bovine serum, 1% L-glutamine-penicillin-streptomycin solution, and 200 μg/ml L-arginine at 37°C in a 5% CO2 atmosphere, and plated at ~105 cells/ml in a volume of 80 μl/well, in 384-well, flat-bottomed, cell culture-treated microtiter plates until confluency and subsequently exposed to serial dilutions of the Gram-positive stimuli. Concurrent to LPS stimulation, serially diluted concentrations of test compounds were added to the cell medium and left to incubate overnight for 16h. Positive- (LPS stimulation only) and negative-controls (J774.A1 medium only) were included in each experiment. Nitrite concentrations were measured adding 50 μl of supernatant to equal volumes of Griess reagents (50 μl/well; 0.1% NED solution in ddH2O and 1% sulfanilamide, 5% phosphoric acid solution in ddH2O) and incubating for 15 minutes at room temperature in the dark. Absorbance at 535 nm was measured using a Molecular Devices Spectramax M2 multifunction plate reader.
3.5. Multiplexed cytokine assays
100 μl aliquots of fresh whole blood, anticoagulated with heparin, obtained by venipuncture from healthy human volunteers with informed consent and as per guidelines approved by the Human Subjects Experimentation Committee were exposed to equal volumes of graded concentrations stimuli diluted in saline for 4h in a 96-well microtiter plate as described previously [26, 61]. The effect of the compounds on modulating cytokine production was examined using a FACSArray multiplexed flow-cytometric bead array (CBA) system (Becton-Dickinson-Pharmingen, San Jose, CA). The system uses a sandwich ELISA-on-a-bead principle [62, 63], and is comprised of 6 populations of microbeads that are spectrally unique in terms of their intrinsic fluorescence emission intensities (detected in the FL3 channel of a standard flow cytometer). Each bead population is coated with a distinct capture antibody to detect six different cytokines concurrently from biological samples. The human inflammation CBA kit includes the following analytes: TNF-α, IL-1β, IL-6, IL-8, IL-10, and IL-12p70; the human chemokine kit allows the quantitative measurement of Interleukin-8 (CXCL8/IL-8), Regulated upon Activation, Normal T-cell Expressed, and Secreted (CCL5/RANTES), Monokine-induced by Interferon-γ (CXCL9/MIG), Monocyte Chemoattractant Protein-1 (CCL2/MCP-1), and Interferon-γ-induced Protein-10 (CXCL10/IP-10). The beads were incubated with 30 μl of sample, and the cytokines of interest were first captured on the bead. After washing the beads, a mixture of optimally paired second antibodies conjugated to phycoerythrin was added which forms a fluorescent ternary complex with the immobilized cytokine, the intensity (measured in the FL2 channel) of which is proportional to the cytokine concentration on the bead. The assay was performed according to protocols provided by the vendor. Standard curves were generated using recombinant cytokines provided in the kit. The data were analyzed in the CBA software suite that is integral to the FACSArray system. The CBA multiplexed assay was also used to quantify cytokine production in J774.A1 cells using the mouse inflammation CBA kit which includes the following analytes: TNF-α, IL-6, IL-10, macrophage chemotactic protein-1 (MCP-1), IFN-γ, and IL-12p70. Chemokine (MIP-1α, MIG, RANTES) production in 23ScCR cells were examined using mouse Flex kits (BD Biosciences) and analyzed using FCAP software.
3.6. Phosflow™ flow cytometric assay for p38MAPK
1 ml aliquots of fresh whole blood, anticoagulated with heparin were incubated with 25 μl an equal volume of graded concentrations of stimuli diluted in saline for 15 minutes at 37°C. Erythrocytes were lysed and leukocytes were fixed in one step by mixing 200 μl of the samples in 4 ml pre-warmed Whole Blood Lyse/Fix Buffer (Becton-Dickinson Biosciences, San Jose, CA). After washing the cells at 500 g for 8 minutes in CBA buffer, the cells were permeabilized in ice-cold methanol for 30 min, washed twice in CBA buffer and transferred to a Millipore MultiScreen BV 1.2μ filter plate and stained with either phycoerythrin (PE)-conjugated mouse anti-p38MAPK (pT180/pY182; BD Biosciences) mAb, or a matched PE-labeled mouse IgG1 κ isotype control mAb for 60 minutes. The cells were washed twice in the plate by aspiration as per protocols supplied by the vendor. Cytometry was performed using a BD FACSArray instrument in the single-color mode for PE acquisition on 20,000 gated events. Post-acquisition analyses were performed using FlowJo v 7.0 software (Treestar, Ashland, OR).
3.7. CD11b flow cytometric assay
1 ml aliquots of fresh anticoagulated whole blood were incubated with 25 μL of graded dilutions of the various Gram-positive stimuli for 1 hour at 37°C. This resulted in final concentrations of 1 μg/mL of stimulus at the highest concentration. Negative (saline) controls were included in each experiment. Samples were placed on ice for 15 minutes before 20 μL of anti-CD11b/Mac-1 antibody (Becton-Dickinson) were added to each sample tube and allowed to incubate on ice for 30 minutes. This 0°C incubation step prevented internalization of antibody, and assured staining of only extracellularly expressed CD11b. Erythrocytes were lysed and leukocytes were fixed in one step by mixing 200 μL of the samples in 4 mL pre-warmed Whole Blood Lyse/Fix Buffer (Becton-Dickinson Biosciences, San Jose, CA). After washing the cells twice at 200 g for 5 minutes in CBA buffer, the cells were transferred to a 96-well plate. Flow cytometry was performed using a BD FACSArray instrument in the single-color mode for PE acquisition on 20,000 gated events. Post-acquisition analyses were performed using FlowJo v 7.0 software.
4. Results
4.1. Confirmation of specificity of TLR2 activity
Contamination with trace quantities of LPS in bacterial products is a common problem [64] and, in order to rule out false-positives and spurious results, we first verified that the Gram-positive stimuli were endotoxin-free by examining the NF-κB induction potency in the HEK-Blue-4 cell assay. As shown in Fig. 1A, LTA purchased from Sigma showed significant TLR4 agonistic activity, presumably due to LPS contamination, and was therefore excluded from all further experiments. Purified n-butanol isolated LTA (from InvivoGen), PGN, PAM2CSK4, FSL-1, and PAM3CSK4 were all devoid of detectable LPS at concentrations of up to 10 μg/ml (Fig. 1A). We further verified that the TLR2-agonistic activities of the Gram-positive stimuli were not inhibited in the HEK-Blue-2 assay by polymyxin B, a specific LPS sequestrant [65, 66], even at a concentration of 2.5 mM (data not shown). PAM2CSK4 and FSL-1 are synthetic diacylated lipopeptides which have been reported to be agonists of heterodimers of TLR2/TLR6 [67, 68] whereas PAM3CSK4, a synthetic triacylated lipopeptide, was thought to be a TLR2/TLR1 [69, 70] agonist, although structural studies show that recognition of both di- and tri-acyl species require TLR2 [71]. Consistent with the recent findings of Buwitt-Beckmann et al., [72] in virtually all of the experiments described below, all three lipopeptides behaved virtually indistinguishably and, for the sake of simplicity, only results obtained with PAM2CSK4 will be shown except in cases where significant differences between the diacyl and triacyl species were observed. In the NF-κB induction assay using TLR2-expressing cells (HEK-Blue-2 cell assay, Fig. 1B), PAM2CSK4 (as well as FSL-1 and PAM3CSK4, not shown) were very potent, exhibiting discernible stimulatory activities at 100 pg/ml, whereas LTA and PGN were significantly less active. As expected LPS, the prototype TLR4 agonist, did not show any NF-κB-inducing activity (Fig. 1B).
4.2. Comparison of TLR2-agonistic activity in murine macrophage cell-lines
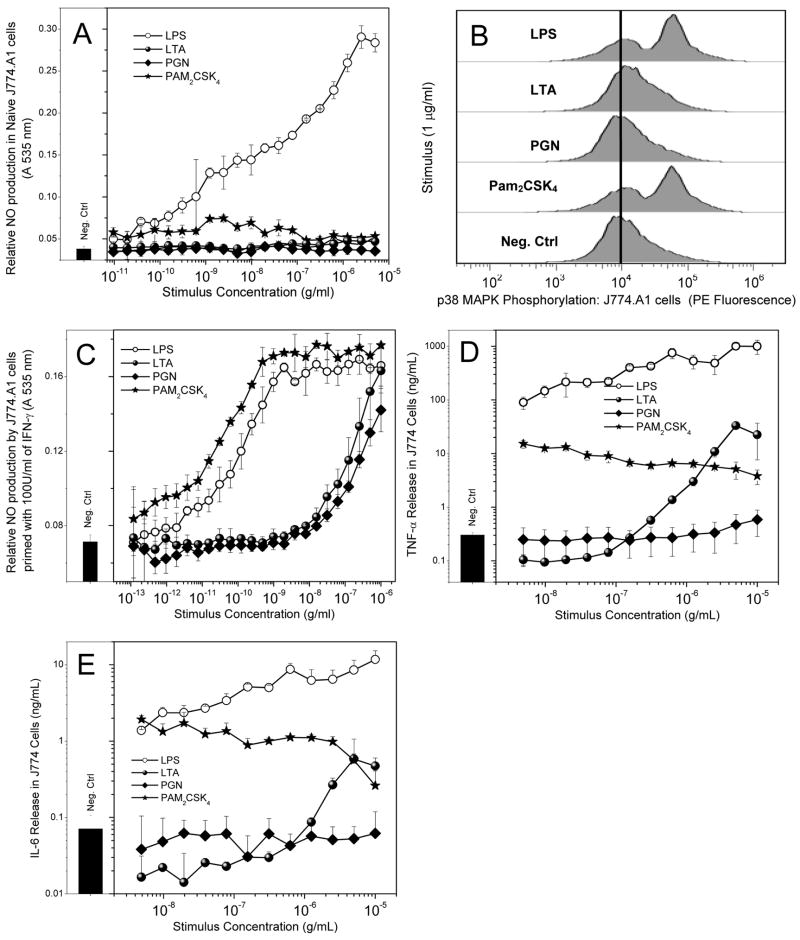
Next, we compared the activities of the Gram-positive stimuli in murine macrophage cell-lines. In LPS-responsive J774.A1 cells, only LPS evoked the production of significant levels of NO (measured as nitrite, Fig. 2A), although exposure to PAM2CSK4 (as well as FSL-1 and PAM3CSK4, not shown), appeared to slightly enhance NO production while PGN and LTA were devoid of stimulatory activity (Fig 2A). However, stimulation of J774.A1 cells at concentrations of 1 μg/ml with either LPS or PAM2CSK4, but not LTA nor PGN, resulted in phosphorylation of p38 MAPK) as analyzed by flow-cytometry using p38MAPK-specific (pT180/pY182) antibodies (Fig. 2B. Since IFN-γ is known to upregulate inducible nitric oxide synthase [73], we reexamined the NO-inducing properties in J774.A1 cells primed with 100 U/ml of IFN-γ for 8h prior to stimulation. IFN-γ priming resulted in robust responses to both LPS as well as PAM2CSK4, with similar IC50 values (concentrations inducing half-maximal responses) of 100 pg/ml and 212 pg/ml, respectively (Fig. 2C). LTA and PGN were much weaker, inducing comparable NO output at about 1 μg/ml. In initial proinflammatory cytokine release assays, only LPS appeared to induce TNF-α and IL-6 responses in standard log-linear plots. On closer examination in log-log plots, however, it became apparent that this was merely due to the rather large differences in the relative amounts of cytokines induced by LPS and the Gram-positive stimuli. Although the absolute levels of PAM2CSK4-induced TNF-α (Fig. 2D) and IL-6 (Fig. 2E) are much lower than that evoked by LPS, we found the lipopeptides to be very potent, causing significant cytokine induction at 5 ng/ml, the lowest concentration tested. Similar results were obtained with FSL-1 and PAM3CSK4 (data not shown). LTA, on the other hand, was found to be considerably less potent than either LPS or PAM2CSK4, showing significant activity only in the μg/ml concentration range (Figs. 2D and 2E).
Fig. 2.
(A) Nitric oxide production (measured as nitrite) in naïve murine macrophage J774A.1 cells treated overnight with various ligands of interest was measured using the Griess assay. The cells exhibit a robust dose-dependent response to LPS. No significant response is observed with the other ligands. (B) Phosphorylation of p38 MAPK in J774A.1 cells. Cells were treated with 1 μg/mL of various ligands and incubated for 15 minutes. Cells were then fixed, permeabilized, and stained with anti-phospho-p38 MAPK antibody-PE conjugate. Cells were washed and analyzed via flow cytometry. (C) NO production in IFN-γ-primed J774A.1 cells. Cells were primed with 1000 U/ml of IFN-γ on day 1, and subsequently treated with the various ligands overnight on Day 2. (D) TNF-α and (E) IL-6 cytokine release by J774A.1 cells upon 4-hour challenge by TLR ligands determined by subsequent cytometric bead array assay.
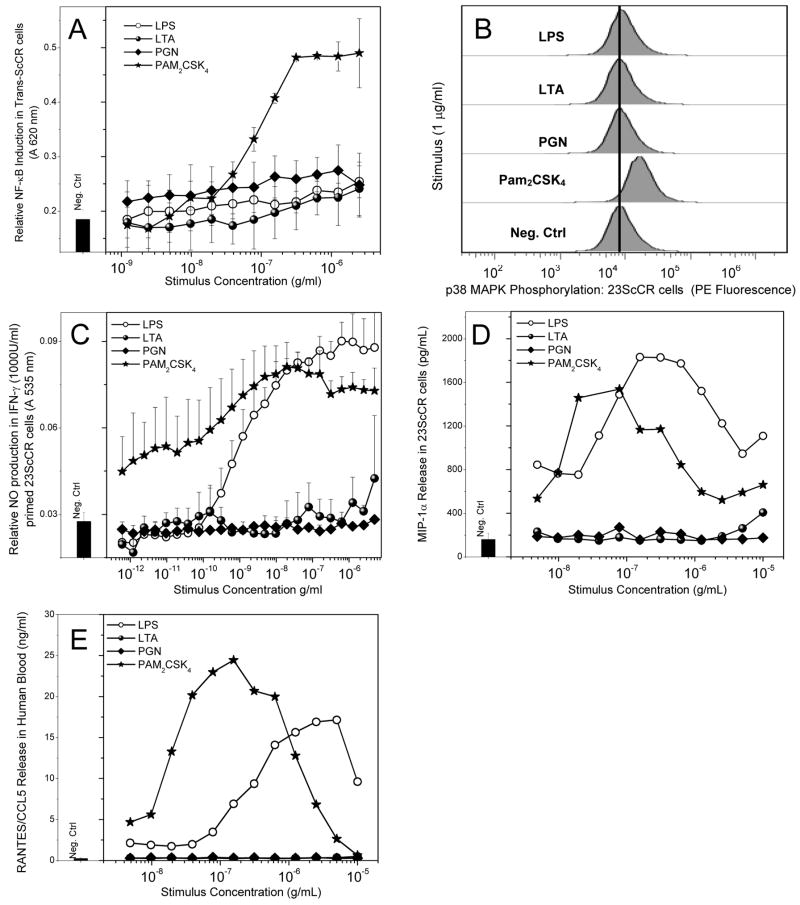
We wished to examine if similar patterns of responsiveness to the various Gram-positive stimuli would hold true in the LPS non-responsive 23ScCR cell-line [74], which is homozygous for a null-mutation of TLR4 [75]. In all of these experiments, LPS was used a control stimulus. In 23ScCR cells stably expressing an NF-κB-sAP reporter gene (Trans-ScCR cells), only the lipopeptides induced sAP expression (Fig. 3). Similarly, exposure to only lipopeptides, but not to any of the other TLR agonists resulted in elevated p38 MAPK phosphorylation (Fig. 3B). As expected, PAM2CSK4 (as well as FSL-1 and PAM3CSK4, not shown) induced substantial levels of NO in IFN-γ-primed ScCR cells; somewhat to our surprise, we found that LPS also behaved as an agonist in ScCR cells that are null for TLR4 (Fig. 3C). We verified that LPS was not contaminated with trace quantities of a TLR2 agonist by showing that LPS, but not the lipopeptides, could be inhibited by polymyxin B (data not shown). Neither LPS nor any of the Gram-positive stimuli induced any measurable proinflammatory cytokines such as TNF-α, IL-6, IL-8 or IL-12. However, both LPS and the lipopeptides induced the production of MIP-1α and RANTES/CCL5 in a bimodal manner characterized by a dose-dependent increase in the initial phase, followed by inhibition at higher stimulus concentrations (Figs. 3D and 3E). Neither PGN nor LTA were active in any of the ScCR assays.
Fig. 3.
(A) NF-κB induction in 23 ScCR cells stably transfected with the pNifty plasmid expressing the NF-κB-sAP reporter gene. (B) Phosphorylation of p38 MAPK in murine macrophage 23 ScCR cells, analyzed by flow cytometry. (C) NO production was tested in 23 ScCR cells primed with IFN-γ (1000 Units/ml). (D) MIP-1α release in 23 ScCR cells upon treatment with the ligands for 4 hours, measured by CBA. (E) RANTES/CCL5 chemokine release in human blood was measured following 8 hour challenge with the ligands of interest diluted serially 1:2. The serum was analyzed for amount of chemokine released via cytometric bead array. Robust, biphasic responses are observed in the LPS- and Pam2CSK4-treated samples. Representative results from three independent experiments performed on blood samples from different donors are shown in D and E.
4.3. Comparison of TLR2-agonistic activity in human blood
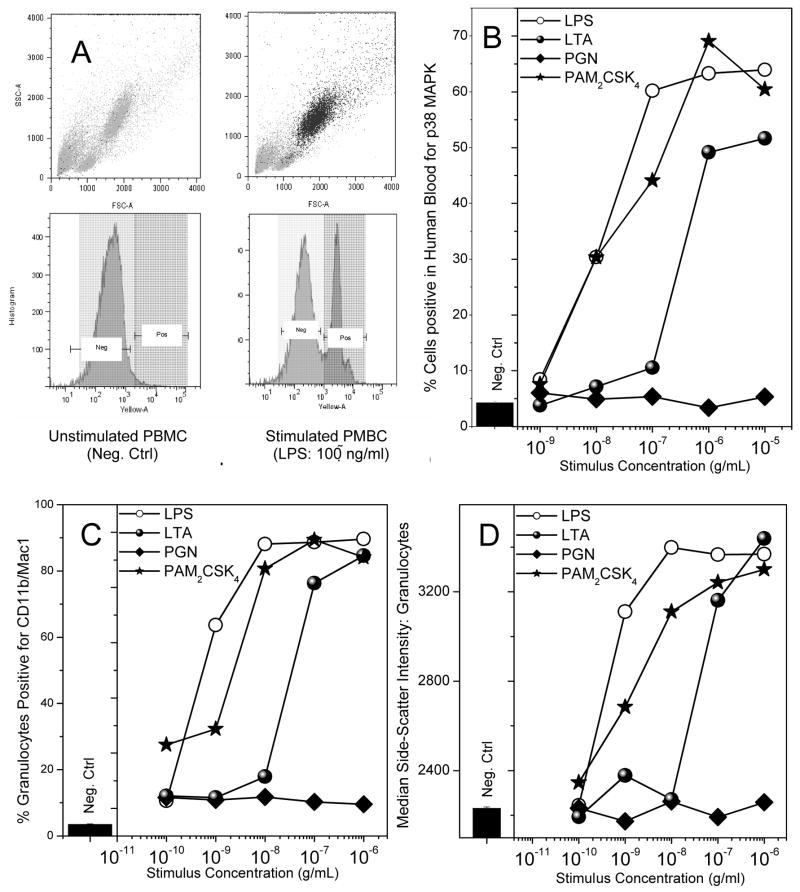
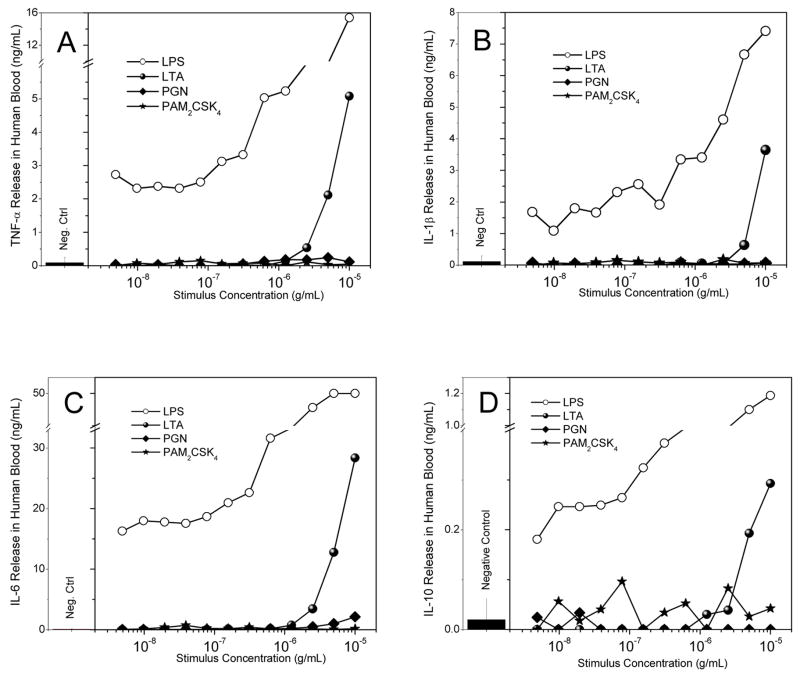
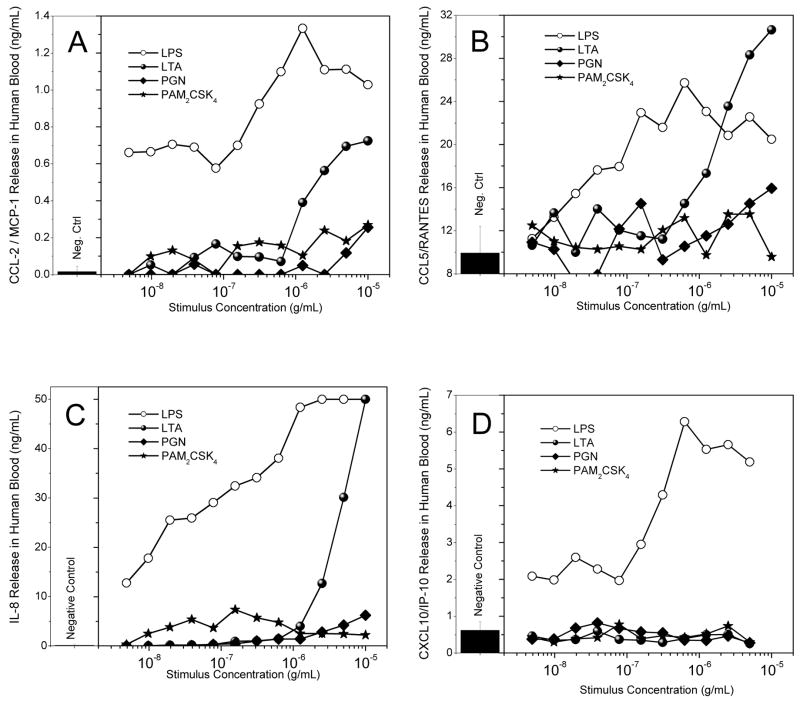
We elected to compare the activities of Gram-positive stimuli with those of LPS in whole human blood rather than isolated mononuclear cells or differentiated macrophages. We examined p38 MAPK phosphorylation, CD11b expression, and cytokine and chemokine release. LPS and the lipopeptides were roughly equipotent in inducing p38 MAPK phosphorylation with IC50 values of about 10 ng/ml (Fig. 4B). Most of the activated cell population mapped directly to the granulocyte fraction (Figs. 4A). The potency of LTA was about an order of magnitude lower, while PGN was inactive (Fig. 4B). A similar dose-response profile was observed for the upregulation of cell-surface expression of CD11b [76] on granulocytes. Interestingly, we found that changes in CD11b expression were exactly mirrored by increases in size of the granulocytes as evidenced by increased side-scatter intensities (Fig. 4D), analogous to previous reports of changes in cell shape of granulocytes [77]. While the lipopeptides as well as LTA were active in inducing an activated phenotype in neutrophils, only LTA, but not the lipopeptides, were effective in inducing the cytokines, TNF-α, IL-1β, IL-6, and IL-10 (Fig. 5A–D), as well as the chemokines MCP-1/CCL-2, CCL5/RANTES, IL-8 (Fig. 6A–C), although the relative levels of these mediators are considerably lower than that induced by LPS. None of the Gram-positive stimuli induced any detectable CXCL10/IP-10, however (Fig. 6D).
Fig. 4.
(A) Depiction of negative control (left; medium alone) and positive control (right; LPS-treated) flow cytometric forward/side scatter and p38 MAPK abundance histogram plots of human blood samples. A marked increase in p38 MAPK signal was observed in the granulocyte population. (B) p38 MAPK phosphorylation in whole human blood treated with 1:10 serial dilutions of the ligands. Blood samples were exposed to stimuli for 15 minutes, and cells were fixed, permeabilized, stained with anti-p38 MAPK antibody-PE conjugate, and analyzed by flow cytometry. (C) Up-regulation of the surface marker CD11b in granulocytes upon treatment with the various ligands of interest. Whole human blood was treated with the ligands under investigation, serially diluted 1:10, for 1 hour. Cells were then stained with anti-CD11b/Mac-1 antibody for 30 minutes on ice, fixed, washed, and analyzed via flow cytometry. (D) Dose-dependent increase in median side-scatter intensity of the granulocyte population in human blood treated with the ligands of interest was carried out on the samples from (C). A robust increase in the median-side scatter intensity, corresponding to increase in cellular granularity, in those samples which were stimulated with LPS and Pam2CSK4 was observed. Representative results from three independent experiments performed on blood samples from different donors are shown in B, C and D.
Fig. 5.
Cytokine release in human blood was verified and quantified via use of the cytometric bead array assay. Human blood was treated with the ligands of interest, serially diluted 1:2, for 8 hours. Serum was tested for released amounts of TNF-α (A), IL-1β (B), IL-6 (C), and IL-10 (D). Representative results from three independent experiments performed on blood samples from different donors are shown.
Fig. 6.
Chemokine release in human blood was verified and quantified via use of the cytometric bead array assay in blood treated with the ligands of interest, serially diluted 1:2, for 8 hours. Serum was tested for released amounts of CCL-2/MCP-1 (A), CCL5/RANTES (B), IL-8 (C), and CXCL10/IP-10 (D). Representative results from three independent experiments performed on blood samples from different donors are shown.
5. Discussion
Although more than twenty years have elapsed since formal proof was obtained showing that the structurally conserved lipid A moiety constitutes the major endotoxic principle of Gram-negative enterobacterial lipopolysaccharides [10, 11], aspects of structure-activity relationships underlying immunostimulatory versus proinflammatory activities in lipid A analogues are just beginning to be understood [78]. As with the availability of chemically defined lipid A analogues, the recent syntheses of LTA [79, 80] and lipopeptide part-structures [81, 82] have been pivotal in delineating the biological responses to these TLR2 ligands. Although the development of specific TLR knockout mouse strains [83, 84] has made an enormous impact on the field of innate immunity, significant differences between murine and non-rodent species exist not only in receptor specificity to TLR ligands, but also in the cellular responses to them. As has been observed with TLR4 ligands such as taxol [85, 86], leptospiral lipid A [87], lipid IVa, an enterobacterial lipid A part-structure [88], and E5531, a synthetic lipid A analogue [89], recent evidence suggests that significant inter-species differences exist for TLR2 also, as exemplified by variations in specificities for lipopeptide recognition in chimeric TLR constructs [90]. Furthermore, the coupling of these pattern recognition receptors to downstream adaptor molecules also appear to be distinct as shown by disparities in clinical outcomes in humans with IRAK-4 deficiency versus the susceptibility to pathogens in knockout mice [91].
Our objective was to identify the major immunostimulatory and proinflammatory component(s) of the Gram-positive bacterial envelope in the human system so that an attempt may be made to design and evaluate specific antagonists or sequestrants for the ‘Gram-positive endotoxin’ as we have with LPS [25–27, 92]. We were also interested in comparing human and murine responses to TLR2 agonists so that appropriate in vivo or ex vivo assays could be developed to aid in screening compounds. We therefore compared the NF-κB-, cytokine- and chemokine-inducing activities of the TLR2 ligands, LTA, PGN, and lipopeptides, in murine macrophage cell-lines as well as in human blood ex vivo.
In LPS-responsive murine J774.A1 macrophages, we observed that O, O′ di-acyl and O, O′,N-tri-acyl lipopeptides as well as LPS were agonistic as adjudged by activation of p38 MAPK, the dose-dependent induction of NO in IFN-γ-primed cells, and the production of TNF-α and IL-6 (Fig. 2). LTA, but not PGN induced cytokine production in J774 cells, but at much higher concentrations. We ruled out contamination of LTA with trace amounts of LPS by verifying that the agonistic activity of LTA could not be inhibited even using high concentrations of PMB, a specific LPS sequestrant [65, 66] (data not shown). In order to compare these stimuli in the context of an absent functional TLR4, we examined the responses in murine 23ScCR cells [93]. A clear parallel between LPS and the lipopeptides was observed with respect to NF-κB induction and p38 MAPK phosphorylation (Fig. 3A and B). Unexpectedly, IFN-γ-primed 23ScCR cells responded robustly to LPS and lipopeptides in producing NO (Fig. 3C), but did not produce any detectable TNF-α, IL-1β, or IL-6 (data not shown). We have earlier observed the secretion of MCP-1 in 23ScCR cells and C3H/HeJ-derived macrophages in response to LPS; furthermore, we have detected a well-defined surge of circulatory MCP-1 in the absence of any detectable TNF-α, IL-1β, or IL-6, in C3H/HeJ mice challenged with LPS (unpublished), which we had been unable to explain adequately. Surmising that a non-TLR4/MyD88-independent pathway may be operational, we examined the effect of the TLR2 ligands on MIP-1α and RANTES/CCL5, the production of both of which are thought to involve the TRIF/Mal/TIRAP pathway [94–98]. LPS as well as lipopeptides induced a bimodal response of the chemokines in 23ScCR cells, while PGN and LTA were found to be inactive (Fig. 3D and E). The hitherto prevalent view that the recognition of enterobacterial LPS is mediated solely via TLR4 has been challenged recently by experiments demonstrating that trimyristoylated lipopeptides as well as highly purified enterobacterial LPS are sensed by both murine TLR2 and TLR4 [99], highlighting the promiscuous specificity of these pattern-recognition receptors. Although our results do not formally implicate TLR2 as the receptor mediating the recognition of both LPS and lipopeptides in murine 23ScCR cells, a non-TLR4 apparatus signaling presumably via the TRIF pathway appears plausible. In support of this conjecture, we have observed that HEK-Blue-2™ cells expressing human TLR2, as well as Trans-ScCR cells respond vigorously to highly purified diphosphoryl lipid A from E. coli as well as Re chemotype LPS from S. minnesota (unpublished). In the case of HEK-Blue-2™ cells we observed not only trans-activation of NF-κB, but also the secretion of IL-8, but not of TNF-α, IL-1β, or IL-6; with Trans-ScCR cells, the activation of NF-κB is paralleled by a dose-dependent production of MIP-1α (Fig. 3D), MCP-1, and RANTES/CCL5 chemokines, but not of any of the above-mentioned proinflammatory cytokines (data not shown). It appears possible, therefore, that defined chemotypes of LPS and lipid A may indeed signal through TLR2, albeit via a non-MyD88-dependent pathway. This hypothesis could best be tested in TLR4/MyD88 double-knockout mice.
In human blood stimulated ex vivo with the various TLR agonists, LPS, PAM2CSK4, as well as LTA stimulate p38 MAPK phosphorylation (Fig. 4 A and B). The activity of the tri-acyl PAM3CSK4 lipopeptide was lower than the di-acyl lipopeptide by two orders of magnitude (data not shown) as has been reported in the literature [100]. The majority of stimulated cells map to the granulocyte population, consistent with earlier reports [101–104]. Concomitant with p38 MAPK phosphorylation, we also observed a dose-dependent upregulation of surface CD11b (Fig. 4C) and CD18 (data not shown), which was accompanied by altered neutrophil morphology, manifested as an increased side-scatter in flow-cytometry experiments (Fig. 4D). Altered neutrophil shape, assessed by light microscopy, upon stimulation with the lipopeptide MALP-2 has been reported [105, 106]; these morphological changes were also shown to be associated with augmented phagocytic activity as well as enhanced oxidative burst in response to fMLP using Rhodamine 123 [107, 108]. We have also obtained data confirming the priming effect to a subsequent stimulus (opsonized zymosan) using the Amplex Red [109] assay (data not shown).
Despite the similarity of the lipopeptides and LTA to LPS with respect to neutrophil activation, only LTA was found to stimulate the production of the proinflammatory cytokines, TNF-α, IL-1β, or IL-6, and the anti-inflammatory cytokine IL-10 in whole human blood (Fig. 5). The potency of LTA in cytokine induction was much lower than that of LPS, however. This was anticipated in light of the fact that LTA binds to, and is attenuated by serum components such as lipoproteins [110] and LPS-binding protein [111], resulting in much weaker activity in whole blood compared to isolated mononuclear cells [112]. Pretreatment of human blood with the lipopeptides followed by stimulation with LTA did not result in any amplification of cytokine responses, ruling out additive or synergistic effects of the two TLR2 ligands (data not shown). In contrast to LTA, neither PGN nor the lipopeptides showed appreciable cytokine-inducing activity (Fig. 5). Similarly, LTA, but not PGN or the lipopeptides induced the release of the chemokines MCP-1, CCL5/RANTES, IL-8, and CXCL10 (Fig. 6). These results are somewhat at variance to those obtained by Wilde et al., [113] who showed that the MALP-2 lipopeptide was equipotent on a gravimetric basis with LPS in inducing IL-8 secretion; however, those studies were performed with isolated neutrophils while we have used whole human blood. It is to be noted that, analogous to LTA, the cytokine-inducing activities of the lipopeptides PAM3CSK4 and FSL-1 are known to be significantly obtunded in the presence of serum [114]. Further experiments on the effect of serum and of GM-CSF, the latter being known to upregulate TLR2 [115], on isolated neutrophils are being planned.
Taken together, these data point to distinct differences in the responses of murine and human cells to known Gram-positive-derived TLR2 agonists. Of the TLR2 ligands examined, only PGN appears to be quiescent in both murine and human cells. While the role of the NOD proteins underscore the importance of intracellular recognition of PGN [116], the role of PGN as an inflammogen in the interstitial or extracellular compartments is yet to be resolved adequately [117, 118], but attention may be called to recent reports of the association of aseptic peritonitis with PGN contamination in peritoneal dialysis fluid [119].
Although LPS and the lipopeptides are highly comparable in their immunostimulatory and proinflammatory profiles in murine cells, the similarities become less clear in human blood. Both LTA and the lipopeptides stimulate human neutrophils, but only LTA appears to induce cytokine and chemokine release. The demonstration of LPS-like activities in synthetic LTA notwithstanding [120–122], there continues to be considerable disagreement as to the “Gram-positive endotoxin”. Trace contamination of LTA with lipopeptides has been thought to contribute to the observed ‘endotoxin-like’ activity even in highly purified LTA preparations [123–126]. However, the clear-cut dichotomy between the murine and human responses that we have observed, and the complete absence of response to LTA in the TLR4-deficient ScCR cells would be inconsistent with this view. Our data lend support to the somewhat contentious [127–130] notion that LTA, but not the lipopeptides, is the major proinflammatory cytokine-inducing TLR2 agonist in human blood ex vivo. However, this is still an incomplete view given that endothelial responses to PAMPs tend to be distinctive [131], and the signaling pathways downstream of TLRs diverge significantly between myeloid and non-myeloid cells [132]. A comparison of responses in endothelial, mesothelial, and epithelial-derived cells may shed light on some of these issues.
Acknowledgments
Grant Support: This work was supported from NIH grant 1R03AI055725.
Abbreviations
- CBA
cytometric bead array
- FSL-1
S-[2,3-bis (palmitoyloxy)- (2RS)propyl]-Cys-Gly-Asp-Pro-Lys-His-Pro-Lys-Ser-Phe
- IC50
concentration inducing half-maximal response
- IFN-γ
interferon-γ
- IL-1β
interlekin-1β
- IL-6
interleukin-6
- IL-8
interleukin-8
- LPS
lipopolysaccharide
- LTA
lipoteichoic acid
- MAPK
mitogen-activated protein kinase
- NO
nitric oxide
- PAM2CSK4
S-[2,3-bis (palmitoyloxy)- (2RS)-propyl]-R-cysteinyl-S-seryl-S-lysyl-S-lysyl-S-lysyl-S-lysine
- PAM3CSK4
N-palmitoyl-S-[2,3-bis (palmitoyloxy)- (2RS)-propyl]-R-cysteinyl-S-seryl-S-lysyl-S-lysyl-S-lysyl-S-lysine
- PAMP
pathogen-associated molecular pattern
- PE
phycoerythrin
- PGN
peptidoglycan
- TLR
Toll-like receptor
- TNF-α
tumor necrosis factor-α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29:530–8. doi: 10.1007/s00134-003-1662-x. [DOI] [PubMed] [Google Scholar]
- 2.Felty AR, Keefer CS. Bacillus coli sepsis: A clinical study of twenty-eight cases of bloodstream infection by the colon bacillus. JAMA. 1924;82:1430–3. [Google Scholar]
- 3.Gelfand JA, Shapiro L. Cytokines and sepsis: pathophysiology and therapy. New Horizons. 1993;1:13–22. [PubMed] [Google Scholar]
- 4.Gasche Y, Pittet D, Sutter P. Outcome and prognostic factors in bacteremic sepsis. In: Sibbald WJ, Vincent JL, editors. Clinical trials for treatment of sepsis. Springer-Verlag; Berlin: 1995. pp. 35–51. [Google Scholar]
- 5.Centers for Diseases Control. Increases in national hospital discharge survey rates for septicemia- United States, 1979–1987. MMWR. 1990;39:31–4. [PubMed] [Google Scholar]
- 6.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 7.Moss M, Martin GS. A global perspective on the epidemiology of sepsis. Intensive Care Med. 2004;30:527–9. doi: 10.1007/s00134-004-2182-z. [DOI] [PubMed] [Google Scholar]
- 8.Cross A, Opal SM. Therapeutic intervention in sepsis with antibody to endotoxin: is there a future? J Endotoxin Res. 1994;1:57–9. [Google Scholar]
- 9.Rietschel ET, Kirikae T, Schade FU, Mamat U, Schmidt G, Loppnow H, Ulmer AJ, Zähringer U, Seydel U, Di Padova F, et al. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 1994;8:217–25. doi: 10.1096/fasebj.8.2.8119492. [DOI] [PubMed] [Google Scholar]
- 10.Galanos C, Lüderitz O, Rietschel ET, Westphal O, Brade H, Brade L, Freudenberg MA, Schade UF, Imoto M, Yoshimura S, Kusumoto S, Shiba T. Synthetic and natural Escherichia coli free lipid A express identical endotoxic activities. Eur J Biochem. 1985;148:1–5. doi: 10.1111/j.1432-1033.1985.tb08798.x. [DOI] [PubMed] [Google Scholar]
- 11.Imoto M, Yoshimura H, Kusumoto S, Shiba T. Total synthesis of lipid A, active principle of bacterial endotoxin. Proc Japan Acad Sci. 1984;60:285–8. [Google Scholar]
- 12.Bone RC. Gram-positive organisms and sepsis. Arch Intern Med. 1994;154:26–34. [PubMed] [Google Scholar]
- 13.Bone RC. How gram-positive organisms cause sepsis. J Crit Care. 1993;8:51–9. doi: 10.1016/0883-9441(93)90033-h. [DOI] [PubMed] [Google Scholar]
- 14.Thornsberry C. Epidemiology of staphylococcal infections--a USA perspective. J Chemother. 1994;6(Suppl 261–5):5. [PubMed] [Google Scholar]
- 15.Cockerill FR, III, Hughes JG, Vetter EA, Mueller RA, Weaver AL, Ilstrup DM, Rosenblatt JE, Wilson WR. Analysis of 281,797 consecutive blood cultures performed over an eight-year period: trends in microorganisms isolated and the value of anaerobic culture of blood. Clin Infect Dis. 1997;24:403–18. doi: 10.1093/clinids/24.3.403. [DOI] [PubMed] [Google Scholar]
- 16.Valles J, Leon C, Alvarez-Lerma F. Nosocomial bacteremia in critically ill patients: a multicenter study evaluating epidemiology and prognosis. Spanish Collaborative Group for Infections in Intensive Care Units of Sociedad Espanola de Medicina Intensiva y Unidades Coronarias (SEMIUC) Clin Infect Dis. 1997;24:387–95. doi: 10.1093/clinids/24.3.387. [DOI] [PubMed] [Google Scholar]
- 17.Elting LS, Rubenstein EB, Rolston KVI, Bodey GP. Outcomes of bacteremia in patients with cancer and neutropenia: observations from two decades of epidemiological and clinical trials. Clin Infect Dis. 1997;25:247–59. doi: 10.1086/514550. [DOI] [PubMed] [Google Scholar]
- 18.Safdar A, Rodriguez GH, Balakrishnan M, Tarrand JJ, Rolston KV. Changing trends in etiology of bacteremia in patients with cancer. Eur J Clin Microbiol Infect Dis. 2006;25:522–6. doi: 10.1007/s10096-006-0173-4. [DOI] [PubMed] [Google Scholar]
- 19.Kragsbjerg P, Holmberg H, Vikerfors T. Dynamics of blood cytokine concentrations in patients with bacteremic infections. Scand J Infect Dis. 1996;28:391–8. doi: 10.3109/00365549609037926. [DOI] [PubMed] [Google Scholar]
- 20.Feezor RJ, Oberholzer C, Baker HV, Novick D, Rubinstein M, Moldawer LL, Pribble J, Souza S, Dinarello CA, Ertel W, Oberholzer A. Molecular characterization of the acute inflammatory response to infections with gram-negative versus gram-positive bacteria. Infect Immun. 2003;71:5803–13. doi: 10.1128/IAI.71.10.5803-5813.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu SL, Chen HW, Yang PC, Peck K, Tsai MH, Chen JJ, Lin FY. Differential gene expression in gram-negative and gram-positive sepsis. Am J Respir Crit Care Med. 2004;169:1135–43. doi: 10.1164/rccm.200211-1278OC. [DOI] [PubMed] [Google Scholar]
- 22.Kragsbjerg P, Soderquist B, Holmberg H, Vikerfors T, Danielsson D. Production of tumor necrosis factor-alpha and interleukin-6 in whole blood stimulated by live Gram-negative and Gram-positive bacteria. Clin Microbiol Infect. 1998;4:129–34. doi: 10.1111/j.1469-0691.1998.tb00375.x. [DOI] [PubMed] [Google Scholar]
- 23.Tietze K, Dalpke A, Morath S, Mutters R, Heeg K, Nonnenmacher C. Differences in innate immune responses upon stimulation with gram-positive and gram-negative bacteria. J Periodontal Res. 2006;41:447–54. doi: 10.1111/j.1600-0765.2006.00890.x. [DOI] [PubMed] [Google Scholar]
- 24.Elson G, Dunn-Siegrist I, Daubeuf B, Pugin J. Contribution of Toll-like receptors to the innate immune response to Gram-negative and Gram-positive bacteria. Blood. 2007;109:1574–83. doi: 10.1182/blood-2006-06-032961. [DOI] [PubMed] [Google Scholar]
- 25.David SA. Towards a rational development of anti-endotoxin agents: novel approaches to sequestration of bacterial endotoxins with small molecules. J Molec Recognition. 2001;14:370–87. doi: 10.1002/jmr.549. [DOI] [PubMed] [Google Scholar]
- 26.Miller KA, Suresh Kumar EVK, Wood SJ, Cromer JR, Datta A, David SA. Lipopolysaccharide Sequestrants: Structural Correlates of Activity and Toxicity in Novel Acylhomospermines. J Med Chem. 2005;48:2589–99. doi: 10.1021/jm049449j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen TB, Adisechan A, Suresh Kumar EVK, Balakrishna R, Kimbrell MR, Miller KA, Datta A, David SA. Protection from Endotoxic Shock by EVK-203, a Novel Alkylpolyamine Sequestrant of Lipopolysaccharide. Bioorg Med Chem. 2007;15:5694–709. doi: 10.1016/j.bmc.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sil D, Shrestha A, Kimbrell MR, Nguyen TB, Adisechan AK, Balakrishna R, Abbo BG, Malladi S, Miller KA, Short S, Cromer JR, Arora S, Datta A, David SA. Bound to Shock: Protection from Lethal Endotoxemic Shock by a Novel, Nontoxic, Alkylpolyamine Lipopolysaccharide Sequestrant. Antimicrob Agents Chemother. 2007 doi: 10.1128/AAC.00200-07. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vollmer W, Holtje JV. The architecture of the murein (peptidoglycan) in gram-negative bacteria: vertical scaffold or horizontal layer(s)? J Bacteriol. 2004;186:5978–87. doi: 10.1128/JB.186.18.5978-5987.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dmitriev BA, Toukach FV, Holst O, Rietschel ET, Ehlers S. Tertiary structure of Staphylococcus aureus cell wall murein. J Bacteriol. 2004;186:7141–8. doi: 10.1128/JB.186.21.7141-7148.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burge RE, Fowler AG, Reaveley DA. Structure of the peptidogylcan of bacterial cell walls. I J Mol Biol. 1977;117:927–53. doi: 10.1016/s0022-2836(77)80006-5. [DOI] [PubMed] [Google Scholar]
- 32.Baddiley J. Bacterial cell walls and membranes. Discovery of the teichoic acids. Bioessays. 1989;10:207–10. doi: 10.1002/bies.950100607. [DOI] [PubMed] [Google Scholar]
- 33.Coley J, Duckworth M, Baddiley J. The occurrence of lipoteichoic acids in the membranes of gram-positive bacteria. J Gen Microbiol. 1972;73:587–91. doi: 10.1099/00221287-73-3-587. [DOI] [PubMed] [Google Scholar]
- 34.Stadelmaier A, Morath S, Hartung T, Schmidt RR. Synthesis of the first fully active lipoteichoic acid. Angew Chem Int Ed Engl. 2003;42:916–20. doi: 10.1002/anie.200390243. [DOI] [PubMed] [Google Scholar]
- 35.Deininger S, Stadelmaier A, von Aulock S, Morath S, Schmidt RR, Hartung T. Definition of structural prerequisites for lipoteichoic acid-inducible cytokine induction by synthetic derivatives. J Immunol. 2003;170:4134–8. doi: 10.4049/jimmunol.170.8.4134. [DOI] [PubMed] [Google Scholar]
- 36.Fischer W. Lipoteichoic acid and lipids in the membrane of Staphylococcus aureus. Med Microbiol Immunol. 1994;183:61–76. doi: 10.1007/BF00277157. [DOI] [PubMed] [Google Scholar]
- 37.Fischer W. Lipoteichoic acid and lipids in the membrane of Staphylococcus aureus. Med Microbiol Immunol. 1994;183:61–76. doi: 10.1007/BF00277157. [DOI] [PubMed] [Google Scholar]
- 38.Kostyal DA, Butler GH, Beezhold DH. A 48-kilodalton Mycoplasma fermentans membrane protein induces cytokine secretion by human monocytes. Infect Immun. 1994;62:3793–800. doi: 10.1128/iai.62.9.3793-3800.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henderson B, Poole S, Wilson M. Bacterial modulins: a novel class of virulence factors which cause host tissue pathology by inducing cytokine synthesis. Microbiol Rev. 1996;60:316–41. doi: 10.1128/mr.60.2.316-341.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muhlradt PF, Kiess M, Meyer H, Sussmuth R, Jung G. Isolation, structure elucidation, and synthesis of a macrophage stimulatory lipopeptide from Mycoplasma fermentans acting at picomolar concentration. J Exp Med. 1997;185:1951–8. doi: 10.1084/jem.185.11.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muhlradt PF, Meyer H, Jansen R. Identification of S-(2,3-dihydroxypropyl)cystein in a macrophage-activating lipopeptide from Mycoplasma fermentans. Biochemistry. 1996;35:7781–6. doi: 10.1021/bi9602831. [DOI] [PubMed] [Google Scholar]
- 42.Fischer W. Lipoteichoic acid and lipids in the membrane of Staphylococcus aureus. Med Microbiol Immunol. 1994;183:61–76. doi: 10.1007/BF00277157. [DOI] [PubMed] [Google Scholar]
- 43.Metzger J, Jung G, Bessler WG, Hoffmann P, Strecker M, Lieberknecht A, Schmidt U. Lipopeptides containing 2-(palmitoylamino)-6,7-bis(palmitoyloxy) heptanoic acid: synthesis, stereospecific stimulation of B-lymphocytes and macrophages, and adjuvanticity in vivo and in vitro. J Med Chem. 1991;34:1969–74. doi: 10.1021/jm00111a008. [DOI] [PubMed] [Google Scholar]
- 44.Lex A, Wiesmuller KH, Jung G, Bessler WG. A synthetic analogue of Escherichia coli lipoprotein, tripalmitoyl pentapeptide, constitutes a potent immune adjuvant. J Immunol. 1986;137:2676–81. [PubMed] [Google Scholar]
- 45.Dziarski R, Gupta D. The peptidoglycan recognition proteins (PGRPs) Genome Biol. 2006;7:232. doi: 10.1186/gb-2006-7-8-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dziarski R, Gupta D. Staphylococcus aureus peptidoglycan is a toll-like receptor 2 activator: a reevaluation. Infect Immun. 2005;73:5212–6. doi: 10.1128/IAI.73.8.5212-5216.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schroder NW, Morath S, Alexander C, Hamann L, Hartung T, Zahringer U, Gobel UB, Weber JR, Schumann RR. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J Biol Chem. 2003;278:15587–94. doi: 10.1074/jbc.M212829200. [DOI] [PubMed] [Google Scholar]
- 48.Opitz B, Schroder NW, Spreitzer I, Michelsen KS, Kirschning CJ, Hallatschek W, Zahringer U, Hartung T, Gobel UB, Schumann RR. Toll-like receptor-2 mediates Treponema glycolipid and lipoteichoic acid-induced NF-kappaB translocation. J Biol Chem. 2001;276:22041–7. doi: 10.1074/jbc.M010481200. [DOI] [PubMed] [Google Scholar]
- 49.Buwitt-Beckmann U, Heine H, Wiesmuller KH, Jung G, Brock R, Ulmer AJ. Lipopeptide structure determines TLR2 dependent cell activation level. FEBS J. 2005;272:6354–64. doi: 10.1111/j.1742-4658.2005.05029.x. [DOI] [PubMed] [Google Scholar]
- 50.Spohn R, Buwitt-Beckmann U, Brock R, Jung G, Ulmer AJ, Wiesmuller KH. Synthetic lipopeptide adjuvants and Toll-like receptor 2--structure-activity relationships. Vaccine. 2004;22:2494–9. doi: 10.1016/j.vaccine.2003.11.074. [DOI] [PubMed] [Google Scholar]
- 51.Nahori MA, Fournie-Amazouz E, Que-Gewirth NS, Balloy V, Chignard M, Raetz CR, Saint GI, Werts C. Differential TLR recognition of leptospiral lipid A and lipopolysaccharide in murine and human cells. J Immunol. 2005;175:6022–31. doi: 10.4049/jimmunol.175.9.6022. [DOI] [PubMed] [Google Scholar]
- 52.Erridge C, Pridmore A, Eley A, Stewart J, Poxton IR. Lipopolysaccharides of Bacteroides fragilis, Chlamydia trachomatis and Pseudomonas aeruginosa signal via toll-like receptor 2. J Med Microbiol. 2004;53:735–40. doi: 10.1099/jmm.0.45598-0. [DOI] [PubMed] [Google Scholar]
- 53.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 54.Takeuchi O, Akira S. Signaling pathways activated by microorganisms. Curr Opin Cell Biol. 2007;19:185–91. doi: 10.1016/j.ceb.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 55.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–51. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 56.Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 57.Akira S, Takeda K. Functions of toll-like receptors: lessons from KO mice. C R Biol. 2004;327:581–9. doi: 10.1016/j.crvi.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 58.Morath S, Geyer A, Hartung T. Structure-function relationship of cytokine induction by lipoteichoic acid from Staphylococcus aureus. J Exp Med. 2001;193:393–7. doi: 10.1084/jem.193.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite and [15-N] nitrate in biological fluids. Anal Biochem. 1982;126:131. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 60.Greiss P. Bemerkungen zu der abhandlung der H.H. Weselsky und Benedikt “Ueber einige azoverbindungen”. Chem Ber. 1879;12:426–7. [Google Scholar]
- 61.Burns MR, Wood SJ, Miller KA, Nguyen T, Cromer JR, David SA. Lysine-spermine conjugates: hydrophobic polyamine amides as potent lipopolysaccharide sequestrants. Bioorg Med Chem. 2005;13:2523–36. doi: 10.1016/j.bmc.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 62.Cook EB, Stahl JL, Lowe L, Chen R, Morgan E, Wilson J, Varro R, Chan A, Graziano FM, Barney NP. Simultaneous measurement of six cytokines in a single sample of human tears using microparticle-based flow cytometry: allergics vs. non-allergics. J Immunol Methods. 2001;254:109–16. doi: 10.1016/s0022-1759(01)00407-0. [DOI] [PubMed] [Google Scholar]
- 63.Funato Y, Baumhover H, Grantham-Wright D, Wilson J, Ernst D, Sepulveda H. Simultaneous measurement of six human cytokines using the Cytometric Bead Array System, a multiparameter immunoassay system for flow cytometry. Cytometry Res. 2002;12:93–103. [Google Scholar]
- 64.Gao JJ, Xue Q, Zuvanich EG, Haghi KR, Morrison DC. Commercial preparations of lipoteichoic acid contain endotoxin that contributes to activation of mouse macrophages in vitro. Infect Immun. 2001;69:751–7. doi: 10.1128/IAI.69.2.751-757.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bhattacharjya S, David SA, Mathan VI, Balaram P. Polymyxin B nonapeptide: Conformations in water and in the lipopolysaccharide-bound state determined by two-dimensional NMR and molecular dynamics. Biopolymers. 1997;41:251–65. [Google Scholar]
- 66.David SA, Balasubramanian KA, Mathan VI, Balaram P. Analysis of the binding of polymyxin B to endotoxic lipid A and core glycolipid using a fluorescent displacement probe. Biochim Biophys Acta. 1992;1165:147–52. doi: 10.1016/0005-2760(92)90180-4. [DOI] [PubMed] [Google Scholar]
- 67.Morr M, Takeuchi O, Akira S, Simon MM, Muhlradt PF. Differential recognition of structural details of bacterial lipopeptides by toll-like receptors. Eur J Immunol. 2002;32:3337–47. doi: 10.1002/1521-4141(200212)32:12<3337::AID-IMMU3337>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 68.Takeuchi O, Sato S, Horiuchi T, Hoshino K, Takeda K, Dong Z, Modlin RL, Akira S. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J Immunol. 2002;169:10–4. doi: 10.4049/jimmunol.169.1.10. [DOI] [PubMed] [Google Scholar]
- 69.Okusawa T, Fujita M, Nakamura J, Into T, Yasuda M, Yoshimura A, Hara Y, Hasebe A, Golenbock DT, Morita M, Kuroki Y, Ogawa T, Shibata K. Relationship between structures and biological activities of mycoplasmal diacylated lipopeptides and their recognition by toll-like receptors 2 and 6. Infect Immun. 2004;72:1657–65. doi: 10.1128/IAI.72.3.1657-1665.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Spohn R, Buwitt-Beckmann U, Brock R, Jung G, Ulmer AJ, Wiesmuller KH. Synthetic lipopeptide adjuvants and Toll-like receptor 2--structure-activity relationships. Vaccine. 2004;22:2494–9. doi: 10.1016/j.vaccine.2003.11.074. [DOI] [PubMed] [Google Scholar]
- 71.Jin MS, Kim SE, Heo JY, Lee ME, Kim HM, Paik SG, Lee H, Lee JO. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell. 2007;130:1071–82. doi: 10.1016/j.cell.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 72.Buwitt-Beckmann U, Heine H, Wiesmuller KH, Jung G, Brock R, Akira S, Ulmer AJ. TLR1- and TLR6-independent recognition of bacterial lipopeptides. J Biol Chem. 2006;281:9049–57. doi: 10.1074/jbc.M512525200. [DOI] [PubMed] [Google Scholar]
- 73.Weisz A, Cicatiello L, Esumi H. Regulation of the mouse inducible-type nitric oxide synthase gene promoter by interferon-gamma, bacterial lipopolysaccharide and NG-monomethyl-L-arginine. Biochem J. 1996;316(Pt 1):209–15. doi: 10.1042/bj3160209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lorenz E, Patel DD, Hartung T, Schwartz DA. Toll-like receptor 4 (TLR4)-deficient murine macrophage cell line as an in vitro assay system to show TLR4-independent signaling of Bacteroides fragilis lipopolysaccharide. Infect Immun. 2002;70:4892–6. doi: 10.1128/IAI.70.9.4892-4896.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Poltorak A, He X, Smirnova I, Liu MY, Huffel CV, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi CP, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–8. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 76.Wilde I, Lotz S, Engelmann D, Starke A, van Zandbergen G, Solbach W, Laskay T. Direct stimulatory effects of the TLR2/6 ligand bacterial lipopeptide MALP-2 on neutrophil granulocytes. Med Microbiol Immunol. 2007;196:61–71. doi: 10.1007/s00430-006-0027-9. [DOI] [PubMed] [Google Scholar]
- 77.Wilde I, Lotz S, Engelmann D, Starke A, van Zandbergen G, Solbach W, Laskay T. Direct stimulatory effects of the TLR2/6 ligand bacterial lipopeptide MALP-2 on neutrophil granulocytes. Med Microbiol Immunol. 2007;196:61–71. doi: 10.1007/s00430-006-0027-9. [DOI] [PubMed] [Google Scholar]
- 78.Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;316:1628–32. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- 79.Deininger S, Stadelmaier A, von Aulock S, Morath S, Schmidt RR, Hartung T. Definition of structural prerequisites for lipoteichoic acid-inducible cytokine induction by synthetic derivatives. J Immunol. 2003;170:4134–8. doi: 10.4049/jimmunol.170.8.4134. [DOI] [PubMed] [Google Scholar]
- 80.Stadelmaier A, Morath S, Hartung T, Schmidt RR. Synthesis of the first fully active lipoteichoic acid. Angew Chem Int Ed Engl. 2003;42:916–20. doi: 10.1002/anie.200390243. [DOI] [PubMed] [Google Scholar]
- 81.Reutter F, Jung G, Baier W, Treyer B, Bessler WG, Wiesmuller KH. Immunostimulants and Toll-like receptor ligands obtained by screening combinatorial lipopeptide collections. J Pept Res. 2005;65:375–83. doi: 10.1111/j.1399-3011.2005.00242.x. [DOI] [PubMed] [Google Scholar]
- 82.Muhlradt PF, Kiess M, Meyer H, Sussmuth R, Jung G. Isolation, structure elucidation, and synthesis of a macrophage stimulatory lipopeptide from Mycoplasma fermentans acting at picomolar concentration. J Exp Med. 1997;185:1951–8. doi: 10.1084/jem.185.11.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 84.Akira S, Takeda K. Functions of toll-like receptors: lessons from KO mice. C R Biol. 2004;327:581–9. doi: 10.1016/j.crvi.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 85.Kawasaki K, Akashi S, Shimazu R, Yoshida T, Miyake K, Nishijima M. Involvement of TLR4/MD-2 complex in species-specific lipopolysaccharide-mimetic signal transduction by Taxol. J Endotoxin Res. 2001;7:232–6. [PubMed] [Google Scholar]
- 86.Byrd-Leifer CA, Block EF, Takeda K, Akira S, Ding A. The role of MyD88 and TLR4 in the LPS-mimetic activity of Taxol. Eur J Immunol. 2001;31:2448–57. doi: 10.1002/1521-4141(200108)31:8<2448::aid-immu2448>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 87.Nahori MA, Fournie-Amazouz E, Que-Gewirth NS, Balloy V, Chignard M, Raetz CR, Saint GI, Werts C. Differential TLR recognition of leptospiral lipid A and lipopolysaccharide in murine and human cells. J Immunol. 2005;175:6022–31. doi: 10.4049/jimmunol.175.9.6022. [DOI] [PubMed] [Google Scholar]
- 88.Lien E, Means TK, Heine H, Yoshimura A, Kusumoto S, Fukase K, Fenton MJ, Oikawa M, Qureshi N, Monks B, Finberg RW, Ingalls RR, Golenbock DT. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J Clin Invest. 2000;105:497–504. doi: 10.1172/JCI8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bryant CE, Ouellette A, Lohmann K, Vandenplas M, Moore JN, Maskell DJ, Farnfield BA. The cellular Toll-like receptor 4 antagonist E5531 can act as an agonist in horse whole blood. Vet Immunol Immunopathol. 2007;116:182–9. doi: 10.1016/j.vetimm.2007.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Omueti KO, Beyer JM, Johnson CM, Lyle EA, Tapping RI. Domain exchange between human toll-like receptors 1 and 6 reveals a region required for lipopeptide discrimination. J Biol Chem. 2005;280:36616–25. doi: 10.1074/jbc.M504320200. [DOI] [PubMed] [Google Scholar]
- 91.Picard C, Puel A, Bonnet M, Ku CL, Bustamante J, Yang K, Soudais C, Dupuis S, Feinberg J, Fieschi C, Elbim C, Hitchcock R, Lammas D, Davies G, Al Ghonaium A, Al Rayes H, Al Jumaah S, Al Hajjar S, Al Mohsen IZ, Frayha HH, Rucker R, Hawn TR, Aderem A, Tufenkeji H, Haraguchi S, Day NK, Good RA, Gougerot-Pocidalo MA, Ozinsky A, Casanova JL. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science. 2003;299:2076–9. doi: 10.1126/science.1081902. [DOI] [PubMed] [Google Scholar]
- 92.Sil D, Shrestha A, Kimbrell MR, Nguyen TB, Adisechan AK, Balakrishna R, Abbo BG, Malladi S, Miller KA, Short S, Cromer JR, Arora S, Datta A, David SA. Bound to Shock: Protection from Lethal Endotoxemic Shock by a Novel, Nontoxic, Alkylpolyamine Lipopolysaccharide Sequestrant. Antimicrob Agents Chemother. 2007 doi: 10.1128/AAC.00200-07. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lorenz E, Patel DD, Hartung T, Schwartz DA. Toll-like receptor 4 (TLR4)-deficient murine macrophage cell line as an in vitro assay system to show TLR4-independent signaling of Bacteroides fragilis lipopolysaccharide. Infect Immun. 2002;70:4892–6. doi: 10.1128/IAI.70.9.4892-4896.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hirotani T, Yamamoto M, Kumagai Y, Uematsu S, Kawase I, Takeuchi O, Akira S. Regulation of lipopolysaccharide-inducible genes by MyD88 and Toll/IL-1 domain containing adaptor inducing IFN-β. Biochem Biophys Res Commun. 2005;328:383–92. doi: 10.1016/j.bbrc.2004.12.184. [DOI] [PubMed] [Google Scholar]
- 95.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–3. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 96.Bowie A, O’Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: signal generators for pro-inflammatory interleukins and microbial products. J Leukoc Biol. 2000;67:508–14. doi: 10.1002/jlb.67.4.508. [DOI] [PubMed] [Google Scholar]
- 97.Miggin SM, O’Neill LA. New insights into the regulation of TLR signaling. J Leukoc Biol. 2006;80:220–6. doi: 10.1189/jlb.1105672. [DOI] [PubMed] [Google Scholar]
- 98.O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–64. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 99.Spiller S, Dreher S, Meng G, Grabiec A, Thomas W, Hartung T, Pfeffer K, Hochrein H, Brade H, Bessler W, Wagner H, Kirschning CJ. Cellular recognition of trimyristoylated peptide or enterobacterial lipopolysaccharide via both TLR2 and TLR4. J Biol Chem. 2007;282:13190–8. doi: 10.1074/jbc.M610340200. [DOI] [PubMed] [Google Scholar]
- 100.Wilde I, Lotz S, Engelmann D, Starke A, van Zandbergen G, Solbach W, Laskay T. Direct stimulatory effects of the TLR2/6 ligand bacterial lipopeptide MALP-2 on neutrophil granulocytes. Med Microbiol Immunol. 2007;196:61–71. doi: 10.1007/s00430-006-0027-9. [DOI] [PubMed] [Google Scholar]
- 101.Lotz S, Starke A, Ziemann C, Morath S, Hartung T, Solbach W, Laskay T. Beta-lactam antibiotic-induced release of lipoteichoic acid from Staphylococcus aureus leads to activation of neutrophil granulocytes. Ann Clin Microbiol Antimicrob. 2006;5:15. doi: 10.1186/1476-0711-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lotz S, Aga E, Wilde I, van Zandbergen G, Hartung T, Solbach W, Laskay T. Highly purified lipoteichoic acid activates neutrophil granulocytes and delays their spontaneous apoptosis via CD14 and TLR2. J Leukoc Biol. 2004;75:467–77. doi: 10.1189/jlb.0803360. [DOI] [PubMed] [Google Scholar]
- 103.Wilde I, Lotz S, Engelmann D, Starke A, van Zandbergen G, Solbach W, Laskay T. Direct stimulatory effects of the TLR2/6 ligand bacterial lipopeptide MALP-2 on neutrophil granulocytes. Med Microbiol Immunol. 2007;196:61–71. doi: 10.1007/s00430-006-0027-9. [DOI] [PubMed] [Google Scholar]
- 104.Hayashi F, Means TK, Luster AD. Toll-like receptors stimulate human neutrophil function. Blood. 2003;102:2660–9. doi: 10.1182/blood-2003-04-1078. [DOI] [PubMed] [Google Scholar]
- 105.Wilde I, Lotz S, Engelmann D, Starke A, van Zandbergen G, Solbach W, Laskay T. Direct stimulatory effects of the TLR2/6 ligand bacterial lipopeptide MALP-2 on neutrophil granulocytes. Med Microbiol Immunol. 2007;196:61–71. doi: 10.1007/s00430-006-0027-9. [DOI] [PubMed] [Google Scholar]
- 106.Hayashi F, Means TK, Luster AD. Toll-like receptors stimulate human neutrophil function. Blood. 2003;102:2660–9. doi: 10.1182/blood-2003-04-1078. [DOI] [PubMed] [Google Scholar]
- 107.Wilde I, Lotz S, Engelmann D, Starke A, van Zandbergen G, Solbach W, Laskay T. Direct stimulatory effects of the TLR2/6 ligand bacterial lipopeptide MALP-2 on neutrophil granulocytes. Med Microbiol Immunol. 2007;196:61–71. doi: 10.1007/s00430-006-0027-9. [DOI] [PubMed] [Google Scholar]
- 108.Hayashi F, Means TK, Luster AD. Toll-like receptors stimulate human neutrophil function. Blood. 2003;102:2660–9. doi: 10.1182/blood-2003-04-1078. [DOI] [PubMed] [Google Scholar]
- 109.Zhou M, Diwu Z, Panchuk-Voloshina N, Haugland RP. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal Biochem. 1997;253:162–8. doi: 10.1006/abio.1997.2391. [DOI] [PubMed] [Google Scholar]
- 110.Levels JH, Abraham PR, van Barreveld EP, Meijers JC, van Deventer SJ. Distribution and kinetics of lipoprotein-bound lipoteichoic acid. Infect Immun. 2003;71:3280–4. doi: 10.1128/IAI.71.6.3280-3284.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mueller M, Stamme C, Draing C, Hartung T, Seydel U, Schromm AB. Cell activation of human macrophages by lipoteichoic acid is strongly attenuated by lipopolysaccharide-binding protein. J Biol Chem. 2006;281:31448–56. doi: 10.1074/jbc.M605966200. [DOI] [PubMed] [Google Scholar]
- 112.Mattsson E, Hartung T, Morath S, Egesten A. Highly purified lipoteichoic acid from Staphylococcus aureus induces procoagulant activity and tissue factor expression in human monocytes, but is a weak inducer in whole blood: Comparison with peptidoglycan. Infect Immun. 2004;72:4322–6. doi: 10.1128/IAI.72.7.4322-4326.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wilde I, Lotz S, Engelmann D, Starke A, van Zandbergen G, Solbach W, Laskay T. Direct stimulatory effects of the TLR2/6 ligand bacterial lipopeptide MALP-2 on neutrophil granulocytes. Med Microbiol Immunol. 2007;196:61–71. doi: 10.1007/s00430-006-0027-9. [DOI] [PubMed] [Google Scholar]
- 114.Hashimoto M, Furuyashiki M, Kaseya R, Fukada Y, Akimaru M, Aoyama K, Okuno T, Tamura T, Kirikae T, Kirikae F, Eiraku N, Morioka H, Fujimoto Y, Fukase K, Takashige K, Moriya Y, Kusumoto S, Suda Y. Evidence of immunostimulating lipoprotein existing in the natural lipoteichoic acid fraction. Infect Immun. 2007;75:1926–32. doi: 10.1128/IAI.02083-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hayashi F, Means TK, Luster AD. Toll-like receptors stimulate human neutrophil function. Blood. 2003;102:2660–9. doi: 10.1182/blood-2003-04-1078. [DOI] [PubMed] [Google Scholar]
- 116.Tattoli I, Travassos LH, Carneiro LA, Magalhaes JG, Girardin SE. The Nodosome: Nod1 and Nod2 control bacterial infections and inflammation. Semin Immunopathol. 2007 doi: 10.1007/s00281-007-0083-2. [DOI] [PubMed] [Google Scholar]
- 117.Dziarski R, Gupta D. Staphylococcus aureus peptidoglycan is a toll-like receptor 2 activator: a reevaluation. Infect Immun. 2005;73:5212–6. doi: 10.1128/IAI.73.8.5212-5216.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem. 1999;274:17406–9. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 119.Martis L, Patel M, Giertych J, Mongoven J, Taminne M, Perrier MA, Mendoza O, Goud N, Costigan A, Denjoy N, Verger C, Owen WF., Jr Aseptic peritonitis due to peptidoglycan contamination of pharmacopoeia standard dialysis solution. Lancet. 2005;365:588–94. doi: 10.1016/S0140-6736(05)17908-2. [DOI] [PubMed] [Google Scholar]
- 120.Deininger S, Stadelmaier A, von Aulock S, Morath S, Schmidt RR, Hartung T. Definition of structural prerequisites for lipoteichoic acid-inducible cytokine induction by synthetic derivatives. J Immunol. 2003;170:4134–8. doi: 10.4049/jimmunol.170.8.4134. [DOI] [PubMed] [Google Scholar]
- 121.Morath S, Geyer A, Hartung T. Structure-function relationship of cytokine induction by lipoteichoic acid from Staphylococcus aureus. J Exp Med. 2001;193:393–7. doi: 10.1084/jem.193.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Stadelmaier A, Morath S, Hartung T, Schmidt RR. Synthesis of the first fully active lipoteichoic acid. Angew Chem Int Ed Engl. 2003;42:916–20. doi: 10.1002/anie.200390243. [DOI] [PubMed] [Google Scholar]
- 123.Hashimoto M, Furuyashiki M, Kaseya R, Fukada Y, Akimaru M, Aoyama K, Okuno T, Tamura T, Kirikae T, Kirikae F, Eiraku N, Morioka H, Fujimoto Y, Fukase K, Takashige K, Moriya Y, Kusumoto S, Suda Y. Evidence of immunostimulating lipoprotein existing in the natural lipoteichoic acid fraction. Infect Immun. 2007;75:1926–32. doi: 10.1128/IAI.02083-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hashimoto M, Tawaratsumida K, Kariya H, Kiyohara A, Suda Y, Krikae F, Kirikae T, Gotz F. Not lipoteichoic acid but lipoproteins appear to be the dominant immunobiologically active compounds in Staphylococcus aureus. J Immunol. 2006;177:3162–9. doi: 10.4049/jimmunol.177.5.3162. [DOI] [PubMed] [Google Scholar]
- 125.Hashimoto M, Tawaratsumida K, Kariya H, Aoyama K, Tamura T, Suda Y. Lipoprotein is a predominant Toll-like receptor 2 ligand in Staphylococcus aureus cell wall components. Int Immunol. 2006;18:355–62. doi: 10.1093/intimm/dxh374. [DOI] [PubMed] [Google Scholar]
- 126.Hashimoto M, Yasuoka J, Suda Y, Takada H, Yoshida T, Kotani S, Kusumoto S. Structural feature of the major but not cytokine-inducing molecular species of lipoteichoic acid. J Biochem (Tokyo) 1997;121:779–86. doi: 10.1093/oxfordjournals.jbchem.a021653. [DOI] [PubMed] [Google Scholar]
- 127.Hashimoto M, Furuyashiki M, Kaseya R, Fukada Y, Akimaru M, Aoyama K, Okuno T, Tamura T, Kirikae T, Kirikae F, Eiraku N, Morioka H, Fujimoto Y, Fukase K, Takashige K, Moriya Y, Kusumoto S, Suda Y. Evidence of immunostimulating lipoprotein existing in the natural lipoteichoic acid fraction. Infect Immun. 2007;75:1926–32. doi: 10.1128/IAI.02083-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hashimoto M, Tawaratsumida K, Kariya H, Kiyohara A, Suda Y, Krikae F, Kirikae T, Gotz F. Not lipoteichoic acid but lipoproteins appear to be the dominant immunobiologically active compounds in Staphylococcus aureus. J Immunol. 2006;177:3162–9. doi: 10.4049/jimmunol.177.5.3162. [DOI] [PubMed] [Google Scholar]
- 129.Hashimoto M, Yasuoka JI, Suda Y, Takada H, Yoshida T, Kotani S, Kusumoto S. Structural feature of the major but not cytokine-inducing molecular species of lipoteichoic acid. J Biochem Tokyo. 1997;121:779–86. doi: 10.1093/oxfordjournals.jbchem.a021653. [DOI] [PubMed] [Google Scholar]
- 130.von Aulock S, Hartung T, Hermann C. Comment on “Not lipoteichoic acid but lipoproteins appear to be the dominant immunobiologically active compounds in Staphylococcus aureus”. J Immunol. 2007;178:2610–1. doi: 10.4049/jimmunol.178.5.2610. [DOI] [PubMed] [Google Scholar]
- 131.Into T, Kanno Y, Dohkan J, Nakashima M, Inomata M, Shibata K, Lowenstein CJ, Matsushita K. Pathogen recognition by Toll-like receptor 2 activates Weibel-Palade body exocytosis in human aortic endothelial cells. J Biol Chem. 2007;282:8134–41. doi: 10.1074/jbc.M609962200. [DOI] [PubMed] [Google Scholar]
- 132.Andreakos E, Sacre SM, Smith C, Lundberg A, Kiriakidis S, Stonehouse T, Monaco C, Feldmann M, Foxwell BM. Distinct pathways of LPS-induced NF-kappa B activation and cytokine production in human myeloid and nonmyeloid cells defined by selective utilization of MyD88 and Mal/TIRAP. Blood. 2004;103:2229–37. doi: 10.1182/blood-2003-04-1356. [DOI] [PubMed] [Google Scholar]